Abstract
Background
Asparaginase and steroids can cause hypertriglyceridemia in children with acute lymphoblastic leukemia (ALL). There are no guidelines for screening or management of patients with severe hypertriglyceridemia (>1000 mg/dL) during ALL therapy.
Patients and Methods
Fasting lipid profiles were obtained prospectively at 4 time-points for 257 children consecutively enrolled on a frontline ALL study. Risk factors were evaluated by the exact chi-square test. Details of adverse events and management of hypertriglyceridemia were extracted retrospectively.
Results
Eighteen of 257 (7%) patients developed severe hypertriglyceridemia. Older age and treatment with higher doses of asparaginase and steroids on the standard/high-risk arm were significant risk factors. Severe hypertriglyceridemia was not associated with pancreatitis after adjustment for age and treatment arm or with osteonecrosis after adjustment for age. However, patients with severe hypertriglyceridemia had a 2.5 to 3 times higher risk of thrombosis compared to patients without, albeit the difference was not statistical significant. Of the 30 episodes of severe hypertriglyceridemia in 18 patients, 7 were managed conservatively while the others with pharmacotherapy. Seventeen of 18 patients continued to receive asparaginase and steroids. Triglyceride levels normalized after completion of ALL therapy in all 12 patients with available measurements.
Conclusion
Asparaginase- and steroid-induced transient hypertriglyceridemia can be adequately managed with dietary modifications and close monitoring without altering chemotherapy. Patients with severe hypertriglyceridemia were not at increased risk of adverse events, with a possible exception of thrombosis. The benefit of pharmacotherapy in decreasing symptoms and potential complications requires further investigation.
Keywords: Hypertriglyceridemia, Childhood ALL, Asparaginase, Thrombosis
Introduction
In children with acute lymphoblastic leukemia (ALL), rational use of risk-adapted multidrug chemotherapy regimens and improved supportive care has led to 5-year survival rates of 90% or more [1, 2]. However, effective therapy can cause adverse interactions and complications. For example, co-administration of asparaginase and steroid can cause significant changes in serum lipid levels [3]. Cases of children with ALL have been reported with triglyceride and cholesterol levels as high as 20,600 mg/dL (normal: <130 mg/dL) and 1640 mg/dL (normal: <200 mg/dL), respectively [4].
Hypertriglyceridemia in children treated for ALL is believed to be under-diagnosed, but transient and generally benign [4]. However, triglyceride levels >1,000 mg/dL in the general population increases the risk of acute pancreatitis [5, 6]. In addition, hypertriglyceridemia-induced hyperviscosity syndrome can lead to thromboembolic events [7, 8]. Lipid derangements may also contribute to the development of steroid-induced osteonecrosis [9, 10]. Data on the prevalence, risk factors and complications of severe hypertriglyceridemia in children treated for ALL remain very limited and there is no consensus regarding the management of this condition.
Approximately 0.2% of healthy children in the United States have severe hypertriglyceridemia (>500 mg/dL) [11], but its prevalence can be as high as 8–16% in children with ALL (>1000 mg/dL) [3, 4, 12]. A study on children with ALL showed no association between triglyceride levels and age or gender [12]. However, a systematic evaluation of potential risk factors and complications has not been performed because of the small number of patients studied. There are no clear recommendations on screening patients for hypertriglyceridemia or for continuing asparaginase, steroids or their combination during severe hypertriglyceridemia [13, 14]. Occasionally, life-threatening emergencies have warranted plasmapheresis [15]. However, for asymptomatic patients or in those with milder symptoms, therapy has ranged from observation and dietary modification alone, [13] to steroid omission, [14] or pharmacotherapy with omega-3 fatty acids (FA), [16] fibrates, [12] statins, [17] heparin [12] or insulin [18].
In this study, we report the prevalence, describe the course and review the management of patients with severe hypertriglyceridemia. We also identify risk factors and potential complications associated with this condition in a large cohort of patients treated uniformly for ALL at a single institution.
Methods
Patients
From October 2008 through December 2011, 258 children with newly diagnosed ALL were consecutively enrolled on the Total Therapy XVI study (NCT00549848) at St Jude Children’s Research Hospital, Memphis, TN [19]. All patients were prospectively screened for dyslipidemia except for 1 patient who died early during remission induction therapy. The study was approved by the institutional review board. Informed consent at enrollment from the parents or guardians and assent from patients, when appropriate, were obtained.
Treatment
In brief, therapy comprised of three phases: remission induction, consolidation and continuation which included two blocks of re-induction therapy (Figure 1). Table 1 provides details of steroid and asparaginase use in the study. Patients received 3000 units/m2/dose of peg-asparaginase during induction and were randomized to receive 2500 units/m2 or 3500 units/m2/dose post-induction. During the continuation and re-induction phases, patients on the standard/high-risk arm received uninterrupted peg-asparaginase every other week for 29 weeks (cumulative dose 37,500–52,500 units/m2) and patients on the low-risk arm received peg-asparaginase twice during each of the two re-induction phases (cumulative dose 10,000–14,000 units/m2). Prednisone was used during induction and dexamethasone post-induction.
Figure 1.
