Abstract
Background
Growth regulating factors (GRFs) have been shown to play important roles in plant growth and development. GRF genes represent a large multigene family in plants. Recently, genome-wide structural and evolutionary analyses of the GRF gene families in Arabidopsis, rice, and maize have been reported. Chinese cabbage (Brassica rapa L. ssp. pekinensis) is one of the most important vegetables for agricultural production, and a full genome assembly for this plant has recently been released. However, to our knowledge, the GRF gene family from Chinese cabbage has not been characterized in detail.
Results
In this study, genome-wide analysis was carried out to identify all the GRF genes in Chinese cabbage. Based on the complete Chinese cabbage genome sequence, 17 nonredundant GRF genes, named BrGRFs, were identified and classified into six groups. Phylogenetic analysis of the translated GRF protein sequences from Chinese cabbage, Arabidopsis, and rice indicated that the Chinese cabbage GRF proteins were more closely related to the GRF proteins of Arabidopsis than to those of rice. Expression profile analysis showed that the BrGRF genes had uneven transcript levels in different organs or tissues, and the transcription of most BrGRF genes was induced by gibberellic acid (GA3) treatment. Additionally, over-expression of BrGRF8 in transgenic Arabidopsis plants increased the sizes of the leaves and other organs by regulation of cell proliferation.
Conclusions
The data obtained from this investigation will contribute to a better understanding of the characteristics of the GRF gene family in Chinese cabbage, and provide a basis for further studies to investigate GRF protein function during development as well as for Chinese cabbage-breeding programs to improve yield and/or head size.
Electronic supplementary material
The online version of this article (doi:10.1186/1471-2164-15-807) contains supplementary material, which is available to authorized users.
Keywords: Chinese cabbage, Expression, Gene family, GRF, Transgenic lines
Background
Growth regulating factors (GRFs) are plant-specific proteins that play important roles in regulating plant growth and development. A GRF gene was first identified in rice where it encodes a protein that functions in regulating gibberellic acid (GA)-induced stem elongation [1].
The deduced protein products of GRF genes contain two conserved domains in the N-terminal regions, the QLQ and WRC domains. The QLQ domain interacts with GRF interacting factors (GIF) and the resulting complex acts as a transcriptional co-activator [2], while the WRC domain comprises a functional nuclear localization signal (NLS) and a zinc-finger motif that functions in DNA binding [3].
Currently, the GRF gene family consists of nine Arabidopsis thaliana genes [3], 12 Oryza sativa genes [4], 14 Zea mays genes [5], and 10 Brachypodium distachyon genes [6]. In these plants, the GRF genes are strongly expressed in actively growing and developing tissues, such as shoot tips, flower buds, and immature leaves, but weakly expressed in mature tissues or organs. GRF genes have been reported to act as positive regulators of leaf size through the promotion and/or maintenance of cell proliferation activity in leaf primordia [2, 3, 7, 8]. In Brassica napus, GRF2 was found to enhance seed oil production by regulating cell number and plant photosynthesis [9]. GRF genes may act by regulating cell proliferation through the suppression of KNOX gene expression [10], which inhibits GA biosynthesis in the S-adenosyl methionine (SAM) cycle by down-regulating the key biosynthetic gene GA20 oxidase [11–15], or by controlling the level of GA2 oxidase 1, which degrades GA [16]. Recently, many studies have reported the involvement of GRF genes in the regulation of flower development [17–19].
Chinese cabbage (Brassica rapa L. ssp. pekinensis) is one of the most important vegetables for agricultural production in Asia and, although it originated in China, it is increasingly popular in other countries around the world. Yield enhancement is now one of the critical targets of plant breeding programs focused on genetic improvement. Understanding the functions of GRF genes in regulating organ size in Chinese cabbage will achieve this objective. However, knowledge about the molecular features of GRF genes in Chinese cabbage is limited. Therefore, identifying and characterizing GRF genes in Chinese cabbage is of great interest.
In this study, 17 putative GRF genes of Chinese cabbage were identified from the Brassica database (http://brassicadb.org/brad/) [20]. The expression patterns of these BrGRFs and the molecular features of the translated BrGRF proteins were analyzed, and the function of BrGRF8 was studied further. The results indicated that GRF genes had higher expression levels in immature organs or tissues than in mature ones, and their transcription was induced by gibberellic acid (GA3). Further analysis showed that cell proliferation was enhanced in transgenic plants.
Results
Identification of the BrGRFs
Based on the recently sequenced B. rapa line Chiifu genome and annotated genes [20], 17 BrGRFs were identified from the Brassica database (http://brassicadb.org/brad/) and designated BrGRF1–BrGRF17 according to their distribution in the genome (Table 1). The coding sequence (CDS) lengths of the BrGRFs varied widely; BrGRF15 was the longest (1617 bp) and BrGRF11 was the shortest (1089 bp). The intron/exon structures of the BrGRFs were determined by aligning the CDSs to the genomic sequence. The results indicated that all the BrGRF gene sequences contained introns in their CDSs. The number of introns varied from one to nine (Figure 1B); however, most of the genes (10 out of 17) had three introns, followed by four introns (3 out of 17), two introns (2 out of 17), and one and nine introns (1 each out of 17). This result was similar to the findings from previous studies on Arabidopsis (a dicot) where most AtGRFs contained three introns (7 out of 9) [4], and on rice (a monocot) where most OsGRFs had two introns (6 out of 12), followed by three introns (4 out of 12), and four introns (2 out of 12). Additionally, the number of introns in the CDSs of GRF genes in the same subfamily was different. For example, BrGRF4, BrGRF15, and BrGRF17, which belong to subfamily A (Figure 1A), had three, nine, and three introns respectively.
Table 1.
