Abstract
Improving the antitumor potency of current oncolytic adenoviruses represents one of the major challenges in development of these viruses for clinical use. We have generated an oncolytic adenovirus carrying the safety-enhancing E1AΔ24 deletion, the potency-enhancing T1 mutation, and the infectivity-enhancing fiber RGD modification. The results of in vitro cytotoxicity assays on 15 human cancer cell lines derived from different tumor types demonstrated that ORCA-010 is more potent than Ad5-Δ24RGD or ONYX-015. As ORCA-010 will initially be developed for the treatment of prostate cancer, selectivity experiments were performed using primary human prostate cells. ORCA-010 killed cancer cells more effectively than these primary human cells. In both primary prostate fibroblasts and epithelial cells, ORCA-010 was as safe as Ad5-Δ24RGD. Evaluation of ORCA-010 in in vivo xenograft tumor models in nude mice showed that ORCA-010 significantly inhibited growth of prostate, lung, and ovarian tumors and conferred prolonged survival of tumor-bearing animals. Furthermore, we observed a substantial increase in infectious viral particles in tumors injected with ORCA-010. The number of infectious viral particles increased after treatment and infectious particles remained present up to at least 4 weeks posttreatment. Intratumoral virus replication was associated with substantial necrosis and fibrosis. In conclusion, ORCA-010 is more potent than earlier generation oncolytic adenoviruses, without demonstrating increased toxicity. ORCA-010 exerted strong in vivo antitumor activity and is therefore a suitable candidate for clinical evaluation.
Introduction
Despite extensive research and development toward new diagnostics and therapeutics, cancer still is one of the leading causes of death worldwide. The complexity of the disease and frequent inherent or acquired resistance against treatment form great challenges to achieve complete cures. To increase the cure rate for cancer patients, treatment options based on new mechanisms of action are urgently needed. Oncolytic viruses have attracted considerable attention as a promising approach for the treatment of cancer. Owing to their ability to replicate within the cancer cell, oncolytic viruses have unique pharmacokinetic properties that differentiate them from conventional small molecule or biological therapeutics. More than 10 different virus groups are currently being explored for the development of virotherapies (Russel et al., 2012). Of these, adenovirus is most widely used.
Oncolytic adenoviruses are engineered to specifically replicate in and kill malignant cells. To ensure that the virus selectively replicates in cancer cells, different strategies have been used (Alemany, 2007). Two paradigm examples of oncolytic adenoviruses are ONYX-015 (Bischoff et al., 1996) and A5-Δ24 (Fueyo et al., 2000). To ensure cancer cell–selective replication, ONYX-015 carries a deletion of the E1B55K gene and Ad5-Δ24 carries a small deletion in the E1A gene. Previous studies have shown that viruses with the Δ24 mutation have superior oncolytic potency compared with ONYX-015 (Heise et al., 2000; Lockley et al., 2006).
The Δ24 mutation is a 24-nucleotide deletion in the CR2 domain of E1A, which results in abolishment of the binding of E1A to the host cell retinoblastoma tumor suppressor protein (pRb). During wild-type adenovirus replication in normal cells, the binding of E1A to pRb is required to ensure release of the cell cycle transcription factor E2F-1. Release of E2F-1 forces the cell into cell cycle (S phase) and promotes replication of the adenovirus by driving expression of the viral E2 gene region. Conversely, loss of this E1A function renders the adenovirus replication incompetent in normal cells with a functional pRb pathway. In contrast, viruses with the Δ24 mutation replicate efficiently in tumor cells where sequestration of pRb by E1A is not necessary, because in most cancers the pRb/p16 pathway is defective (Fueyo et al., 2000; Heise et al., 2000).
Although evidence of biological activity was obtained in clinical trials, overall the observed efficacy of oncolytic adenoviruses as single agents has been modest. Hence, improvement of the potency of oncolytic adenoviruses is required to achieve the desired clinical efficacy. To accomplish this, several strategies have been taken. A first approach is the insertion of transgenes, leading to augmented therapeutic efficacy. Various genes have been incorporated into the adenovirus backbone for this purpose, such as genes encoding tumor suppressor p53 (Van Beusechem et al., 2002), various prodrug-converting enzymes (Wildner et al., 1999), and immune modulators (Lei et al., 2009). A second approach has been the isolation of mutant adenovirus variants with augmented replication in cancer cells through genetic bioselection. Using this approach, AdT1 virus expressing a mutant E3/19K protein lacking the endoplasmic reticulum retention domain was isolated (Gros et al., 2008). The mutant E3/19K-T1 protein relocates to the plasma membrane and induces membrane permeabilization causing enhanced release of progeny virus from infected cancer cells. A third approach has been to promote oncolytic adenovirus entry into cancer cells by changing or expanding its tropism. A particularly effective modification has been the insertion of an RGD motif in the adenovirus fiber protein (Suzuki et al., 2001). The RGD motif consists of a 9-amino acid peptide (CDCRGDCFC) that binds to integrins (particularly αvβ3 or αvβ5). Insertion of this RGD motif in the HI loop of the fiber protein of adenovirus results in enhanced infectivity in a number of different cancer cell lines (Dimitriev et al., 1998; Suzuki et al., 2001).
