Abstract
As a basis for venous thromboembolism (VTE) prophylaxis after traumatic brain injury (TBI), we have previously published an algorithm known as the Parkland Protocol. Patients are classified by risk for spontaneous progression of hemorrhage with chemoprophylaxis regimens tailored to each tier. We sought to validate this schema. In our algorithm, patients with any of the following are classified “low risk” for spontaneous progression: subdural hemorrhage ≤8 mm thick; epidural hemorrhage ≤8 mm thick; contusions ≤20 mm in diameter; a single contusion per lobe; any amount of subarachnoid hemorrhage; or any amount of intraventricular hemorrhage. Patients with any injury exceeding these are “moderate risk” for progression, and any patient receiving a monitor or craniotomy is “high risk.” From February 2010 to November 2012, TBI patients were entered into a dedicated database tracking injury types and sizes, risk category at presentation, and progression on subsequent computed tomgraphies (CTs). The cohort (n=414) was classified as low risk (n=200), moderate risk (n=75), or high risk (n=139) after first CT. After repeat CT scan, radiographic progression was noted in 27% of low-risk, 53% of moderate-risk, and 58% of high-risk subjects. Omnibus analysis of variance test for differences in progression rates was highly significant (p<0.0001). Tukey's post-hoc test showed the low-risk progression rate to be significantly different than both the moderate- and high-risk arms; no difference was noted between the moderate- and high-risk arms themselves. These criteria are a valid tool for classifying TBI patients into two categories of risk for spontaneous progression. This supports tailored chemoprophylaxis regimens for each arm.
Key words: : progression, TBI, validation, venous thromboembolism
Introduction
The optimal timing for the initiation of venous thromboembolism (VTE) prophylaxis in patients with traumatic brain injury (TBI) is controversial. National guidelines are frustratingly vague on this topic, with the Brain Trauma Foundation simply stating that low-molecular-weight heparin or low-dose unfractionated heparin should be used in conjunction with a means of mechanical prophylaxis at some point in a TBI patient's convalescence, and that chemical prophylaxis carries a risk for propagation of brain injury.1 The only other recent, major guidelines to guide caregivers in initiating chemoprophylaxis after TBI are those of the American College of Chest Physicians, who suggest that mechanical prophylaxis be used until the risks of intracranial bleeding expansion are felt to have abated enough that pharmacological prophylaxis can be safely initiated.2 Our group has previously shown that TBI is a heterogeneous population of injuries with regard to the risk of spontaneous progression of intracranial injury.3 If the risk for expansion of hemorrhage is heterogeneous, it stands to reason that the time to stabilization of hemorrhage is as well as would be the time frame for the safe initiation of chemoprophylaxis. In recognition of this spectrum of risk, we have constructed and promulgated a theoretical treatment algorithm for VTE prophylaxis after TBI, which we are calling the “Parkland Protocol.”4,5 Based on a modification of injury patterns initially put forth by Berne and Norwood,6–8 our original protocol and its subsequent iterations classify TBI patients as low, moderate, or high risk for spontaneous progression of their intracranial hemorrhage (ICH) pattern and tailor a VTE prophylaxis regimen to each arm (Fig. 1). The aim of this study was to internally validate the existence of three tiers of risk for spontaneous progression of ICH within the Parkland Protocol.
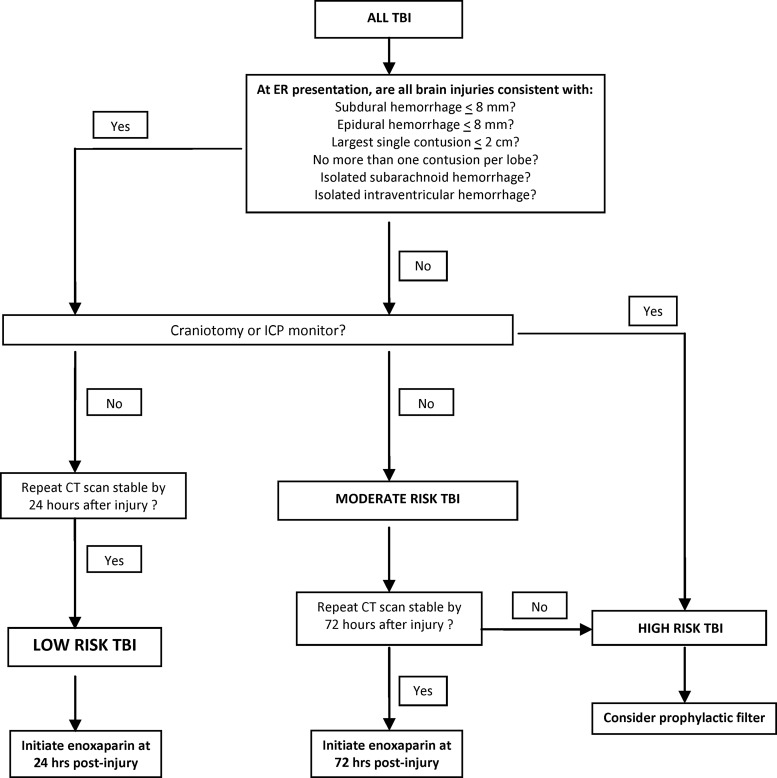
FIG. 1.