Chinese cabbage BrGRF gene family
| Gene | Accession no. | Chr. (strand) | Start/stop codon | CDS (bp) | GC content (%) | Length a (aa) | MW a (kDa) | pI a |
|---|---|---|---|---|---|---|---|---|
| BrGRF1 | Bra011781 | A01 (+) | 585376/587110 | 1356 | 47.49 | 451 | 49.44 | 9.29 |
| BrGRF2 | Bra013767 | A01 (+) | 7684376/7685880 | 1116 | 47.13 | 371 | 40.90 | 6.44 |
| BrGRF3 | Bra021521 | A01 (-) | 24798925/24802342 | 1236 | 42.23 | 411 | 46.77 | 7.38 |
| BrGRF4 | Bra028541 | A02 (-) | 969253/971390 | 1101 | 46.50 | 366 | 40.79 | 8.86 |
| BrGRF5 | Bra022667 | A02 (-) | 8154404/8155533 | 1044 | 47.51 | 347 | 37.98 | 8.67 |
| BrGRF6 | Bra023066 | A03 (-) | 8479530/8481703 | 1107 | 50.77 | 368 | 40.35 | 8.70 |
| BrGRF7 | Bra000575 | A03 (-) | 11850224/11852523 | 1557 | 48.43 | 518 | 55.45 | 9.03 |
| BrGRF8 | Bra001532 | A03 (+) | 16888039/16889424 | 1092 | 43.59 | 363 | 41.14 | 8.69 |
| BrGRF9 | Bra019244 | A03 (+) | 25506702/25508111 | 1128 | 48.23 | 375 | 40.72 | 6.62 |
| BrGRF10 | Bra017851 | A03 (-) | 30925406/30927659 | 1344 | 47.99 | 447 | 48.57 | 9.29 |
| BrGRF11 | Bra017240 | A04 (-) | 15818605/15820821 | 1089 | 50.23 | 362 | 40.01 | 6.76 |
| BrGRF12 | Bra039334 | A04 (+) | 18570071/18571766 | 1281 | 45.43 | 426 | 47.79 | 9.32 |
| BrGRF13 | Bra005268 | A05 (+) | 4422082/4424566 | 1167 | 50.99 | 388 | 42.73 | 8.25 |
| BrGRF14 | Bra027384 | A05 (-) | 20638410/20639958 | 1176 | 43.11 | 391 | 44.08 | 8.23 |
| BrGRF15 | Bra019640 | A06 (-) | 5345436/5350074 | 1617 | 43.66 | 538 | 60.82 | 8.25 |
| BrGRF16 | Bra015184 | A07 (-) | 2559826/2562310 | 1203 | 43.72 | 400 | 45.76 | 9.01 |
| BrGRF17 | Bra006956 | A09 (-) | 26481602/26483701 | 1149 | 46.56 | 382 | 42.86 | 6.81 |
aLength, MW, and pI refer to the translated BrGRF proteins.
Figure 1.
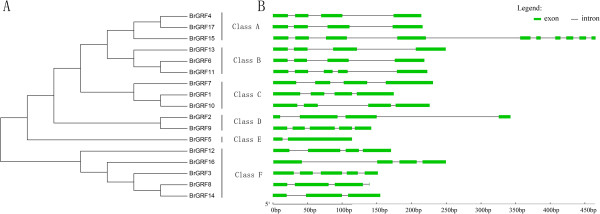
Chinese cabbage BrGRF gene family. (A) Phylogenetic relationships among the translated BrGRF proteins. (B) Intron/exon structure of the BrGRF genes.
Simple sequence repeat (SSR) markers are useful for a variety of applications in plant genetic mapping and molecular breeding because of their genetic codominance, abundance, dispersal throughout the genome, multi-allelic variation, high reproducibility, and high level of polymorphisms [21]. In this study, 16 SSR markers, including nine di-, six tri-, and one hexa-nucleotide motifs were detected in the 17 identified BrGRFs using the online SSR identification tool SSRIT (Table 2). Further, BrGRF10, BrGRF13, BrGRF15, and BrGRF16 had two SSR markers each, BrGRF2–BrGRF6, BrGRF11, BrGRF12, and BrGRF17 genes had only one SSR marker each, and BrGRF1, BrGRF7–BrGRF9, and BrGRF14 genes had no SSR markers. Of the detected SSRs in these genes, seven were detected in exons and nine were detected in introns.
Table 2.
Simple sequence repeats (SSRs) predicted in the BrGRF s
| Gene | Motif | No. of repeats | SSR start | SSR end | Length a | Intron/exon |
|---|---|---|---|---|---|---|
| BrGRF2 | cta | 10 | 641 | 670 | 3418 | exon |
| BrGRF3 | cat | 5 | 103 | 117 | 1505 | exon |
| BrGRF4 | ag | 13 | 1383 | 1408 | 2138 | intron |
| BrGRF5 | tcatcc | 4 | 87 | 110 | 1130 | exon |
| BrGRF6 | tc | 6 | 487 | 498 | 2174 | intron |
| BrGRF10 | gct | 5 | 1572 | 1586 | 2254 | exon |
| BrGRF10 | cat | 6 | 1959 | 1976 | 2254 | exon |
| BrGRF11 | caa | 5 | 1806 | 1820 | 2217 | exon |
| BrGRF12 | ca | 6 | 395 | 406 | 1696 | intron |
| BrGRF13 | tc | 6 | 504 | 515 | 2485 | intron |
| BrGRF13 | ca | 6 | 1800 | 1811 | 2485 | intron |
| BrGRF15 | tc | 6 | 517 | 528 | 4639 | intron |
| BrGRF15 | at | 7 | 3723 | 3736 | 4639 | intron |
| BrGRF16 | ta | 11 | 1354 | 1375 | 2485 | intron |
| BrGRF16 | tc | 13 | 1462 | 1487 | 2485 | intron |
| BrGRF17 | gaa | 5 | 1969 | 1983 | 2160 | exon |
aLength of the gene from the start codon to the stop codon in the genomic sequence. SSRs were identified using SSRIT (http://archive.gramene.org/db/markers/ssrtool).
The BrGRFs were unevenly distributed across the chromosomes (Figure 2), which is similar to previous results in Arabidopsis and rice [4]. The chromosome 03, 01, 02 and 04 has five, three, two and two genes, respectively, whereas the chromosome 06, 07 and 09 each includes only one.
Figure 2.
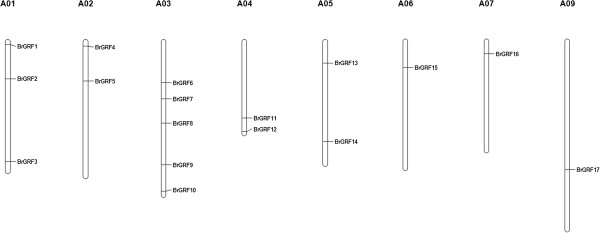
Locations of the BrGRF genes on the Chinese cabbage chromosomes. The chromosome number is indicated at the top of each chromosome representation.
