Abstract
CONTEXT
Cardiac glycosides of plant origin are implicated in toxic ingestions that may result in hospitalization and are potentially lethal. The utility of commonly available digoxin serum assays for detecting foxglove and oleander ingestion has been demonstrated, but no studies have evaluated the structurally similar convallatoxin found in Convallaria majalis (lily of the valley) for rapid laboratory screening, nor has digoxin immune Fab been tested as an antidote for this ingestion.
OBJECTIVE
We aimed to (1) evaluate multiple digoxin assays for cross-reactivity to convallatoxin, (2) identify whether convallatoxin could be detected in vivo at clinically significant doses and (3) determine whether digoxin immune Fab could be an effective antidote to convallatoxin.
MATERIALS AND METHODS
Cross-reactivities of purified convallatoxin and oleandrin with five common digoxin immunoassays were determined. Serum from mice challenged with convallatoxin was tested for apparent digoxin levels. Binding of convallatoxin to digoxin immune Fab was determined in vitro.
RESULTS
Both convallatoxin and oleandrin were detectable by a panel of commonly used digoxin immunoassays, but cross-reactivity was variable between individual assays. We observed measurable apparent digoxin levels in serum of convallatoxin intoxicated mice at sublethal doses. Convallatoxin demonstrated no binding by digoxin immune Fab.
CONCLUSION
Multiple digoxin immunoassays detect botanical cardiac glycosides including convallatoxin and thus may be useful for rapid determination of severe exposures, but neutralization of convallatoxin by digoxin immune Fab is unlikely to provide therapeutic benefit.
Keywords: botanical cardiac glycosides, convallotoxin, lily of the valley, oleandrin, digoxin, digoxin immunoassays
Introduction
Lily of the valley (Convallaria majalis) is a common low-growing perennial garden and woodland plant native to the temperate climates of Eurasia which has naturalized to North America. It has been considered one of the most potent cardiotoxic plants because it produces at least 38 cardiac glycosides, most notably convallatoxin1-3. These cardiac glycosides inhibit the sodium-potassium ATPase and cause digitalis-like effects. Clinical manifestations of Convallaria exposure vary from mild gastrointestinal symptoms including vomiting and diarrhea to terminal cardiac dysfunction with bradycardia and arrhythmias1, 2. In the United States, more than 250 exposures to lily of the valley are reported to poison control centers each year and as many as 15% of these patients present for medical care5. Lethal exposures in animals1, 5, 6 and symptomatic exposures in humans7, 8 have been well described.
Given the structural similarity of convallatoxin with digoxin (Fig. 1), we hypothesized that commonly available digoxin immunoassays would cross-react with convallatoxin. Previous studies have demonstrated cross-reactivity of older digoxin immunoassays for botanical digitoxin9 and oleandrin, the major cardiac glycoside in Nerium oleander10-12. In this study, we tested a panel of five digoxin immunoassays in current clinical use for cross-reactivity to convallatoxin and oleandrin. We chose assays that represent a combination of frequently utilized suppliers, instruments and methodologies based on the College of American Pathologists (CAP) proficiency testing survey.
Figure 1. Structural similarity of botanic cardiac glycosides with digoxin.
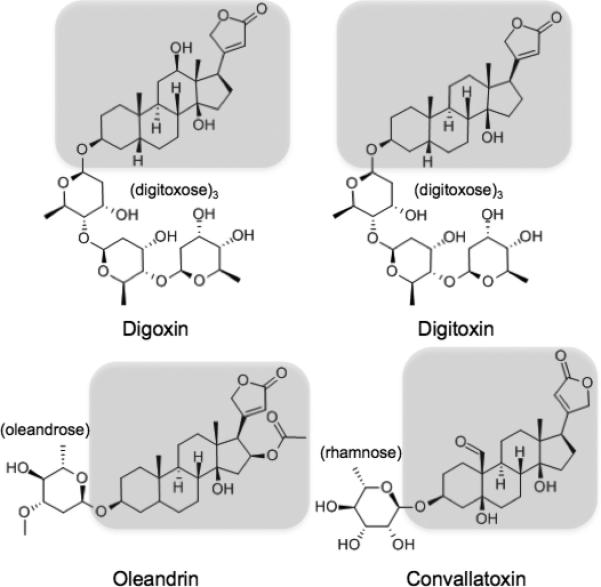
Common backbone highlighted with variable glycosides digitoxose (digoxin and digitoxin), oleandrose (oleandrin) and rhamnose (convallatoxin).
