Abstract
Objective
Treatment of myocardial infarction (MI) within the first 1–2 hours with a thrombolytic agent, percutaneous coronary intervention, or an αIIbβ3 antagonist decreases mortality and the later development of heart failure. We previously reported on a novel small molecule αIIbβ3 antagonist, RUC-2, that has a unique mechanism of action. We have now developed a more potent and more soluble congener of RUC-2, RUC-4, designed to be easily administered intramuscularly (IM) by autoinjector to facilitate its use in the pre-hospital setting. Here we report the properties of RUC-4 and the antiplatelet and antithrombotic effects of RUC-2 and RUC-4 in animal models.
Approach and Results
RUC-4 was ~20% more potent than RUC-2 in inhibiting human ADP-induced platelet aggregation and much more soluble in aqueous solutions (60–80 mg/ml). It shared RUC-2’s specificity for αIIbβ3 vs αVβ3, did not prime the receptor to bind fibrinogen, or induce changes in β3 identified by a conformation-specific monoclonal antibody. Both RUC-2 and RUC-4 prevented FeCl3-induced thrombotic occlusion of the carotid artery in mice and decreased microvascular thrombi in response to laser injury produced by human platelets infused into transgenic mice containing a mutated von Willebrand factor that reacts with human, but not mouse platelets. IM injection of RUC-4 in non-human primates at 1.9 and 3.85 mg/kg led to complete inhibition of platelet aggregation within 15 minutes, with dose-dependent return of platelet aggregation after 4.5–24 hours.
Conclusions
RUC-4 has favorable biochemical, pharmacokinetic, pharmacodynamic, antithrombotic, and solubility properties as a pre-hospital therapy of MI, but the possibility of increased bleeding with therapeutic doses remains to be evaluated.
Keywords: αIIbβ3, platelet, myocardial infarction
Introduction
The platelet αIIbβ3 receptor plays an important role in both hemostasis and thrombosis by virtue of it being required for platelet aggregation.1 It is a validated target for antiplatelet therapy having been found to be efficacious in reducing the risk of complications of percutaneous coronary interventions (PCI) in patients with ST-segment Elevated Myocardial Infarction (STEMI) in multiple randomized studies.2 Currently, there are three approved αIIbβ3 antagonists, abciximab, a recombinant chimeric Fab fragment of the monoclonal antibody 7E3, and two small molecule inhibitors, eptifibatide and tirofiban, both of which are patterned after the R(K)GD sequence found in some αIIbβ3 ligands and in snake venoms and peptides that bind to the receptor’s ligand binding pocket.3,4 All three antagonists require intravenous (IV) administration and are associated with thrombocytopenia in a small percentage of recipients, most commonly with abciximab.5 Early administration of these agents to patients having STEMI is associated with improved outcomes,6–13 but this strategy has not been adopted widely because of the difficulty of administering the drugs in the pre-hospital period by Emergency Medical Service (EMS) personnel. Attempts to develop oral αIIbβ3 antagonists that might be more easily administered failed in trials of chronic therapy because of lack of efficacy, an increased risk of death with some agents, an increased risk of bleeding, and infrequent thrombocytopenia.14,15 It has been proposed that the thrombocytopenia associated with these agents is caused in part by their inducing the receptor to undergo a major conformational change that exposes neoepitopes to which some patients have pre-formed antibodies.5,14,15 In fact, two of the oral agents associated with increased mortality, xemilofiban and orbofiban were reported to expose a ligand-induced binding site (LIBS) epitope on the β3 subunit,16 but variable results have been reported with other LIBS antibodies and other αIIbβ3 antagonists.16–19 Similarly, the paradoxical increase in mortality has been proposed to result from their inducing the receptor to adopt the high affinity ligand binding conformation, thereby priming the receptor to bind ligand when the drug dissociates from the receptor.14,15,17,20–22 However, priming by αIIbβ3 antagonists has only been reported with purified receptor or when platelets are fixed in the presence of the αIIbβ3 antagonist and then the antagonist is washed away.23–27 An alternative explanation for the paradoxical increase in mortality with the oral agents is the increased bleeding associated with these drugs,27,28 which likely reflects their narrow therapeutic window, since such events commonly lead to cessation of antiplatelet therapy. Moreover, oral agents are problematic when administered early to STEMI patients since absorption is poor and erratic. In fact, there are data with all of the approved oral P2Y12 antagonists demonstrating marked delays in the onset of action, even with high loading doses.29–31 Thus, intramuscular (IM) administration is preferable since it assures absorption without the technical challenges associated with IV administration under emergency conditions in the field.32