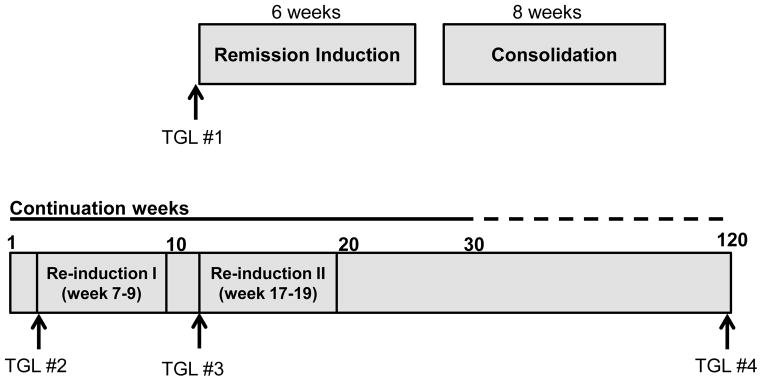
Schematic representation of total XVI therapy.
Arrows represent the timing of screening triglyceride measurements.
Table 1.
Steroids and asparaginase administered to patients enrolled on Total XVI
| Treatment Phase | Drug | Low risk | Standard/high risk | ||
|---|---|---|---|---|---|
| Dose | Total Dose | Dose | Total Dose | ||
| Remission Induction | Prednisone | 40 mg/m2 for 28 days | 1,120 mg/m2 | * Same | * Same |
| Peg-asparaginase | 3,000 units/m2, 1 dose | 3,000 units/m2 | 3,000 units/m2, 1–2 doses | 3,000–6,000 units/m2 | |
| Continuation (weeks 1–6 and 10–16) | Dexamethasone | 8 mg/m2/day for 5 days × 3 pulses | 120 mg/m2 | 12 mg/m2/day for 5 days × 3 pulses | 180 mg/m2 |
| Peg-asparaginase | None | None | 2,500 vs. 3,500 units/m 2, 11 doses | 27,500 vs. 38,500 units/m2 | |
| Re-induction I (continuation weeks 7–9) and Re-induction II (continuation weeks 17–19) | Dexamethasone | 8 mg/m2 for 15 days | 120 mg/m2 | 8 mg/m2 for 15 days | 120 mg/m2 |
| Peg-asparaginase | 2,500 vs. 3,500 units/m2, 4 doses | 10,000 vs. 14,000 units/m2 | Same | Same | |
| Continuation (Total XV weeks 21–28; Total XVI weeks 21–29)^ | Dexamethasone | 8 mg/m2 for 5 days every 4 weeks | 80 mg/m2 | 12 mg/m2 × 5 days every 4 weeks | 120 mg/m2 |
| Peg-asparaginase | None | None | 2,500 vs. 3,500 units/m2, 5 doses | 12,500 vs. 17,500 units/m2 | |
Patients with early T-cell precursor ALL received dexamethasone 10 mg/m2/day from days 1–21, 2 mg/m2/day from days 22–24 and 2 mg/m2/day from days 25–28 (Total dose 230 mg/m2).
Second dose of peg-asparaginase was given if minimal residual disease was ≥ 1% on Day 15
Subsequently, patients received 5-day pulses of dexamethasone every 4 weeks until week 100 of continuation.
Lipid Screening
Fasting lipid profiles comprised serum triglycerides, cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels and were measured prospectively at diagnosis, start of re-inductions I and II and end of therapy. In patients with severe hypertriglyceridemia, additional measurements were obtained when clinically indicated and repeated at least weekly in most patients until <1000 mg/dL. Assays for measurement of triglycerides, cholesterol and HDL were performed on a Cobas 600 analyzer using enzymatic/colorimetric methods (Roche Diagnostics, Indianapolis IN). LDL cholesterol was calculated according to the Friedewald formula [20]. In this report, we focused on patients with severe hypertriglyceridemia defined as a fasting triglyceride level >1000 mg/dL (11.4 mmol/L) which corresponds to a grade 4 adverse event according to the Common Terminology Criteria for Adverse Events version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE). A recurrent episode of severe hypertriglyceridemia was defined as a triglyceride level >1000 mg/dL after reduction to ≤1000 mg/dL for ≥7 days.
Assessment of Risk Factors and Potential Toxicities of Severe Hypertriglyceridemia
Data on patient characteristics, details of therapy, adverse events and management of hypertriglyceridemia were extracted retrospectively from medical records and study databases. For 2–19 year-old patients, body mass index percentiles were calculated and used to define obesity (www.cdc.gov/obesity/childhood). Dyslipidemia was classified according to the guidelines of the National Cholesterol Education Program [21, 22].
Statistical Analyses
To study risk factors associated with the development of severe hypertriglyceridemia, we compared data from patients with triglyceride levels >1000 mg/dL and all other patients using the exact chi-square test. Clinical factors significantly associated with risk of developing severe hypertriglyceridemia in univariate logistic regression models were subsequently included in multiple logistic regression models to identify independent risk factors. Data were analyzed by SAS v9.2 (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
Of the 257 children, 18 (7%) had at least one episode of severe hypertriglyceridemia. Table 2 shows clinical characteristics of these 18 patients (median age 11 years; range 7–18 years). Of the 14 patients for whom family history was available, 10 (71%) had a history of cardiovascular disease. At diagnosis, 4 (22%) patients were obese (BMI ≥95th percentile for age) and 4 (22%) were overweight (BMI 85th–94th percentile). Fifteen (83%) patients had elevated baseline triglyceride levels (≥130 mg/dL), 5 (28%) patients had elevated baseline total cholesterol (median 120 mg/dL; range 97–305 mg/dL), 3 (17%) patients had elevated LDL (median 73 mg/dL; range 39–199 mg/dL) and all patients had decreased HDL (median 19 mg/dL; range 7–35 mg/dL). Seventeen (94%) patients were treated on the standard/high-risk arm of their respective study.
Table 2.