The original iteration of the Parkland Protocol before validation. Three tiers of risk for radiographic progression of intracranial hemorrhage patterns existed in this version of the protocol, each with a tailored recommendation for thromboprophylaxis. TBI, traumatic brain injury; ICP, intracranial pressure; CT, computed tomography.
Methods
We performed an institutional review board (IRB)-approved (UTSW IRB #STU 062012-023) retrospective review of all patients presenting to our urban level 1 trauma center with ICH between February 2010 and November 2012. During this time, all patients presenting with ICH were prospectively entered into a dedicated TBI database, which, in addition to standard demographics, tracked intracranial injury type and size, Parkland Protocol risk category at presentation and at final disposition, any clinical change in neurological exam, any radiographic progression on subsequent computed tomography (CT) scans, and the timing and type of VTE prophylaxis administration. All head CT scans were reread by a neurosurgeon or radiologist, and it was this interpretation that served as the data source. For the purposes of this investigation, we excluded those patients who only had one CT scan throughout their clinical course as well as those who were on warfarin at the time of their injury. Subjects on antiplatelet therapy at the time of their injury were included in the sample. During the time period of this study, it was our local standard of care to not perform duplex screening of the extremities of asymptomatic TBI patients. Duplex ultrasounds were performed based on the physical finding of a swollen extremity, whereas CT angiography was performed for the finding of idiopathic hypoxia and/or tachycardia.
Patients were assigned to one of the Parkland Protocol's three risk categories at presentation. These risk categories represent the risk of spontaneous progression of the ICH pattern before initiation of pharmacological anticoagulation for VTE prophylaxis. Low-risk TBI are those patients with any or all of the following: a subdural hemorrhage ≤8 mm at its thickest point, epidural hemorrhage ≤8 mm thick, no more than a single parenchymal contusion per lobe, all contusions ≤20 mm in their greatest diameters, and/or any amount of subarachnoid hemorrhage or intraventricular hemorrhage. Moderate-risk TBI included any injury larger than the low-risk criteria, but not undergoing craniotomy or monitor placement. High-risk TBI were those patients with any TBI that underwent craniotomy or monitor placement.
Repeat CT scans were performed between 6 and 24 h after injury at the discretion of the attending trauma surgeon or neurosurgeon at the point of care in a nonstandardized fashion, either for clinical concerns of progression or for routine screening for progression. Progression on head CT scan was defined as any enlargement of an existing lesion or the development of a new lesion. The incidence and timing of radiographic progression before chemoprophylaxis initiation was recorded for each category.
Because our goal was to validate the modified Berne-Norwood criteria as predictors of injury progression, we focused our analysis on spontaneous progression between the time of initial placement in one of the three risk categories and the subsequent CT scan. Per protocol, patients may or may not be reclassified in a different risk category if they experience progression on their second CT scan (e.g., a low-risk patient showing radiographic progression on subsequent CT scans will be reclassified as moderate risk, but a moderate-risk who progresses would only be reclassified as high risk if they subsequently underwent monitor placement or craniotomy). Therefore, progression, rather than reclassification, was the endpoint of the study.
An omnibus analysis of variance (ANOVA) was performed to assess for significant differences in spontaneous progression rates among the three risk categories overall, which was followed by a Tukey's post-hoc comparison. Descriptive statistics were calculated for the incidence and time frame of pharmacological VTE prophylaxis initiation. SAS version 9.3 (Cary, NC) was used for all statistical analyses.
