Abstract
Background
The dopamine D4 receptor gene (DRD4) has been implicated in psychiatric disorders in which deficits of self-regulation are a prominent feature (e.g., attention-deficit hyperactivity disorder and substance use disorders) and in dopamine D4 receptor insensitivity within prefrontal regions of the brain. Our hypothesis was that carriers of 7-repeats in the Variable Number of Tandem Repeats (VNTR) of DRD4 (7R+) would recruit prefrontal brain regions involved in successful inhibitory control to a lesser degree than non-carriers (7R−) and demonstrate less inhibitory control as confirmed by observation of locally reduced blood oxygenation level dependent (BOLD) % signal change and lower accuracy while performing “No-Go” trials of a Go/No-Go task.
Methods
Participants (age=18, n=62, 33 females) were recruited from the general population of the St. Louis, Missouri region. Participants provided a blood or saliva sample for genotyping, completed drug and alcohol-related questionnaires and IQ testing, and performed a Go/No-Go task inside of a 3T fMRI scanner.
Results
Go/No-Go task performance did not significantly differ between 7R+ and 7R− groups. Contrast of brain activity during correct “No-Go” trials with a non-target letter baseline revealed significant BOLD activation in a network of brain regions previously implicated in inhibitory control including bilateral dorsolateral prefrontal, inferior frontal, middle frontal, medial prefrontal, subcortical, parietal/temporal, and occipital/cerebellar brain regions. Mean BOLD % signal change during “No-Go” trials was significantly modulated by DRD4 genotype, with 7R+ showing a lower hemodynamic response than 7R− in right anterior prefrontal cortex/inferior frontal gyrus, left premotor cortex, and right occipital/cerebellar areas. Follow-up analyses suggested that 7-repeat status accounted for approximately 5–6% of the variance in the BOLD response during “No-Go” trials.
Discussion
The DRD4 7-repeat allele may alter dopaminergic function in brain regions involved in inhibitory control. When individuals must inhibit a prepotent motor response, presence of this allele may account for 5–6% of the variance in BOLD signal in brain regions critically associated with inhibitory control, but its influence may be associated with a greater effect on brain than on behavior in 18-year-olds from the general population.
Keywords: dopamine, DRD4, executive function, fMRI, inhibitory control, response inhibition
1. Introduction
Executive function refers to a collection of cognitive abilities that support goal-directed behavior. Cognitive abilities thought to contribute substantially to executive function include the ability to orient to goal-relevant stimuli (attention), hold information online while performing goal-directed actions (working memory), shift between sets of automatic behaviors (cognitive flexibility), imagine the steps necessary to reach a goal in the future (planning), and cancel a prepotent motor response on demand (inhibitory control). Dopamine is thought to play an important role in executive function. For example, individuals with Parkinson’s Disease or attention deficit/hyperactivity disorder (ADHD) have notable dopaminergic deficiencies and impairment in executive function (Bidwell, McClernon, & Kollins, 2011; Dirnberger & Jahanshahi, 2013; Kudlicka, Clare, & Hindle, 2011; Swanson, Baler, & Volkow, 2011), supporting the view that intact executive function depends on normal dopaminergic functioning.
The etiology of dopamine-deficiency-associated executive impairment and related psychiatric disorders in which deficits of inhibitory control are prominent (e.g., ADHD, substance abuse disorders, conduct disorder, etc.) may be related to genes, environment, or to an interaction between environment and genes. For example, twin, family, and adoption studies suggest that ADHD is substantially heritable. One review considered the results of 20 studies of heritability and concluded that 76% of the variance in ADHD diagnosis may be under genetic control (Faraone, et al., 2005). The most replicated genetic association with ADHD is with a Variable Number of Tandem Repeats (VNTR) of a 48 bp sequence located in exon 3 of DRD4 (Faraone, et al., 2005; Gizer, Ficks, & Waldman, 2009; Li, Sham, Owen, & He, 2006), a gene that encodes the dopamine D4 receptor (Ding, et al., 2002; E. Wang, et al., 2004). The 48-bp fragment is repeated between 2 to 11 times, and the most common variants (2-repeats, 4-repeats and 7-repeats) are represented in over 90% of the general population (E. T. Wang, Kodama, Baldi, & Moyzis, 2006). Meta-analyses have confirmed that individuals with 7-repeats of this bp sequence in exon 3 of DRD4 are at increased risk of developing ADHD (Faraone, et al., 2005; Gizer, et al., 2009; Li, et al., 2006). However, this finding has not been replicated in all studies that have examined this association, and the estimated odds ratio associated with the risk for ADHD is only small to moderate (1.1–1.4) (Faraone, et al., 2005; Gizer, et al., 2009; Li, et al., 2006). Similarly, variation at the DRD4 VNTR locus has been increasingly implicated in addiction-related phenotypes (McGeary, 2009).
Greater density of dopamine D4 receptors has reportedly been observed in prefrontal regions of the human brain compared to other brain regions (Meador-Woodruff, et al., 1996; Mulcrone & Kerwin, 1997). Prefrontal brain regions have been identified as being critically involved in executive functions including inhibitory control (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Drewe, 1975; Iversen & Mishkin, 1970; Picton, et al., 2007). Previous biochemical studies have suggested that the DRD4 7-repeat variant impacts the length of D4 receptor's third cytoplasmic loop. As this region is responsible for G-protein coupling and activation of intracellular responses to dopamine release through reducing intracellular cAMP levels, the DRD4 7-repeat variant has been posited to be associated with a less sensitive D4 receptor (Asghari, et al., 1995; Van Tol, et al., 1992). More recent work suggests that differences in dopamine function may additionally be due to the inability of the 7-repeat variant of the D4 receptor to heterodimerize with D2 receptors affecting signal transduction pathways such as the mitogen-activated protein kinases pathway (Borroto-Escuela, et al., 2011; Gonzalez, et al., 2012). As such, the exact mechanisms by which dopamine function may be affected in DRD4 7-repeat carriers remain unclear at this time. Nevertheless, all of these lines of evidence support the view that genetic variation in the VNTR of the DRD4 gene is associated with dysfunction of the dopamine D4 receptor within prefrontal regions of the human brain and that variation at this genetic locus could potentially account for prefrontally mediated dopamine-deficiency-associated impairment of inhibitory control and related neuropsychiatric symptoms.
However, studies that have investigated whether variation in the DRD4 VNTR polymorphism is associated with poorer performance on tasks that require intact executive function have produced heterogeneous findings. Neuropsychological studies of children and adults with ADHD have reported that 7-repeat carriers (7R+) may perform more poorly (Kieling, Roman, Doyle, Hutz, & Rohde, 2006; Langley, et al., 2004) or better (Johnson, et al., 2008; Swanson, et al., 2000) on tasks of executive function than non-carriers of the 7-repeat allele (7R−) whereas other studies have reported no difference between these groups (Barkley, Smith, Fischer, & Navia, 2006; Konrad, et al., 2010). There are several potential explanations for the heterogeneity in findings across studies including administration of very different neuropsychological tests purported to measure the same cognitive construct, failure to control for developmental changes in cognitive performance that may interact with ADHD, stimulant treatment, the presence of comorbid conditions, and varying sample sizes (Kebir & Joober, 2011).
When the association between inhibitory control and variation in the DRD4 VNTR 7-repeat polymorphism has been examined in non-clinical samples, the results of these studies have shown mixed findings with some studies reporting that 7R+ carriers perform more poorly on measures of inhibitory control than 7R− (Altink, et al., 2011; Congdon, Lesch, & Canli, 2008), and other studies reporting no differences between the groups (Barkley, et al., 2006; Colzato, van den Wildenberg, Van der Does, & Hommel, 2010; Cummins, et al., 2012; Heinzel, et al., 2012; Johnson, et al., 2008; Kramer, et al., 2009). Administration of different neuropsychological instruments across studies, heterogeneity in age groups examined within and across studies, and differences in the way that the allele carriers were grouped may account for the mixed findings. For example, it is notable that the two studies that reported associations in this literature used the Stop Signal task, a task that provides an estimate of the average latency to inhibition of a prepotent response (stop signal reaction time, SSRT), with groups of adolescents and adults (Altink, et al., 2011; Congdon & Canli, 2008). By contrast, when variants of the Continuous Performance Test (CPT) or the Go/No-Go task were administered to groups of healthy 7R+ and 7R−, the two groups did not differ on accuracy during “No-Go” trials or on rate of commission errors (Barkley, et al., 2006; Colzato, et al., 2010; Heinzel, et al., 2012; Johnson, et al., 2008; Kramer, et al., 2009), the traditional measures of inhibitory control associated with these tasks (Strauss, Sherman, & Spreen, 2006). Almost all of the studies that administered the Go/No-Go or the CPT task either neglected to report accuracy or commission rates during “No-Go” trials or they reported commission error rates very close to floor (i.e., 0%) calling into question whether their version of the CPT or the Go/No-Go challenged inhibitory control within the age range studied. Although Kramer and colleagues administered both “easy” and “hard” versions of a Go/No-Go task and reported that healthy volunteers ranging in age between 20 and 30 years of age made commission errors on approximately 23% of “No-Go” trials, they reportedly found no difference in commission error rates between 7R+ and 7R− (Kramer, et al., 2009). Because this study compared the behavioral performance of small groups of homozygous 7R+ (n=10) and homozygous 4-repeat carriers (n=10), it is difficult to compare the findings of this study with others in this literature as other studies collected larger samples and compared carriers of at least one 7-repeat allele with 7R−. Thus, the relationship between this DRD4 VNTR polymorphism and performance on inhibitory control tasks remains an open question for future research.