Patient characteristics
| Pt | Age(years)/Gender | Race | Cardiovascular disease in family | ALL subtype | Risk arm* | BMI at diagnosis kg/m2 (percentile) | TGL at diagnosis (mg/dL) | Peak TGL (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 7/M | W | Yes | B | Low | 15.4 (25–50) | 59 | 1619 |
| 2 | 14/F | W | Yes | B | Standard | 24.4 (85–90) | 492 | 1573 |
| 3 | 8/M | W | Yes | B | Standard | 21.7 (>95) | 151 | 6630 |
| 4 | 11/M | W | Yes | T | Standard | 17.5 (50–75) | 152 | 1146 |
| 5 | 8/F | W | No | B | Standard | 15.2 (25–50) | 81 | 4897 |
| 6 | 11/M | A | Yes | B | Standard | 18.8 (75–85) | 171 | 3730 |
| 7 | 18/M | W | Yes | T | Standard | 24.8 (75–85) | 288 | 5420 |
| 8 | 10/F | W | Unknown | T | Standard | 20.27 (85–90) | 239 | 2709 |
| 9 | 18/M | B | Yes | B | Standard | 33.3 (>95) | 160 | 1729 |
| 10 | 7/M | W | Yes | T | Standard | 23.2 (>95) | 658 | 1647 |
| 11 | 11/F | W | Yes | B | Standard | 15.7 (10–25) | 441 | 2399 |
| 12 | 15/M | B | Unknown | B | Standard | 19.9 (50–75) | 75 | 3578 |
| 13 | 8/F | W | No | B | Standard | 15.4 (25–50) | 204 | 1721 |
| 14 | 14/M | W | Yes | B | Standard | 22.3 (75–85) | 175 | 5489 |
| 15 | 12/M | W | Unknown | T | Standard | 20.9 (85–90) | 130 | 2066 |
| 16 | 7/M | W | No | B | Standard | 15.5 (50–75) | 202 | 2606 |
| 17 | 13/M | W | Unknown | T | Standard | 28.1 (>95) | 150 | 1982 |
| 18 | 13/M | W | No | T | Standard | 22.1 (85–90) | 153 | 1660 |
BMI: body mass index; TGL: triglyceride level, F: female; M: male; W: white; B: Black; A: Asian; NOS: not otherwise specified.
For information on risk stratification, see reference 19
Episodes of Severe Hypertriglyceridemia
The 18 patients had 30 isolated episodes of severe hypertriglyceridemia (Table 3). Twenty-eight (93%) episodes occurred within 2 weeks of receiving asparaginase and steroids concurrently, 1 episode after steroid alone and 1 after asparaginase alone. The most common symptoms were diarrhea (N=7), abdominal pain (N=6), fatigue (N=6), nausea/vomiting (N=5) and headache (N=4). One patient had transient blurred vision. No symptoms were reported for 15 (50%) episodes. Pseudohyponatremia and hepatic transaminitis (≥grade 2, i.e. ≥3 x normal) were noted during 19 and 10 episodes respectively. Evidence of a hemolyzed blood sample (hyperkalemia and elevated lactic dehydrogenase in a lipemic sample) was present in 11 episodes. In 21 of 30 (70%) episodes, triglyceride levels were measured at least weekly until <1000 mg/dL. The median duration of these 21 episodes was 7 days (range 1–42 days)
Table 3.
Episodes of severe hypertriglyceridemia
| Pt | Episode | Timing of therapy |
Preceding steroid, asp |
Peak TGL mg/dL |
Days until TGL <1000 mg/dL |
Symptoms | Abnormalities on laboratory tests |
Adverse event (time from peak TGL) |
Medications (duration in days) |
Subsequent asparaginase |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1st 2nd |
Induction Day 24 Induction Day 32 |
Pred, peg-asp Pred |
1619 1127 |
3 1 |
Headache None |
HBS | Cerebral vasculitis (7 days pre) | Cholestyramine (12) Cholestyramine (continue) |
Yes Yes |
| 2* | 1st | Re-induction I Day 1 | Dex, peg-asp | 1573 | NA | Headache, nausea | – | Yes | ||
| 3* | 1st 2nd |
Re-induction II Day 1 Continuation, Week 25 |
Dex, peg-asp Peg-asp |
6630 1454 |
17 NA |
Fatigue, diarrhea, nausea Fatigue, diarrhea |
Transaminitis, hyponatremia HBS Transaminitis |
O-3 FA (74), Fibrate (21) O-3 FA (continue) |
Yes Yes |
|
| 4 | 1st | Re-induction II Day 1 | Dex, peg-asp | 1146 | NA | Diarrhea, abdominal pain | – | Yes | ||
| 5 | 1st 2nd 3rd |
Re-induction I Day 20 Continuation Week 14 Continuation Week 20 |
Dex, peg-asp Dex, peg-asp Dex, peg-asp |
3937 4897 1282 |
22 36 NA |
Fever, nausea, abdominal pain Abdominal pain None |
Hyponatremia Hyponatremia |
O-3 FA (7) Fibrate (30) – |
Yes Yes Yes |
|
| 6* | 1st | Re-induction I Day 12 | Dex, peg-asp | 3730 | 4 | Fatigue, headache | Transaminitis | Pancreatitis (56 days post) | – | Yes (stopped after pancreatitis) |
| 7* | 1st 2nd |
Induction Day 24 Continuation Week 5 |
Pred, peg-asp Dex, peg-asp |
5420 2062 |
16 2 |
Fatigue, emesis, diarrhea, abdominal pain Fatigue, diarrhea |
Hyponatremia, transaminitis Transaminitis |
O-3 FA (365), Fibrate (365) O-3 FA, Fibrate (continue) |
Yes Yes |
|
