Abstract
Renal toxicity is a concern in HIV-infected children receiving antiretrovirals. However, the prevalence [1.7%; 95% confidence interval (CI): 1.0–2.6%] and incidence of kidney dysfunction (0.17 cases/100 person-years; 95% CI: 0.04–0.30) were rare in this multicenter cohort study of 1,032 perinatally HIV-infected Latin American and Caribbean children followed from 2002 to 2011.
Introduction
Combined antiretroviral treatment (cART) for infants during their first year(s) of life provides better clinical, immunological, and virological outcomes when compared to deferred treatment.1 Consequently children are remaining healthy and living longer. However, certain noninfectious complications, such as kidney diseases, are still common.2
The incidence of kidney disease in the HIV-infected pediatric population varies according to the population and endpoint studied. In an earlier cohort study of 2,102 HIV-infected children in the United States (18.2% with HIV RNA<400 copies/ml), 22% had at least one persistent renal laboratory abnormality during their follow-up; 15% had elevated creatinine and 8% had persistent proteinuria.3 Persistent renal dysfunction was commonly reported among patients of Hispanic/Latino ethnicity in this study. In another earlier cohort study in Miami, the frequency of proteinuria was 33% among 286 HIV-infected children and 11.2% had nephrotic range proteinuria; the mortality rate was higher among patients with proteinuria.4 In contrast, the rate of proteinuria was lower in a more recent cohort study of HIV-infected youth in the United States.5
Drug-associated nephropathy is also common among HIV-infected children using antiretrovirals, such as tenofovir disoproxil fumarate (TDF) and indinavir. However, the impact of these drugs on HIV-related nephropathy is uncertain, since most studies are underpowered to investigate drug-related adverse events.3,4 In a study of 448 children, more than 3 years of TDF use was independently associated with proteinuria.5 Yet, TDF is being widely used, and is considered one of the first line nucleoside reverse transcriptase inhibitors (NRTIs) for use in children globally.6
The aim of this study was to examine the prevalence and incidence of kidney dysfunction in a cohort of HIV-infected children from Latin American and Caribbean countries, and to evaluate whether renal function declined over time in this cohort.
Materials and Methods
Data were extracted from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International Site Development Initiative (NISDI) Pediatric/PLACES (Pediatric Latin American Countries Epidemiologic Study) prospective cohort study.7 HIV-infected children were systematically followed at 6 month intervals from 2002 to 2011, with medical history (including diagnoses, hospitalizations, medications, and vaccinations), physical examination, laboratory evaluations (including hematology, flow cytometry, and standard biochemical assays), growth parameters, HIV viral load, morbidity evaluation, and mortality status collected. Self-report of antiretroviral adherence was collected only from those subjects enrolled to the PLACES protocol, which contributed less than half of the eligible study population. The NISDI protocols were approved by the ethical review board at each clinical site, the institutional review boards at the sponsoring institution (NICHD), and the data management center (Westat), as well as the Brazilian National Ethics Committee (CONEP). Parents/guardians provided written informed consent for participation in the study.
In this protocol, 1,032 perinatally infected children were enrolled; the average length of follow-up was 37 months, with a retention rate of over 90%. At enrollment, the children ranged in age from <1 to 21 years; 55% were female, 70% were from Brazil, and 30% had experienced at least one CDC class C category event. 7
Patients who did not have serum creatinine measured during study follow-up were excluded from this analysis.
Kidney dysfunction was defined on the basis of an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, computed using the Schwartz formula.8 Nephrotoxicity was defined as a Grade 1 or higher creatinine level [creatinine ≥1.1 times the upper limit of normal (ULN)], based on the DAIDS toxicity table (http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf). For purposes of analysis, the onset of kidney dysfunction or creatinine toxicity was defined by the first occurrence, with the prevalence determined on the basis of the first available eGFR or creatinine measure. Incident cases were defined among nonprevalent cases on the basis of a single measure meeting the outcome definitions; persistence of kidney dysfunction was also examined.