Results
The study population consisted of 414 subjects who had ICH and at least one repeat CT scan of the head. At the time of admission in the emergency department, 200 patients were classified as low risk, 75 as moderate risk, and 139 as high risk according to the original iteration of the Parkland Protocol (Table 1). In this sample, radiographic progression before initiation of pharmacological prophylaxis occurred in 29% of low-risk patients, 53% of moderate-risk patients, and 58% of high-risk patients. The overall ANOVA test was highly significant (p<0.0001). Tukey's post-hoc test indicated that the low-risk category differed from the moderate- and high-risk groups, and that the latter two did not differ from one another (Table 2).
Table 1.
Descriptive Statistics for the Three Categories of Brain Injuries as Classified at the Admission in the Emergency Department
| Statistic | Low risk (n=200) | Moderate risk (n=75) | High risk (n=139) |
|---|---|---|---|
| Age, years | 42.6±20.3 | 46.9±21.7 | 37.5±21.8 |
| Female/male, % | 31/69 | 20/80 | 17/83 |
| Blunt/penetrating, % | 99/1 | 93/7 | 95/5 |
| Injury Severity Score | 23.8±10.7 | 25.3±11.3 | 31.8±11.4 |
| Head and neck AIS | 3.8±0.7 | 4.2±0.6 | 4.5±0.7 |
| Abdomen AIS | 0.6±1.2 | 0.5±1.1 | 0.7±1.2 |
| Chest AIS | 1.3±1.6 | 1.3±1.6 | 1.7±1.7 |
| Emergency department GCS | 11.7±4.4 | 11.1±4.2 | 6.5±3.9 |
| Packed red cells at 24 h | 0.7±2.1 | 0.4±0.7 | 1.6±2.9 |
| ICU Length of stay, days | 4.5±6.4 | 5.6±6.0 | 9.5±8.1 |
| Hospital length of stay, days | 10.2±13.2 | 11.2±9.3 | 19.2±16.4 |
| Mortality, % | 4 | 9 | 25 |
AIS, Abbreviated Injury Score; GCS, Glasgow Coma Score; ICU, intensive care unit.
Table 2.
Analysis of Variance Omnibus Testing Demonstrated a Significant Intergroup Difference
| Low risk (n=200) | Moderate risk (n=75) | High risk (n=139) | Omnibus p | |
|---|---|---|---|---|
| Radiographic progression rate (%) | 29 | 53 | 58 | <0.0001 |
| Distinct group membership | (+) | (−) | (−) |
Post-hoc testing showed that the low-risk group comprised a distinct subgroup, whereas no significant difference was found between the moderate- and high-risk groups.
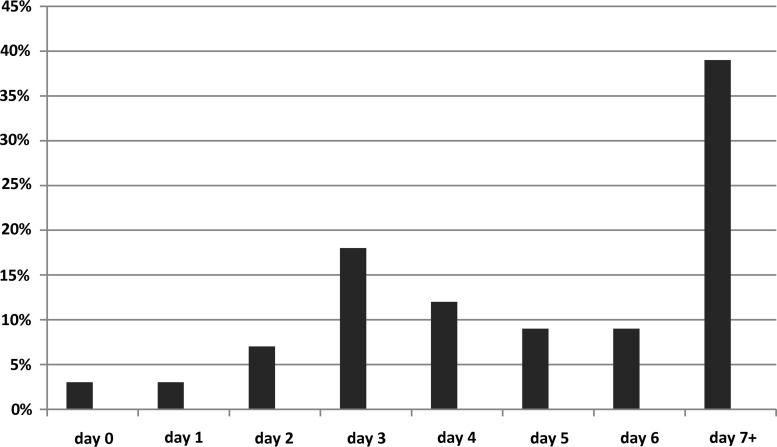
A subset analysis of a group created by pooling the moderate- and high-risk categories demonstrated that 36% of these subjects received no prophylactic anticoagulation before discharge or death. Of the remaining 64% (n=186), a bimodal distribution was observed when considering the day after injury on which prophylactic anticoagulation was initiated, with the most common being days 3 and 7 or greater. Note that the data in Figure 2 consist only of those patients in the newly created high-risk category (n=186). For those patients receiving prophylaxis, it was initiated as follows: 5.0±4.7 days for the overall cohort; 5.0±4.9 days for the low-risk patients; 5.0±4.1 for the moderate-risk patients; and 5.1±5.5 for the high-risk patients.
FIG. 2.
Day after injury that subjects in the validated Parkland Protocol's newly created high-risk category had thromboprophylaxis initiated (n=186) among those subjects in whom it was started. Peaks were noted at days 3 and 7 or beyond.