| 8 | 1st | Re-induction I Day 1 | Dex, peg-asp | 2709 | 19 | Fatigue, diarrhea, abdominal pain, headache, blurred vision | Transaminitis, hyponatremia | Pancreatitis (75 days post) | O-3 FA (30) | Yes (stopped after pancreatitis) |
| 9 | 1st 2nd |
Re-induction I Day 8 Re-induction II Day 1 |
Dex, peg-asp Dex, peg-asp |
1507 1729 |
42 7 |
None None |
O-3 FA (350) O-3 FA (continue), Fibrate (180) |
Yes Yes |
||
| 10* | 1st | Re-induction I Day 1 | Dex, peg-asp | 1647 | 7 | None | Thrombosis: DVT (86 days post) | O-3 FA (7) | Yes | |
| 11 | 1st | Re-induction I Day 13 | Dex, peg-asp | 2399 | 7 | Abdominal pain, fever | HBS | O-3 FA (14) | Only 1 dose (discontinued for hyperbilirubinemia) | |
| 12 | 1st 2nd |
Re-induction I Day 1 Re-induction II Day 1 |
Dex, peg-asp Dex, peg-asp |
3059 3578 |
NA NA |
None None |
HBS | O-3 FA (120) O-3 FA (continue) |
Yes | |
| 13 | 1st | Re-induction I Day 1 | Dex, peg-asp | 1721 | NA | None | – | Yes | ||
| 14 | 1st 2nd 3rd |
Re-induction I week 1 Continuation Week 22 Continuation Week 32 |
Dex, peg-asp Dex, peg-asp Dex, peg-asp |
5489 2036 1790 |
17 25 7 |
None Fever Nausea, emesis |
Hyponatremia, HBS Hyponatremia, HBS HBS |
Thrombosis: DVT, PE (2 days pre) | O-3 FA (77), Fibrate (74) O-3 FA (153), Fibrate (153) O-3 FA, Fibrate (continue) |
Yes Yes (held 1 dose) No (none due) |
| 15 | 1st | Re-induction II Day 1 | Dex, peg-asp | 2066 | NA | None | Thrombosis: DVT, PE (69 days pre) | – | Yes (held 1 dose) | |
| 16 | 1st 2nd 3rd 4th |
Re-induction I Day 8 Continuation, Week 15 Re-induction II Day 14 Continuation Week 30 |
Dex, peg-asp Dex, peg-asp Dex, peg-asp Dex, peg-asp |
1801 1169 1216 2606 |
42 7 5 7 |
None None None Fatigue, diarrhea, back pain |
Transaminitis, hyponatremia HBS Transaminitis, HBS Transaminitis, hyponatremia, HBS |
O-3 FA (180), Fibrate (90) O-3 FA, Fibrate (continue) O-3 FA, Fibrate (continue) O-3 FA (continue), Fibrate (35) |
Yes Yes Yes No (none due) |
|
| 17* | 1st | Re-induction II Day 1 | Dex, peg-asp | 1982 | 14 | None | Transaminitis, HBS | O-3 FA (365), Fibrate (71) | Yes | |
| 18* | 1st | Re-induction II Day 1 | Dex, peg-asp | 1660 | NA | None | Thrombosis: cortical vein (70 days pre) | – | Yes |
TGL: triglyceride; Pred: prednisone; Dex: dexamethasone; Asp: asparaginase; NA: not available; PE: pulmonary embolism; DVT: deep venous thrombosis; O-3FA: omega-3 fatty acids; HBS: hemolyzed blood sample
Patients who developed symptomatic osteonecrosis during therapy
Management of Severe Hypertriglyceridemia
All patients were advised to follow a low-fat diet. Seven episodes were managed conservatively while 8 episodes were managed with omega-3 FA alone, one with fibrate alone (due to intolerance to omega-3 FA) and 12 with a combination of omega-3 FA and fibrate. For 5 of the latter 12 episodes, a fibrate was added after omega-3 FA did not lower triglyceride levels. One patient received cholestyramine (2 episodes).
Seventeen patients continued the planned asparaginase and/or steroids. Asparaginase was permanently discontinued after one additional dose for patient #11 because of recurrent conjugated hyperbilirubinemia (possibly related to the combination of asparaginase, doxorubicin and vincristine during induction and re-induction). One dose of peg-asparaginase was held for patients #14 and #15 during acute thromboembolic episodes. Eight patients had more than one episode of severe hypertriglyceridemia: 5 patients had 2 episodes, 2 patients had 3 episodes and 1 patient had 4 episodes. After completion of ALL therapy, triglyceride levels were available for 12 patients and had normalized in all.
Toxicities in Patients with Severe Hypertriglyceridemia
Significant toxicities possibly associated with severe hypertriglyceridemia were thromboembolism in 4 patients and pancreatitis in 2.
Thromboembolism
Four of 18 (22%) patients with severe hypertriglyceridemia developed thrombosis (including 2 patients with pulmonary embolism) compared to 17 of 239 (7.1%) patients without (P=0.02). However, this comparison did not reach statistical significance in multivariable analysis (P=0.1), possible due to small numbers. In analyses limited to patients treated on the standard/high-risk arm, the incidence of thrombosis was 2.4 times higher in patients with severe hypertriglyceridemia (4 of 17 patients; 23.5%) compared to the others (14 of 143 patients; 9.8%).