With the aim to generate an oncolytic adenovirus with further enhanced anticancer potency, we here combine the efficacy-enhancing E3/19K-T1 and fiber RGD elements with the Δ24 mutation conferring cancer cell selectivity in an Ad5 backbone. We evaluate the lytic replication of the new virus ORCA-010 on malignant and nonmalignant cells in vitro and in three different xenograft tumor models in vivo. We demonstrate that ORCA-010 has superior oncolytic potency compared with earlier generation oncolytic adenoviruses. The data presented here show that ORCA-010 exhibits desirable features for use as an oncolytic agent in future clinical trials.
Materials and Methods
Cells
FaDu, UM-SCC-11B, UM-SCC-14C, UM-SCC-22A, and VU1131 head and neck squamous cell carcinoma cell lines, SK-Mel-28 melanoma cells, A549 lung adenocarcinoma cells, NIH OVCAR-3 ovarian cancer cells, MDA-MB-231 breast cancer cells, Hep3B hepatocellular carcinoma cells, U2OS osteosarcoma cells, HCT 116 colorectal carcinoma cells, and 911 AdE1-complementing human embryonic retinoblasts (Fallaux et al., 1996) were cultured in DMEM (Lonza). The prostate cancer cell lines PC-3, LNCaP, and DU-145 were cultured in RPMI 1640 (Lonza). All media were supplemented with 10% FBS (Greiner) and 0.5% pen/strep (Lonza). Cells were split twice a week and incubated at 37°C with 5% CO2. Primary human prostate epithelial cells and primary human prostate fibroblasts were purchased from Sciencell and cultured in a cell-specific medium supplemented with 10% FBS and 0.5% pen/strep (Sciencell). Cells were split at 90% confluency and were cultured at 37°C with 5% CO2.
Viruses
Wild-type adenovirus serotype 5 (Ad5) was provided by Prof. Rob Hoeben (Leiden University Medical Center, The Netherlands). The oncolytic adenoviruses ONYX-015, Ad5-Δ24, and Ad5-Δ24RGD have been described before. ONYX-015 carries an E1B-55K deletion mutation (Bischoff et al., 1996). Ad5-Δ24 (Fueyo et al., 2000) contains a 24 bp deletion at position 923–946 of the E1A gene (E1AΔ24) in Ad5. Ad5-Δ24RGD carries the same E1A modification and a cyclic RGD insertion in the HI loop of the fiber (Suzuki et al., 2001). To generate new oncolytic adenoviruses Ad5-Δ24T1 and Ad5-Δ24RGD-T1 (ORCA-010), homologous recombination (HR) technology in the yeast strain YPH-857 was used with the LiAc high-efficiency transformation protocol (Gietz et al., 2007).
The oncolytic adenovirus plasmid pAd5-Δ24T1, with the E1AΔ24 mutation and the potency-enhancing E3/19K-T1 mutation, was generated by HR between a 14 kb AleI-AleI cut fragment from plasmid pAdE3/19K-445A (Gros et al., 2008) containing the E3/19K-T1 mutation and pAd5-Δ24RGD (Suzuki et al., 2001) from which we excised the 27.5 kb PacI-SrfI fragment containing the RGD motif. To generate pAd5-Δ24RGD-T1 comprising the E1AΔ24 deletion, E3/19K-T1 mutation, and the RGD motif insertion in the HI loop of the fiber, HR was done with a 26 kb PmeI-PacI fragment containing the RGD motif of pAd415-Δ24RGD-T1 and pAd5-Δ24T1, from which we excised the 29.5 kb NdeI-NdeI fragment carrying the wild-type fiber gene. Viruses were generated by lipofectamine 2000 transfection (Invitrogen) of PacI linearized plasmids and propagation in A549 cells.
For generation of concentrated virus stocks, virus was purified using the Vivapure adenopack 100 kit (Sartorius Stedim Biotech S.A.) and stored in a 10 mM Tris (pH 7), 25 mM NaCl buffer containing 2.5% w/v glycerol. E1AΔ24, T1, and RGD modifications were confirmed in all plasmids and viruses by sequence analysis. Restriction analysis was performed on plasmids and viruses to verify that Ad5-Δ24T1 and ORCA-010 are derived from the adenovirus reference material (Sugarman et al., 2003).
Infectious virus titers were determined with an antihexon immunocytochemistry (ICC) assay using the Adeno-X Rapid Titer Kit (Clontech) according to the manufacturer's protocol. The following adaptations were made to this protocol: instead of HEK293 cells, 911 cells were used, and volumes of reagents were adjusted for use in 96-well plate format. Determination of virus particle titers in purified preparations was done spectrophotometrically using the GeneQuant spectrophotometer (Amersham Pharmacia) (Maizel et al., 1968), using a conversion factor of 1.1×1012 viral particles per absorbance unit at 260 nm.