Conserved domains and motifs in the predicted BrGRF proteins
The modular structures of GRF proteins have been studied thoroughly in Arabidopsis [3], rice [4], maize [5], and Brachypodium distachyon [6]. Based on this information, we used the MEME web server (http://meme.nbcr.net/meme/cgi-bin/meme.cgi) to analyze the domain distribution in BrGRFs. Motif 2, specified as the QLQ domain, was predicted to be present in 16 of the 17 BrGRFs, BrGRF3 was the exception (Figure 3). We found that although BrGRF3 could encode the QLQ domain, the deletion of two adenine residues at positions 62 and 63 bp in the BrGRF3 sequence compared with the BrGRF8 sequence caused a frameshift that introduced a TAG stop codon that truncated the BrGRF3 protein sequence (Figure 4). Motif 1, specified as the WRC domain, was predicted in all 17 BrGRF proteins. The results also indicated that AtGRF9 and BrGRF12 contained a second motif 1 downstream of the first. Additionally, motif 3, specified as the GGPL domain, was observed in 12 of the 17 BrGRF proteins, including BrGRF1, BrGRF2, BrGRF4–BrGRF7, BrGRF9–BrGRF11, BrGRF13, BrGRF15, and BrGRF17 (Figure 3).
Figure 3.
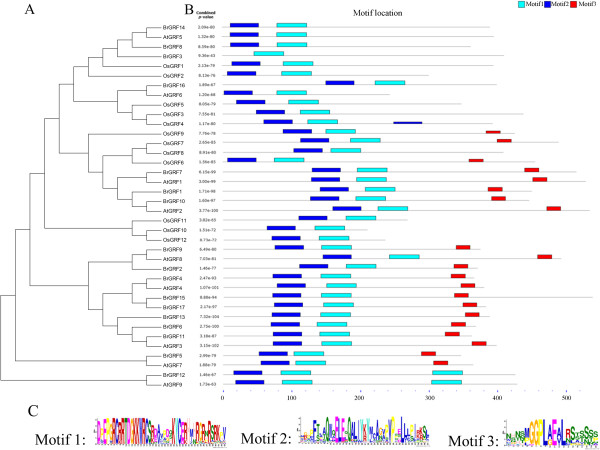
Conserved domains and motifs in BrGRF proteins. (A) Phylogenetic tree of Chinese cabbage, rice, and Arabidopsis GRF proteins. (B) Distribution of conserved motifs in Chinese cabbage, rice, and Arabidopsis GRF proteins. The GRF protein sequences of Chinese cabbage, Arabidopsis, and rice were obtained from the BRAD (http://brassicadb.org/brad/), TAIR (http://www.arabidopsis.org/) and TIGR (http://www.tigr.org/) databases, respectively. (C) Sequence logos of the predicted domains in the BrGRF protein sequences.
Figure 4.
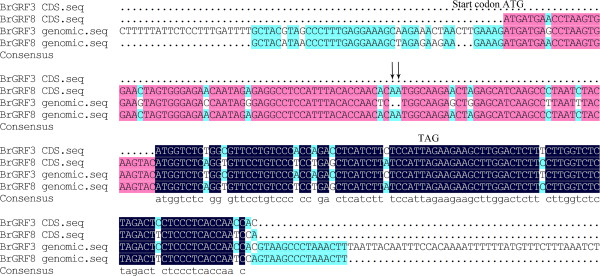
Multiple sequence alignment of the CDS and genomic sequences of BrGRF3 and BrGRF8 .
Phylogenetic relationships and duplication events of the BrGRFs
Classifying genes and constructing a phylogeny are important for the functional analysis of a gene family. A phylogenetic tree of the 17 predicted BrGRFs, 12 rice GRFs (OsGRF) and nine Arabidopsis GRFs (AtGRF) was constructed using MEGA4.1 with the bootstrap–neighbor-joining method. The 17 BrGRF proteins were classified into six subgroups: A, BrGRF1, 7, and 10; B, BrGRF2 and 9; C, BrGRF4, 6, 11, 13, 15, and 17; D, BrGRF5; E, BrGRF12; F, BrGRF3, 8, 14 and 16 (Figure 1A). The phylogenetic tree suggested that the BrGRF proteins were closest to the AtGRF proteins and more distant from the OsGRF proteins (Figure 3A). The phylogenetic tree also indicated a number of putative homologous pairs: BrGRF14/AtGRF5 (identity 82.84%), BrGRF16/AtGRF6 (identity 46.42%), BrGRF7/AtGRF1 (identity 85.05%), BrGRF10/AtGRF2 (identity 66.48%), BrGRF9/AtGRF8 (identity 56.54%), BrGRF4/AtGRF4 (identity 79.79%), BrGRF11/AtGRF3 (identity 77.04%), BrGRF5/AtGRF7 (identity 70.70%), and BrGRF12/AtGRF9 (identity 70.09%) (Figure 3A). Additionally, by comparing the MEME and phylogenetic tree analyses, we found that these homologous pairs usually had similar motif structures; for example, the BrGRF12/AtGRF9 pair had two WRC domains in their N-terminus (Figure 3A and 3B).
Analyzing the duplication events that may have occurred in the Chinese cabbage genome during evolution will help in understanding the evolutionary mechanisms of the BrGRFs. Paralogous relationships for these BrGRFs have been displayed in http://brassicadb.org/brad/. The results indicated that there were three set of triplicated genes in the Chinese cabbage genome: BrGRF3, 8, and 14 in the F block; BrGRF6, 11, and 13 in the J block; and BrGRF15, 4, and 17 in the N block (Table 3). The other BrGRF genes were either duplicated or singletons. No tandem duplication event was detected in the BrGRF family.
Table 3.