The trend in eGFR measures during study follow-up was examined using a generalized estimating equations (GEE) model.9 This approach enabled fitting regression models to the eGFR data that account for possible correlations among the repeated measures obtained for study participants, yet does not require that there be equal numbers of eGFR measures obtained for each participant. The trend was modeled as a linear association, as well as a polynomial association (inclusion of quadratic and cubic terms) to allow for a possible curvilinear relationship for the change in eGFR over time. The best fit to the data was determined by the QIC (Quasilikelihood under the Independence model Criterion),10 analogous to the AIC (Akaike's Information Criterion) statistic used for comparing model fit.
Results
The study population consisted of 1,032 perinatally HIV-infected children; one patient did not have any creatinine measurements, leaving 1,031 children for this investigation.
At enrollment, the mean (±standard deviation) age of participants was 5.9 (±3.7) years and 53% were female. Other characteristics of the study population at enrollment are given in Table 1.
Table 1.
Demographic Characteristics of the Study Population
| Characteristic at study enrollment | N (%) | Mean (SD) |
|---|---|---|
| Country | ||
| Argentina | 71 (6.9) | |
| Brazil | 712 (69.1) | |
| Mexico | 148 (14.4) | |
| Peru | 78 (7.6) | |
| Jamaica | 22 (2.1) | |
| Age (years) | 5.9 (3.7) | |
| Gender | ||
| Female | 550 (53.3) | |
| Male | 481 (46.7) | |
| HIV viral load (copies/ml) | ||
| >400 | 661 (64.4) | |
| ≤400 | 365 (35.6) | |
| CD4 count (cells/mm3) | ||
| <200 | 35 (3.4) | |
| 200–499 | 168 (16.5) | |
| ≥500 | 815 (80.1) | |
| CD4 percent | ||
| <15% | 88 (9.5) | |
| 15–24% | 256 (27.7) | |
| ≥25% | 579 (62.7) | |
| ARV regimen | ||
| No ARVs | 206 (20.0) | |
| NNRTI-containing regimen, no PIs | 178 (17.3) | |
| PI-containing regimena | 542 (52.6) | |
| Other ARV regimen | 105 (10.2) | |
| CDC disease classification | ||
| N | 115 (11.2) | |
| A | 245 (23.8) | |
| B | 316 (30.7) | |
| C | 352 (34.2) | |
| Duration of ARV use (years) | 3.5 (2.9) | |
| HIV RNA (log10 transformed) | 3.5 (1.4) | |
Of the 542 chlidren receiving combination therapy containing a PI, 265 (48.9%) were receiving a regimen containing lopinavir/ritonavir, seven (1.3%) were receiving indinavir and none atazanavir; two patients received TDF with lopinavir/ritonavir.
SD, standard deviation; ARV, antiretroviral; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate.
During the study, 17 (1.6%) participants died. The primary cause of death and underlying cause/contributing factors were abstracted from medical records for 16 of the subjects who died; renal disease was not indicated as a primary or underlying cause of death for any of them.
There were 17 of 1,028 participants [1.7%; 95% confidence interval (CI): 1.0–2.6%] with prevalent kidney dysfunction; height was not measured at the baseline visit for three participants preventing their eGFR from being calculated. Only one patient persisted with kidney dysfunction at the next available measure. Three of 1,000 participants (only one also had prevalent kidney dysfunction) with laboratory standards available for defining the ULN, required for applying the DAIDS toxicity criteria, presented with prevalent Grade ≥1 nephrotoxicity (0.3%; 95% CI: 0.1–0.9%): two patients presented with Grade 2 toxicity and one with Grade 4. None of these toxicities persisted with the next available creatinine measure.
Excluding subjects with prevalent kidney dysfunction based on eGFR (n=17), seven of the remaining 1,011 subjects had incident dysfunction (0.7%; 95% CI: 0.3–1.4%). The incidence of kidney dysfunction was 0.17 cases/100 person-years of follow-up (95% CI: 0.04–0.30). None of the incident cases had an eGFR value below 60 for the next available measure.