Although the serum concentration of convallatoxin required to produce symptoms in humans is unknown, in animal models toxic convallatoxin levels are in the range of 10-100 μg/mL based on dose and volume of distribution13. In our study, we utilized a mouse model that had been previously demonstrated to show physiologic similarity to humans13 to assess the potential clinical viability of the digoxin assay for samples taken from living organisms.
Oleandrin14, 15 and plant-sourced digitoxin16 have been shown to be neutralized by digoxin immune Fab, suggesting that the structurally similar convallatoxin may respond to this antidote. In this study, we assessed the ability of digoxin immune Fab to bind convallatoxin in vitro. Together, the objectives of our study were to evaluate multiple digoxin assays for cross-reactivity to convallatoxin, identify whether convallatoxin could be detected in vivo at clinically significant doses and determine whether digoxin immune Fab could be an effective antidote to convallatoxin.
Materials and Methods
Reagents
Convallatoxin (≥65% purity), oleandrin (≥95% purity), and digoxin (≥95% purity, analytical standard grade) were purchased from Sigma Chemicals (St. Louis, MO) and standard solutions were prepared in ethanol. DigiFab (40 mg vial also containing sodium acetate and mannitol) was obtained from BTG Pharmaceuticals (BTG Pharmaceuticals, West Conshohocken, PA) and reconstituted at 10mg/mL in sterile water. Pooled human serum was prepared from discarded clinical specimens and determined to be free from digoxin and digoxin-like immunoreactive substances before use in experiments.
Digoxin Immunoassays
We tested the chemiluminescent immunoassay (CIA) on the Siemens Immulite 2000 analyzer, chemiluminescent microparticle immunoassays (CMIA) on the ci8200 Abbott Architect analyzer, the Elecsys electrochemiluminescence immunoassay (ECLIA) on the Roche Cobas e601 analyzer, the latex agglutination assay on the Roche Cobas c501 analyzer, and the microparticle enzyme immunoassay (MEIA) on the Abbott Axsym analyzer. Serum pools were supplemented with convallatoxin (0.05, 0.1, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50, 100 and 500 μg/mL) or oleandrin (1, 10, 50, 100 μg/mL) and apparent digoxin concentration was determined according to the manufacturers’ specifications for each assay. Values were expressed as the mean of duplicates.
In Vivo Experiments in Mice
Ten week old female outbred Swiss Webster mice (National Cancer Institute, Frederick, MD) received a single intraperitoneal injection of convallatoxin in phosphate buffered saline. One mouse received a sham saline injection, 5 mice received 1 mg/kg (10% of the LD50) and 3 mice received 10 mg/kg (LD50)13. Mice were euthanized after ten minutes and serum was separated from clotted whole blood obtained by cardiac puncture. Specimens were diluted in normal mouse serum (Milipore) to obtain a volume sufficient for testing and measurement within the analytical range of the assay. Apparent digoxin was measured by chemiluminescent microparticle immunoassays (CMIA) on the Abbott Architect analyzer, the most sensitive assay for convallatoxin among our panel, as described below. A standard curve was constructed by supplementing normal mouse serum with convallatoxin (1, 0.33, and 0.11 μg/mL) and determining apparent digoxin concentration, which was then used to calculate apparent convallatoxin concentration in the mouse serum samples. All procedures used in this study complied with federal guidelines and were approved by the Yale Animal Care and Use Committee.
DigiFab Binding Experiments
Concentrations of digoxin (10, 40 and 160 ng/mL) and convallatoxin (50, 100 and 400 μg/mL) at and above known toxic levels were prepared in human serum. Toxin containing serum was supplemented with two concentrations of digoxin immune Fab (DigiFab, BTG Pharmaceuticals, West Conshohocken, PA) representative of human blood levels during standard treatment of digoxin overdose: 10 and 25 μg/mL. Convallatoxin was also treated with 100 μg/mL digoxin immune Fab, a concentration well in excess of standard treatment. Specimens were incubated at 37°C for 1 h. Protein free ultrafiltrates were prepared by centrifugation with the Centrifree Ultrafiltation Device (Milipore) for 25 min at 2000rpm. Apparent digoxin (free and total) was measured using the CMIA on the Roche Cobas e601. Experiments were conducted by a single operator using the same batch of centrifuge columns. Values were expressed as the mean of duplicate measurements.