To our knowledge, only one study to date has used fMRI to explore a possible association between variation in the DRD4 7-repeat allele and differences in the neural correlates of executive function (Gilsbach, et al., 2012). Gilsbach and colleagues administered two executive function tasks to 26 healthy children between 8 and 15 years of age (mean age=11.4), one of which challenged inhibitory control (the interference control task). On each trial of the interference control (IC) task, two symbols were simultaneously presented on the screen but one of the two symbols was presented for a longer period of time than the other one (250 to 350 milliseconds longer). On most of the trials (60–80%), the two symbols were visually identical whereas on a minority of the trials, the symbols did not match. Participants were instructed to respond on every trial, and if they judged the two symbols to be visually identical they were instructed to make a button press response on the side where one of the symbols was presented for a longer period of time, but if the two symbols did not match, they were instructed to respond on the side opposite to where one of the symbols was presented for the longer period of time. The authors reported that performance on the IC task was associated with a significant error rate (approximately 22%) in children and adolescents between the ages of 8 and 15. Although they did not observe that 7R+ (n=10) had a higher error rate on this task than 7R− (n=16), they did observe that 7R+ had lower blood oxygenation level dependent response (BOLD) during performance of the IC task than 7R−. Associations with lower BOLD response were observed in the left inferior frontal gyrus (IFG) and left middle frontal gyrus, two brain regions that have been previously associated with inhibitory control. The authors interpreted their findings to be consistent with the idea that 1) D4 receptor function in prefrontal cortex may be less sensitive in DRD4 7R+; 2) that the BOLD response in prefrontal brain regions may be sensitive to this genetically-mediated difference in D4 dopamine sensitivity; and 3) that fMRI studies may be more sensitive than neuropsychological measures in the detection of genetically-mediated alterations of prefrontal function. However, the authors did note limitations to the conclusions of their study due to the small size of their sample. Also, because this fMRI study employed a block design rather than an event-related design, it is possible that this experiment may have also been more vulnerable to confounds such as mixed event types and error-related processing. Lack of behavioral differences within the presence of differences in brain function, however, is not unusual in fMRI studies. Some authors have proposed that genes may have a greater effect on brain functions than they do on behavioral phenotypes (Goldberg & Weinberger, 2004). Furthermore, several groups have argued that when group differences in task-based fMRI BOLD responses are observed within the context of group differences in task performance, interpretation of the differences in BOLD response may be falsely attributed to group differences in brain function when they may actually be epiphenomenal to task performance (Barch, et al., 1997; Church, Petersen, & Schlaggar, 2010; Murphy & Garavan, 2004; Price & Friston, 1999; Schlaggar, et al., 2002).
The goal of the current fMRI study was to use an event-related fMRI design to investigate how the presence of the DRD4 7-repeat variant affects inhibitory control and associated BOLD response in 18-year-old emerging young adults. We decided to examine a sample of 62 emerging young adults ascertained from a general population sample (Anokhin, Golosheykin, Grant, & Heath, 2010) so that we could minimize potential confounds associated with aspects of ADHD diagnosis including comorbid conditions, stimulant effects, and general health issues. We ascertained a group who were 18 years of age to control for developmental changes in performance of an inhibitory control task (Christ, White, Mandernach, & Keys, 2001; Diamond, 1990; Ridderinkhof & van der Molen, 1997), the functional neuroanatomy of inhibitory control (Casey, et al., 1997; Garavan, Hester, Murphy, Fassbender, & Kelly, 2006; Nielson, Langenecker, & Garavan, 2002; Rubia, Smith, Taylor, & Brammer, 2007), and brain metabolism that may be related DRD4 7-repeat status (Volkow, et al., 2013). Examination of emerging young adults also provides a counterpoint to the study by Gilsbach and colleagues as our study can address whether the pattern of neural activation associated with 7-repeats is stable across development into young adulthood. Eighteen years of age is also just prior to the period of time wherein the greatest risk for dependence on substances of abuse occurs. Thus, we also hoped to minimize confounds associated with dependence on substances (Lessov-Schlaggar, et al., 2012) while still potentially describing behavioral and neural correlates of risk for dependence on such substances. We use a Go/No-Go task, the behavioral and neural correlates of which have been previously reported in the literature (Garavan, Ross, & Stein, 1999; Simmonds, Pekar, & Mostofsky, 2008), and, in previous research, we have shown that our implementation of this Go/No-Go task is sensitive to continuing symptoms of ADHD in adulthood (Mulligan, et al., 2011). In our fMRI analyses, we examine hemodynamic BOLD response associated with those trials in which a prepotent motor response tendency was successfully inhibited so that we might avoid confounds associated with potential differences in task performance.
Our hypotheses were derived from previous literature. Based on: 1) biochemical studies suggesting that possessing at least one 7-repeat allele in the VNTR of DRD4 gene is associated with insensitivity of the dopamine D4 receptor; 2) neuroanatomical evidence suggesting that D4 receptors are more likely to be expressed in prefrontal cortex; 3) behavioral genetic evidence suggesting an association between DRD4 VNTR 7-repeat status and a disinhibited psychiatric presentation (e.g., ADHD, substance use disorders); 4) neuropsychological evidence suggesting that prefrontal brain regions are critically involved in inhibitory control; 5) neuropsychopharmacological evidence suggesting that insufficient dopamine is associated with worse performance on tasks of executive function; and 6) a previous fMRI study that observed lower BOLD response during performance of an executive function task in the prefrontal cortex of children and adolescents with 7-repeats in at least one allele of the VNTR of DRD4 compared to individuals without this genetic variant, we hypothesized that individuals with at least one allele associated with 7-repeats of the 48 bp fragment would display a lower percentage of correctly inhibited trials on our Go/No-Go task, and that these individuals would show lower BOLD response during correct “No-Go” trials than non-carriers within areas of prefrontal cortex that have been previously implicated in inhibitory control (e.g., the right inferior frontal gyrus).
2. Materials and Methods
2.1 Participants
Participants were originally identified through the Missouri Family Registry and recruited from the general population to participate in a prospective, longitudinal study of adolescent twins (Anokhin, et al., 2010). Those who participated in the current study protocol were a subset (n=62, age at assessment: M= 18.01 years, S.D. = 0.25; 47.7% females) of the originally ascertained group (original n = 747; age at first assessment: M= 12.52 years, S.D. = 0.20; 47.8% females). As part of the original longitudinal study protocol, they were administered standardized assessments including the second edition of the Kaufman Brief Intelligence Test (K-BIT 2), a screening measure of IQ (Kaufman & Kaufman, 2004), the Barratt Impulsiveness Scale (BIS-11), a self-report measure of trait impulsiveness (Patton, Stanford, & Barratt, 1995), and a semi-structured interview including detailed questions about substance use. Participants were excluded from the current and longitudinal study protocol if they reported a history of serious head trauma or health conditions that would make a laboratory visit or performance of the experimental task infeasible (e.g. severe visual impairment or mental retardation). Those who were under the influence of alcohol or illicit substances were also excluded based on Breathalyzer and urine drug tests. Twin zygosity was assessed through three methods: 1) a standard interview administered to twins’ parents; 2) research assistants’ ratings of the twins’ physical similarity; and 3) a set of 156 DNA markers genotyped in 96% of the participants. Our sample included 25 monozygotic (MZ) and 37 dizygotic (DZ) twins. Eight of the individuals were single twins (twins whose co-twin was unwilling or unable to participate). Additional characteristics of the sample can be found in Table 1. This table shows that the 7R+ and 7R− did not differ with regard to any behavioral or self-report measure including IQ or sex distribution. The Human Research Protection Office at the Washington University School of Medicine approved the study. A written informed consent was obtained from all the participants. Participants were compensated for participation in the study.
Table 1.
Behavioral and self-report measures in 18-year-old DRD4 VNTR 7-repeat noncarriers (7R−) and carriers (7R+)
| 7R− (n=39) | 7R+ (n=23) | |
|---|---|---|
| K-BIT 2 IQ (Standard Scores) | ||
| Verbal | 102.7 (10.9) | 105.7 (14.8) |
| Nonverbal | 100.6 (12.8) | 106.8 (20.3) |
| % Accuracy (Go) | 78.9 (9.9) | 79.6 (11.4) |
| % Accuracy (No-Go) | 77.5 (14.2) | 82.3 (12.6) |
| Go reaction time (ms) | 441.9 (54.1) | 436.0 (49.7) |
| BIS-11 (total) | 59.2 (9.4) | 59.4 (9.9) |
| Alcohol Drinking Volume (drinks/year) | 181.0 (379.1) | 160.3 (250.1) |
| No. of smokers (100+ cigarettes lifetime) | 3 | 2 |
| No. ever smoked marijuana | 8 | 6 |
| No. of Caucasians | 37 | 20 |
| No. of African-Americans | 2 | 3 |
Note. Means (SD) are provided. There were no significant differences between 7R− and 7R+ on any of the measures listed. p < 0.05
2.2 Collection of DNA samples and genotyping of DRD4 VNTR
DNA was collected from either blood or buccal mucosa samples collected from the participants using standard genomic DNA extraction techniques. Genomic DNA was prepared for multiple displacement amplification (MDA) using a kit commercially available from Qiagen, Inc. (Valencia, CA) following manufacturers instructions. The number of 48-bp repeats in the VNTR of exon 3 of DRD4 was genotyped following previously described procedures (LaHoste, et al., 1996). Briefly, primers D4–42 and D4–3 were used to amplify the 48-bp repeat in exon 3 of the DRD4 gene following PCR guidelines (Lichter, et al., 1993). PCR products were visualized by gel electrophoresis and compared to positive controls for the variant alleles. The experimenters who collected the behavioral and fMRI data were blinded to the results of genotyping at the time of data collection.
2.3 Inhibitory control task, Go/No-Go
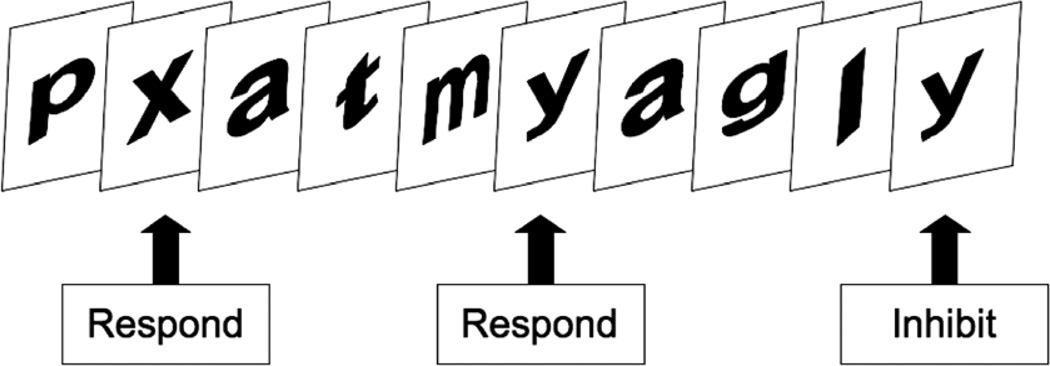
Our version of the Go/No-Go task followed a format that has been described previously (Garavan, et al., 1999; Mulligan, et al., 2011). During three practice runs performed outside of the scanner, participants saw a series of lower case letters presented on a computer screen replacing one another at a rate of 500 ms/letter. In the first two practice runs (2 runs of 250 letters each, 75 targets per run), participants were instructed to make a button press response as quickly and as accurately as possible whenever certain target letters (“x” or “y”) were presented within a series of distracter letters. In the third run, a switch in the rules was made stipulating that participants should only respond to the letters “x” or “y” if the two target letters alternated in the presented series (Figure 1). Following the three practice runs, participants completed six runs of this task inside of the scanner while attempting to conform to the new rule (250 letters per run, 1500 total letters). A 15.5 second rest period was presented at the beginning and end of each run. During these rest periods, participants viewed a fixation cross that was of the same size, font, and coloring as the presented letters (black with white background). Response to targets constituted the “Go” condition of this task whereas the presentation of “lure” letters, to which a prepotent response has been practiced but becomes inappropriate, constituted the “No-Go” condition. When blocks of letters were presented during the six scanning runs and the last practice run, “No-Go” letters were presented in a jittered pseudorandom sequence approximately every ~ 20 seconds and targets approximately every 3.5 seconds. There were a total of 214 “Go” trials and 38 “No-Go” trials across the six runs and an average of 36 “Go” letters and 7 “No-Go” letters per run. Response prepotency was maintained by including five times more valid “Go” letters than “No-Go” letters and through prior instructions that emphasized rapid response. Participants were encouraged to respond while the stimulus was still on screen, but responses within 1000 milliseconds of stimulus onset were considered valid responses.