In vitro cytotoxicity assay
Cytotoxicity assays on human cancer cell lines were performed by infecting 5,000 cells seeded per well in 96-well plates with 2-fold serial virus dilutions ranging from 8×102 to 3.8×10−4 IU/cell for ONYX-015 and 1×102 to 4.8×10−5 IU/cell for all other viruses. Virus replication was allowed to proceed for 5–9 days on Hep3B, A549, U2OS, HCT 116, SK-Mel-28, UM-SCC-22A, LNCaP, NP9, and DU-145 cells and for 10–15 days on UM-SCC-11B, UM-SCC-14C, PC-3, VU1131, MDA-MB-231, and FaDu cells. After incubation, plates were washed with PBS and stained using the BCA assay kit (Pierce). Virus was inactivated by addition of SDS (final concentration 1%). The absorbance was measured at 595 nm using the Benchmark microplate reader (BioRad). For cytotoxicity assays on primary cells, the cells were first grown to quiescence. To this end, 20,000 cells per well were seeded in a 96-well plate and cultured for 7 days with medium change at 4 days postplating. At 6 days postplating, the number of Ki-67-positive cells was determined by ICC staining to verify quiescence. Cells were fixed with 10% formalin, stained with anti-Ki-67 antibody M7240 (Abcam) and secondary HRP-conjugated rabbit antimouse antibody Ab6728 (Abcam), and developed with 3,3′-diaminobenzidine. At 7 days postplating, cells were infected with serial dilutions of virus as described above. Cultures were maintained for a maximum of 10 days, which is the maximum feasible time to culture these cells without change of medium and no significant loss of viability. Plates were washed with PBS and viability was determined with the CellTiter-Blue assay (Promega) and a Tecan Infinite F200 microplate reader at 540 nm excitation and 590 nm emission wavelengths.
The virus concentration required to kill 50% of the cells (EC50) was calculated from the dose–response curves by standard nonlinear regression using a sigmoidal dose–response equation (GraphPad Prism). The inverse of the EC50 (1/EC50) is used as a measure of the cytotoxicity of the different viruses. Results are expressed as a mean (+standard error) of triplicate samples from at least two independent experiments.
In vivo antitumor efficacy
Balb/c nu/nu mice (Charles River Laboratories) were housed in a 12 hr light/dark cycle, with ad libitum access to food and water. All animal procedures were conducted in accordance with the European Community Council directive (86/609/EEC) for the care and use of laboratory animals.
Human PC-3 prostate cancer cells (2×106) or A549 lung cancer cells (1×107) were resuspended in 50 μl of 50% DMEM/50% Matrigel solution (Becton Dickinson) and injected subcutaneously into the flank of 5–6-week-old mice to establish tumor xenografts. The tumor volume was determined 1–3 times a week using a digital caliper. Once tumors reached a volume of 80–150 mm3, the animals were randomized over groups of 7–8 mice and injected intratumorally on 3 subsequent days with purified ORCA-010 virus (total dose 1.3×107, 1.3×108, 1.3×109, or 1.3×1010 VP; VP:IU ratio of 13:1) or diluent without virus (control). To establish NIH OVCAR-3 xenografts, 8–9-week-old Balb/c nu/nu mice were used. Animals underwent whole-body irradiation with 2 Gy γ irradiation before injection of 1×107 NIH OVCAR-3 cells into their flank.
Tumor sizes and animal body weights were recorded 1–3 times per week, and tumor volumes were calculated according to the formula V (mm3)=(4/3×π×[width+length]/4)3. When tumors reached a size of 2,000 mm3, the animals were sacrificed for ethical reasons. PC-3 and A549 tumors were dissected and embedded in paraffin for hematoxylin–eosin staining. Tumor growth was analyzed in GraphPad Prism and the two-tailed Student's t-test was used for comparing the tumor progression in the different treatment groups. Kaplan–Meier survival curves were generated in GraphPad Prism based on number of days required to reach a tumor volume of 2,000 mm3. Statistical analysis was done with a Log-Rank Mantel Cox test comparing survival of treated groups with the control group.
Analysis of intratumoral adenovirus replication
Balb/c nu/nu mice bearing pre-established PC-3 xenografts (121–623 mm3) were injected intratumorally with a single dose of 4.3×109 VP ORCA-010 virus. At 3, 7, 14, 21, and 28 days posttreatment, animals were sacrificed. The tumors were fixed in formalin and embedded in paraffin. Sections of 5 μm were prepared for HE or Masson's trichrome (Polysciences, Inc.) staining or stained for presence of adenovirus using an antihexon antibody. Antigen retrieval was done by heating in Tris/EDTA buffer. Specific monoclonal antihexon antibody (Ab8249; Abcam) and a secondary HRP-labeled rabbit antimouse antibody (Ab6728; Abcam) were used. The sections were developed with 3,3′-diaminobenzidine and counterstained with hematoxylin.