Excluding the three cases with prevalent nephrotoxicity, there were only seven Grade ≥1 toxicities among the remaining 997 participants (0.7%; 95% CI: 0.3–1.4%): two children had Grade 1 toxicity, four had Grade 3 toxicity, and one had Grade 4 toxicity. There were 0.17 cases/100 person-years of follow-up (95% CI: 0.04–0.30). None of the toxicities persisted with the next available creatinine measure. There was little overlap between those with incident kidney dysfunction and those with incident nephrotoxicity with only one participant having both.
During study follow-up, 7.9% (n=81) of the participants ever used TDF. Of those that did, 70% received it for more than 100 days; the median duration of use was 329 days (maximum=1,596 days). At least 80% of the participants were reported to be taking the recommended or higher than recommended dose of TDF. Among the participants aged 12 years or older, one of 30 participants was reported to be taking a lower than recommended dose of TDF; among 44 participants younger than 12 years of age at the time of initial TDF, nine (20.5%) were reported to be receiving less than the recommended dose. In seven patients the TDF dosage was not available in the medical record. No participant on TDF developed kidney dysfunction or nephrotoxicity.
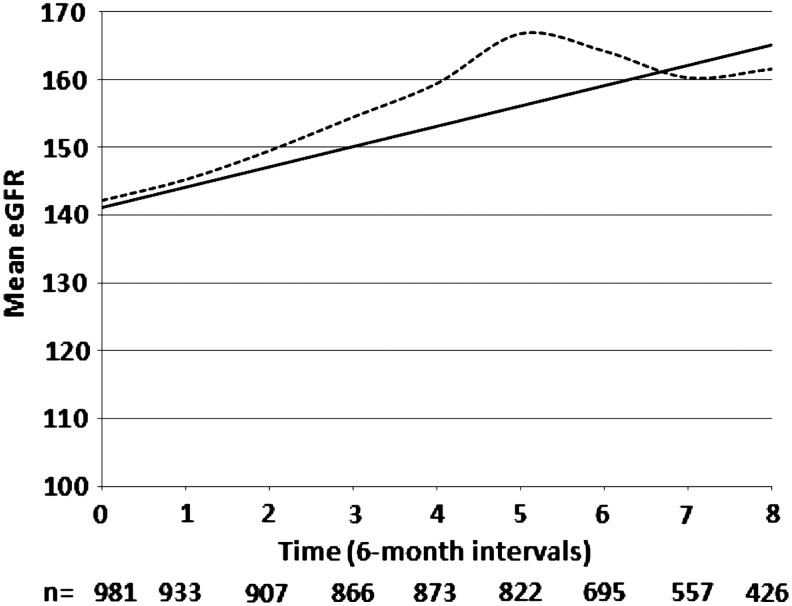
Figure 1 reports the trend in eGFR measures over time, including the means of the raw data, as well as model-based estimates. Due to the substantial drop-off in the sample size after 4 years of follow-up, the modeling was restricted to the baseline and first eight 6-month follow-up visits. The raw data and all of the models demonstrate that eGFR increased over time. Among the different models fit to the data, the linear model was judged the preferred model based on the QIC fit statistic. For this model the predicted increase in mean eGFR per 6-month visit was 3.0 ml/min/1.73 m2 and differed significantly from zero (p<0.0001).
FIG. 1.
Trends in mean estimated glomerular filtration rate (eGFR) over time for observed data and linear model-based estimates.  , raw data;
, raw data;  , linear model.
, linear model.
Discussion
In this prospective cohort study of 1,032 perinatally HIV-infected children, predominantly of Latino ethnicity, the prevalence and cumulative incidence of kidney dysfunction were very low (1.7% and 0.7%, respectively), with almost none persisting over time. These rates are much lower than those seen in two American cohort studies: 15% of elevated creatinine level in one study and 33% of proteinuria in the other.3,4 However, both of those studies spanned the years before and after cART became available, with higher rates of kidney disease, as expected, with less well controlled HIV disease. In addition, both of these cohorts included older children and a large percentage of African Americans who are at increased risk of kidney dysfunction.