Syntenic GRF genes between Arabidopsis and Chinese cabbage
| tPCK Chr a | Block | Arabidopsis gene | Chinese cabbage gene | ||
|---|---|---|---|---|---|
| LF b | MF1 c | MF2 c | |||
| tPCK2 | F | AtGRF5 (AT3G13960) | BrGRF14 | BrGRF3 | BrGRF8 |
| tPCK2 | G | AtGRF6 (AT2G06200) | BrGRF16 | - | - |
| tPCK3 | J | AtGRF3 (AT2G36400) | BrGRF13 | BrGRF11 | BrGRF6 |
| tPCK3 | J | AtGRF9 (AT2G45480) | - | BrGRF12 | - |
| tPCK3 | I | - | - | - | BrGRF7 |
| tPCK4 | U | AtGRF2 (AT4G37740) | BrGRF1 | BrGRF10 | - |
| tPCK4 | U | AtGRF8 (AT4G24150) | BrGRF2 | BrGRF9 | - |
| tPCK5 | Wb | AtGRF7 (AT5G53660) | - | - | BrGRF5 |
| tPCK6 | N | AtGRF4 (AT3G52910) | BrGRF17 | BrGRF15 | BrGRF4 |
The data were downloaded from the Brassica Database (http://brassicadb.org/brad/).
a tPCK Chr, Chromosome of translocation the Proto-Calepineae Karyotype, the ancestral karyotype of the Brassicaceae family. b LF, Less fractioned subgenome. c MF1 and MF2, More fractioned subgenomes.
Putative functional analysis of the BrGRF proteins
The Gene Ontology (GO) database (http://www.geneontology.org/) is an international standardized gene functional classification system that offers a dynamically updated controlled vocabulary that comprehensively describes the properties of genes and their products in any organism under three main categories: biological process, molecular function, and cellular component. In this study, the 17 predicted BrGRFs were assigned GO terms. The results indicated that most of BrGRFs (except BrGRF3, which had no GO hits) were annotated with terms in the same GO groups, including ATP binding; hydrolase activity, acting on acid anhydrides, in phosphorus-containing anhydrides under the molecular function category, regulation of transcription under the biological process category, and nucleus under the cellular component category (Additional file 1). These data implied that these BrGRF proteins could have similar biological functions. BrGRF15 was also annotated with terms related to hydrolase activity, acting on ester bonds; nuclease activity; DNA binding; recombinase activity under the molecular function category, and response to DNA damage stimulus; DNA recombination; DNA repair; nucleobase, nucleoside, nucleotide and nucleic acid metabolic process under the biological process category. This result suggested that BrGRF15 may be involved in nucleic acid metabolic processes.
Expression patterns of the BrGRFs and BrGIFs (GRF interacting factor genes)
GRF proteins play important roles in the growth and development in plants [2, 3, 7–9, 17–19]. To understand which GRF genes may be involved in regulating specific tissue or organ growth in Chinese cabbage, the expression patterns of the BrGRFs in various tissues were investigated by real-time quantitative PCR (RT-qPCR). The results indicated that all 17 BrGRFs had higher expression levels in young leaves compared with in old leaves, except for the BrGRF3 and BrGRF14, which were undetectable in all the tissues examined (Figure 5A). In addition, BrGRF1, 4, 5, 6, 8, and 13 had higher expression levels in buds than in blooming flowers, whereas BrGRF2, 7, 9, 11, 12, 15, and 16 had higher expression levels in blooming flowers than in buds. The expression levels of BrGRF16 were highest in root tissues. The BrGRFs that were expressed mainly in certain organs or tissues might play important roles in the growth and development of these organs or tissues.
Figure 5.
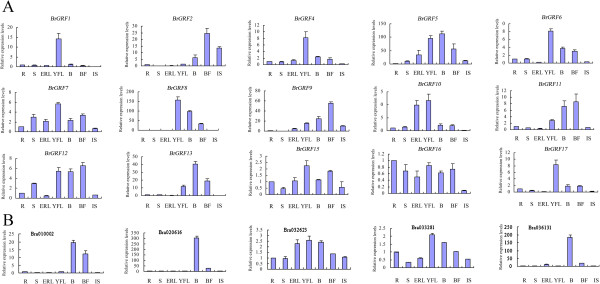
Expression profiles of the BrGRF (A) and BrGIF (B) genes in organs/tissues of Chinese cabbage. Samples were collected from roots (R), stems (S), expanded rossete leaves (ERL), young folding leaves (YFL) beginning to fold at the early folding stage (about 24–25 leaves), buds (B), blooming flowers (BF), and immature siliques (IS) 15 d after fertilization. The analysis was carried out by RT-qPCR.
The response to GA3 treatment was also investigated. Compared with distilled water (DW) treatment, the transcript levels of BrGRF5, 8, 9, 11, 12, 13, 15, 16, and 17 were increased more than 5-fold and BrGRF2, 4, and 7 were increased 2- to 5-fold in response to 100 μM GA3 application (Figure 6A). The expression levels of the other BrGRFs were only slightly affected (less than 2-fold) or not affected by the GA3 treatment.
Figure 6.
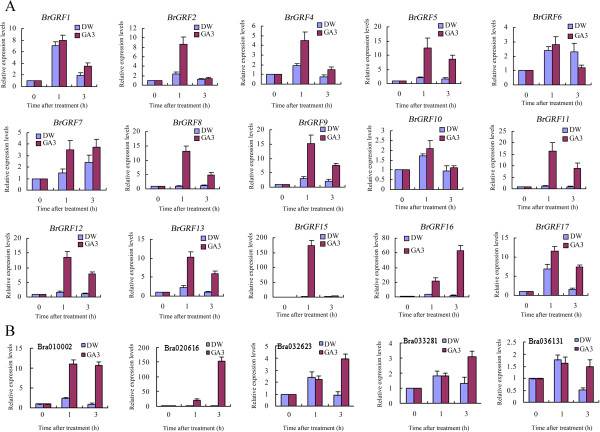
Gene expression of the BrGRF s (A) and BrGIFs (B) in response to GA3 treatment. GA3, 100 μM gibberellic acid treatment; DW, distilled water treatment as the control. The analysis was carried out by RT-qPCR.
GRF and GIF proteins may act as transcription activators or coactivators, respectively, as part of a complex involved in the growth and development in plants [4, 22]. Therefore, the expression patterns of BrGIF genes, including Bra010002, Bra020616, Bra036131, Bra032623 and Bra033281 were also investigated by RT-qPCR.