Results and Discussion
Construction of the new oncolytic adenovirus ORCA-010 (Ad5-Δ24RGD-T1)
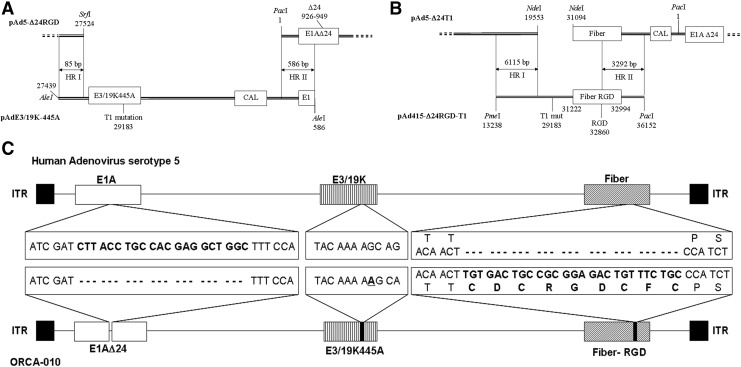
Human adenovirus serotype 5 (Ad5)-derived oncolytic adenovirus ORCA-010 was constructed by introducing the E1AΔ24 mutation providing cancer cell-selective replication (Fueyo et al., 2000), the infectivity-enhancing cyclic-RGD insertion in the fiber (Dimitriev et al., 1998; Suzuki et al., 2001), and the E3/19K-T1 mutation promoting cell lysis and virus progeny spread (Gros et al., 2008) into the Ad5 genome (Fig. 1). Restriction and sequencing analysis of pAd5-Δ24RGD-T1 and DNA isolated from ORCA-010 virus propagated in A549 cells confirmed that the virus carried the relevant E1AΔ24, T1, and RGD modifications in an Ad5 reference material backbone (Sugarman et al., 2003).
FIG. 1.
Construction of ORCA-010 and comparison of genome structures with wild-type Ad5. A schematic representation of the excised DNA fragments used for homologous recombination to generate Ad5-Δ24-T1 (A) and Ad5-Δ24RGD-T1 (B). Homologous recombination occurred in the indicated areas (HRI and HRII). (C) Differences between the DNA sequences of ORCA-010 and Ad5: a 24 nt deletion in the E1A gene encoding 8 amino acids of the pRb-binding domain; a single A insertion at nucleotide position 445 of the E3/19K gene causing a frame shift introducing a premature stop codon resulting in a protein lacking the ER-retention domain (T1 mutation); and a 21 nt insert in the HI-loop of the fiber gene encoding a cyclic RGD-4C integrin-binding domain (amino acid sequence given).
ORCA-010 displays enhanced oncolytic potency in vitro
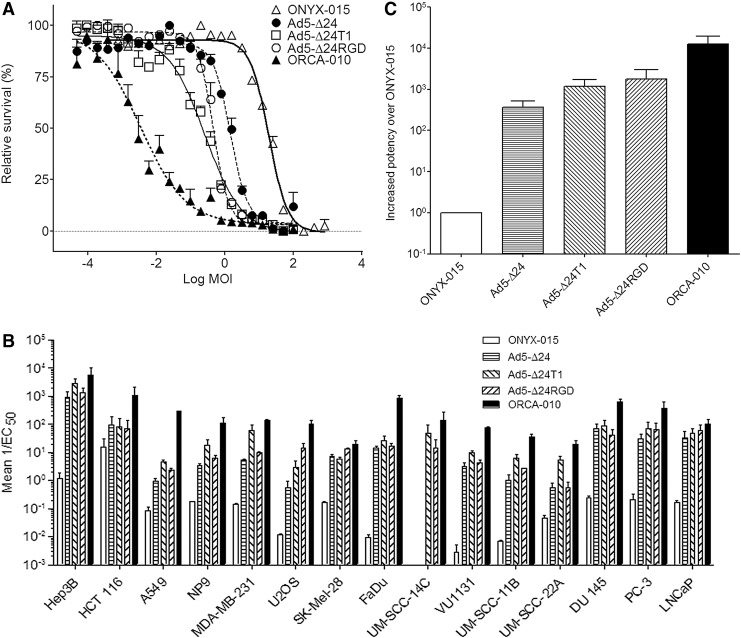
To evaluate the oncolytic potency of ORCA-010, we compared the new virus in a set of in vitro cytotoxicity assays with a panel of earlier generation oncolytic adenoviruses. These viruses included ONYX-015 (Bischoff et al., 1996) that carries an E1B-55K deletion mutation and has been thoroughly evaluated in numerous human clinical trials. Furthermore, Ad5-Δ24, which carries a 24 nt deletion mutation in the Rb-binding domain of E1A and was shown to be a more potent cancer cell killer than ONYX-015 (Heise et al., 2000; Lockley et al., 2006), was used. Finally, to dissect the contribution of the RGD and T1 mutations to ORCA-010's oncolytic properties, Ad5-Δ24RGD (Suzuki et al., 2001) and Ad5-Δ24-T1 each carrying one of these modifications were included. The assays were done on 15 human cancer cell lines from different tissue origins. Cells were infected with a dilution series of the respective viruses and incubated to allow for virus replication. As adenovirus replicates with different efficiency on different cell lines, the culture duration was adapted accordingly. Dose–response curves were made (representative example in Fig. 2A), and the inverse of the virus dose required to reduce cell viability by 50% (1/EC50) was calculated as a measure for oncolytic potency. Figure 2B shows the results obtained for each virus on each cell line. Individual cancer cell lines exhibited considerable differences in susceptibility to lytic adenovirus replication. This could be because of differential expression of the natural Ad5 high-affinity receptor coxsackie adenovirus receptor (CAR) on the cells, or on their inherent differences in supporting adenovirus DNA replication, late gene expression, and/or viral release (van Beusechem et al., 2002; Royds et al., 2006).