We recently described a novel αIIbβ3 antagonist termed RUC-2, a derivative of a smaller compound (RUC-1) identified in a high throughput screen.19,33 RUC-1 and RUC-2 lack a carboxyl group analogous to the carboxyl group in the ligand Asp and in the αIIbβ3 antagonists that coordinates the Mg2+ ion in the β3 subunit’s metal ion adhesion site (MIDAS).4,33 Interactions between the ligand (or antagonist) carboxyl group and the backbone nitrogens in the β1-α1 loop of β3 result in the movement of that loop toward the MIDAS, initiating the dramatic swing-out motion of the β3 hybrid domain that leads to the receptor adopting a high affinity ligand binding conformation.4 In support of this hypothesis, neither RUC-1 nor RUC-2 induced the reorganization of divalent cations in the β3 ligand binding pocket, nor did they induce conformational changes in β3 detectable by a conformation-specific monoclonal antibody or by electron microscopy (EM).19,33,34 Moreover, unlike eptifibatide and tirofiban, neither RUC-1 nor RUC-2 primed the receptor to bind the ligand fibrinogen.19,33,34 RUC-2 is ~100-fold more potent than RUC-1 (IC50s of ~90 nM and 13 µM, respectively) and has a unique mechanism of action, with X-ray crystallography demonstrating that its amine group competes with the MIDAS Mg2+ for binding to the carboxyl of β3 Glu220, thus displacing the Mg2+ and locking the receptor in the inactive conformation.33 As detailed in this paper, RUC-2 has potent antithrombotic effects in an animal model and favorable pharmacokinetics and pharmacodynamics for use in the pre-hospital setting, but it has limited solubility, essentially precluding it from being able to be delivered IM by autoinjector under emergency conditions. As a result, we synthesized congeners of RUC-2 and identified RUC-4, which is slightly more potent than RUC-2 and more than 500-fold more soluble.35 We now report on RUC-4’s properties with regard to specificity, priming, and ability to induce conformational changes in the β3 subunit. We also provide data on its mechanism of action, pharmacokinetics and pharmacodynamics in mice and non-human primates, and antithrombotic properties in mouse models that use both mouse and human platelets. We conclude that RUC-4 has favorable properties for further development for pre-hospital IM therapy of STEMI by autoinjector.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Comparison of the Properties of RUC-2 and RUC-4
The structures and properties of RUC-2 and RUC-4 are presented in Figure 1 and Table 1. RUC-4 differs from RUC-2 in being ~20% more potent as judged by its IC50 for 5 µM ADP-induced platelet aggregation using citrated platelet-rich plasma, and most notably, being more than 500-fold more soluble in aqueous buffer at neutral pH. Since some αIIbβ3 antagonists are more potent when assayed in citrated platelet-rich plasma compared to anticoagulants that do not chelate divalent cations,36,37 we compared the inhibition of ADP-induced platelet aggregation in blood anticoagulated with citrate and blood anticoagulated with bivalirudin. The IC50s were 85 ± 22 nM and 100 ± 23 nM (n=4; p=0.24), respectively, indicating a small effect of citrate.
Figure 1. Structures of RUC-2 and RUC-4.
RUC-2 (top) and RUC-4 (bottom)
Table 1.
Comparison of RUC-2 and RUC-4
| RUC-2 | RUC-4 | |
|---|---|---|
| Molecular weight | 385 | 386 |
| IC50 ADP-induced platelet aggregation (nM) | 95 ± 20 | 57 ± 10* |
| Solubility in aqueous buffer at pH 7.4 (mg/ml) | 0.068–0.092 | 60–80 |
| Reactivity with murine platelets | No | No |
| Specificity for αIIbβ3 vs αVβ3 | Yes | Yes |
| Ability to prime αIIbβ3 to bind fibrinogen | No | No |
| Ability to Induce conformational changes in αIIbβ3 identifiable by LIBS mAb | No | No |
n=8; p<0.001
Given their similarity in IC50, we went on to assess RUC-4’s specificity, ability to induce conformational changes in the β3 subunit, and ability to prime αIIbβ3 to bind the ligand fibrinogen. We used concentrations of each agent expected to completely inhibit platelet aggregation induced by ADP. RUC-4, like RUC-2, demonstrated specificity for αIIbβ3 relative to αVβ3 as shown by its inability to inhibit the binding of cells expressing αVβ3 to vitronectin, while inhibiting the binding of cells expressing αIIbβ3 to fibrinogen (Figure 2). Similarly, like RUC-2, but unlike eptifibatide, RUC-4 did not induce increased binding of mAb AP5, which recognizes a calcium sensitive ligand-induced binding site epitope in the β3 PSI domain (Figure 3). Moreover, RUC-4, like RUC-2, but unlike eptifibatide and an RGD-containing peptide (RGDS), did not prime αIIbβ3 to bind fibrinogen (Figure 4). In the latter studies, we could not directly test that RUC-4 was removed from the receptor by the washing procedures, but we inferred that it was removed because eptifibatide, which has more than 4-fold higher affinity for αIIbβ3 than RUC-4 as judged by its IC50 for platelet aggregation,33 did prime the receptor to bind fibrinogen under the same experimental conditions.