Figure 1. Go/No-Go task.
On this version of the Go/No-Go task, participants were required to respond with a button press whenever specific target letters (“x” and “y”) were presented, but were required to withhold their response whenever nonalternating target letters were presented (“x” followed by “x” or “y followed by “y”). Response to targets (“x” or “y” alternating) constituted the “Go” condition of this task whereas the presentation of “lure” letters to which a prepotent response has been practiced but becomes inappropriate constituted the “No-Go” condition. There were 5 more times as many “No-Go” trials as “Go” trials to maintain prepotent responding.
2.4 Magnetic resonance imaging and processing
Whole-brain imaging was performed on a Siemens TIM TRIO 3 Tesla scanner (Erlangen, Germany) at the Center for Clinical Imaging Research at Washington University in St. Louis. For anatomical reference, high resolution, 3D MP-RAGE anatomic images were collected [TE = 3.16 ms; TR = 2400 ms, 8 flip angle, slice thickness = 1.0 mm, FOV = 25.6 cm, matrix size = 2562] prior to the collection of the BOLD echoplanar data.
To provide whole-brain coverage, BOLD echoplanar images [TE = 27 ms; TR = 2000 ms; 77 flip angle; slice thickness = 4.0 mm; FOV = 25.6 cm; matrix size = 642;] were acquired with 35 slices. For each of the six two-minute and 36 second imaging runs, this procedure yielded 78 whole-brain volumes with a spatial resolution of 4 mm3 per voxel.
Image processing and analyses were performed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Prior to individual analyses, images were spatially registered in 3D space to the third image in the time series using an iterative linear least squares algorithm that achieved least-squares alignment of three translational and three rotational parameters, and activation outside of the brain was removed through edge detection algorithms.
2.5 fMRI analysis
2.5.1 Individual models of functional activity
The time-series data were then modeled using onset times associated with correct “Go” and “No-Go” trials. This was performed within the context of a general linear model by convolving onset times associated with each trial type with the temporal derivative of a γ-variate function representing the event-related hemodynamic BOLD response (M. S. Cohen, 1997). This general linear model yielded a separate estimate of the magnitude of the hemodynamic response associated with correct “Go” and “No-Go” trials within every voxel in the brain with no bias in estimation of the magnitude of hemodynamic response associated with each trial type. Trends in the time series and estimates of motion from each dimension of space were included in the individual model as covariates of no interest. Baseline hemodynamic activity during distracter letters was also allowed to vary freely in the model. Voxelwise calculation of % signal change was achieved by dividing the beta weight associated with a particular trial type of interest (i.e., “No-Go” trials) by an estimate of the baseline provided by the general linear model.
2.5.2 Group activation maps associated with correct “No-Go” trials
Percent signal change data for all participants were then transformed into stereotaxic space (Talaraich & Tourneaux, 1988) and spatially averaged over a 6 mm radius to allow direct voxel-by-voxel statistical comparisons and to compensate for individual differences in brain anatomy. To identify a network of brain regions associated with inhibitory control, estimates of % signal change associated with correct “No-Go” trials were then contrasted against an implicit baseline (non-target letters) using a one-sample, two-tailed t-test comparison (Simmonds, et al., 2008). An uncorrected voxelwise threshold of α = 1.0 × 10−5 was applied to this contrast, and voxels not comprising a cluster of contiguous voxels with a minimum volume of 200 µl were removed. This approach is based on theory suggesting that larger clusters of voxels have less likelihood of being false positive errors (Forman, et al., 1995). A Monte Carlo simulation procedure implemented in AFNI (3dClustSim) allows one to estimate the probability of Type I error due to multiple comparisons on a cluster by cluster basis based on each cluster’s size, the level of smoothness associated with the BOLD data, and 1,000 random image permutation simulations. Application of this procedure suggested that our approach was associated with < 0.1% chance of any of the clusters identified in our analyses as being false positive errors.
2.5.3 fROI analysis
Following voxel-wise analyses, a functional region of interest (fROI) analysis was conducted (Poldrack, 2007). An fROI analysis aims to identify task-associated brain regions prior to testing a study’s hypotheses (Celone, et al., 2006; Mitsis, Iannetti, Smart, Tracey, & Wise, 2008; Mulligan, et al., 2011; Roberts, Nestor, & Garavan, 2009). Benefits include 1) improved statistical power over voxel-wise approaches; 2) reduced potential for Type I error due to the overall number of comparisons made; and 3) hypothesis testing within meaningfully defined regions of interest. Any clusters of voxels showing significant % signal change during correct “No-Go” trials in the voxelwise analyses (as defined by the methods described above in section 2.5.2) were identified as fROIs. Although some authors have argued that the BOLD response observed during “No-Go” trials should be contrasted with the BOLD response observed during “Go” trials rather than with baseline, the latter approach may be more effective in identifying areas of the brain critically involved in inhibitory control when this version of Go/No-Go task is administered (Mulligan, et al., 2011; Simmonds, et al., 2008). In our own study (Mulligan, et al., 2011), this version of the Go/No-Go task was administered to groups of longitudinally-tracked adults with and without continuing symptoms of ADHD. When the BOLD response associated with correct “No-Go” trials was contrasted against the BOLD response associated with correct “Go” trials, significantly greater hemodynamic response during “No-Go” trials was only evident in the parietal lobes whereas contrast of correct “No-Go” trials with implicit baseline (non-target letters) revealed significant activation in prefrontal regions previously identified as being critically related to inhibitory control (e.g., pre-supplementary motor area and the right IFG). Follow-up conjunction and fROI analyses revealed that there was only one region of the brain that was commonly recruited during correct performance of Go and “No-Go” trials, the pre-supplementary motor area (pre-SMA), and that adults with continuing symptoms of ADHD recruited this brain region to a lesser degree than control participants during “No-Go” trials but not during “Go” trials. This raised the possibility that the pre-SMA may have been overlooked as a neural correlate of inhibitory control in previous studies that employed the Go/No-Go task wherein the neural correlates of Go and “No-Go” trials were directly contrasted against one another. Consistent with this interpretation, a meta-analysis of studies that used versions of the Go/No-Go task similar to our own also found significant activation in the pre-SMA when the neural correlates of correct “No-Go” trials were contrasted against baseline (Simmonds, et al., 2008). Following this line of research, fROIs for the current study were defined based on the contrast of % signal change during correct “No-Go” trials with implicit baseline (non-target letters). The degree of % signal change during correct “No-Go” trials was averaged for each participant within the spatial extent of each of the fROIs so that these metrics could be used as dependent variables in statistical analyses. Follow-up analyses included contrasts between 7R+ and 7R− as well as correlational analyses. Correlation analyses included correlation of % signal change during correct “No-Go” trials with key measures such as task performance, as measured by the percentage of correctly inhibited “No-Go” trials, and trait impulsivity, as measured by quantitative scores on the BIS-11.
2.5.4 Common areas of recruitment: “Go” and “No-Go” trials
To reveal brain regions that may have made contributions to both “Go” and “No-Go” trials and to determine if differences in hemodynamic change observed during “No-Go” trials may have been accounted for by differences that may have been present during “Go” trials, a conjunction analysis (i.e., “AND” mask) was also performed following the Minimum Statistic compared to the Conjunction Null method (MS/CN)(Nichols, Brett, Andersson, Wager, & Poline, 2005). This followed a series of steps. First, BOLD % signal change associated with correct “Go” and “No-Go” trials were each contrasted separately against an implicit task baseline (i.e., non-target letters) through use of voxelwise one-sample, two-tailed t-test comparisons at uncorrected α = 1.0 × 10−5 and a minimum cluster volume of 200 µl. Second, maps associated with each trial type were then converted to binary code (1=activated, 0=not activated) and added to one another in order to identify brain regions that were common to both trial types. To perform comparisons that examined potential group differences (7R+ vs. 7R−) in hemodynamic response within each fROI that was identified by this “AND” mask, an averaged BOLD % signal change response for correctly performed “Go” trials was calculated for all voxels within each fROI identified by the conjunction analyses and then compared between groups (7R+ vs. 7R−) using the follow-up contrast procedures described for “No-Go” trials.
2.6 Genetic association analyses
As noted above, we used a sample of 25 MZ and 37 DZ twins. Consequently, observations (% signal change within each fROI) were assumed non-independent due to the nesting of individuals (i.e., each twin from a pair) within families. Our data was comprised of 54 individuals who were members of 27 family units, and eight twins whose co-twin was unable to participate (single twins). Typically, when observations are non-independent, statistical tests computed using the general linear model are positively biased leading to increased probability of Type I errors (J. R. Cohen, Cohen, West, & Aiken, 2003).