FIG. 2.
Comparative cytotoxicity evaluation of ORCA-010 and earlier generation oncolytic adenoviruses. The cytotoxicity of different oncolytic adenoviruses was evaluated in in vitro cytotoxicity assays on 15 different human cancer cell lines. (A) Exemplary dose–response curves obtained on A549 cells. The EC50 values derived from these curves were used to calculate oncolytic cytotoxicity (1/EC50). (B) Mean cytotoxicities of each virus on each cancer cell line, derived from at least two independent experiments. (C) The mean cytotoxicity of each virus on the panel of cancer cell lines was calculated and is given relative to the potency of ONYX-015, which was set at 1. Bars in (B) and (C) depict means; error bars show standard errors of the mean.
On individual cell lines, ORCA-010 was at least as potent or up to 60-fold more potent as the most effective earlier generation virus Ad5-Δ24RGD. Figure 2C shows the average potency enhancement on the panel of cancer cell lines that each Ad5-Δ24-based variant virus exhibited relative to ONYX-015. As can be seen, Ad5-Δ24-based viruses were much more potent than ONYX-015, confirming earlier observations. On average, introduction of the T1 (Ad5-Δ24T1) or RGD (Ad5-Δ24RGD) mutation into Ad5-Δ24 enhanced the potency of the virus approximately 3- and 4-fold, respectively. ORCA-010 that comprises both potency-enhancing modifications displayed an even greater enhanced potency than could be expected from the additive effects of the two modifications. ORCA-010 was on average 33-fold more potent than Ad5-Δ24, 10-fold more potent than Ad5-Δ24T1, and 7-fold more potent than Ad5-Δ24RGD. These results confirm that the T1 mutation and the RGD fiber insertion increase the oncolytic potency of Ad5-Δ24-variant viruses and suggest that when combined they might work synergistically to enhance oncolytic adenovirus potency.
Selectivity of ORCA-010 lytic replication
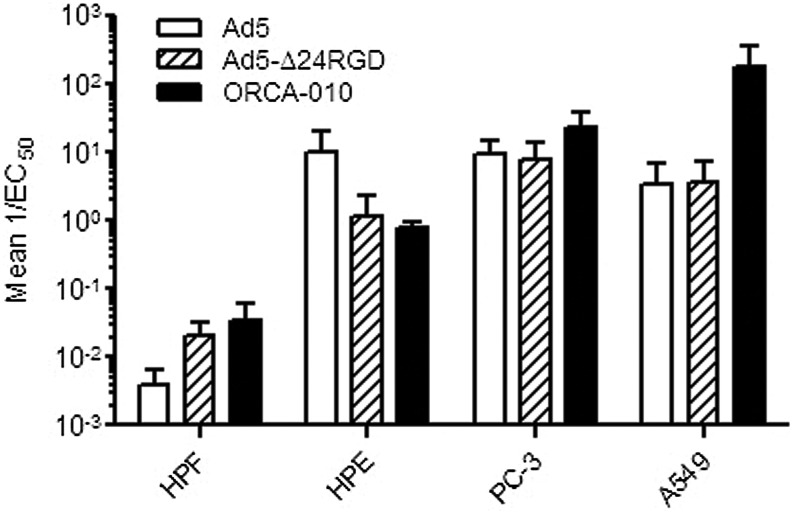
As ORCA-010 will initially be developed for local treatment of prostate cancer, the cells most exposed to the virus upon clinical administration are cells present in the prostate of the patient. Therefore, the selectivity of the virus was evaluated by comparing its in vitro cytotoxic potency on primary human prostate epithelial cells and primary human prostate fibroblasts versus PC-3 and A549 cancer cells. To mimic the in vivo quiescent state, primary cells were cultured until they grew confluent and became quiescent. The quiescent state of the primary cells was confirmed by Ki-67 staining before addition of virus. Quiescent primary cell cultures contained less than 1% Ki-67-positive cells. They could be maintained in culture for another 10 days without loss of viability or increase of percentage of Ki-67-positive cells. However, it cannot be completely ruled out that some proliferation occurred in the primary cell cultures, as it is not possible to fully mimic the in vivo conditions in an in vitro environment. In the in vitro selective cytotoxicity experiments, Ad5 and Ad5-Δ24RGD were included for comparison. As can be seen in Fig. 3, Ad5 and both oncolytic adenoviruses replicated much more efficiently in cancer cells than in quiescent nonmalignant cells. As expected, the two oncolytic viruses were less toxic to prostate epithelial cells than Ad5. In contrast, they were more potent than Ad5 on prostate fibroblasts. This is probably because of the insertion of the integrin-binding RGD motif in the fiber protein of ORCA-010 and Ad5-Δ24RGD mediating more efficient entry of the virus into these cells that express very low levels of the natural Ad5 high-affinity receptor CAR (Hidaka et al., 1999). Importantly, while ORCA-010 displayed enhanced oncolytic potency compared with Ad5 and Ad5-Δ24RGD on PC-3 and A549 cancer cells, its cytotoxicity on nonmalignant prostate cells was comparable to that of Ad5-Δ24RGD. Hence, the T1 mutation in ORCA-010 caused a cancer cell–selective potency enhancement, increasing the therapeutic window.