Using clinical suspicion for VTE as a trigger for diagnostic testing, 12.8% of the overall cohort underwent at least one extremity duplex, 4.8% underwent at least one CT angiogram of the chest, and 1.0% underwent at least one of both. The overall cohort demonstrated a VTE rate of 2.4% (deep vein thrombosis [DVT]=5; pulmonary embolism [PE]=5). No patient was found to have both DVT and PE.
Two hundred thirty-eight subjects were excluded from the analysis because of only having received a single CT of the head during their entire hospital stay. Of this group of patients, 76% were classified as low risk based on their presenting CT of the head done in the emergency department, 20% were moderate risk, and 4% were high risk. Additionally, 51% of this single-CT group were discharged or died by 48 h after injury. Because no repeat CT head was done, the incidence of radiographic progression among these patients is unknown.
Discussion
This investigation internally validated the Parkland Protocol's injury criteria as predictors of two tiers of risk for spontaneous progression of traumatic ICH. This has led us to reconstruct the Parkland Protocol to reflect this reality (Fig. 3). Additionally, a post-hoc review of the newly designated high-risk category showed that, despite an emphasis by our group on earlier chemical thromboprophylaxis after TBI, many of these patients are receiving delayed initiation of prophylaxis, if it occurs at all.
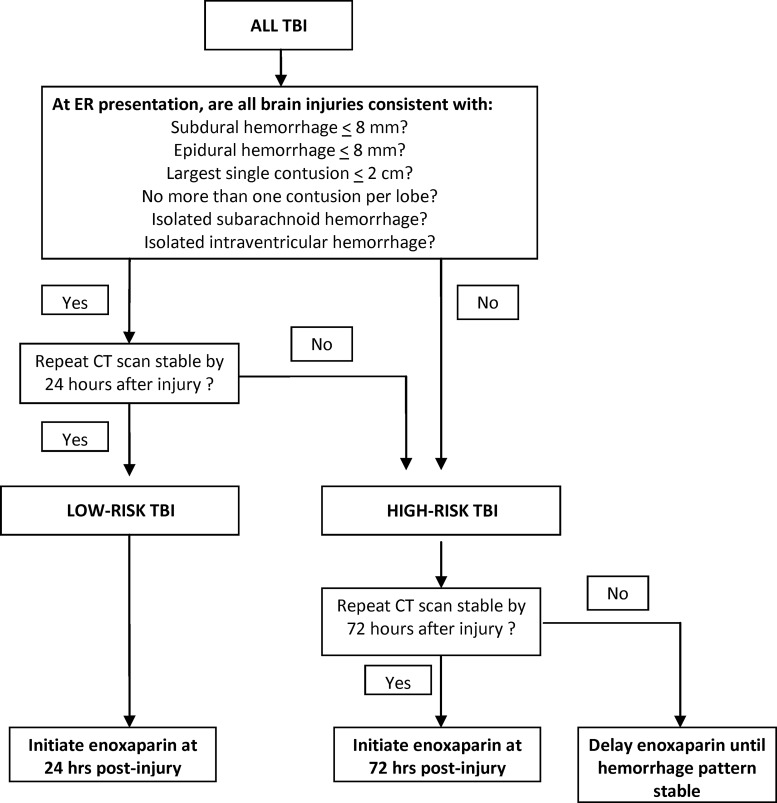
FIG. 3.
Newly validated Parkland Protocol. The protocol's injury patterns at presentation and their behavior over time were found to predict two tiers of risk for radiographic progression of hemorrhage. Whereas the low-risk category and its recommendations for enoxaparin initiation have not changed from the protocol's earlier iterations, the moderate- and high-risk categories have been combined into a single high-risk category based on the results of the present investigation. Additionally, the original protocol's recommendation to consider prophylactic filtration for patients with a craniotomy, monitor, or unstable TBI patterns at 72 h after injury has been dropped based, in part, on our group's performance improvement efforts. TBI, traumatic brain injury; CT, computed tomography.