The results indicated that the Bra010002, Bra020616, and Bra036131 genes had the highest expression levels in buds, while Bra032623 and Bra033281 had the highest expression levels in young leaves (Figure 5B). Additionally, the transcript expression levels of BrGIFs could be increased by GA3 treatment. At 3 h after GA3 treatment, the transcript levels were 11.9-, 199.6-, 4.3-, 2.3-, and 2.8-fold higher for Bra010002, Bra020616, Bra032623, Bra033281 and Bra036131, respectively, compared with their levels after DW treatment (Figure 6B).
Ectopic expression of BrGRF8in Arabidopsis increases organ size
Compared with the other BrGRFs, BrGRF8 had the highest transcription level ratio between immature and mature leaves, implying that it might play an important role in the growth and development of Chinese cabbage leaves. To explore the functions of BrGRF8, we examined the Arabidopsis phenotypes caused by the ectopic expression of this gene. The transgenic line developed larger leaves and other organs compared with the vector control lines. Quantitatively, the dimensions of each of the first 17 leaves (in each plant), including leaf width, leaf length, and petiole length, increased dramatically in the 35S:BrGRF8 transgenic plants (Figure 7) compared with their dimensions in the vector control plants. However, there was little difference between the 35S:BrGRF8 and vector control plants in terms of silique size and seed number per silique (Table 4). The increase in leaf area in the BrGRF8 over-expressers was mediated directly by an increase in cell number because the adaxial epidermal surface cell sizes were normal (Figure 8).
Figure 7.
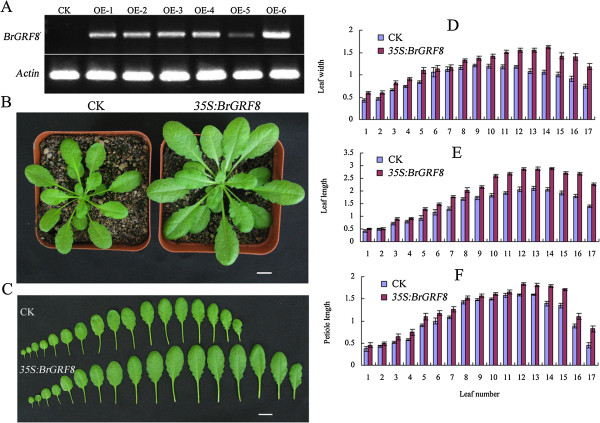
Ectopic expression of BrGRF8 in Arabidopsis. (A) RT-PCR analysis of BrGRF8 expression in different transgenic Arabidopsis lines. (B) 40-day-old vector control (CK) and 35S:BrGRF8 transgenic plants grown under the same conditions. Scale bar: 1 cm. (C) The first 17 leaves of 40-day-old vector control (CK) and 35S:BrGRF8 transgenic plants. Scale bar: 1 cm. Leaf width (D), leaf length (E), and petiole length (F) of 40-day-old vector control (CK) and 35S:BrGRF8 transgenic plants.
Table 4.
Morphology of the 35S:BrGRF8 transgenic Arabidopsis plants
| Variable | Vector control (n) | 35S:BrGRF5(ncpa) |
|---|---|---|
| Hypocotyl length (mm)a | 11.47 ± 1.06 (15) | 15.40 ± 1.30* (15) |
| Seedling length (mm)b | 21.55 ± 3.50 (10) | 29.41 ± 2.69* (10) |
| Silique length (mm) | 12.43 ± 1.00 (20) | 12.73 ± 1.13 (20) |
| Seeds/silique | 47.45 ± 2.06 (20) | 48.15 ± 1.79 (20) |
The measured values are given as mean ± standard error and ‘n’ indicates the number of plants per genotype. The star (*) indicate values that were significantly different (T- test; p <0.05). aSeedlings grown in the dark for 6 days. bRoot length plus hypocotyl length of 20-day-old seedlings grown at 22°C under a 16/8-h (light/dark) photoperiod.
Figure 8.
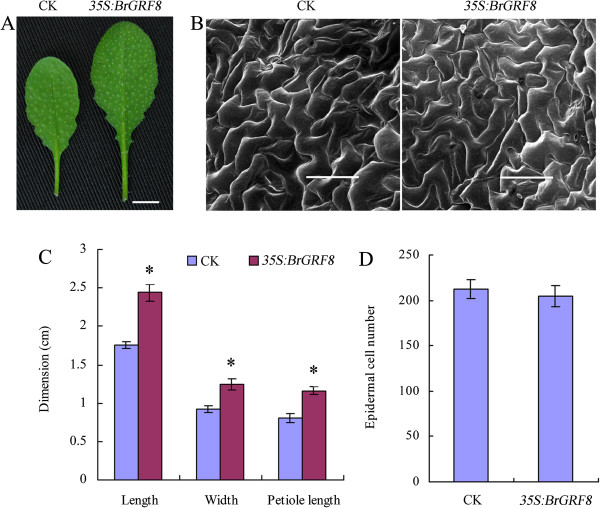
Morphology and histological analysis of fifth rosette leaves of 35-day-old plants. (A) Morphology of the fifth rosette leaves of vector control (CK) and 35S:BrGRF8 transgenic plants. Scale bar: 1 cm. (B) Scanning electron micrographs of the fifth rosette leaves of vector control (CK) and 35S:BrGRF8 transgenic plants. Scale bars: 100 μm. (C) Dimensions of the fifth rosette leaves. Error bars represent ± SD (n = 10). The star (*) indicate values that were significantly different (T-test; p <0.05). (D) Number of epidermal cells per unit area (mm2) in the adaxial surface of fully expanded fifth rosette leaves of vector control (CK) and 35S:BrGRF8 transgenic plants. The middle areas of a half leaf beside the midvein were examined. Error bars represent ± SD (n = 5).
Discussion
Previous studies have shown that GRF genes have positive functions in regulating organ size by promotion and/or maintenance of cell proliferation activity [2, 7, 8]. However, it is not known whether or how GRF proteins in Chinese cabbage regulate organ size. Because GRF proteins in different species may have different regulatory roles during the process of plant development, it is necessary to study the GRF genes in each species to understand the mechanisms of plant organ size control. In this study, a comprehensive set of 17 non-redundant GRF proteins were identified and characterized. Although the B. rapa genome is approximately 4-fold larger than the Arabidopsis genome (485 Mb and125 Mb, respectively), the gene number in B. rapa is only twice that of Arabidopsis (17:9), suggesting that there was extensive gene loss during genome duplication [20, 23].