Another important factor associated with nephropathy in HIV-infected children is the specific drug used. Among HIV-infected children, TDF use is important to consider. Although the most commonly reported TDF nephropathy is proximal tubulopathy, consistent with increased renal phosphate and calcium losses, decreasing eGFR has also been cited.3–5 In our study, where 8% of the children were exposed to TDF, there was no association between TDF use and kidney dysfunction.
In a cohort study of 615 adults, although eGFR improved after cART initiation, it declined in most patients including those with viral suppression, suggesting an effect of cART.10 In our study, we found that eGFR increased over time, although the majority of children did not have viral suppression.
The main limitation of our study is that proteinuria, phosphaturia, and calciuria measurements were not performed. Therefore, nephrotoxicity, including TDF-related nephrotoxicity, could be underestimated. It is important to consider these measures in deciding on the clinical use and monitoring of this drug. Although we do not have accurate adherence data, at least 50% of the patients on cART had a viral load <400 copies/ml, consistent with a good compliance.
In conclusion, this study of a large Latin American cohort of perinatally HIV-infected children demonstrated that kidney dysfunction based on eGFR or nephrotoxicity based on creatinine level was rare and was not seen in the 8% of children who were treated with TDF. Further longitudinal follow-up, including screening for albuminuria and/or proteinuria, is necessary to assess whether dysfunction increases as children enter adolescence and young adulthood with increased cumulative exposure to ARVs, particularly those with a potential for nephrotoxicity such as TDF.
Acknowledgments
Supported by NIH/NICHD contracts N01-HD-3-3345 and N01-HD-8-0001. We would like to thank the participants and their families and the NISDI Executive Committee.
Principal investigators, co-principal investigators, study coordinators, data management center representatives, and NICHD staff (for the NISDI Pediatric Study Group): Brazil: Belo Horizonte: Jorge A. Pinto, Flávia F. Faleiro, Marcelle M. Maia (Universidade Federal de Minas Gerais); Caxias do Sul: Rosa Dea Sperhacke, Nicole Golin, Sílvia Mariani Costamilan (Universidade de Caxias do Sul/Serviço Municipal de Infectologia); Nova Iguacu: Jose Pilotto, Luis Felipe Moreira, Ivete Gomes (Hospital Geral Nova de Iguacu–HIV Family Care Clinic); Porto Alegre: Rosa Dea Sperhacke, Breno Riegel Santos, Rita de Cassia Alves Lira (Universidade de Caxias do Sul/Hospital Conceição); Rosa Dea Sperhacke, Mario Ferreira Peixoto, Elizabete Teles (Universidade de Caxias do Sul/Hospital Fêmina); Rosa Dea Sperhacke, Marcelo Goldani, Carmem Lúcia Oliveira da Silva, Margery Bohrer Zanetello (Universidade de Caxias do Sul/Hospital de Clínicas de Porto Alegre); Regis Kreitchmann, Marcelo Comerlato Scotta, Debora Fernandes Coelho (Irmandade da Santa Casa de Misericordia de Porto Alegre); Ribeirão Preto: Marisa M. Mussi-Pinhata, Maria Célia Cervi, Márcia L. Isaac, Fernanda Tomé Sturzbecher, Bento V. Moura Negrini (Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo); Rio de Janeiro: Ricardo Hugo S. Oliveira, Maria C. Chermont Sapia (Instituto de Puericultura e Pediatria Martagão Gesteira); Esau Custodio Joao, Maria Leticia Cruz, Leon Claude Sidi, Maria Isabel Gouvêa, Mariza Curto Saavedra, Clarisse Bressan, Fernanda Cavalcanti A. Jundi (Hospital dos Servidores do Estado); São Paulo: Regina Celia de Menezes Succi, Daisy Maria Machado (Escola Paulista de Medicina–Universidade Federal de São Paulo); Marinella Della Negra, Wladimir Queiroz, Yu