Triglyceride levels were measured at the time of the thrombotic event for patients #14 and #18 and were 2036 mg/dL and 637 mg/dL respectively. For patient #10, triglyceride levels was 611 mg/dL two days prior, and for patient #15, it was 521 mg/dL 10 days after the thrombotic event. Patients #15 and #18 were not taking lipid-lowering medications, patient #10 was taking omega-3 FA, and patient #14 had stopped taking omega-3 FA and fibrate one month before the event. Low-molecular weight heparin was initiated for all 4 patients and none of them developed further thromboembolic complications. One additional patient (#1) developed cerebral vasculitis attributed to asparaginase and intrathecal methotrexate therapy.
Pancreatitis
The incidence of pancreatitis did not differ between patients with triglyceride levels >1000 mg/dL (2 of 18 patients; 11%), or ≤1000 mg/dL (19 of 239 patients; 8%) (P=0.63). The 2 patients (#6 and #8; 8%) who developed pancreatitis had triglyceride levels of 444 mg/dL and 611 mg/dL respectively, at the time of the acute event. In both patients, acute pancreatitis developed 3 months after peak triglyceride levels (3730 mg/dL and 2709 mg/dL respectively). Osteonecrosis: Symptomatic osteonecrosis (≥ grade 2) developed in 7 of 18 (39%) patients with hypertriglyceridemia and in 27 of 239 (11%) without (P=0.0009). Results remained significant when adjusted for risk arm (P=0.01), but not for age (P=0.6).
Risk Factors for Severe Hypertriglyceridemia
Race or gender did not differ significantly between patients who developed severe hypertriglyceridemia (N=18) and those who did not (N=239) (Table 4). Baseline triglyceride, total cholesterol, LDL and HDL levels at diagnosis did not influence the development of severe hypertriglyceridemia during therapy. Overweight/obesity was more prevalent in patients who developed severe hypertriglyceridemia compared to those who did not (44% versus 31% respectively), but the difference was not statistically significant (P=0.09). Age > 10 years and therapy on the standard/high-risk arm with higher cumulative doses of peg-asparaginase and dexamethasone, each significantly increased the risk of hypertriglyceridemia (P<0.0001 for age and P=0.0035 for therapy arm). The risk of severe hypertriglyceridemia was similar in patients randomized to receive 2500 units/m2 or 3500 units/m2/dose peg-asparaginase.
Table 4.
Risk factors for severe hypertriglyceridemia
| Factor | Total number of patients (%) | Peak TG >1000mg/dL (%) | Peak TG ≤1000 mg/dL (%) | P-value | |
|---|---|---|---|---|---|
| Age at diagnosis | <10 years | 188 (73.2%) | 6 (33.3%) | 182 (76.2%) | <.0001 |
| ≥10 years | 69 (26.8%) | 12 (66.7%) | 57 (23.8%) | ||
| Gender | Male | 145 (56.4%) | 13 (72.2%) | 132 (55.2%) | 0.16 |
| Female | 112 (43.6%) | 5 (27.8%) | 107 (44.8%) | ||
| Race | White | 196 (76.3%) | 15 (83.3%) | 181 (75.7%) | 0.76 |
| Black | 39 (15.2%) | 2 (11.1%) | 37 (15.5%) | ||
| Other | 22 (8.6%) | 1 (5.6%) | 21 (8.8%) | ||
| Initial WBC | <50,000 | 211 (82.1%) | 17 (94.4%) | 194 (81.2%) | 0.16 |
| ≥50,000 | 46 (17.9%) | 1 (5.6%) | 45 (18.8%) | ||
| BMI at diagnosis | Underweight/Healthy | 139 (54.1%) | 10 (55.6%) | 129 (69.4%) | 0.09 |
| Overweight/Obese | 65 (25.3%) | 8 (44.4%) | 56 (30.6%) | ||
| TGL at diagnosis* | Age Specific Normal | 31 (12.1%) | 2 (11.1%) | 29 (12.6%) | 0.38 |
| Age Specific Borderline High | 42 (16.3%) | 1 (5.6%) | 41 (17.7%) | ||
| Age Specific High | 176 (68.5%) | 15 (83.3%) | 161 (69.7%) | ||
| LDL at diagnosis | Normal (<110 mg/dL) | 215 (83.7%) | 13 (81.3%) | 202 (89.8%) | 0.52 |
| Borderline High (110–129 mg/dL) | 11 (4.3%) | 1 (6.3%) | 10 (4.4%) | ||
| High (>130 mg/dL) | 15 (5.8%) | 2 (12.5%) | 13 (5.8%) | ||
| HDL at diagnosis | Low (<40 mg/dL) | 233 (90.7%) | 18 (100.0%) | 215 (93.1%) | 0.51 |
| Borderline Low (40–45 mg/dL) | 10 (3.9%) | 0 (0.0%) | 10 (4.3%) | ||
| Normal (> 45 mg/dL) | 6 (2.3%) | 0 (0.0%) | 6 (2.6%) | ||
| Total cholesterol at diagnosis | Normal (<169 mg/dL) | 219 (85.2%) | 13 (72.2%) | 206 (89.2%) | 0.09 |
| Borderline High (170–199 mg/dL) | 20 (7.8%) | 3 (16.7%) | 17 (7.4%) | ||
| High (> 200 mg/dL) | 10 (3.9%) | 2 (11.1%) | 8 (3.5%) | ||
| Treatment arm | Low-risk | 97 (37.7%) | 1 (5.6%) | 96 (40.2%) | 0.0035 |
| Standard/High-risk | 160 (62.3%) | 17 (94.4%) | 143 (59.8%) | ||
| Peg-asparaginase randomization (LR arm) | 2500 units/m2 | 56 (56.6%) | 1 (100.0%) | 55 (56.1%) | 0.38 |
| 3500 units/m2 | 43 (43.4%) | 0 (0.0%) | 43 (43.9%) | ||
| Peg-asparaginase randomization (SR/HR arm) | 2500 units/m2 | 91 (57.6%) | 9 (52.9%) | 82 (58.2%) | 0.68 |
| 3500 units/m2 | 67 (42.4%) | 8 (47.1%) | 59 (41.8%) | ||
TGL: triglyceride; WBC: white blood cell count; BMI: body mass index; LDL: low-density lipoprotein; HDL: high-density lipoprotein; LR: low-risk; SR: standard-risk; HR: high-risk
- Age 0–9 years: Normal:
- <75; Borderline High: 75–99; High: ≥100
- Age 10–19 years: Normal: <90; Borderline High: 90–129; High: ≥130
Discussion
This study reports the largest cohort of uniformly treated children with ALL who underwent prospective clinical screening for dyslipidemia during therapy. Older children and those treated with higher-risk therapy are at risk for severe hypertriglyceridemia. While severe hypertriglyceridemia is associated with major acute complications in the general population, this could not be confirmed in children with ALL. However, they may have higher rates of thromboembolic events which are already problematic in this vulnerable population.