Pseudogenes are non-functional copies of gene fragments incorporated into the genome either by retrotransposition of mRNA or by duplication of genomic DNA. Pseudogenes are widely distributed in eukaryotic genomes. Sequence alignment showed that the BrGRF3 and BrGRF8 gene sequences were highly similar, and that there was a deletion of two A residues in the BrGRF3 CDS compared with the BrGRF8 CDS that lead to the introduction of a premature stop codon. Because, BrGRF3 gene expression was not detected in the various tissues, even after GA3 treatment, we suggest that BrGRF3 may be a pseudogene in the Chinese cabbage genome.
Phylogenetic analysis of the predicted GRF protein sequences from Chinese cabbage, Arabidopsis, and rice indicated that the BrGRFs shared higher similarity with AtGRFs than with OsGRFs, which is consistent with their evolutionary relationships: Chinese cabbage and Arabidopsis are both dicots of the Brassicaceae family, while rice is a monocot of the Poaceae family. Additionally, the phylogenetic and gene duplication analyses revealed that the BrGRFs contained three triplets, two duplicates, and four singletons. However, none of the BrGRFs exhibited tandem duplication. These results suggest that the expansion of the BrGRFs could be explained by the ancestor genome of B. rapa experiencing a whole-genome triplication and evolving finally into Chinese cabbage [20, 23].
The BrGRFs were expressed mainly in specific organs and tissues, suggesting that they may play important roles in the growth and development of these organs or tissues. We found that the transcript levels of the BrGRF1, 4, 6, 8, 10, 15, and 17 genes were higher in young leaves than in the other tested tissues, suggesting that these genes are involved mainly in the growth and development of young leaves in Chinese cabbage. Thus, improving the expression levels of these genes might help improve Chinese cabbage leaf head yield.
GA3 is involved in various physiological processes in plants and KNOX proteins contribute to the regulation of meristem maintenance by negatively regulating the production of gibberellins [11–16]. Therefore, downregulation of KNOX gene expression at the flanks of the SAM cycle was reported to increase the level of GA, resulting in organized cell proliferation and determination of cell fate [13]. A previous study showed that GRF proteins act as repressors and down-regulators of KNOX gene expression [10]. As reported previously in rice [4], the transcription of most BrGRFs was induced by GA3 treatment. These results suggested that the GRF genes may function in maintaining or promoting cell proliferation in plants by a feedback regulation mechanism, in which the GRF genes positively regulate the production of gibberellins, and GAs in turn upregulate GRF gene expression.
In previous studies, the over-expression of GRF genes in Arabidopsis was found to result in bigger organ size than the vector control [3, 7], while reduction of AtGRF gene expression by the overexpression of the microRNA miR396 in transgenic Arabidopsis caused narrow-leaf phenotypes due to a reduction in cell number [24–26]. Here, we found that the ectopic expression of BrGRF8 also increased leaf size in Arabidopsis. In addition, our histological results revealed a significant increase in cell number but not in cell size in the 35S:BrGRF8 transgenic Arabidopsis plants compared with the vector control plants, suggesting that BrGRF8 may control the growth of plant organs by regulating cell proliferation rather than by enlarging cell volume.
Conclusions
We identified 17 members of the Chinese cabbage GRF gene family that encoded putative GRF proteins that fell into six subfamilies. The phylogenetic relationships among Chinese cabbage, rice, and Arabidopsis GRF genes, suggested that the BrGRFs were more closely allied with AtGRFs than with OsGRFs. Further, phylogenetic and duplication event analysis suggested that whole genome duplication may have been the main contributor to the expansion of the BrGRFs. Additionally, the ectopic expression of BrGRF8 in Arabidopsis positively controlled organ size by regulating cell proliferation. The expression profiles obtained by RT-qPCR showed that the BrGRFs may be involved in immature organ or tissue growth and development via the GA pathway. Together, these data will not only contribute to a further understanding of the characteristics and functions of the GRF family in different species, but will also provide a promising strategy for Chinese cabbage breeding programs to improve yield/head size.
Methods
Identification and analysis of GRF genes in Chinese cabbage
The nucleotide and protein sequences of BrGRFs were identified based on the B. rapa line Chiifu genome sequence (http://brassicadb.org) [20]. The GRF nucleotide sequences were aligned using DNAMAN 6.0.40 (Lynnon Biosoft, USA). Intron/exon structure analysis was performed using the Gene Structure display Server (GSDS) (http://gsds.cbi.pku.edu.cn/). Phylogenetic trees were constructed with the MEGA 4.0 software using the neighbor-joining method and a bootstrap test that was replicated 1000 times [27]. The GC content was calculated by DNASTAR (Madison, WI, USA). The number of amino acids, molecular weight (MW), and theoretical isoelectric point (pI) were computed using the ProtParam tool (http://web.expasy.org/protparam/). The SSR markers were detected using the SSRIT software (http://archive.gramene.org/db/markers/ssrtool) with the parameters adjusted for identification of perfect di-, tri-, tetra-, penta-, and hexa-nucleotide motifs with a minimum of 6, 5, 5, 4 and 4 repeats, respectively. The GO analysis of BrGRF proteins was carried out using the GO term analysis tool in Gramene (http://www.geneontology.org/) developed by the GO consortium Conserved motifs in the full-length amino acid sequences of GRF proteins from Chinese cabbage, Arabidopsis, and rice were identified using MEME [28]. The Arabidopsis and rice GRF protein sequences were downloaded from the Arabidopsis Information Resource (TAIR: http://www.arabidopsis.org/) and the Institute for Genomic Research Rice Genome Annotation project (TIGR: http://www.tigr.org/), respectively.