FIG. 3.
Comparative cytotoxicity evaluation of ORCA-010 on cancer cells and primary prostate cells. Quiescent primary human prostate epithelial (HPE) cells and human prostate fibroblasts (HPF) and A549 and PC-3 cancer cells were infected with serial dilutions of Ad5, Ad5-Δ24RGD, and ORCA-010. Seven days after infection (A549) or 10 days after infection (HPE, HPF, and PC-3), cell viability was measured and used to calculate cytotoxicity (1/EC50). Data are means with SE of at least 2 independent triplicate experiments.
ORCA-010 exhibits antitumor activity against human tumor xenografts in vivo
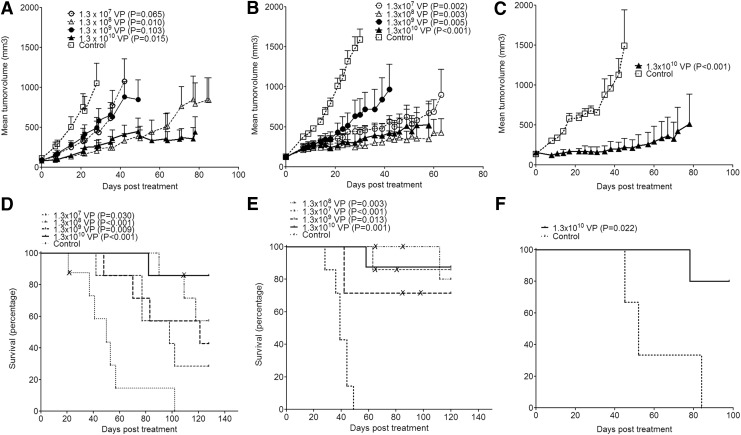
The in vivo antitumor activity of ORCA-010 was investigated in nude mice bearing subcutaneous human prostate cancer (PC-3), lung cancer (A549), or ovarian cancer (NIH OVCAR-3) xenografts. Established tumors were injected with ORCA-010. PC-3 and A549 tumors received dosages of 1.3×107 VP, 1.3×108 VP, 1.3×109 VP, or 1.3×1010 VP; NIH OVCAR-3 tumors were treated only at the highest dose. Treatment with ORCA-010 resulted in significant inhibition of tumor growth compared with vehicle-injected control tumors in all three models (Fig. 4A–C). Although a clear dose–response was not observed, analysis of tumor growth and survival showed that in mice xenografted with prostate tumors, injection of the highest dose of 1.3×1010 VP resulted in the most profound antitumor response. In all models, animals treated intratumorally with ORCA-010 had an increased survival compared with the control animals (Fig. 4D–F). In the highest dose group, 80% of the animals survived until the end of the study with no tumor or a tumor smaller than 2,000 mm3 (i.e., the tumor size defined as humane endpoint requiring sacrifice of the animal). In all dose groups of the PC-3 and A549 models, one or two animals did not bear a detectable tumor at the end of the study. Necropsy and/or histological analysis confirmed that these animals were indeed tumor-free (not shown).
FIG. 4.
Antitumor activity of ORCA-010 in subcutaneous human tumor xenografts in vivo. Mice bearing subcutaneous PC-3 (A, D), A549 (B, E), or NIH OVCAR-3 (C, F) xenografts were injected intratumorally with ORCA-010 at the indicated dosages or with diluent. (A–C) Mean tumor volumes with standard error of treatment groups are given until the first animal of the group had to be sacrificed because its tumor size exceeded 2,000 mm3. At this point, that is, day 28 for PC-3, day 30 for A549, and day 45 for NIH OVCAR-3, significance of growth inhibition was tested. p-Values of each treatment group compared with the diluent-injected group are given in the panel legends. (D–F) Kaplan–Meier curves depicting survival with a tumor smaller than 2,000 mm3. p-Values of treatment groups compared with the diluent group are given. “X” marks indicate animals that had to be sacrificed while bearing a tumor smaller than 2,000 mm3 for ethical reasons, that is, with an open necrotic wound at the site of the tumor.