Severe hypertriglyceridemia occurred in 7% of our patients with ALL and was temporally related to steroid and asparaginase administration as described previously [3, 12]. Corticosteroids increase triglyceride synthesis and also increase the activity of lipoprotein lipase, a key enzyme required for the hydrolysis of triglycerides. On the other hand, asparaginase can inhibit the activity of lipoprotein lipase[23]. When asparaginase and steroids are given together, it is likely that triglyceride-rich lipoproteins are rapidly formed but insufficiently cleared[23]. Patients with severe hypertriglyceridemia may be asymptomatic or develop non-specific symptoms, and thus a high index of suspicion must be maintained, especially during intensive asparaginase and steroid therapy. However, symptoms such as diarrhea, abdominal pain and fatigue can be caused by multiple chemotherapeutic agents and co-morbidities during ALL therapy and are difficult to attribute to hypertriglyceridemia alone. Hypertriglyceridemia should also be suspected in patients with transaminitis and/or hyponatremia of unclear etiology. As ex vivo hemolysis often occurs in lipemic blood samples [24], especially if tubes are not handled gently; samples should be carried by hand to the laboratory instead of using a pneumatic tube system.
Therapy on the standard/high-risk arm with higher doses of asparaginase and steroids and older age (two features that are highly correlated), were significantly associated with a higher risk of severe hypertriglyceridemia. It is recognized that older patients have delayed clearance and increased systemic exposure of steroids given the same dosages compared to younger patients; an observation which may partly explain the association between older age and the development of hypertriglyceridemia [25]. The majority of our patients (218 of 257 patients; 85%) had mild to moderate elevations in baseline triglycerides before chemotherapy was initiated, as reported in patients with ALL and other cancers [26, 27]. In contrast, only 10% of healthy children have triglyceride levels >150 mg/dL [11]. Because hypertriglyceridemia resolves after completion of ALL therapy, it has been speculated that this lipid derangement is a manifestation of an acute phase or immunologic response [27, 28].
Although the association of hypertriglyceridemia with coronary artery disease is well known, [29] its association with venous thromboembolism is unclear and might be related to changes in the fibrinolytic system[30]. A few case reports describe thrombosis in ALL patients with hypertriglyceridemia [7, 12] but in our study, approximately 20% of patients with severe hypertriglyceridemia developed venous thromboembolism. The combination of asparaginase, steroids and triglycerides causes a hypofibrinolytic state in the setting of hyperviscosity which may explain the increased risk of thromboembolism. Whether lowering triglyceride levels with pharmacotherapy or interventions like prophylactic anticoagulants decreases the risk of thrombosis in these patients remains unclear, but the latter approach was advocated in one report and merits further investigation [12].
In adults, severe hypertriglyceridemia is a well-described risk factor for pancreatitis and fibrates are recommended as first-line therapy [5]. Similarly, expert guidelines for children recommend pharmacotherapy and referral to a lipid specialist to prevent pancreatitis [22]. Acute pancreatitis is a well-known complication of leukemia therapy due to the use of asparaginase, steroids and thiopurines [31, 32]. However, in our study and other reports of children with ALL, severe hypertriglyceridemia did not increase the risk of pancreatitis [3, 12].
The association of osteonecrosis with hypertriglyceridemia requires additional investigation. Although the incidence of osteonecrosis was high in patients with severe hypertriglyceridemia in our study, it was primarily related to age; patients ≥10 years old develop both osteonecrosis and hypertriglyceridemia more frequently than younger children. In an animal model of steroid-induced osteonecrosis, significant elevations in cholesterol, triglycerides and free fatty acids occurred concurrently with the onset of osteonecrosis, approximately 1 week after steroid injection and it was proposed that fat embolism contributed to the development of osteonecrosis [9]. In a clinical study of osteonecrosis during childhood ALL therapy, hypercholesterolemia (not hypertriglyceridemia) increased the risk of symptomatic osteonecrosis [10].