Plant materials and GA3 treatment
Chinese cabbage (Brassica rapa L. ssp. pekinensis inbred line Fushanbaotou) plants were grown in a greenhouse at 20 ± 2°C with a photoperiod of 16 h light and 8 h dark. For the GA3 treatment, uniformly sized seedlings were selected when they had developed four fully opened leaves, and then treated with 100 μM GA3 or distilled water (DW). The leaves of the seedlings were harvested after 0, 1, and 3 h of GA3 treatment. For analysis of GRF genes expression in different tissues, roots (R), stems (S), expanded rossete leaves (ERL), young folding leaves (YFL) beginning to fold at the early folding stage (about 24–25 leaves), buds (B), blooming flowers (BF), and immature siliques (IS) 15 d after fertilization were collected. All materials were frozen immediately in liquid nitrogen, and stored at -80°C until RNA isolation.
Real-time quantitative PCR
Total RNA was extracted from each sample using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and treated with RNase-free DNase I (Takara, Dalian, China) for 45 min according to the manufacturer’s protocol. First-strand cDNA was synthesized from 1 μg of total RNA using a PrimeScript 1st Strand cDNA Synthesis Kit (Takara). RT-qPCR was carried out using a SYBR Green Master mix (Takara) on an IQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The gene-specific primers designed for the BrGRF and BrGIF genes are listed in Additional file 2. The actin gene was used as a constitutive expression control in the RT-qPCR experiments. The PCR cycling conditions comprised an initial polymerase activation step of 95°C for 1 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. After each PCR run, a dissociation curve was designed to confirm the specificity of the product and to avoid the production of primer dimers. The relative amounts of the amplification products were calculated by the comparative 2-ΔΔCт method [29].
Transformation of Arabidopsis
To create transgenic plants overexpressing BrGRF8, the full-length cDNA was amplified by PCR with the primers BrGRF8-forward (5′-CGGGATCCATGATGAACCTAAGTGGAACTAGTG-3′) and BrGRF8-reverse (5′-TAGTCGACTCAGCTACCAGTGTCGAGTCTTGAC-3′). The underlined sequences represent restriction sites for BamHI and SalI, respectively. The amplified DNA fragments were cloned into the pGEM-T easy vector (Promega, Madison, WI, USA) and sequenced using an ABI 3730 × l DNA Sequencer (Applied Biosystems, Foster City, CA, USA). Then, the BrGRF8 cDNA in the pGEM-T easy vector was double-digested with BamHI and SalI and subcloned downstream of the CaMV 35S promoter into the binary vector pCAMBIA2300-35S-OCS to construct the plasmid CaMV35S:BrGRF5 (35S:BrGRF8). The pCAMBIA2300-35S-OCS vector without the BrGRF8 cDNA insert was used as a negative vector control. The 35S:BrGRF8 and vector control constructs were transformed separately into Agrobacterium tumefaciens LBA4404 and then the A. tumefaciens-mediated transformation of Arabidopsis was performed via vacuum infiltration [30]. The transgenic plants were screened on 1/2 MS agar plates containing 50 mg/ml kanamycin sulfate, and further verified by reverse transcription PCR. Homozygous T3 generation transgenic plants were used for subsequent analyses.
Histological analysis
Fully expanded fifth leaves of the vector control and 35S:BrGRF8 plants were detached and fixed in 100 mM sodium phosphate buffer (pH 7.0) containing 4% glutaraldehyde for 2 h at room temperature. Following a brief rinse in the buffer, the samples were dehydrated in an ethanol series for 1 h at each gradation. The dehydrated samples were dried in a critical-point dryer with liquid CO2 as the transitional fluid, and examined using a scanning electron microscope (SEM; JEOL JSM-7600 F, Japan) after coating the samples with gold. Epidermal cell size and number of cells on the adaxial side were determined in the middle region of a half leaf near the midvein. At least five leaves from each of the transgenic plants were selected for counting epidermal cell number in a fixed area on the SEM images.
Electronic supplementary material
Additional file 1: Putative functions and cellular localizations of GRF proteins in Chinese cabbage. (DOC 38 KB)
Additional file 2: Primers used in real-time quantitative PCR. (DOCX 15 KB)
Acknowledgements
This work was supported by the National Nature Science Foundation of China (31101553), the Prospect of Shandong Seed Project, China (2012lzgcshucaizy), Modern Agricultural Industrial Technology System Funding of Shandong Province, China (SDAIT-02-022-04), the National High Technology Research and Development Program, China (2012AA100103009), and the Promotive Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province, China (BS2010SW027).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JG and FW conceived and designed the experiments. FW, NQ, QD, and JL performed the experiments. YZ and HL analyzed the data. JG and FW drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Fengde Wang, Email: wfengde@163.com.
Nianwei Qiu, Email: nianweiqiu@163.com.
Qian Ding, Email: dingqian87@126.com.
Jingjuan Li, Email: lijj0620@163.com.
Yihui Zhang, Email: zyh_0923@163.com.
Huayin Li, Email: 1275424711@qq.com.
Jianwei Gao, Email: jianweigao3@yahoo.com.