Results
Cross-Reactivity of Digoxin Immunoassays for Purified Convallatoxin and Oleandrin
Measurable apparent digoxin concentrations were observed when digoxin-free serum was supplemented with concentrations of convallatoxin in and below the reported range of human and veterinary Convallaria toxicity5, 6, 7 for all five assays tested (Fig. 2). The sensitivity of these assays varied considerably with the Abbott Architect showing the highest sensitivity for detecting convallatoxin.
Figure 2. Cross-reactivity of digoxin immunoassays for purified convallatoxin.
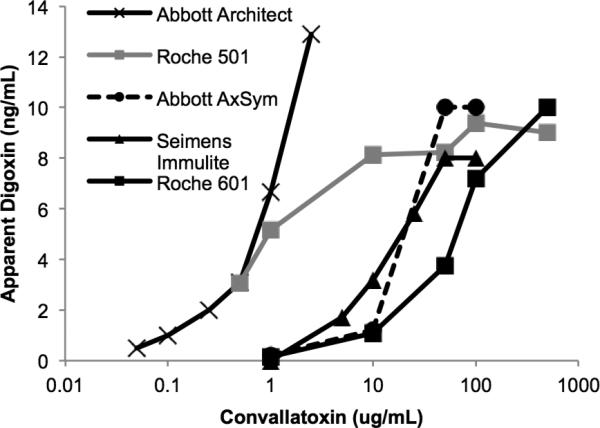
Digoxin-free serum was supplemented with varying concentrations of convallatoxin and apparent digoxin was determined using the indicated immunoassays. Means of two measurements are shown.
In agreement with a prior study by Dasgupta et al10, serum supplemented with oleandrin produced measurable apparent digoxin levels using the Abbott AxSym (Fig. 3). In addition, the four newer assays in our panel also detected oleandrin. Convallatoxin produced a measurable apparent digoxin level at 0.05 ug/mL, whereas a much higher concentration of oleandrin (1 ug/mL) was required to produce a measurable apparent digoxin value.
Figure 3. Cross-reactivity of digoxin immunoassays for purified oleandrin.
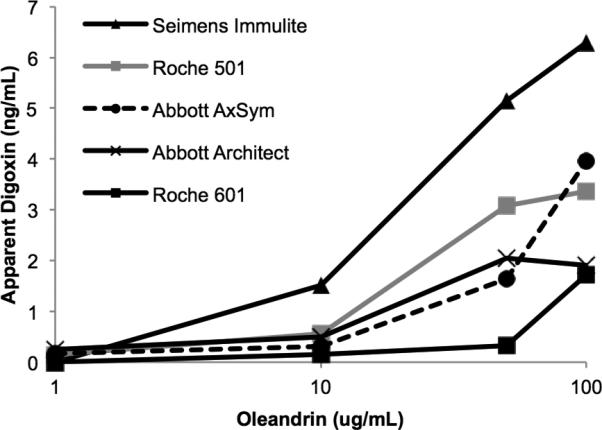
Digoxin-free serum was supplemented with varying concentrations of oleandrin and apparent digoxin was determined using the indicated immunoassays. Means of two measurements are shown.
Detection of Convallatoxin in Intoxicated Mice
Of the systems we tested, the Abbott Architect digoxin assay yielded the highest sensitivity for convallatoxin. Using that platform, we identified that convallatoxin could be readily detected in serum during in vivo intoxication in a mouse model. Convallatoxin administered by intraperitoneal injection at doses ranging from the 10 mg/kg LD50, to 10% of the LD50 produced significant apparent serum digoxin measurements in the majority of animals (Fig. 4). One sample in the 10% LD50 was undetectable.
Figure 4. Detection of convallatoxin in the serum of intoxicated mice.