Our study has some limitations. The absence of a screening time-point during or at the end of remission induction therapy did not give us the opportunity to investigate the incidence and complications hypertriglyceridemia when patients were receiving prednisone instead of dexamethasone in combination with asparaginase. Since the majority of episodes of hypertriglyceridemia occurred after dexamethasone and asparaginase given together, it is plausible that dexamethasone may be more potent than prednisone in causing hypertriglyceridemia. The number of patients with adverse events is low, making it difficult to determine definite associations. Thus, additional studies are needed to investigate the contribution of hypertriglyceridemia in the development of toxicities such as thromboembolism. Also, the management of severe hypertriglyceridemia was not uniform and differed by treating physicians. Because of low patient numbers and lack of adequate information on duration of episodes, it is unclear whether pharmacotherapy, either single agent or in combination, could reduce symptoms and adverse events potentially related to hypertriglyceridemia. Fibrates may help reduce triglyceride levels rapidly and decrease the risk of hypertriglyceridemia-associated complications [5, 33]. However, fibrates should be used with extreme caution in children with ALL because of their potential hepatotoxicity, especially in combination with other hepatotoxic chemotherapeutic agents. Randomized trials of early interventions, either dietary modifications in patients at risk, or pharmacotherapy in patients with severe hypertriglyceridemia are needed to study the benefit of these interventions.
In summary, severe hypertriglyceridemia, an untoward effect of asparaginase and steroid therapy can cause troublesome symptoms and laboratory abnormalities. Severe hypertriglyceridemia during ALL therapy is transient and rarely associated with significant acute complications other than a possible increased risk of thrombosis. Given the risk of the underlying malignancy, steroids or asparaginase should not be held for severe hypertriglyceridemia and can be continued under close observation. Although routine screening is likely not required in clinical practice, long-term follow up of patients with history of severe hypertriglyceridemia is recommended to better understand additional therapy-related risk factors for the development of metabolic syndrome and cardiovascular disease which are significant concerns in ALL survivors [34, 35].
Highlights.
Older age and higher doses of asparaginase are risk factors for hypertriglyceridemia
Hypertriglyceridemia can be adequately managed without altering ALL therapy
Hypertriglyceridemia was not associated with pancreatitis in children with ALL
Acknowledgments
Funding: This work was supported by the National Institutes of Health grant GM92666 (MVR), P30-CA021765 and the American Lebanese Syrian Associated Charities (all authors).
The authors thank Vani Shankar from the Department of Scientific Editing, St Jude Children’s Research Hospital for assistance with editing the manuscript
Footnotes
Conflicts of interest statement
No conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14):1663–9. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons SK, Skapek SX, Neufeld EJ, Kuhlman C, Young ML, Donnelly M, et al. Asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Blood. 1997;89(6):1886–95. [PubMed] [Google Scholar]
- 4.Steinherz PG. Transient, severe hyperlipidemia in patients with acute lymphoblastic leukemia treated with prednisone and asparaginase. Cancer. 1994;74(12):3234–9. doi: 10.1002/1097-0142(19941215)74:12<3234::aid-cncr2820741224>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969–89. doi: 10.1210/jc.2011-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195–203. doi: 10.1097/01.mcg.0000436438.60145.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietel V, Buhrdel P, Hirsch W, Korholz D, Kiess W. Cerebral sinus occlusion in a boy presenting with asparaginase-induced hypertriglyceridemia. Klin Padiatr. 2007;219(2):95–6. doi: 10.1055/s-2007-921455. [DOI] [PubMed] [Google Scholar]
- 8.Rosenson RS, Shott S, Tangney CC. Hypertriglyceridemia is associated with an elevated blood viscosity Rosenson: triglycerides and blood viscosity. Atherosclerosis. 2002;161(2):433–9. doi: 10.1016/s0021-9150(01)00656-6. [DOI] [PubMed] [Google Scholar]
- 9.Kabata T, Kubo T, Matsumoto T, Hirata T, Fujioka M, Takahashi KA, et al. Onset of steroid-induced osteonecrosis in rabbits and its relationship to hyperlipaemia and increased free fatty acids. Rheumatology (Oxford) 2005;44(10):1233–7. doi: 10.1093/rheumatology/keh721. [DOI] [PubMed] [Google Scholar]
- 10.Kawedia JD, Kaste SC, Pei D, Panetta JC, Cai X, Cheng C, et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117(8):2340–7. doi: 10.1182/blood-2010-10-311969. quiz 2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christian JB, Juneja MX, Meadowcroft AM, Borden S, Lowe KA. Prevalence, characteristics, and risk factors of elevated triglyceride levels in US children. Clin Pediatr (Phila) 2011;50(12):1103–9. doi: 10.1177/0009922811414286. [DOI] [PubMed] [Google Scholar]
- 12.Cohen H, Bielorai B, Harats D, Toren A, Pinhas-Hamiel O. Conservative treatment of L-asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54(5):703–6. doi: 10.1002/pbc.22305. [DOI] [PubMed] [Google Scholar]