A lack of evidence on the earliest safe time point for the initiation of prophylaxis after TBI led our group to take this question up several years ago. In reviewing the literature, a commonality of most studies that considered this topic was that ICH was generally considered to be a binary phemonenon (i.e., present or absent).9–18 An exception to this general rule was the work of Berne and Norwood, who had promulgated a set of intracranial injury patterns that could safely receive enoxaparin 30 mg subcutaneously beginning 24 h after injury if a repeat CT scan of the head was stable.6–8 Using their work as a starting point, our group modified these criteria and created the Parkland Protocol. This original version of the protocol classified TBI patients as being at low, moderate, or high risk for the spontaneous progression of their injury pattern and tailored a VTE prophylaxis regimen to each arm.4,5
In 2012, we published a study of 245 patients with at least two CT scans of the head who had been classified and followed per this original iteration of the Parkland Protocol between February 2010 and March 2011.3 In that study, we found that spontaneous progression of hemorrhage occurred in 25% of subjects in the low-risk arm (n=136), 43% of moderate-risk subjects (n=42), and 64% of high-risk subjects (n=67). Chi-square testing showed an overall significant difference between arms for the rate of spontaneous progression between the three arms, but intergroup differences were not pursued.
During the time of this study, however, our local standard of care for the practice of repeat CT scanning of the head after TBI was undergoing a gradual evolution away from mandatory repeat scanning for all patients to clinical observation, with scan repetition only for neurological deterioration. After publishing on the safety of this practice19 in 145 subjects, our group adopted this practice of selective rescanning for high-GCS (Glasgow Coma Scale) patients as our default. The result of this new strategy was that fewer repeat CT scans were being done without any decriment in neurological outcomes.
What remained unknown, however, was this new practice's effect on the Parkland Protocol (which is based on both the injury at presentation as well as its behavior over time on subsequent CT scans). We recognized immediately that fewer patients would qualify for prophylaxis treatment by the pathway because there would be no second scan to take them through the progressions of the protocol to the endpoint of the timing of enoxaparin initiation. This was not as troublesome as first thought, however, because we saw that many of these patients had a high GCS and were therefore often capable of early ambulation with a lesser need for anticoagulation. It also stood to reason that because we performed fewer scans on asymptomatic patients and more of them on patients who were in the midst of a neurological decompensation, the rate of spontaneous progression on repeat scanning would intuitively increase. Because our group's acceptance of selective scanning continued to increase during that series of 245 patients, and because we had not formally validated the Parkland Protocol at that time, we undertook the present investigation to internally validate the Parkland Protocol with an expanded cohort of 414 subjects.
Compared with our 2012 report, we did see increases in the rates of spontaneous progression in the low- (25–29%) and moderate-risk (43–53%) arms. The original iteration of the protocol's high-risk arm consisted primarily of those patients who had undergone placement of an intracranial monitor and/or craniotomy. Interestingly, this group's progression rate actually declined from 65% in our 2012 study to 58% in the present investigation. It should be noted that this finding in the high-risk arm cannot be said to be affected by a policy of selective scanning because, by definition, all of these patients receive at least one repeat CT scan after their neurosurgical intervention. The practical cumulative effect, however, is that no statistical difference currently exists between the (previous) moderate- and high-risk arms, and that the newly validated Parkland Protocol consists of two arms, which we are now calling low and high risk.
Our group has conducted a pilot randomized trial on the low-risk arm, which we called the “Delayed versus Early Enoxaparin Prophylaxis (DEEP) I” study.20 In DEEP I, subjects in the low-risk arm with stable injury patterns on repeat scan at 24 h after injury were randomized to receive enoxaparin 30 mg subcutaneously or placebo in a double-blind fashion from 24 to 96 h after injury. After enrolling 62 subjects, no clinical progressions of hemorrhage were noted in either group. Radiographic progression of hemorrhage was observed in 6% of enoxaparin subjects and 4% of placebo patients. Having demonstrated feasibility with DEEP I, our group is now turning our attention to DEEP II, which will be a pilot study on the Parkland Protocol's newly created high-risk arm.
One of our first tasks in constructing DEEP II was the choice of time points for our intervention that would have equipoise. In the present study, we were surprised to see that, despite our group's efforts to initiate earlier chemoprophylaxis on those patients who would now qualify for our newly created high-risk group, only 64% of subjects in this cohort actually received anticoagulation. Further, those patients who did receive anticoagulation did so with great variability (Fig. 2). During the time period of the present study, at least 19 different surgical intensivists, anesthesiologists, neurointensivists, and neurosurgical attending physicians provided care to TBI patients in the Parkland Surgical Intensive Care Unit (SICU) and were therefore responsible for giving input on the timing of prophylaxis initiation. Whereas the results of DEEP I promoted widespread acceptance of enoxaparin at 24 h after injury in low-risk patients among our extended group of SICU providers, these results suggest that consensus has been harder to reach for larger injury patterns. To that end, for those high-risk patients with stable injury patterns at 72 h after injury, we feel that equipoise exists for randomizing patients to starting enoxaparin at 72 h versus 7 days after injury and have designed the study with those as the time points for our intervention.