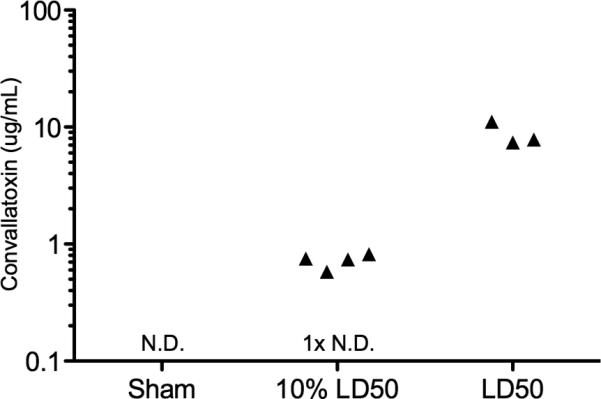
Convallatoxin was administered by intraperitoneal injection at the LD50 for mice (10mg/kg; n=3) or 10% of the LD50 (1mg/kg; n=5). One mouse received a sham saline injection. Apparent digoxin was measured in the serum after 10 minutes using the Abbott Architect assay. Convallatoxin levels were calculated based on a standard curve. N.D. not detected.
Convallatoxin is Not Bound by Digoxin Immune Fab
Digoxin immune Fab effectively bound digoxin at physiologically relevant concentrations, as demonstrated by the absence of free digoxin after incubation with Digoxin immune Fab (Fig. 5). Effective neutralization was observed even at the highest digoxin (160ng/mL) and lowest antibody (10μg/mL) concentrations tested. There was also significant binding of digoxin to normal serum proteins, as demonstrated by a 25% reduction in free digoxin versus total levels in the absence of therapeutic antibody. In contrast, convallatoxin demonstrated little binding to normal serum proteins and no neutralization by digoxin immune Fab, even at antidote concentrations 10-fold above the usual therapeutic dose (Fig. 6).
Figure 5. Digoxin immune FAB binds digoxin.
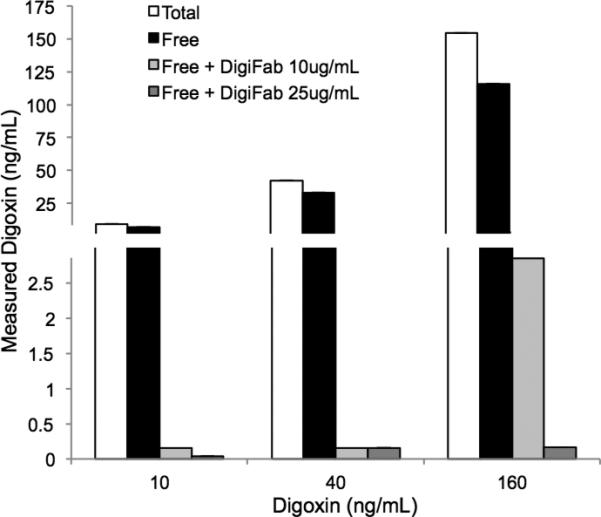
Serum supplemented with digoxin was incubated with digoxin immune Fab as indicated. Digoxin was measured in an unmanipulated sample (total). Protein bound drug was removed by ultrafiltration and remaining unbound (free) digoxin was measured. Note that effective binding of digoxin by DigiFab necessitated a broken y-axis to accommodate the range of measured values. Means of two measurements are shown.
Figure 6. Digoxin immune FAB does not bind convallatoxin.
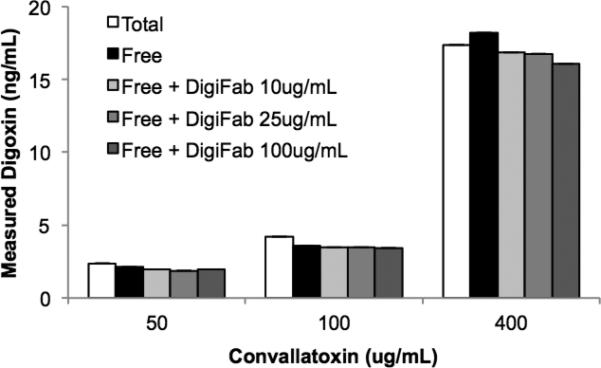
Serum supplemented with convallatoxin was incubated with digoxin immune Fab as indicated. Apparent digoxin was measured in an unmanipulated sample (total). Protein bound toxin was removed by ultrafiltration and apparent digoxin was measured as a surrogate for remaining unbound (free) convallatoxin. Means of two measurements are shown.