ORCA-010 replicates in PC-3 tumor xenografts
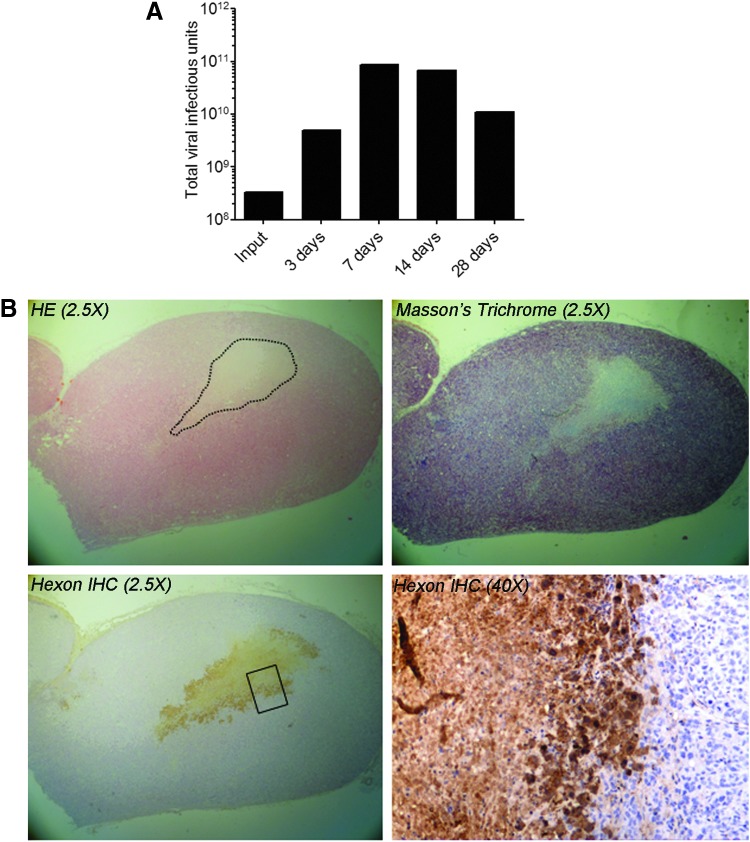
The in vivo replication and intratumoral spread of ORCA-010 was investigated in animals bearing subcutaneous PC-3 xenografts. Animals were treated with a single intratumoral injection of 3.3×108 IU of ORCA-010 and were sacrificed at different time points after injection. Dissected tumors were used to determine the infectious viral titer by ICC assay (Fig. 5A) and to visualize viral spread by immunohistochemistry (Fig. 5B and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum). As can be seen in Fig. 5A, infectious adenovirus could be recovered from injected tumors at all tested time points. The total virus load in tumors was always higher than the input dose. At days 7 and 14, the virus load was 200 times higher than the injected dose. It could thus be concluded that ORCA-010 replicated in PC-3 tumors in vivo and that viable virus was retained in PC-3 tumors until at least 28 days posttreatment. Immunohistochemical analysis on tumor tissue sections confirmed the presence of hexon-positive cells in tumors at days 3, 7, and 14. Figure 5B shows a tumor analyzed 7 days after injection. Virus-injected tumors contained large necrotic areas (indicated in the HE-stained section) with fibrosis (Masson's trichrome staining). Hexon-positive cells were found primarily in the viable tumor tissue surrounding necrotic areas. This suggested that the necrosis was caused by the replicating virus.
FIG. 5.
ORCA-010 replication upon intratumoral injection in PC-3 prostate tumor xenografts in vivo. Tumors were injected with 4.3×109 VP (3.3×108 IU) ORCA-010 and collected at 3, 7, 14, and 28 days postinjection. (A) Tumors were homogenized and total infectious virus content (IU) was determined by immunocytochemistry titration assay. (B) (Immuno)histochemical analysis of a tumor collected at 7 days after ORCA-010 injection. Sections were stained with hematoxylin–eosin (HE), with Masson's trichrome (staining connective tissue blue), or with an antibody against the adenovirus hexon protein counterstained with hematoxylin (hexon IHC), as indicated. Pictures were taken at 2.5- and 40-fold magnification, as indicated. The dotted line in the HE staining indicates the border between viable tumor tissue and necrotic tissue. The rectangle in the hexon IHC at 2.5-fold magnification indicates an approximation of the magnified area shown in the 40-fold magnification. Hexon protein-positive cells are stained brown.