To account for potential non-independence of brain activation between twins, we used a simple two-level multilevel model implemented in HLM7 (S.W. Raudenbush, Bryk, & Congdon, 2011). Raudenbush and Bryk reported that restricted maximum likelihood (REML) has better small sample performance (when estimating variance components) compared to full maximum likelihood (S. W. Raudenbush & Bryk, 2002). Consequently, we used REML to estimate model parameters. The two-level, fixed intercept, random slopes regression model and its parameters are defined below.
| yij = β0j + β1j * (Carrier Statusij) + εij | Level 1 |
| β0j = γ00 | Level 2 |
| β1j = γ10 + r1j | |
| Where, | |
| Level 1 | |
| yij | was the % signal change in BOLD in a region for individual i in family j. |
| β0j | was the Y-intercept for family unit j. |
| β1j | was the linear association (i.e., slope) of BOLD % signal change with 7-repeat allele carrier status for family unit j. |
| Carrier Statusij | was a dummy variable that distinguished whether individual i in family unit j was a 7-repeat carrier (coded 1) or non-carrier (coded 0). |
| εij | was the error for individual i in family unit j. Our model assumed that the variance of the errors, σε2, (within-family error variance; level 1 error variance) was the same for all of the family units. |
| Level 2 | |
| γ00 | was the grand mean (across sample) Y-intercept. |
| γ10 | was the grand mean slope (association of the 7-repeat allele with BOLD % signal change). This was our parameter of interest. |
| rj | was the residual slope for family unit j. Each residual slope was the difference between the slope for family unit j and the grand mean slope (rj = γ10 - β1j). Variance among family unit slopes, Var(rj), was assumed to be uncorrelated with level 1 error variance (σε2). |
3. Results
3.1 Genotyping
The sample sizes associated with the two experimental groups were as follows: 7R+ (n = 23) and 7R− (n = 39). There was an approximately equal distribution of the 7-repeat allele across gender in our sample (7R+: 11 males, 12 females; and 7R−: 18 males, 21 females). The 7R+ and 7R− groups were not different with respect to the proportion of self-reported Caucasians to African-Americans (Table 1). The allele frequency of the 7-repeat allele in the sample was 0.209. This estimate is consistent with estimates of the worldwide prevalence of 7-repeat allele (Chang, Kidd, Livak, Pakstis, & Kidd, 1996; Ding, et al., 2002) and with estimates of its prevalence in individuals of mixed European descent (Chang, et al., 1996; Kidd, Pakstis, & Yun, 2014) provided by studies with larger sample sizes. The genotype frequencies were as follows: no 7-repeat alleles=0.629 (n=39), one 7-repeat allele=0.323 (n=20), 7-repeat homozygotes=0.048 (n=3). Genotypes were in Hardy-Weinberg equilibrium (HWE). Because our sample contained related individuals, one representative from each family was randomly selected to test deviation from HWE. To avoid biases related to small sample size and the presence of multiple rare alleles, genotype frequencies were tested using the exact test with likelihood ratio as the test statistic (Engels, 2009). This was performed using ExactoHW (http://www.genetics.org/cgi/content/full/genetics.109.108977/DC1). A published genotype classification scheme for the DRD4 exon 3 VNTR was employed (Das, Tan, & Easteal, 2010). Results suggested the probability of deviation from HWE in a sample with our observed allele frequencies was p=0.51.
3.2 Behavioral measures
The 7R+ and 7R− did not differ with regard to % accuracy of correct responses during “Go” trials (the number of responses where the participant pressed the button within 1000 milliseconds of onset of a “Go” trial divided by the total number of “Go” trials presented), % accuracy of responses withheld during “No-Go” trials (the number of events where no response was recorded within 1000 milliseconds of presentation of a “No-Go” trial divided by the total number of “No-Go” trials presented), or mean reaction time to “Go” trials (Table 1). There was no significant difference between 7R+ and 7R− with regard to the mean number of responses successfully withheld during “No-Go” trials [mean (standard deviation): 7R+ = 31.3 (4.8), 7R− = 29.5 (5.4)] or with regard to the minimum (7R+ = 17, 7R− = 16) or maximum (7R+ = 38, 7R− = 38) number of responses successfully withheld during “No-Go” trials. Furthermore, the two groups did not differ with regard to K-BIT 2 Standard Score estimates of Verbal or Non-Verbal IQ (Table 1).
3.3 Self-report measures
The 7R+ did not differ from 7R− with regard to the proportion of individuals who reported having smoked greater than 100 cigarettes in their lifetime, the proportion of individuals who reported ever having smoked marijuana, their score on BIS-11, or the self-reported number of drinks per year (Table 1).
3.4 fMRI BOLD activation patterns associated with correct “No-Go” trials
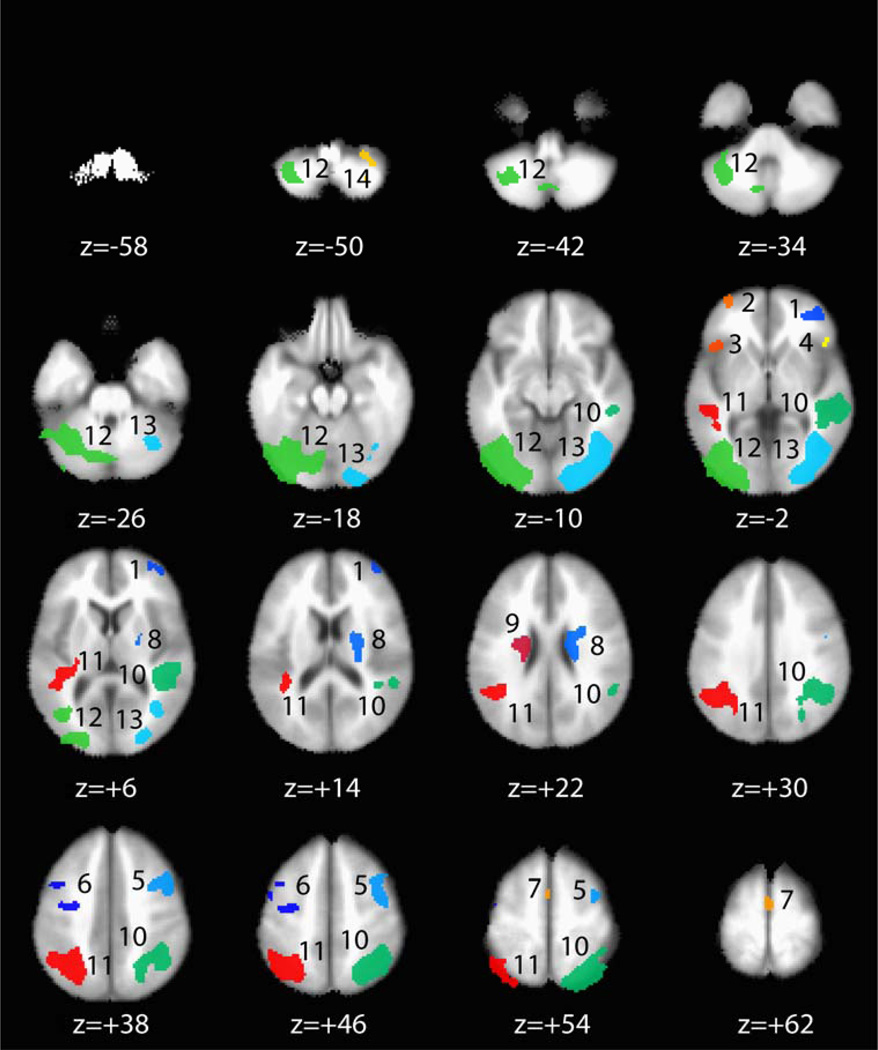
Contrast of % signal change during correct “No-Go” trials with implicit baseline (non-target letters) revealed a bilateral pattern of brain activity in 14 distinct brain regions including anterior prefrontal (BA 10), inferior frontal (BA 47), medial prefrontal (pre-SMA), middle frontal (BA 6/8), parietal/temporal, striatal (caudate and putamen), and occipital/cerebellar brain regions in addition to a distinct region in right cerebellum (Table 2, Figure 2).
Table 2.
Modulation of BOLD % signal change during “No-Go” trials by 7-repeat carrier status.
| Brain Region | µl | x | y | z | γ10 | SE | df | t | p |
|---|---|---|---|---|---|---|---|---|---|
| “No-Go” > Baseline | |||||||||
| Frontal | |||||||||
| (1) R Anterior Prefrontal Cortex/IFG (BA 10/47) | 2894 | 39 | 52 | 1 | −0.11 | 0.05 | 34 | −2.14 | 0.04* |
| (2) L Anterior Prefrontal Cortex (BA 10) | 670 | −33 | 60 | −4 | −0.05 | 0.06 | 34 | −0.95 | 0.35 |
| (3) L IFG (BA 47, orbitalis) | 688 | −44 | 20 | 0 | −0.08 | 0.05 | 34 | −1.75 | 0.09 |
| (4) R IFG (BA 45, triangularis) | 235 | 49 | 27 | 1 | −0.09 | 0.05 | 34 | −1.83 | 0.08 |
| (5) R Middle Frontal Gyrus (BA 6/8) | 4854 | 47 | 15 | 39 | −0.04 | 0.03 | 34 | −1.70 | 0.10 |
| (6) L Middle Frontal Gyrus (BA 6) | 2450 | −32 | −8 | 45 | −0.05 | 0.02 | 34 | −2.32 | 0.03* |
| (7) R Medial Frontal Gyrus (BA 6 pre-SMA/SMA) | 670 | 1 | −4 | 61 | −0.04 | 0.03 | 34 | −1.43 | 0.16 |
| Subcortical | |||||||||
| (8) R Caudate/Putamen | 4115 | 21 | −6 | 18 | −0.01 | 0.02 | 34 | −0.74 | 0.46 |
| (9) L Caudate | 1893 | −21 | −10 | 21 | −0.02 | 0.02 | 34 | −1.41 | 0.17 |
| Parietal/Temporal | |||||||||
| (10) R Parietal/Temporal Gyrus (BA 7/40/22/21) | 29217 | 26 | −67 | 50 | −0.05 | 0.02 | 34 | −1.97 | 0.06 |
| (11) L Parietal/Temporal Gyrus (BA 7/40/22/21) | 22001 | −27 | −60 | 43 | −0.04 | 0.02 | 34 | −1.84 | 0.07 |
| Occipital/Cerebellar | |||||||||
| (12) L Occipital Gyrus/Cerebellum (BA 18/17) | 41741 | −27 | −88 | −5 | −0.05 | 0.03 | 34 | −1.72 | 0.10 |
| (13) R Occipital Gyrus/Cerebellum (BA 18/17) | 20536 | 26 | −85 | −1 | −0.07 | 0.03 | 34 | −2.22 | 0.03* |
| (14) R Cerebellum | 629 | 29 | −37 | −51 | −0.08 | 0.04 | 34 | −2.16 | 0.04* |
Note. Brain regions are defined by voxelwise comparison of BOLD % signal change during correct “No-Go” trials with baseline (non-target letters). All brain regions listed passed a voxelwise α of p < 1.0 × 10−5, and a cluster threshold of 200 µl. Regions of interest are labeled and numbered as in Figure 2 and 3 and follow the convention of the Talaraich atlas (Talaraich and Tourneaux, 1988). Coordinates represent distance in mm from anterior commissure: × right (+)/left(−); y anterior (+)/posterior (−); z superior (+)/inferior (−). Abbreviations used in the table are as follows: R = right; L = Left, BA = Brodmann areas; IFG = Inferior Frontal Gyrus; SMA = Supplementary Motor Area. γ10 is the parameter estimate of the effects of DRD4 VNTR 7-repeat status on BOLD % signal change (±SE, df) during correct “No-Go” trials. Statistical significance (t- and p-values ) was computed after controlling for potential non-independence between twins as in Figure 3. Negative t values represent 7R+ < 7R−.
p <.05.