Ching Lian (Instituto de Infectologia Emilio Ribas); Mexico: Mexico City: Noris Pavía-Ruz, Dulce Morales-Pérez, Karla Ojeda-Diezbarroso (Hospital Infantil de México Federico Gómez); Peru: Lima: Jorge O. Alarcón Villaverde (Instituto de Medicina Tropical “Daniel Alcides Carrión”–Sección de Epidemiologia, UNMSM), María Castillo Díaz (Instituto Nacional de Salud del Niño), Mary Felissa Reyes Vega (Instituto de Medicina Tropical “Daniel Alcides Carrión”– Sección de Epidemiologia, UNMSM); Data Management and Statistical Center: Yolanda Bertucci, Laura Freimanis Hance, René Gonin, D. Robert Harris, Roslyn Hennessey, Margot Krauss, Sue Li, Karen Megazzini, Orlando Ortega, James Korelitz, Sharon Sothern de Sanchez, Sonia K. Stoszek, Qilu Yu (Westat, Rockville, Maryland); NICHD: Rohan Hazra, Lynne M. Mofenson, George K. Siberry (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland).
Supported by NICHD Contract N01-HD-3-3345 (2002–2007) and by NICHD Contract HHSN267200800001C (NICHD Control: N01-HD-8-0001) (2007–2012).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chiappini E, Galli L, Tovo PA, Gabiano C, Gattinara GC, Guarino A, Badolato R, Giaquinto C, Lisi C, and de Martino M, for the Italian Register for HIV Infection in Children: Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS 2006;20:207–215 [DOI] [PubMed] [Google Scholar]
- 2.Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, and Van Dyke RB, for the Pediatric AIDS Clinical Trials Group 219/219C Team: Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr 2010;53:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andiman WA, Chernoff MC, Mitchell C, et al. : Incidence of persistent renal dysfunction in human immunodeficiency virus-infected children: Associations with the use of antiretrovirals, and other nephrotoxic medications and risk factors. Pediatr Infect Dis J 2009;28:619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaparro AI, Mitchell CD, Abitol CL, et al. : Proteinuria in children infected with human immunodeficiency virus. J Pediatr 2008;152:844–849 [DOI] [PubMed] [Google Scholar]
- 5.Purswani M, Patel K, Kopp JB, Seage GR, 3rd, Chernoff MC, Hazra R, Siberry GK, Mofenson LM, Scott GB, and Van Dyke RB, for the Pediatric HIVAIDS Cohort Study: Tenofovir treatment duration predicts proteinuria in a multi-ethnic United States cohort of children and adolescents with perinatal HIV-1 infection. Pediatr Infect Dis J 2013;32:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havens P. and Essajee S: Technical update on treatment optimization, use of tenofovir in HIV-infected children and adolescents: A public health perspective. WHO, June2012. http://apps.who.int/iris/bitstream/10665/70944/1/9789241503822_eng.pdf Accessed May30, 2013 [Google Scholar]
- 7.Hazra R, Stoszek SK, Freimanis Hance L, Pinto J, Marques H, Peixoto M, Alarcon J, Mussi-Pinhata M, and Serchuck L, for the NISDI Pediatric Study Group 2008: Cohort Profile: NICHD International Site Development Initiative (NISDI): A prospective, observational study of HIV-exposed and HIV-infected children at clinical sites in Latin American and Caribbean countries. Int J Epidemiol 2009;38:1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz GJ, Brion LP, and Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin N Am 1987;34:571–590 [DOI] [PubMed] [Google Scholar]
- 9.Pan W: Akaike's information criterion in generalized estimating equations, Biometrics 2001;57:120–125 [DOI] [PubMed] [Google Scholar]
- 10.Choi AI, Shlipak MG, Hunt PW, Martin JN, and Deeks SG: HIV-infected persons continue to lose kidney function despite successful antiretroviral therapy. AIDS 2009;23:2143–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]