Discussion
Our findings demonstrate the potential utility of digoxin immunoassays for rapid evaluation of lily of the valley (Convallaria majalis) exposure. Each of the digoxin immunoassays tested in this study demonstrated significant cross-reactivity for convallatoxin, the major cardiac glycoside in lily of the valley, at concentrations in and below the reported range of in vivo Convallaria toxicity5-7. Cross-reactivity of older generation digoxin immunoassays for oleandrin has been previously demonstrated10-12 and our findings extend these observations to common assays in current use. The degree of cross-reactivity varied among the individual assays and cross-reactivity for oleandrin did not correlate with that of convallatoxin. These differences are likely related to the nature of the binding sites of the individual antibodies used in each assay as well as the assay methodology. Although rigorous characterization of immunoassay interference due to cross-reactants should be performed in the presence of the primary analyte, we elected not to test cross-reactivity for convallatoxin in the presence of digoxin because the majority of intoxications are in pediatric patients and pets, populations that would not be expected to use digoxin therapeutically.
We were able to detect apparent digoxin levels not only in convallatoxin supplemented serum in vitro, but in the serum of intoxicated mice, even at sublethal doses. Although these studies further suggest that the cardiac glycosides of lily of the valley may be detectable in the serum of humans and pets exposed through ingestion, a limitation of this study was our inability to obtain samples from convallatoxin intoxicated patients.
We were surprised that despite binding to antibodies in digoxin immunoassays, convallatoxin displays no detectable binding to the digoxin immune Fab product, DigiFab. This may be related to (1) differences in molar concentrations needed for toxicity, (2) the chemical properties of convallatoxin in serum, or (3) different antigenic sensitivities between the assay antibodies and the antidote antibody fragment. Toxic convallatoxin (MW 551 g/mol) levels are in the range of 10-100 μg/mL13 (18-180 μM), while the generally accepted threshold of digoxin (MW 781 g/mol) toxicity is 2 ng/mL (2.6 nM). The larger molar concentration of convallatoxin may not be effectively bound by the Fab, even at the 10-fold antidote dose, given the large molarity difference. Arguing against this theory is that oleandrin's (MW 577) estimated toxic concentration of 10 μg/mL (17 μM) is similar to convallatoxin and oleandrin is effectively bound by digoxin immune Fab14, 15. We are unable to detect lower concentrations of convallatoxin with our current assays, and thus cannot assess digoxin immune Fab binding at molar concentrations comparable with digoxin.
With regard to differing chemical properties, we noticed little binding of convallatoxin to normal serum proteins, in contrast to digoxin and oleandrin14, as well as moderately increased hydrophobicity of convallatoxin compared to digoxin during reconstitution. This physical property may affect both protein binding and antidote binding to convalatoxin, relative to digoxin or oleandrin. In addition, convallatoxin may not share the specific epitope recognized by DigiFab brand digoxin immune Fab. Impurities in the commercially available convallatoxin preparation used in our experiments could represent other cardiac glycosides found in Convallaria that may contribute to digoxin immunoassay reactivity and clinical toxicity, but are not bound by DigiFab. We cannot exclude the therapeutic effectiveness of the other commercially available therapeutic digoxin neutralizing antibody, DigiBind. Given the wide apparent range of convallatoxin affinity revealed by the various digoxin immunoassays, it remains possible that DigiBind may effectively bind convallatoxin, although this product is not currently available in the United States.
Conclusions
Multiple digoxin immunoassays can detect the botanical cardiac glycoside convallatoxin in ranges consistent with animal toxicity, and thus may be useful for rapid detection of clinically significant exposures. Neutralization of convallatoxin by DigiFab is unlikely to provide therapeutic benefit. Locally validated cross-reactivity for convallatoxin of the available on-site digoxin immunoassay could prepare clinicians to effectively discern the severity of Convallaria exposure or ingestion.
Acknowledgements
We appreciate the advice of Herbert Malkus, and assistance of Maria Galanakis, Florecita Santos and Jennifer Trick in running the digoxin assays.
Footnotes
Declaration of Interest
Suzanne Ward and BTG Pharmaceuticals provided the DigiFab digoxin immune Fab. This study was funded by the American Academy of Clinical Toxicology Lampe-Kunkel Award and a Connecticut College of Emergency Physicians Resident Research Grant.