Figure 2. Brain Regions Activated During Correct “No-Go” Trials.
Colored areas indicate brain regions (fROIs) demonstrating significantly greater BOLD % signal change during correct “No-Go” trials than at baseline (voxelwise α = 1.0 × 10−5; minimum cluster volume threshold = 200 µl; < 0.1 % chance that any fROI identified through this method is a false positive). Each distinct fROI that was identified through this method was assigned a separate color and number. Regions are numbered as in Table 2 and Figure 3. Axial slices display the functional data in stereotaxic space (Talaraich & Tourneaux, 1988) moving from inferior to superior in 8 mm increments to show subcortical as well as cortical activity patterns associated with correct “No-Go” trials. Distance from the anterior commissure in mm (z) is depicted below each slice. Functional data is overlayed on a group-averaged T1 image to reflect inter-individual variability in brain anatomy.
3.5 Associations between DRD4 VNTR 7-repeat status and BOLD % signal change
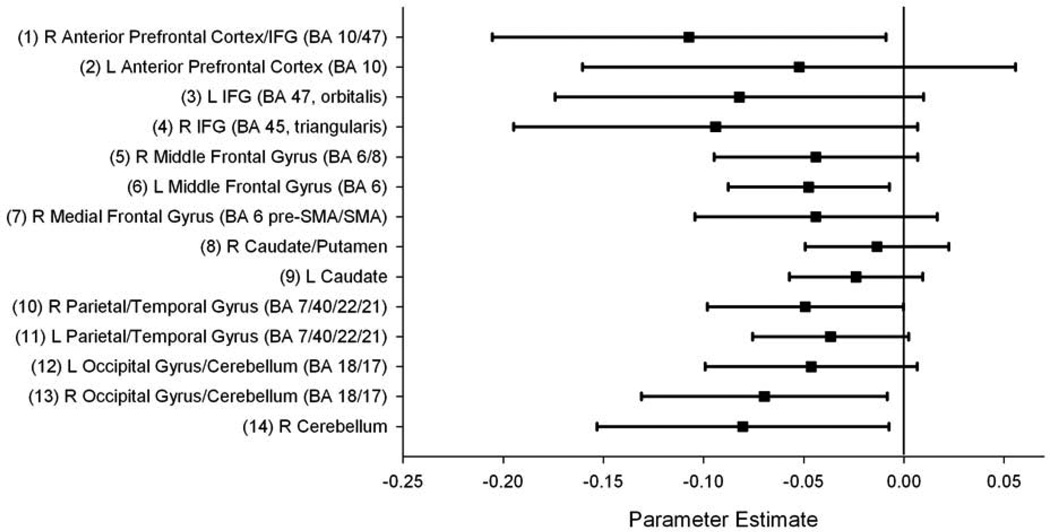
Associations between 7-repeat status and BOLD % signal change in each of the 14 fROIs during correct “No-Go” trials are shown in Table 2. Also shown are the results of statistical tests. Results suggest that during “No-Go” trials, % signal change in right IFG extending into anterior frontal cortex (BA 47/10), left middle frontal gyrus (BA 8), right occipital gyrus extending into the cerebellum, and a distinct region in the right cerebellum were significantly associated with 7-repeat carrier status (Figure 3). Trends for association were also observed between right IFG (pars triangularis), right middle frontal gyrus, bilateral parietal lobe extending into the temporal lobe, and left occipital gyrus extending into the cerebellum. All of the coefficients (γ10) were negative indicating that compared to 7R−, 7R+ showed reduced BOLD % signal change during correct “No-Go” trials.
Figure 3. Effects of DRD4 VNTR 7-Repeat status on BOLD % Signal Change During Correct “No-Go” trials.
Parameter estimates are displayed for each fROI indicating the magnitude and direction of the effect of 7-repeat status on BOLD % signal change (± 95% confidence intervals) during correct “No-Go” trials. A negative parameter estimate indicates lower % signal change during “No-Go” trials in the presence of a 7-repeat allele. Numbers on the x-axis correspond to parameter estimates provided by the multilevel model after correcting for possible non-independence between twins (γ10). Numbers and labels on the y-axis correspond to fROI information provided in Table 2 and Figure 2. Those regions whose 95% confidence intervals did not overlap with 0 were significantly associated with DRD4 VNTR 7-repeat status (Table 2).
3.5.1 Genetic association robustness analyses
We conducted additional analyses to determine whether or not the associations of right middle occipital gyrus, right IFG extending into anterior frontal cortex (BA 47/10), left middle frontal gyrus (BA 8), and the right cerebellum were robust to potential confounding variables: sex, race, and zygosity status. To do so, we entered each as a covariate into the level-one model (such that in separate analyses, each level one-model comprised two predictor variables (carrier status; treated as a random effect) and sex, zygosity or race (treated as fixed effects, due to too few degrees of freedom available to compute level-one error variance with the random effect included). Only zygosity was associated with BOLD % signal change in the right cerebellum (but not with the other regions), t(adjusted df = 25) = −2.76, p < .05. However, zygosity status accounted for variance in right cerebellum that was mostly non-overlapping with variance that was accounted for by carrier status; the association of carrier status with BOLD % signal change remained statistically significant, t(adjusted df = 34) = −2.23, p < .05, when zygosity was included in the model. The covariate-adjusted association of carrier status with right cerebellum was −0.0786. For the other three regions, none of the covariates were associated with BOLD % signal change, and our results remained unchanged.
3.5.2 % variance explained by carrier status
Following Snidjers & Bosker (Snijders & Bosker, 1999), we report variance explained by carrier status. To do so, we used the formula:
where the numerator comprised the variance components defined above that included carrier status in the model (conditional model), and the denominator comprised the same variance components with carrier status not in the model (unconditional model). 7-repeat carrier status significantly accounted for 6.02% of the variance in BOLD % signal change in right middle occipital gyrus, 5.55% of the variance in right IFG extending into anterior frontal cortex (BA 47/10), 4.84% of the variance in left middle frontal gyrus (BA 8), and 5.66% of the variance in right cerebellum.
3.5.3 Correlations between BOLD % signal change during “No-Go” trials and behavior
Correlational analyses did not suggest that there were significant associations between BOLD % signal change during correct “No-Go” trials and accuracy of performance on “No-Go” trials. Furthermore, significant associations were not observed between BOLD % signal change during correct “No-Go” trials and trait impulsiveness as measured by BIS-11 score.
3.6 % Common activation patterns observed during “Go” and “No-Go” trials and follow-up contrasts of BOLD % signal change (7R+ vs. 7R−) in commonly recruited brain regions
The results of the conjunction analysis using the MS/CN method showed that there were four regions in which participants demonstrated common activation during the correct performance of “Go” and “No-Go” trials: right caudate/putamen (x=21, y=−4, z=18, 1,888 µl), left caudate (x=−20, y=−9, z=21, 1,080 µl), left pre-SMA/SMA (x=−1, y=−1, z=57, 384 µl), and the left cerebellum (x=−5, y=−70, z=−18; 256 µl). Follow-up analyses of average BOLD % signal change within these brain regions suggested that the 7R+ group did not demonstrate significantly different BOLD % signal change than the 7R− group during correct “Go” trials within any brain region that was commonly recruited during correct performance of “Go” and “No-Go” trials. As none of these brain regions were the regions in which a difference in BOLD % signal change was observed between 7R+ and 7R− during “No-Go” trials (Table 2, Figures 2 & 3) and no differences were observed between 7R+ and 7R− during “Go” trials within any of these brain regions, this suggests that differences in BOLD % signal change observed between 7R+ and 7R− during “No-Go” trials could not be attributed to differences in brain response observed during “Go” trials.
4. Discussion
The goal of the present study was to evaluate the effect of a functional polymorphism in the dopamine D4 receptor gene on regional changes in hemodynamic activity occurring during inhibition of a prepotent motor response. Specifically, we tested the hypothesis that 18-year-old 7R+ carriers might demonstrate lower fMRI BOLD % signal change in prefrontal cortex and worse performance than 7R− during performance of “No-Go” trials of a Go/No-Go task. Such a finding would be consistent with the hypothesis that possessing a 7-repeat allele might place individuals at greater risk for the development of associated psychiatric conditions involving a disinhibited presentation such as continuing symptoms of ADHD and development of substance abuse disorders. The results of the current study suggested that 7R+ did not demonstrate worse performance than 7R− on the Go/No-Go task even though both groups responded inappropriately on approximately 20% of “No-Go” trials. However, our group of 7R+ demonstrated significantly lower BOLD % signal change than 7R− in four out of 14 brain regions that were recruited during successful inhibition of a prepotent motor response including two brain regions that were located in the prefrontal cortex: the right anterior prefrontal cortex extending into the right IFG and an area in left middle frontal gyrus (BA 6). Furthermore, significant associations between the presence of a 7-repeat allele and lower BOLD % signal change were also observed in other brain regions that were activated during correct inhibition of a motor response including a right occipital lobe region that extended into the cerebellum and a distinct area in right cerebellum. Trends for an association between 7-repeat status and lower BOLD % signal change were also observed in another right IFG region (pars triangularis), a right middle frontal gyrus region (BA 6/8), bilateral parietal areas that extended into the temporal lobes, and in a left occipital region that extended into the cerebellum. Differences in event-related hemodynamic activity between 7R+ and 7R−during “No-Go” trials could not be accounted for by differences in age, sex, zygosity, IQ, trait impulsivity as measured by the BIS-11, the number of alcoholic drinks reportedly consumed per year, the ratio of smokers to non-smokers in the sample, the ratio of those who ever tried to those who never tried marijuana in the sample, or to differences in the hemodynamic response during “Go” trials.
Insufficient sample size could be a possible explanation for the lack of behavioral differences in performance of the Go/No-Go task, but zero out of five previous studies that used the CPT or Go/No-Go tasks in samples of healthy participants found an association between DRD4 7-repeat status and performance on “No-Go” trials even though participants in these studies ranged between 5 and 55 years of age and the sample sizes in these studies ranged between 10 and >100 participants in each group (Barkley, et al., 2006; Colzato, et al., 2010; Heinzel, et al., 2012; Johnson, et al., 2008; Kramer, et al., 2009). Cummins and colleagues also observed no association between DRD4 7-repeat status and SSRT when the Stop Signal task was administered to 405 undergraduate participants (Cummins, et al., 2012). However, this study did not examine whether DRD4 7-repeat status was associated with differences in event-related fMRI BOLD signal. Such associations were only examined within a subsample of participants who had participated in the fMRI protocol (n=50) when genetic variants first demonstrated an association with performance on the Stop Signal task (SSRT) within the entire sample (n=405). Although Gilsbach and colleagues also observed that 7R+ and 7R− groups between the ages of 8 and 15 years of age performed similarly on the IC task, this null behavioral finding was observed within the context of observed differences in recruitment of prefrontal brain regions during fMRI task performance. One possible explanation for these results would follow from neuropsychological theory (Lezak, Howiesen, Bigler, & Tranel, 2012) suggesting that performance on “No-Go” trials of Go/No-Go tasks would only be impacted if certain prefrontal regions thought to be critically involved in inhibitory control were substantially affected by DRD4 7-repeat status. Support for this theory would require evaluation of the degree to which certain brain regions that are thought to be critically involved in inhibitory control are affected by the presence of a DRD4 7-repeat allele during inhibition of a prepotent motor response. We address this hypothesis within the context of reviewing our fMRI findings.