Creation of a new iteration of the Parkland Protocol with only low- and high-risk arms led to an additional change: the dropping of the recommendation for consideration of a prophylactic vena cava filter for high-risk patients, as was observed in the protocol's early stages. As our group began to consider the preponderance of the evidence suggesting that prophylactic filtration does not lower the rate of symptomatic PE21–23 as well as literature suggesting that some of what we are calling PEs may actually be pulmonary thrombi forming in the lung,24,25 our providers began to become increasingly conservative with placement of these devices after TBI. This culminated in a local quality improvement (QI) project, which showed that an evolution in our prophylactic filter placement policy from very liberal to very restrictive did not affect our symptomatic PE rates after trauma (2009–2010=0.7%; 2010–2011=0.9%; 2011–2012=0.6%; unpublished data). Based on these results, we have dropped the recommendation for consideration of filter placement after TBI in favor of enoxaparin after radiographic stabilization of the injury pattern whenever that may occur.
Our results are notable for the finding of a VTE rate of 2.4%. It should be mentioned that this is in the context of using overt clinical symptoms as a trigger for diagnostic testing. Though some would argue that these are the only VTEs that matter, this view of the disease process does not take into account the sequelae of DVT and, in particular, the decriment in quality of life associated with the development of post-thrombotic syndrome. Post-thrombotic syndrome has been shown to develop in as many as 30% of patients with DVT26 and, when mild, is associated with quality-of-life scores below those of age-matched patients with arthritis, chronic lung disease, and diabetes. When severe, quality of life drops off below that of patients with angina, cancer, and congestive heart failure.26 In short, an acutely asymptomatic DVT is not a benign entity.
Finally, whereas much of our work to date has centered on pushing the time frame for prophylaxis initiation to increasingly earlier points, we have been far less vigilant about ensuring the completeness of the regimen once initiated. Evidence has emerged that missing as little as a single dose of anticoagulation markedly increases the risk of VTE development.27 Our future clinical practice and scientific efforts will have to account for this finding.
As with any observational study, the results should be interpreted in light of possible biases. First, the interpretation of CT scans as worse/not worse was done by a single reading from a study neurosurgeon or radiologist, leading to the possibility of an individual's consistent under- or overcalling as a confounder. Second, the fact that our group practices evolved over the time period of the study in regard to repetition of scans, prophylaxis initiation, and prophylactic filter placement also opens it to potential issues with performance and chronology biases. Last, management by the Parkland Protocol is suggested, but not mandated, among the many physicians providing TBI care in our multi-disciplinary group (some of whom are coauthors on this article). This has the clear potential for a selection bias in regard to the timing of prophyalxis initiation. As a result of these limitations, the group of TBI patients to whom these results are most clearly generalizable are those patients who have multiple CT scans. Though we freely concede that patients receiving a single scan are still a significant percentage of the overall TBI population (37% in our study), the issue of prophyalxis is less pressing for many of these patients because a slim majority are dead or home by 48 h after injury. Of the remaining patients who have longer hospital stays with only a single CT scan, one should use marked caution in extrapolating our results to these patients.
In conclusion, the Parkland Protocol's injury criteria are valid predictors of two tiers of risk for radiographic progression of ICH while patients remain naïve to anticoagulants. Though evidence exists to support the initiation of enoxaparin at 24 h after injury in low-risk TBI patients with stable injury patterns, considerable variation exists on the timing of chemoprophylaxis in high-risk patients. The next step in our group's work on this subject will consist of a pilot randomized trial examining the feasibility and safety of chemoprophylaxis initiation at 72 h after injury in high-risk patients with stable injury patterns.