- 13.Berrueco R, Rives S, Lopez-Garcia VS, Catala A, Toll T, Estella J. Very high hypertriglyceridemia induced: is plasmapheresis needed? Pediatr Blood Cancer. 2011;57(3):532. doi: 10.1002/pbc.23177. [DOI] [PubMed] [Google Scholar]
- 14.Tong WH, Pieters R, van der Sluis IM. Successful management of extreme hypertriglyceridemia in a child with acute lymphoblastic leukemia by temporarily omitting dexamethasone while continuing asparaginase. Pediatr Blood Cancer. 2012;58(2):317–8. doi: 10.1002/pbc.23266. [DOI] [PubMed] [Google Scholar]
- 15.Solano-Paez P, Villegas JA, Colomer I, Gutierrez MD, Fernandez-Teijeiro A. L-Asparaginase and steroids-associated hypertriglyceridemia successfully treated with plasmapheresis in a child with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33(3):e122–4. doi: 10.1097/MPH.0b013e3181faf7a1. [DOI] [PubMed] [Google Scholar]
- 16.Bostrom B. Successful management of extreme hypertriglyceridemia from pegaspargase with omega-3. Pediatr Blood Cancer. 2012;59(2):350. doi: 10.1002/pbc.24108. [DOI] [PubMed] [Google Scholar]
- 17.Lashkari HP, Lancaster D, Atra A, Champion MP, Taj MM. Symptomatic severe hypertriglyceridaemia with asparaginase therapy in acute lymphoblastic leukaemia (ALL) and lymphoblastic lymphoma: is rechallenging safe? Int J Hematol. 2011;94(6):571–5. doi: 10.1007/s12185-011-0966-9. [DOI] [PubMed] [Google Scholar]
- 18.Lawson EB, Gottschalk M, Schiff DE. Insulin infusion to treat severe hypertriglyceridemia associated with pegaspargase therapy: a case report. J Pediatr Hematol Oncol. 2011;33(2):e83–6. doi: 10.1097/MPH.0b013e3181f46c22. [DOI] [PubMed] [Google Scholar]
- 19.Pui CH, Campana D, Sandlund JT, Bhojwani D, Evans WE, Relling MV, et al. Treatment of childhood acute lymphoblastic leukemia without cranial irradiation. Ann Hematol. 2011;90 (Suppl 1):S61–63. [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 21.NCEP. National Cholesterol Education Program: highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89(3):495–501. [PubMed] [Google Scholar]
- 22.Daniels SR, Benuck I, Christakis DA, Dennison BA, Gidding SS, Gillman MW, et al. Full report of the expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents (NIH Publication No. 12-7486) National Heart, Lung, and Blood Institute; 2012. pp. 1–181. [Google Scholar]
- 23.Hoogerbrugge N, Jansen H, Hoogerbrugge PM. Transient hyperlipidemia during treatment of ALL with L-asparaginase is related to decreased lipoprotein lipase activity. Leukemia. 1997;11(8):1377–9. doi: 10.1038/sj.leu.2400703. [DOI] [PubMed] [Google Scholar]
- 24.Dimeski G, Mollee P, Carter A. Increased lipid concentration is associated with increased hemolysis. Clin Chem. 2005;51(12):2425. doi: 10.1373/clinchem.2005.058644. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Panetta JC, Cai X, Yang W, Pei D, Cheng C, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008;26(12):1932–9. doi: 10.1200/JCO.2007.13.8404. [DOI] [PubMed] [Google Scholar]
- 26.Bielecka-Dabrowa A, Hannam S, Rysz J, Banach M. Malignancy-associated dyslipidemia. Open Cardiovasc Med J. 2011;5:35–40. doi: 10.2174/1874192401105010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moschovi M, Trimis G, Apostolakou F, Papassotiriou I, Tzortzatou-Stathopoulou F. Serum lipid alterations in acute lymphoblastic leukemia of childhood. J Pediatr Hematol Oncol. 2004;26(5):289–93. doi: 10.1097/00043426-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Scribano D, Baroni S, Pagano L, Zuppi C, Leone G, Giardina B. Return to normal values of lipid pattern after effective chemotherapy in acute lymphoblastic leukemia. Haematologica. 1996;81(4):343–45. [PubMed] [Google Scholar]
- 29.Murad MH, Hazem A, Coto-Yglesias F, Dzyubak S, Gupta S, Bancos I, et al. The association of hypertriglyceridemia with cardiovascular events and pancreatitis: a systematic review and meta-analysis. BMC Endocr Disord. 2012;12:2. doi: 10.1186/1472-6823-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Schouwenburg IM, Mahmoodi BK, Gansevoort RT, Muntinghe FL, Dullaart RP, Kluin-Nelemans HC, et al. Lipid levels do not influence the risk of venous thromboembolism. Results of a population-based cohort study. Thromb Haemost. 2012;108(5):923–9. doi: 10.1160/TH12-06-0426. [DOI] [PubMed] [Google Scholar]
- 31.Samarasinghe S, Dhir S, Slack J, Iyer P, Wade R, Clack R, et al. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2013;162(5):710–3. doi: 10.1111/bjh.12407. [DOI] [PubMed] [Google Scholar]
- 32.Nitsche C, Maertin S, Scheiber J, Ritter CA, Lerch MM, Mayerle J. Drug-induced pancreatitis. Curr Gastroenterol Rep. 2012;14(2):131–8. doi: 10.1007/s11894-012-0245-9. [DOI] [PubMed] [Google Scholar]
- 33.Manlhiot C, Larsson P, Gurofsky RC, Smith RW, Fillingham C, Clarizia NA, et al. Spectrum and management of hypertriglyceridemia among children in clinical practice. Pediatrics. 2009;123(2):458–65. doi: 10.1542/peds.2008-0367. [DOI] [PubMed] [Google Scholar]
- 34.Jung HS, Myung SK, Kim BS, Seo HG. Metabolic syndrome in adult cancer survivors: a meta-analysis. Diabetes Res Clin Pract. 2012;95(2):275–82. doi: 10.1016/j.diabres.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 35.Oudin C, Simeoni MC, Sirvent N, Contet A, Begu-Le Coroller A, Bordigoni P, et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood. 2011;117(17):4442–8. doi: 10.1182/blood-2010-09-304899. [DOI] [PubMed] [Google Scholar]