Our sample of 18-year-old twins demonstrated a bilateral pattern of brain activation while correctly inhibiting a motor response. Significant bilateral activation was observed in anterior prefrontal (BA 10), inferior frontal (45/47), middle frontal (BA 6/8), striatal (caudate/putamen), parietal/temporal, and occipital/cerebellar brain regions. Numerous studies that have investigated the neural correlates of inhibitory control have reportedly found significant brain activation in inferior frontal, middle frontal, striatal, and parietal brain regions when Stop-Signal and Go/No-Go tasks are administered (Aron, 2010; Chambers, Garavan, & Bellgrove, 2009; Simmonds, et al., 2008). Furthermore, a meta-analysis showed that activation can be observed in temporal, occipital, and cerebellar brain regions when a version of the Go/No-Go task similar to our own is administered to groups of healthy adults and the neural correlates of “No-Go” trials are contrasted with implicit baseline (Simmonds, et al., 2008). Our version of the Go/No-Go task required use of working memory (remembering the character of the previous target) to successfully complete the task. As such, some studies have purported that the large activation patterns that were observed in dorsolateral prefrontal and parietal areas could be related to the event-related demands on working memory that were associated with administration of our version of the Go/No-Go task (Mostofsky, et al., 2003; Simmonds, et al., 2008). However, other studies have suggested that working memory and inhibitory control may depend on common neural substrates that include the dorsolateral prefrontal and parietal brain regions identified (Hester, Murphy, & Garavan, 2004), and that dependence on common brain regions may account for behavioral decrement in inhibitory control performance under increased working memory demand (Hester & Garavan, 2005). As such, the brain regions that were identified as activated during correct “No-Go” trials in our study may have described areas involved in inhibitory control in addition to those involved in working memory or they may describe the neural correlates of a cognitive control system that is responsible for multiple executive functions while that system is engaged in inhibitory control.
In the present study, associations between DRD4 VNTR 7-repeat status and reduced BOLD % signal change during “No-Go” trials were observed in the right anterior prefrontal cortex/IFG (BA 10/BA 47), middle frontal gyrus (BA 6/8), the occipital lobe/cerebellum (BA 17, 18), and a distinct region in the cerebellum whereas no such associations were observed in striatal regions. Due to lack of a specific ligand, there is still controversy with regard to the regional distribution of D4 receptors in the human brain (McGeary, 2009). However, several studies that have used indirect methods have reported that the density of D4 receptors is highest in prefrontal cortex relative to other brain regions. For example, one study used RT-PCR on human brain tissue (post-mortem) to characterize D4 receptor expression levels (Mulcrone & Kerwin, 1997). Results suggested relatively increased expression levels in areas of frontal lobe, including middle frontal gyrus and cingulate gyrus, the temporal lobe, the hippocampus, the amygdala, the occipital lobe, and cerebellum whereas relatively reduced levels of expression were observed in substantia nigra, caudate, globus pallidus, and the parietal lobe. The results of the present study are highly consistent with this previous report.
When our sample of 18-year-old twins successfully inhibited a prepotent motor response tendency, they demonstrated a pattern of significant brain activity in regions of prefrontal cortex considered critical for inhibitory control including the right IFG and the pre-SMA. Lesion, transcranial magnetic stimulation (TMS), single cell recording, and functional neuroimaging studies suggest that intact function of the right IFG and pre-SMA is critical for intact inhibitory control (Aron, 2010; Chambers, et al., 2009). The results of the present study further revealed that when a prepotent response tendency is successfully inhibited, lower BOLD % signal change may be observed in DRD4 VNTR 7R+ in an area of prefrontal cortex extending from the right IFG (pars orbitalis, BA 47) into the anterior prefrontal cortex (BA 10) (region 1, Table 2, Figure 2). Anterior prefrontal cortex (BA 10) is reliably activated during successful “No-Go” trials when this version of Go/No-Go task is administered (Garavan, et al., 2006; Simmonds, et al., 2008). The results of one study indicated that the extent to which the anterior prefrontal cortex is recruited during inhibitory control may be modulated by the degree to which participants are prepared to inhibit a prepotent response tendency (Kelly, et al., 2004), potentially implicating this brain region in proactive processes related to inhibitory control (Aron, 2010). Recent resting state functional connectivity studies also suggest that the anterior prefrontal cortex, IFG, and medial prefrontal cortex may form a circuit related to task control (Power & Petersen, 2013). Based on this previous literature, we expected that if the right IFG were associated with DRD4 VNTR status it would also be associated with poorer performance on “No-Go” trials of our Go/No-Go task. Contrary to our expectations, we observed that even though DRD4 VNTR 7-repeat status was associated with lower BOLD % signal change in the right IFG/anterior prefrontal cortex (BA 47/10), lower hemodynamic response in this brain region was not associated with poorer “No-Go” trial performance. However, follow-up analyses also revealed that only 5.6% of the variance in BOLD % signal change observed during correct “No-Go” trials could be accounted for by DRD4 VNTR 7-repeat status in this brain region. Thus, the degree to which the right IFG was affected by DRD4 VNTR 7-repeat status may not have been sufficient to produce gross deficits in inhibitory control. This interpretation would be consistent with the results of the study conducted by Gilsbach and colleagues who in their study of healthy children and adolescents also found lower task-related BOLD response in IFG to be associated with DRD4 7-repeat status but also found no evidence for an association between DRD4 7-repeat status and performance on the IC task (Gilsbach, et al., 2012). These findings would seemingly support the view that the effect of DRD4 7-repeat status on inhibitory control and its neural correlates may be subtle.
Although our group of adolescents exhibited significant BOLD % signal change in the pre-SMA/SMA (region 7, Table 2, Figure 2; BA 6) during successful inhibition of a motor response, change in BOLD signal was not associated with DRD4 7-repeat status in this brain region. In our previous study that examined the neural correlates of inhibitory control in a sample of longitudinally-tracked adults showing continuing symptoms of ADHD, we observed that our ADHD group recruited the pre-SMA to a lesser degree than our age-, education-, and IQ-matched control group while successfully performing “No-Go” trials on this version of the Go/No-Go task (Mulligan, et al., 2011). Taken within the context of the results of this previous study, our findings suggest that less recruitment of the pre-SMA during successful inhibitory control may be more related to continuing symptoms of ADHD than it may be to DRD4 7-repeat status per se.
In our sample of 18-year-old participants, DRD4 VNTR 7R+ demonstrated lower BOLD % signal change than 7R− in the left middle frontal gyrus (BA 6) while they correctly inhibited a prepotent motor response. Gilsbach and colleagues also observed lower BOLD response in the left premotor cortex of children and adolescents who possessed at least one 7-repeat allele while they performed an executive function task (IC task). Significant increases in fMRI BOLD response have been repeatedly observed in the left premotor cortex (middle frontal gyrus, BA 6) when participants successfully inhibit a prepotent motor response tendency on “No-Go” trials of Go/No-Go and Stop-Signal tasks. Recent models of inhibitory control suggest that the premotor cortex may either be involved in motor planning during inhibition of a motor response or it may be involved in signaling cancellation of a motor response through an indirect pathway involving subcortical brain regions (Aron, 2010; Chambers, et al., 2009). One study investigated the role the premotor cortex may play in inhibition of a prepotent motor response tendency within the context of a transcranial magnetic stimulation study (Chambers, et al., 2007). Administering repetitive transcranial magnetic stimulation and a Stop Signal Task to a group of healthy adults, the investigators found that stimulation of the left dorsal premotor cortex during performance of “No-Go” trials was not associated with worsening of Go-related reaction time or with longer SSRT, suggesting that the left premotor cortex may not be critically related to execution of a motor response or to inhibitory control. Garavan has remarked that TMS studies might be useful for identifying brain regions necessary but not sufficient for inhibitory control whereas functional neuroimaging studies might identify brain regions sufficient but not necessary for inhibitory control (Garavan, et al., 2006). However, the brain region stimulated in the aforementioned study was ~20 mm superior to the brain region activated in the present study. Furthermore, in the present study, DRD4 VNTR 7-repeat status only accounted for 4.8% of the variance in “No-Go” related BOLD % signal change in left premotor cortex. Thus, lower BOLD % signal change in the left premotor cortex of DRD4 VNTR 7R+ may not have been associated with worse performance during “No-Go” trials on our version of the Go/No-Go task because: 1) it may not be critically associated with response inhibition; 2) it may influence inhibitory control indirectly; 3) or it may not affect DRD4 VNTR status to the degree that it impairs performance on “No-Go” trials on this version of the Go/No-Go task.
While correctly inhibiting a prepotent motor response, our sample of 18-year-old DRD4 7R+ demonstrated lower BOLD % signal change than 7R− in a right occipital brain region that extended into the right cerebellum. Significant activation in the occipital lobe has been consistently observed when Go/No-Go tasks similar to the one used in the present study are administered to samples of healthy adults (Simmonds, et al., 2008). Recruitment of occipital lobe during “No-Go” trials was likely related to identification of a task-relevant stimulus. Although our group of DRD4 VNTR 7R+ demonstrated significantly lower BOLD % signal change than 7R−, DRD4 7-repeat status only accounted for 6.02% of the inhibition-related BOLD response, consistent with the observation that 7R+ and 7R− did not differ in “No-Go” trial accuracy. However, because the functional ROI that was examined included areas of the cerebellum as well as the occipital lobe, an association that was located in the cerebellum may have driven differences in the task-related BOLD response observed within this fROI.