References
- 1.Cortinovis C, Caloni F. Epidemiology of intoxication of domestic animals by plants in Europe. Vet J. 2013;197(2):163–8. doi: 10.1016/j.tvjl.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Nelson LSSR, Balick MJ. Handbook of poisonous and Injurious Plants. Springer Sciences; New York, NY: 2007. pp. 24pp. 133–134. [Google Scholar]
- 3.Radford DJ, Gillies AD, Hinds JA, Duffy P. Naturally occurring cardiac glycosides. Med J Aust. 1986;144(10):540–544. doi: 10.5694/j.1326-5377.1986.tb112283.x. [DOI] [PubMed] [Google Scholar]
- 4.Krenzelok EP, Jacobsen T, Aronis J. Lily-of-the-valley (Convallaria majalis) exposures: are the outcomes consistent with the reputation? (Abstract) Clinical Toxicology. 1996;34:601. [Google Scholar]
- 5.Atkinson KJ, Fine DM, Evans TJ, Khan S. Suspected lily-of-the-valley (Convallaria majalis) toxicosis in a dog. J Vet Emerg Crit Care. 2008;18(4):399–403. [Google Scholar]
- 6.Moxley RA, Schneider NR, Steinegger DH, Carlson MP. Apparent toxicosis associated with lily-of-the-valley (Convallaria majalis) ingestion in a dog. J Am Vet Med Assoc. 1989;195(4):485–487. [PubMed] [Google Scholar]
- 7.Alexandre J, Foucault A, Coutance G, Scanu P, Milliez P. Digitalis intoxication induced by an acute accidental poisoning by lily of the valley. Circulation. 2012;125(8):1053–1055. doi: 10.1161/CIRCULATIONAHA.111.044628. [DOI] [PubMed] [Google Scholar]
- 8.Edgerton PH. Symptoms of digitalis-like toxicity in a family after accidental ingestion of lily of the valley plant. J Emerg Nurs. 1989;15(3):220–223. [PubMed] [Google Scholar]
- 9.Hoffman BF BT. Digitalis and Allied Cardiac Glycosides. In: Gilman AG, R. T., Nies AS, Taylor P, editors. Goodman and Gilman's The Pharmaceutical Basis of Therapeutics. 814-839. Pergamon Press; New York: 1990. [Google Scholar]
- 10.Dasgupta A, Datta P. Rapid detection of oleander poisoning using digoxin immunoassays: comparison of five assays. Ther Drug Monit. 2004;26(6):658–663. doi: 10.1097/00007691-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta A, Klein K, Risin SA, Actor JK. Rapid detection of oleander poisoning by Dimension Vista digoxin assay (Flex Reagent Cartridge) J Clin Lab Anal. 2011;25(2):105–109. doi: 10.1002/jcla.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasgupta A, Risin SA, Reyes M, Actor JK. Rapid detection of oleander poisoning by Digoxin III, a new Digoxin assay: impact on serum Digoxin measurement. Am J Clin Pathol. 2008;129(4):548–553. doi: 10.1309/CC6791DFF20QPCX3. [DOI] [PubMed] [Google Scholar]
- 13.Forster W, Sziegoleit W, Guhlke I. Comparative study of some extracardiac effects of cardiac glycosides. Archives Internationales de Pharmacodynamie et de Therapie. 1965;165-182 [PubMed] [Google Scholar]
- 14.Dasgupta A, Emerson L. Neutralization of cardiac toxins oleandrin, oleandrigenin, bufalin, and cinobufotalin by digibind: monitoring the effect by measuring free digitoxin concentrations. Life Sci. 1998;63(9):781–788. doi: 10.1016/s0024-3205(98)00333-6. [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta A, Hart AP. Rapid detection of oleander poisoning using fluorescence polarization immunoassay for digitoxin. Effect of treatment with digoxin-specific Fab antibody fragment (ovine) Am J Clin Pathol. 1997;108(4):411–416. doi: 10.1093/ajcp/108.4.411. [DOI] [PubMed] [Google Scholar]
- 16.Woolf AD, Wenger T, Smith TW, Lovejoy FH. The use of digoxin-specific Fab fragments for severe digitalis intoxication in children. N Engl J Med. 1992;326(26):1739–1744. doi: 10.1056/NEJM199206253262604. [DOI] [PubMed] [Google Scholar]