Therapeutic utility and perspectives for clinical development of ORCA-010
As shown here, the new oncolytic virus ORCA-010, containing a combination of the efficacy-enhancing E3/19K-T1 and fiber RGD elements in the Ad5-Δ24 backbone, exhibited superior oncolytic potency compared with Ad5-Δ24, Ad5-Δ24RGD, and the first-generation oncolytic adenovirus ONYX-015. The T1 mutation and the RGD motif independently enhanced the oncolytic potency and when combined displayed a supra-additive effect. Further studies showed that ORCA-010 kills cancer cells more effectively than primary nonmalignant cells. Despite the enhanced potency, ORCA-010 was as selective as Ad5-Δ24RGD, which was shown to be safe for use in humans up to 3×1012 viral particles (Kimball et al., 2010; Pesonen et al., 2012; Clemens Dirven, personal communication). Moreover, intratumoral injection of ORCA-010 in human prostate, ovarian, and lung cancer tumors xenografted in immune-deficient mice resulted in significant inhibition of tumor growth and prolonged survival. Taken together, these data support progression of ORCA-010 toward further preclinical safety testing in anticipation of a first clinical trial. In this respect, recent data of an ongoing clinical trial of Ad5-Δ24RGD in patients with glioblastoma multiforme are very encouraging. It was reported that half of the patients who received a single intratumoral injection of Ad5-Δ24RGD (DNX-2401) as first-line therapy showed stabilization or partial or complete regression of their disease (Pol et al., 2013). Here, we have shown that ORCA-010 has superior oncolytic potency compared with Ad5-Δ24RGD in a wide variety of cancer types. Altogether, this warrants further clinical development of ORCA-010, in particular for cancers with a high medical need for local tumor control. Therefore, ORCA-010 will first be developed for treatment of patients with locally recurrent prostate cancer.
Supplementary Material
Acknowledgments
We thank Hedy van Wijngaarden for technical assistance with the in vitro cytotoxicity and animal experiments. We thank Dr. R. Brakenhoff (VUmc, The Netherlands) for providing the HNSCC cell lines. We are grateful to Dr. R. Rozendaal (VUmc, The Netherlands) for evaluation of the tumor sections.
Author Disclosure Statement
W.D., J.-W.H.v.G., and K.Y.A. are employees of ORCA Therapeutics B.V.; J.J.M.M. is CEO and shareholder of ORCA Therapeutics B.V.; R.A. is a scientific advisor of ORCA Therapeutics B.V. and CSO and shareholder of VCN Biosciences S.L.
References
- Alemany R. (2007). Cancer selective adenoviruses. Mol. Aspects Med. 28, 42–58 [DOI] [PubMed] [Google Scholar]
- Bischoff J.R., et al. (1996). An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274, 373–376 [DOI] [PubMed] [Google Scholar]
- Dimitriev I., et al. (1998). An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackie virus and adenovirus-independent cell entry mechanism. J. Virol. 72, 9706–9713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallaux F.J., et al. (1996). Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum. Gene Ther. 7, 215–222 [DOI] [PubMed] [Google Scholar]
- Fueyo J., et al. (2000). A mutant oncolytic adenovirus targeting the Rb pathway produces anti- glioma effect in vivo. Oncogene 19, 2–12 [DOI] [PubMed] [Google Scholar]
- Gietz R.D., and Schiestl R.H. (2007). High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 31–34 [DOI] [PubMed] [Google Scholar]
- Gros A., et al. (2008). Bioselection of a gain of function mutation that enhances adenovirus 5 release and improves its antitumoral potency. Cancer Res. 68, 8928–8937 [DOI] [PubMed] [Google Scholar]
- Heise C., et al. (2000). An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 6, 1134–1139 [DOI] [PubMed] [Google Scholar]
- Hidaka C., et al. (1999). CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J. Clin. Invest. 103, 579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball K.J., et al. (2010). A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin. Cancer Res. 16, 5277–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N., et al. (2009). An oncolytic adenovirus expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors. Cancer Gene Ther. 16, 33–43 [DOI] [PubMed] [Google Scholar]
- Lockley M., et al. (2006). Activity of the adenoviral E1A deletion mutant dl922-947 in ovarian cancer: comparison with E1A wild-type viruses, bioluminescence monitoring, and intraperitoneal delivery in icodextrin. Cancer Res. 66, 989–998 [DOI] [PubMed] [Google Scholar]
- Maizel J.V., Jr., White D.O., and Scharff M.D. (1968). The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 36, 115–125 [DOI] [PubMed] [Google Scholar]
- Pesonen S., et al. (2012). Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int. J. Cancer 130, 1937–1947 [DOI] [PubMed] [Google Scholar]
- Pol J.G., et al. (2013). Panorama from the oncolytic virotherapy summit. Mol. Ther. 21, 1814–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royds J.A., et al. (2006). p53 promotes adenoviral replication and increases late viral gene expression. Oncogene 25, 1509–1520 [DOI] [PubMed] [Google Scholar]
- Russell S.J., Peng K.W., and Bell J.C. (2012). Oncolytic virotherapy. Nat. Biotechnol. 30, 658–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman B.J., et al. (2003). The complete nucleic acid sequence of the adenovirus type 5 reference material (ARM) genome. BioProcess J. 2, 27–33 [Google Scholar]
- Suzuki K., et al. (2001). A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin. Cancer Res. 7, 120–126 [PubMed] [Google Scholar]
- Van Beusechem V.M., et al. (2002). Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res. 62, 6165–6171 [PubMed] [Google Scholar]
- Wildner O., Blaese R.M., and Morris J.C. (1999). Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 59, 410–413 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.