Our sample of 18-year-old DRD4 7R+ also demonstrated lower BOLD % signal change than 7R− in a brain region located exclusively within the right cerebellum while correctly inhibiting a prepotent motor response tendency. Gilsbach and colleagues also observed an association between DRD4 VNTR 7-repeat status and lower BOLD response in the cerebellum when their sample of children and adolescents performed an fMRI task requiring the estimation and comparison of time epochs (Gilsbach, et al., 2012). Consistent with the results of the present study, those investigators observed that 7R+ demonstrated less BOLD response in the cerebellum than 7R− during performance of this task despite equivalent task performance. A meta-analysis of studies using versions of the Go/No-Go task similar to our own reported that healthy adults activate the cerebellum during correct inhibition of a prepotent motor response even under conditions involving limited working memory demand (Simmonds, et al., 2008) suggesting that the cerebellum plays a fundamental role in inhibitory control. Greater recruitment of the cerebellum during inhibition of a motor response has been observed in adults compared to children (Rubia, et al., 2007) and in cocaine dependent samples compared to healthy controls (Hester & Garavan, 2004). These findings suggest that BOLD response in the cerebellum during motor inhibition is sensitive to changes in development and task difficulty. Follow-up analyses within the present study suggested that 5.66% of the variance in BOLD % signal change observed during correct inhibition of a prepotent motor response could be accounted for by DRD4 7-repeat status within the context of equivalent age and performance on the task. Thus, the role of the cerebellum in inhibitory control remains unclear although aspects of timing related to inhibitory control remain an interesting area of investigation for future studies.
There are notable limitations to this study. Although our sample size was large for a typical neuropsychology or fMRI study, it was small by behavioral genetics standards. We attempted to balance control of possible confounds (i.e., possible similarity in task-related brain response between family members or the possible influence of ethnic heterogeneity or sex differences on task-related brain response) with consideration of issues related to sample size and maximal power surrounding our genetic association analyses. Future studies should attempt to replicate our findings in studies with higher sample sizes. Higher sample sizes would also allow for more precise characterization of family-related, ethnicity-related, and sex-related influences on the neural correlates of inhibitory control. Despite these limitations, it is remarkable that associations between DRD4 VNTR 7-repeat status and lower BOLD % signal change were observed in prefrontal brain regions during the performance of “No-Go” trials of our Go/No-Go task as was predicted by our hypotheses. Also, Gilsbach and colleagues (2012) reported associations between DRD4 VNTR 7-repeat status and lower BOLD response in similar brain regions (i.e., inferior frontal gyrus, left middle frontal gyrus, and cerebellum) when a sample of children and adolescents performed an executive function task. This suggests that the association between DRD4 VNTR 7-repeat status and the neural correlates of inhibitory control may remain stable from childhood into young adulthood.
Some authors have advanced hypotheses suggesting that neural functioning related to inhibitory control may depend greatly on DRD4 VNTR 7-repeat status and its interaction with specific environmental factors (Belsky, et al., 2009; Neuman, et al., 2007) or that its effects are accentuated depending on a particular genetic background (Congdon, et al., 2008) or that different loci within the DRD4 gene may also have an influence (e.g., DRD4 −521C/T SNP) or that variation in entirely different genes may have a comparable influence (e.g., COMT). Our sample was relatively small (n=62) and was ascertained from a general population sample. Furthermore, there was limited molecular genetic information collected in relation to those individuals who participated in the longitudinal design. Due to these limitations, we were unable to appropriately address the latter hypotheses within a more atheoretical approach. Thus, we acknowledge the possibility that variation at other loci within the DRD4 gene, in other genes, in relation to gene-environment interactions, or due to gene-gene interactions might also be associated with the neural correlates of inhibitory control within 18-year-olds.
For example, a previous study reported that when a sample of healthy adults between the ages of 18 and 30 performed the Stop Signal Task, greater BOLD response could be observed in the right inferior frontal gyrus of individuals who possessed at least one Met allele in the COMT gene compared to a group that was homozygous for the Val allele (Congdon, Constable, Lesch, & Canli, 2009). The authors interpreted the lower BOLD response in those with two Val alleles to reflect sub-optimal availability of dopamine for neurotransmission due to the greater activity of the enzyme produced by the Val allele in degrading dopamine compared to that of the enzyme produced by the Met allele. They also noted that the COMT gene’s association with the BOLD response within the right inferior frontal gyrus was not just related to the inhibitory-control-related “Stop” trials. The association could also be observed in relation to BOLD response during “Go” trials suggesting that it was related to the function of more general attentional or motor systems. In the present study, associations between DRD4 VNTR 7-repeat status and task-related BOLD response were observed during inhibitory-control-related “No-Go” trials, but when the possibility of an association between BOLD response and DRD4 7-repeat status during “Go” trials was examined within the spatial extent of brain regions activated during correct “No-Go” trials no such association was found. This suggests a specific association between DRD4 VNTR 7-repeat status and neurophysiological response during inhibitory control.
In the present study, possessing at least one allele in which there were 7-repeats in the VNTR of the DRD4 gene was associated with lower BOLD response in prefrontal and cerebellar brain regions during “No-Go” trials, but no differences in behavioral performance of the Go/No-Go task were observed between carriers and non-carriers of this allele. Also, there were no correlations observed between task-related BOLD response observed during “No-Go” trials and indices of performance on the Go/No-Go task. In the absence of such correlations or differences in behavioral performance on the task, less recruitment of prefrontal and cerebellar brain regions could be interpreted as evidence of greater “neural efficiency” in DRD4 VNTR 7-repeat carriers as these individuals recruited prefrontal and cerebellar regions to a lesser degree while achieving a similar level of accuracy on the Go/No-Go task. Considering literature reporting an association between DRD4 VNTR 7-repeat status and 1) dysfunctional dopamine D4 receptor G-protein coupling; 2) inability to heterodimerize with D2 receptors; and 3) a variety of disinhibited neuropsychiatric presentations, we consider this explanation to be less likely. Nevertheless, this hypothesis cannot be ruled out based on the results of the present study, and future studies should explore this hypothesis using methods that can more directly measure receptor function and neural efficiency.
Investigation of all of these hypotheses within future studies will be important as these studies may one day elucidate how genetic and environmental factors combine to produce risk for psychiatric phenotypes at the neurobiological level. However, we believe that reports such as our own are also important as they are hypothesis-driven and might reveal specific information regarding the effect size, neuroanatomical expression, and direction of effects associated with the DRD4 7-repeat gene in relation to regional brain function ultimately helping to explain the mechanisms by which variation at these loci increase the risk for associated neuropsychiatric disorders.
In summary, we observed that DRD4 VNTR 7-repeat status was associated with lower BOLD % signal change in prefrontal areas and in occipital/cerebellar brain regions during inhibition of a premotor motor response tendency in a sample of 18-year-old twins recruited from a general population-based sample. This difference in brain activity was not associated with differences in behavioral performance or with differences in age, sex, zygosity, IQ, trait impulsivity, the number of alcoholic drinks reportedly consumed per year, the ratio of smokers to non-smokers in the sample, or the ratio of those who ever tried to those who never tried marijuana in the sample. Although inhibitory-control-related brain response was associated with DRD4 VNTR 7-repeat status in a brain region that is considered critical for inhibitory control (right IFG), it only accounted for approximately 5% of the variance in this brain region, and there were no differences in behavioral performance of a Go/No-Go task between 7R+ and 7R− groups. fMRI brain response in prefrontal brain regions during inhibitory control may be influenced by DRD4 VNTR 7-repeat status, but this may not penetrate to the level of behavior in a general population sample. Whether the level of contribution from this genetic variant might change under varying environmental conditions or in certain clinical populations remains an important area for future research that may ultimately inform personalized treatment.
Highlights.
We recruited a sample of 62 18-year-old twins from a general population sample.
DRD4 VNTR 7-repeat status is not associated with Go/No-Go task performance.
DRD4 VNTR 7-repeat status is associated with fMRI BOLD % signal change.
DRD4 7-repeat carriers exhibit lower BOLD % signal change during “No-Go” trials.
Lower BOLD % signal change is observed in prefrontal and cerebellar brain regions.