Acknowledgments
This work was supported by a pilot grant awarded by the UT Southwestern Clinical Translational Science Initiative, which is itself funded by a National Institutes of Health grant (no. 1 KL2 RR024983-01) titled, “North and Central Texas Clinical and Translational Science Initiative” (Robert Toto, MD, principal investigator). This work was presented as a poster at the September 2013 Annual Meeting of the AAST in San Francisco, California.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bratton S.L., Chestnut R.M., Ghajar J., McConnell-Hammond F.F., Harris O.A., Hartl R., Manley G.T., Nemecek A., Newell D.W., Rosenthal G., Schouten J., Shutter L., Timmons S.D., and Ullman J.S. (2007). V. Deep vein thrombosis prohylaxis. J. Neurotrauma 24, S32–S36 [DOI] [PubMed] [Google Scholar]
- 2.Gould M.K., Garcia D.A., Wren S.M., Karanicolas P.J., Arcelus J.I., Heit J.A., and Samama C.M. (2012). Prevention of VTE in nonorthopeic surgical patients: Antithrombotic therapy and prevention of thrombosis, 9th edition American College of Chest Physicians evidence based clinical practice guidelines. Chest 141, 227S–277S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phelan H.A., Eastman A.L., Madden C., Aldy K., Berne J., Norwood S.H., Scott W.C., Bernstein I., Pruitt J., Butler G., Rogers L., and Minei J.P. (2012). TBI risk stratification at presentation: a prospective study of the incidence and timing of radiographic worsening in the Parkland Protocol. J. Trauma Acute Care Surg. 73, S122–S127 [DOI] [PubMed] [Google Scholar]
- 4.Phelan H.A. (2012). Pharmacologic venous thromboembolism prophylaxis after TBI: a critical literature review. J. Neurotrauma 29, 1821–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phelan H.A. (2013). Venous thromboembolism after traumatic brain injury. Semin. Thromb. Hemost. 39, 541–548 [DOI] [PubMed] [Google Scholar]
- 6.Norwood S.H., McAuley C.E., Berne J.D., Vallina V.L., Kerns D.B., Grahm T.W., and McLarty J.W. (2001). A potentially expanded role for enoxaparin in preventing venous thromboembolism in high risk blunt trauma patients. J. Am. Coll. Surg. 192, 161–167 [DOI] [PubMed] [Google Scholar]
- 7.Norwood S.H., McAuley C.E., Berne J.D., Vallina V.L., Kerns D.B., Grahm T.W., and Short K. (2002). Prospective evaluation of the safety of enoxaparin prophylaxis for venous thromboembolism in patients with intracranial hemorrhagic injuries. Arch. Surg. 137, 696–702 [DOI] [PubMed] [Google Scholar]
- 8.Norwood S.H., Berne J.D., Rowe S.A., Villarreal D.H., and Ledlie J.T. (2008). Early venous thromboembolism prophylaxis with enoxaparin in patients with blunt traumatic brain injury. J. Trauma 65, 1021–1027 [DOI] [PubMed] [Google Scholar]
- 9.Cothren C., Smith W., Moore E., and Morgan S. (2007). Utility of once-daily dose of low molecular weight heparin to prevent VTE in multisystem trauma patients. World J. Surg. 31, 98–104 [DOI] [PubMed] [Google Scholar]
- 10.Koehler D., Shipman J., Davidson M., and Guillamondegui O. (2011). Is early venous thromboembolism prophylaxis safe in trauma patients with intracranial hemorrhage? J. Trauma 70, 324–329 [DOI] [PubMed] [Google Scholar]
- 11.Kurtoglu M., Yanar H., Bilsel Y., Guloglu R., Kizilirmak S., Buyukkurt D., and Granit V. (2004). Venous thromboembolism prophylaxis after head and spinal trauma: Intermittent pneumatic compression devices versus low molecular weight heparin. World J. Surg. 28, 807–811 [DOI] [PubMed] [Google Scholar]
- 12.Depew A., Hu C., Nguyen A., and Driessen N. (2008). Thromboembolic prophylaxis in blunt traumatic intracranial hemorrhage: a retrospective review. Am. Surg. 74, 906–911 [PubMed] [Google Scholar]
- 13.Dudley R., Aziz I., Bonnici A., Saluja R., Lamoureux J., Kalmovitch B., Gursahaney A., Razek T., Maleki M., and Marcoux J. (2010). Early venous thromboembolic event prophylaxis in traumatic brain injury with low-molecular-weight heparin: risks and benefits. J. Neurotrauma 27, 2165–2172 [DOI] [PubMed] [Google Scholar]