References
- 1.van der Knaap E, Kim JH, Kende H. A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth. Plant Physiol. 2000;122:695–704. doi: 10.1104/pp.122.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Kende H. A transcriptional coactivator, AtGIF1, is involved in regulated leaf growth and morphology in Arabidopsis. Proc Natl Acad Sci U S A. 2004;101:13374–13379. doi: 10.1073/pnas.0405450101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JH, Chi D, Kenda H. The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J. 2003;36:94–104. doi: 10.1046/j.1365-313X.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi D, Kim JH, Kende H. Whole genome analysis of the OsGRF gene family encoding plant-specific putative transcription activators in rice (Oryza sativa L.) Plant Cell Physiol. 2004;45:897–904. doi: 10.1093/pcp/pch098. [DOI] [PubMed] [Google Scholar]
- 5.Zhang DF, Li B, Jia GQ, Zhang TF, Dai JR, Li JS, Wang SC. Isolation and characterization of genes encoding GRF transcription factors and GIF transcriptional coactivators in Maize (Zea mays L.) Plant Sci. 2008;175:809–817. doi: 10.1016/j.plantsci.2008.08.002. [DOI] [Google Scholar]
- 6.Fïlïz E, Koç İ, Tombuloğlu H. Genome-wide identification and analysis of growth regulating factor genes in Brachypodium distachyon: in silico approaches. Turk J Biol. 2014;38:296–306. doi: 10.3906/biy-1308-57. [DOI] [Google Scholar]
- 7.Horiguchi G, Kim GT, Tsukaya H. The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J. 2005;43:68–78. doi: 10.1111/j.1365-313X.2005.02429.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Lee BH. GROWTH-REGULATING FACTOR4 of Arabidopsis thaliana is required for development of leaves, cotyledons, and shoot apical meristem. J Plant Biol. 2006;49:463–468. doi: 10.1007/BF03031127. [DOI] [Google Scholar]
- 9.Liu J, Hua W, Yang HL, Zhan GM, Li RJ, Deng LB, Wang XF, Liu GH, Wang HZ. The BnGRF2 gene (GRF2-like gene from Brassica napus) enhances seed oil production through regulating cell number and plant photosynthesis. J Exp Bot. 2012;63(10):3727–3740. doi: 10.1093/jxb/ers066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuijt SJH, Greco R, Agalou A, Shao J, Hoen CCJ, Overnäs E, Osnato M, Curiale S, Meynard D, van Gulik R, de Faria Maraschin S, Atallah M, de Kam RJ, Lamers GEM, Guiderdoni E, Rossini L, Meijer AH, Ouwerkerk PBF. Interaction between the GRF and KNOX families of transcription factors. Plant Physiol. 2014;164(4):1952–1966. doi: 10.1104/pp.113.222836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusaba S, Fukumoto M, Honda C, Yamaguchi I, Sakamoto T, Kano-Murakami Y. Decreased GA1 content caused by the overexpression of OSH1 is accompanied by suppression of GA 20-oxidase gene expression. Plant Physiol. 1998;117:1179–1184. doi: 10.1104/pp.117.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka-Ueguchi M, Itoh H, Oyama N, Koshioka M, Matsuoka M. Overexpression of a tobacco homeobox gene, NTH15, decreases the expression of a gibberellin biosynthetic gene encoding GA 20-oxidase. Plant J. 1998;15:391–400. doi: 10.1046/j.1365-313X.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto T, Kamiya N, Ueguchi-Tanaka M, Iwahori S, Matsuoka M. KNOX homeodomain protein directly suppresses the expression of a gibberellins biosynthetic gene in the tobacco shoot apical meristem. Genes Dev. 2001;15:581–590. doi: 10.1101/gad.867901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hay A, Kaur H, Phillips A, Hedden P, Hake S, Tsiantis M. The gibberellins pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr Biol. 2002;12:1557–1565. doi: 10.1016/S0960-9822(02)01125-9. [DOI] [PubMed] [Google Scholar]
- 15.Rosin FM, Hart JK, Horner HT, Davies PJ, Hannapel DJ. Overexpression of a knotted-like homeobox gene of potato alters vegetative development by decreasing gibberellin accumulation. Plant Physiol. 2003;132:106–117. doi: 10.1104/pp.102.015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolduc N, Hake S. The maize transcription factor KNOTTED1 directly regulates the gibberellin catabolism gene ga2ox1. Plant Cell. 2009;21:1647–1658. doi: 10.1105/tpc.109.068221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang F, Liang G, Liu D, Yu D. Arabidopsis MiR396 mediates the development of leaves and flowers in transgenic tobacco. J Plant Biol. 2009;52:475–481. doi: 10.1007/s12374-009-9061-7. [DOI] [Google Scholar]
- 18.Liang G, He H, Li Y, Wang F, Yu D. Molecular mechanism of miR396 mediating pistil development in Arabidopsis thaliana. Plant Physiol. 2014;164(1):249–258. doi: 10.1104/pp.113.225144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baucher M, Moussawi J, Vandeputte OM, Monteyne D, Mol A, Pérez-Morga D, Jaziri MEI. A role for the miR396/GRF network in specification of organ type during flower development, as supported by ectopic expression of Populus trichocarpa miR396c in transgenic tobacco. Plant Biol. 2013;15(5):892–898. doi: 10.1111/j.1438-8677.2012.00696.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, Bai Y, Mun JH, Bancroft I, Cheng F, Huang S, Li X, Hua W, Wang J, Wang X, Freeling M, Pires JC, Paterson AH, Chalhoub B, Wang B, Hayward A, Sharpe AG, Park BS, Weisshaar B, Liu B, Li B, Liu B, Tong C, Song C, Duran C, et al. The genome of the mesopolyploid crop species Brassica rapa. Nat Genet. 2011;43:1035–1039. doi: 10.1038/ng.919. [DOI] [PubMed] [Google Scholar]
- 21.Powell W, Machray GC, Provan J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1996;1:215–222. doi: 10.1016/1360-1385(96)86898-1. [DOI] [Google Scholar]
- 22.Treich I, Cairns BR, de los Santos T, Brewster E, Carlson M. SNF11, a new component of the yeast SNF–SWI complex that interacts with a conserved region of SNF2. Mol Cell Biol. 1995;15:4240–4248. doi: 10.1128/mcb.15.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng F, Mandáková T, Wu J, Xie Q, Lysak MA, Wang X. Deciphering the diploid ancestral genome of the Mesohexaploid Brassica rapa. Plant Cell. 2013;25:1541–1554. doi: 10.1105/tpc.113.110486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D, Song Y, Chen Z, Yu D. Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol Plant. 2009;136(2):223–236. doi: 10.1111/j.1399-3054.2009.01229.x. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez RE, Mecchia MA, Debernardi JM, Schommer C, Weigel D, Palatnik JF. Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development. 2010;137:103–112. doi: 10.1242/dev.043067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Gu X, Xu D, Wang W, Wang H, Zeng M, Chang Z, Huang H, Cui X. miR396-targeted AtGRF transcription factors are required for coordination of cell division and differentiation during leaf development in Arabidopsis. J Exp Bot. 2011;62(2):761–773. doi: 10.1093/jxb/erq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 28.Bailey TL, Elkan C. The value of prior knowledge in discovering motifs with MEME. Proc Int Conf Intell Syst Mol Biol. 2005;3:21–29. [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCт method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Paris Life Sci. 1993;316:1194–1199. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Putative functions and cellular localizations of GRF proteins in Chinese cabbage. (DOC 38 KB)
Additional file 2: Primers used in real-time quantitative PCR. (DOCX 15 KB)