Acknowledgements
We would like to thank Arpana Agrawal, Ph.D. and Blythe Janowiak, Ph.D. for their very helpful comments in relation to the genetic and biochemical models associated with this project. This study was supported by grants from the National Institute on Drug Abuse (NIDA) R01 DA01889, K02 DA027096, and R03 DA27125 to A.A., the McDonnell Center for Systems Neuroscience (McDCSN) to A.R., NIDA grant T32 DA007261 (T.Cicero), NIDA grant R25 DA027995 (P. Madden & J. Rice), and a grant from the ABMRF: The Foundation for Alcohol Research to S.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- Altink ME, Rommelse NN, Slaats-Willemse DI, Vasquez AA, Franke B, Buschgens CJ, Fliers EA, Faraone SV, Sergeant JA, Oosterlaan J, Buitelaar JK. The dopamine receptor D4 7-repeat allele influences neurocognitive functioning, but this effect is moderated by age and ADHD status: an exploratory study. World J Biol Psychiatry. 2011;13:293–305. doi: 10.3109/15622975.2011.595822. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Developmental and genetic influences on prefrontal function in adolescents: a longitudinal twin study of WCST performance. Neurosci Lett. 2010;472:119–122. doi: 10.1016/j.neulet.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2010;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Smith KM, Fischer M, Navia B. An examination of the behavioral and neuropsychological correlates of three ADHD candidate gene polymorphisms (DRD4 7+, DBH TaqI A2, and DAT1 40 bpVNTR) in hyperactive normal children followed to adulthood. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:487–498. doi: 10.1002/ajmg.b.30326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, McClernon FJ, Kollins SH. Cognitive enhancers for the treatment of ADHD. Pharmacol Biochem Behav. 2011;99:262–274. doi: 10.1016/j.pbb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Van Craenenbroeck K, Romero-Fernandez W, Guidolin D, Woods AS, Rivera A, Haegeman G, Agnati LF, Tarakanov AO, Fuxe K. Dopamine D2 and D4 receptor heteromerization and its allosteric receptor-receptor interactions. Biochem Biophys Res Commun. 2011;404:928–934. doi: 10.1016/j.bbrc.2010.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A Developmental Functional MRI Study of Prefrontal Activation during Performance of a Go-No-Go Task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, Kamke M, Mattingley JB. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. J Neurophysiol. 2007;98:3638–3647. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Hum Genet. 1996;98:91–101. doi: 10.1007/s004390050166. [DOI] [PubMed] [Google Scholar]
- Christ SE, White DA, Mandernach T, Keys BA. Inhibitory control across the life span. Dev Neuropsychol. 2001;20:653–669. doi: 10.1207/S15326942DN2003_7. [DOI] [PubMed] [Google Scholar]
- Church JA, Petersen SE, Schlaggar BL. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum Brain Mapp. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Cohen P, West S, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd edition ed. Mahwah, N.J: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WP, Van der Does AJ, Hommel B. Genetic markers of striatal dopamine predict individual differences in dysfunctional, but not functional impulsivity. Neuroscience. 2010;170:782–788. doi: 10.1016/j.neuroscience.2010.07.050. [DOI] [PubMed] [Google Scholar]
- Congdon E, Canli T. A neurogenetic approach to impulsivity. J Pers. 2008;76:1447–1484. doi: 10.1111/j.1467-6494.2008.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Constable RT, Lesch KP, Canli T. Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biol Psychol. 2009;81:144–152. doi: 10.1016/j.biopsycho.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cummins TD, Hawi Z, Hocking J, Strudwick M, Hester R, Garavan H, Wagner J, Chambers CD, Bellgrove MA. Dopamine transporter genotype predicts behavioural and neural measures of response inhibition. Mol Psychiatry. 2012;17:1086–1092. doi: 10.1038/mp.2011.104. [DOI] [PubMed] [Google Scholar]
- Das D, Tan X, Easteal S. Effect of model choice in genetic association studies: DRD4 exon III VNTR and cigarette use in young adults. Am J Med Genet B Neuropsychiatr Genet. 2010;156B:346–351. doi: 10.1002/ajmg.b.31169. [DOI] [PubMed] [Google Scholar]
- Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases of, inhibitory control in reaching. Ann N Y Acad Sci. 1990;608:637–669. doi: 10.1111/j.1749-6632.1990.tb48913.x. discussion 669–676. [DOI] [PubMed] [Google Scholar]
- Ding YC, Chi HC, Grady DL, Morishima A, Kidd JR, Kidd KK, Flodman P, Spence MA, Schuck S, Swanson JM, Zhang YP, Moyzis RK. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proc Natl Acad Sci U S A. 2002;99:309–314. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. J Neuropsychol. 2013;7:193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- Drewe EA. Go - no go learning after frontal lobe lesions in humans. Cortex. 1975;11:8–16. doi: 10.1016/s0010-9452(75)80015-3. [DOI] [PubMed] [Google Scholar]
- Engels WR. Exact tests for Hardy-Weinberg proportions. Genetics. 2009;183:1431–1441. doi: 10.1534/genetics.109.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res Mol Brain Res. 2006;1105:130–142. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsbach S, Neufang S, Scherag S, Vloet TD, Fink GR, Herpertz-Dahlmann B, Konrad K. Effects of the DRD4 genotype on neural networks associated with executive functions in children and adolescents. Dev Cogn Neurosci. 2012;2:417–427. doi: 10.1016/j.dcn.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Rangel-Barajas C, Peper M, Lorenzo R, Moreno E, Ciruela F, Borycz J, Ortiz J, Lluis C, Franco R, McCormick PJ, Volkow ND, Rubinstein M, Floran B, Ferre S. Dopamine D4 receptor, but not the ADHD-associated D4.7 variant, forms functional heteromers with the dopamine D2S receptor in the brain. Mol Psychiatry. 2012;17:650–662. doi: 10.1038/mp.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S, Dresler T, Baehne CG, Heine M, Boreatti-Hummer A, Jacob CP, Renner TJ, Reif A, Lesch KP, Fallgatter AJ, Ehlis AC. COMT × DRD4 epistasis impacts prefrontal cortex function underlying response control. Cereb Cortex. 2012;23:1453–1462. doi: 10.1093/cercor/bhs132. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Working memory and executive function: the influence of content and load on the control of attention. Mem Cognit. 2005;33:221–233. doi: 10.3758/bf03195311. [DOI] [PubMed] [Google Scholar]
- Hester R, Murphy K, Garavan H. Beyond common resources: the cortical basis for resolving task interference. Neuroimage. 2004;23:202–212. doi: 10.1016/j.neuroimage.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Experimental Brain Research. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Kelly SP, Robertson IH, Barry E, Mulligan A, Daly M, Lambert D, McDonnell C, Connor TJ, Hawi Z, Gill M, Bellgrove MA. Absence of the 7-repeat variant of the DRD4 VNTR is associated with drifting sustained attention in children with ADHD but not in controls. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:927–937. doi: 10.1002/ajmg.b.30718. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, Second Edition. Bloomington, MN: Pearson, Inc; 2004. [Google Scholar]
- Kebir O, Joober R. Neuropsychological endophenotypes in attention-deficit/hyperactivity disorder: a review of genetic association studies. Eur Arch Psychiatry Clin Neurosci. 2011;261:583–594. doi: 10.1007/s00406-011-0207-5. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. Eur J Neurosci. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Kidd KK, Pakstis AJ, Yun L. An historical perspective on “The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus”. Hum Genet. 2014;133:431–433. doi: 10.1007/s00439-013-1386-0. [DOI] [PubMed] [Google Scholar]
- Kieling C, Roman T, Doyle AE, Hutz MH, Rohde LA. Association between DRD4 gene and performance of children with ADHD in a test of sustained attention. Biol Psychiatry. 2006;60:1163–1165. doi: 10.1016/j.biopsych.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Konrad K, Dempfle A, Friedel S, Heiser P, Holtkamp K, Walitza S, Sauer S, Warnke A, Remschmidt H, Gilsbach S, Schafer H, Hinney A, Hebebrand J, Herpertz-Dahlmann B. Familiality and molecular genetics of attention networks in ADHD. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:148–158. doi: 10.1002/ajmg.b.30967. [DOI] [PubMed] [Google Scholar]
- Kramer UM, Rojo N, Schule R, Cunillera T, Schols L, Marco-Pallares J, Cucurell D, Camara E, Rodriguez-Fornells A, Munte TF. ADHD candidate gene (DRD4 exon III) affects inhibitory control in a healthy sample. BMC Neurosci. 2009;10:150. doi: 10.1186/1471-2202-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudlicka A, Clare L, Hindle JV. Executive functions in Parkinson's disease: systematic review and meta-analysis. Mov Disord. 2011;26:2305–2315. doi: 10.1002/mds.23868. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, Kennedy JL. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry. 1996;1:121–124. [PubMed] [Google Scholar]
- Langley K, Marshall L, van den Bree M, Thomas H, Owen M, O'Donovan M, Thapar A. Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. Am J Psychiatry. 2004;161:133–138. doi: 10.1176/appi.ajp.161.1.133. [DOI] [PubMed] [Google Scholar]
- Lessov-Schlaggar CN, Lepore RL, Kristjansson SD, Schlaggar BL, Barnes KA, Petersen SE, Madden PA, Heath AC, Barch DM. Functional neuroimaging study in identical twin pairs discordant for regular cigarette smoking. Addict Biol. 2012;18:98–108. doi: 10.1111/j.1369-1600.2012.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howiesen DB, Bigler ED, Tranel D. Neuropsychological assessment. USA: Oxford University Press; 2012. [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum Mol Genet. 2006;15:2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- Lichter JB, Barr CL, Kennedy JL, Van Tol HH, Kidd KK, Livak KJ. A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Hum Mol Genet. 1993;2:767–773. doi: 10.1093/hmg/2.6.767. [DOI] [PubMed] [Google Scholar]
- McGeary J. The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: a review. Pharmacol Biochem Behav. 2009;93:222–229. doi: 10.1016/j.pbb.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL, Watson SJ. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology. 1996;15:17–29. doi: 10.1016/0893-133X(95)00150-C. [DOI] [PubMed] [Google Scholar]
- Mitsis GD, Iannetti GD, Smart TS, Tracey I, Wise RG. Regions of interest analysis in pharmacological fMRI: how do the definition criteria influence the inferred result? Neuroimage. 2008;40:121–132. doi: 10.1016/j.neuroimage.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, Courtney SM, Calhoun VD, Kraut MA, Denckla MB, Pekar JJ. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Res Cogn Brain Res. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Mulcrone J, Kerwin RW. The regional pattern of D4 gene expression in human brain. Neurosci Lett. 1997;234:147–150. doi: 10.1016/s0304-3940(97)00702-7. [DOI] [PubMed] [Google Scholar]
- Mulligan RC, Knopik VS, Sweet LH, Fischer M, Seidenberg M, Rao SM. Neural correlates of inhibitory control in adult attention deficit/hyperactivity disorder: Evidence from the Milwaukee longitudinal sample. Psychiatry Research: Neuroimaging. 2011;194:119–129. doi: 10.1016/j.pscychresns.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Garavan H. Artifactual fMRI group and condition differences driven by performance confounds. Neuroimage. 2004;21:219–228. doi: 10.1016/j.neuroimage.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Neuman RJ, Lobos E, Reich W, Henderson CA, Sun LW, Todd RD. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biol Psychiatry. 2007;61:1320–1328. doi: 10.1016/j.biopsych.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Petersen SE. Control-related systems in the human brain. Curr Opin Neurobiol. 2013;23:223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8:102–108. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models, applications and data analysis methods. 2nd ed. ed. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 7 for Windows. Skokie, IL: Scientific Software International, Inc; 2011. [Google Scholar]
- Ridderinkhof KR, van der Molen MW. Mental resources, processing speed, and inhibitory control: a developmental perspective. Biol Psychol. 1997;45:241–261. doi: 10.1016/s0301-0511(96)05230-1. [DOI] [PubMed] [Google Scholar]
- Roberts GM, Nestor L, Garavan H. Learning and memory deficits in ecstasy users and their neural correlates during a face-learning task. Brain Res. 2009;1292:71–81. doi: 10.1016/j.brainres.2009.07.040. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. Third Edition ed. New York, New York: Oxford University Press; 2006. [Google Scholar]
- Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36:207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Oosterlaan J, Murias M, Schuck S, Flodman P, Spence MA, Wasdell M, Ding Y, Chi HC, Smith M, Mann M, Carlson C, Kennedy JL, Sergeant JA, Leung P, Zhang YP, Sadeh A, Chen C, Whalen CK, Babb KA, Moyzis R, Posner MI. Attention deficit/hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. Proc Natl Acad Sci U S A. 2000;97:4754–4759. doi: 10.1073/pnas.080070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaraich J, Tourneaux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- Van Tol HH, Wu CM, Guan HC, Ohara K, Bunzow JR, Civelli O, Kennedy J, Seeman P, Niznik HB, Jovanovic V. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358:149–152. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Klein N, Wong C, Swanson JM, Shumay E. Association between dopamine D4 receptor polymorphism and age related changes in brain glucose metabolism. PLoS One. 2013;8:e63492. doi: 10.1371/journal.pone.0063492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Ding YC, Flodman P, Kidd JR, Kidd KK, Grady DL, Ryder OA, Spence MA, Swanson JM, Moyzis RK. The genetic architecture of selection at the human dopamine receptor D4 (DRD4) gene locus. Am J Hum Genet. 2004;74:931–944. doi: 10.1086/420854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Kodama G, Baldi P, Moyzis RK. Global landscape of recent inferred Darwinian selection for Homo sapiens. Proc Natl Acad Sci U S A. 2006;103:135–140. doi: 10.1073/pnas.0509691102. [DOI] [PMC free article] [PubMed] [Google Scholar]