- 14.Levy A., Salottolo R., Bar-Or R., Offner P., Mains C., Sullivan M., and Bar-Or D. (2010). Pharmacologic thromboprophlaxis is a risk factor for hemorrhage progression in a subset of patients with traumatic brain injury. J. Trauma 68, 886–894 [DOI] [PubMed] [Google Scholar]
- 15.Minshall C., Eriksson E., Leon S., Doben A., McKinzie B., and Fakhry S. (2011). Safety and efficacy of heparin or enoxaparin prophylaxis in blunt trauma patients with a head abbreviated injury severity score >2. J. Trauma 71, 396–400 [DOI] [PubMed] [Google Scholar]
- 16.Salottolo K., Offner P., Levy A., Mains C., Slone D., and Bar-Or D. (2011). Interrupted pharmacologic thromboprophylaxis increases VTE in traumatic brain injury. J. Trauma 70, 19–26 [DOI] [PubMed] [Google Scholar]
- 17.Scudday T., Brasel K., Webb T., Codner P., Somberg L., Weigelt J., Herrmann D, and Peppard W. (2011). Safety and efficacy of prophylactic anticoagulation in patients with traumatic brain injury. J. Am. Coll. Surg. 213, 148–154 [DOI] [PubMed] [Google Scholar]
- 18.Reiff D., Haricharan R., Bullington N., Griffin R., McGwin G., andRue L., III. (2009). Traumatic brain injury is associated with the development of deep vein thrombosis independent of pharmacologic prophylaxis. J. Trauma 66, 1436–1440 [DOI] [PubMed] [Google Scholar]
- 19.Abdelfattah K.R., Eastman A.E., Aldy K., Wolf S.E., Minei J.P., Scott W.W., Madden C., Rickert K.L., and Phelan H.A. (2012). A prospective evaluation of the use of routine repeat cranial CT scans in patients with intracranial hemorrhage and GCS Score of 13–15. J. Trauma Acute Care Surg. 73, 685–688 [DOI] [PubMed] [Google Scholar]
- 20.Phelan H.A., Wolf S.A., Norwood S.H., Aldy K., Brakenridge S.C., Eastman A.L., Madden C.J., Nakonezny P.A., Yang L., Chason D.P., Arbique G., Berne J., and Minei J.P. (2012). A randomized, double-blinded, placebo-controlled pilot trial of anticoagulation in low-risk TBI: the Delayed vs Early Enoxaparin Prophylaxis I (DEEP I) study. J. Trauma Acute Care Surg. 73, 1434–1441 [DOI] [PubMed] [Google Scholar]
- 21.Cherry R.A., Nichols P.A., Snavely T.M., David M.T., and Lynch F.C. (2008). Prophylactic IVC filters: do they make a difference in trauma patients? J. Trauma 65, 544–548 [DOI] [PubMed] [Google Scholar]
- 22.McMurtry A.L., Owings J.T., Anderson J.T., Battistella F.D., and Gosselin R. (1999). Increased use of prophylactic vena cava filters in trauma patients failed to decrease overall incidence of pulmonary embolism. J. Am. Coll. Surg. 189, 314–320 [DOI] [PubMed] [Google Scholar]
- 23.Antevil J.L., Sise M.J., Sack D.I., Sasadeusz K.J., Swanson S.M., Rivera L., Lome B.R., Weingarten K.E., and Kaminski S.S. (2006). Retrievable vena cava filters for preventing pulmonary embolism in trauma patients: a cautionary tale. J. Trauma 60, 35–40 [DOI] [PubMed] [Google Scholar]
- 24.Knudson M.M., Gomez D., Haas B., Cohen M.J., and Nathens A.B. (2011). Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann. Surg. 254, 625–632 [DOI] [PubMed] [Google Scholar]
- 25.Brakenridge S.C., Henley S., Golden R., Kashner T.M., Phelan H.A., Cohen M.J., Sperry J., Moore E.E., Minei J.P., Cuschieri J., and Maier R. (2013). Comparing clinical predictors of deep venous thrombosis vs pulmonary embolus after severe blunt traumatic injury: a new paradigm for post-traumatic VTE? J. Trauma Acute Care Surg. 74, 1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn S.R., Shbaklo H., Lamping D.L., Holcroft C.A., Shrier I., Miron M.J., Roussin A., Desmarais S., Joyal F., Kassis J., Solymoss S., Desjardins L., Johri M., and Ginsberg J.S. (2008). Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J. Thromb. Haemost. 6, 1105–1112 [DOI] [PubMed] [Google Scholar]
- 27.Louis S.G., Sato M., Geraci T., Anderson R., Cho S.D., Van P.Y., Barton J.S., Riha G.M., Underwood S., Differding J., Watters J.M., and Schreiber M.A. (2014). Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 149, 365–370 [DOI] [PubMed] [Google Scholar]