Figure 2. The effects of RUC-2 and RUC-4 on the adhesion of cells expressing αIIbβ3 to fibrinogen and cells expressing αVβ3 to vitronectin.
HEK293 cells expressing αIIbβ3 were incubated for 15 minutes at room temperature with buffer (control; Con), mAb 10E5 (anti-αIIbβ3; 40 µg/ml), EDTA (10 mM), mAb LM609 (20 µg/ml), RUC-2 (100 µM) or RUC-4 (300 µM) and then 100,0000 were added to microtiter wells precoated with fibrinogen at 50 µg/ml in HEPES-modified Tyrode’s (HMBT) buffer containing 1 mM MgCl2 and 2 mM CaCl2. After 1 hour at 37°C, the wells were washed and the adherent platelets quantified by detecting their acid phosphatase activity. Similarly, HEK293 cells expressing αVβ3 were added to wells precoated with vitronectin (5 µg/ml) in buffer containing 1 mM MgCl2 and adhesion quantified as indicated for cells expressing αIIbβ3. Data shown are mean ± SD for 4 separate analyses.
Figure 3. The effects of RUC-2 and RUC-4 on the binding of the ligand-induced binding site mAb AP5.
Platelets from blood collected into acid-citrate dextrose were washed with HMBT buffer containing 1 µM PGE1 and resuspended to 2.5 × 105 platelets/µl in HMBT containing 1 mM MgCl2 and 2 mM CaCl2. After adding buffer (control; C), EDTA (10 mM), eptifibatide (Epti; 10 µM), RUC-2 (100 µM), or RUC-4 (100 µM) and incubating for 15 min. mAb AP5, labeled with Alexa488, was added and incubated for another 30 minutes. Samples were then diluted, washed and analyzed by flow cytometry. Data shown are mean ± SD for 4 separate analyses.
Figure 4. The effects of RUC-2 and RUC-4 on priming platelet αIIbβ3 to bind fibrinogen.
Platelets were washed as per the studies on AP5 binding and resuspended at 1 × 105/µl in HMBT buffer containing 1 mM MgCl2 and 2 mM CaCl2. After adding buffer (control; Con), EDTA (10 mM), eptifibatide (1 µM), RGDS peptide (100 µM), RUC-2 (100 µM), or RUC-4 (300 µM) the platelets were incubated for 20 minutes at room temperature and then fixed with equal volume of 2% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4. for 40 minutes. The paraformaldehyde was quenched with glycine (5 mM) in PBS and then washed X3 with HMBT buffer and resuspended in HMBT with 1 mM MgCl2 and 2 mM CaCl2. Fibrinogen (200 µg/ml) labeled with Alexa488 was then added and after 30 minutes of incubation at room temperature, the platelets were washed and analyzed by flow cytometry. Data shown are mean ± SD for 3 separate analyses.
Molecular Dynamics Simulation Studies of RUC-4’s Mechanism of Action
The structural basis of RUC-4’s higher potency compared to RUC-2 was evaluated by molecular dynamics simulations. Detailed analysis of representative snapshots from the 50 ns simulation trajectories of RUC-2-αIIbβ3 (Figure 5A) and RUC-4-αIIbβ3 (Figure 5B) showed that RUC-4 binds to the protein the way RUC-2 does. Stable interactions that were identified during the simulations of both the RUC-2 and RUC-4 complexes (Figure SI) included: a) a direct hydrogen bond between the piperazine nitrogen and one of the oxygens of the side chain carboxyl group of αIIb D224, b) a direct hydrogen bond between the primary amine and one of the oxygens of the side chain carboxyl group of β3 E220, as well as with the backbone carbonyl oxygen of β3 A218, c) a direct hydrogen bond between the phenylacetamide nitrogen with the backbone carbonyl oxygen of β3 N215, d) a π-π stacking interaction between RUC-4's fused ring and the αIIb Y190 aromatic ring, and e) a water-mediated hydrogen bond between the carbonyl group in the compound’s fused ring and the side chain carboxyl group of αIIb D232. No additional, direct interactions were found between RUC-4 and the protein compared to RUC-2. However, an additional water-mediated interaction between the extra nitrogen in the RUC-4's phenyl ring and the oxydryl group of the β3 residue Y166 was observed during the RUC-4 simulations (Figure 5B), providing a structural rationale for the slightly higher affinity of RUC-4 compared to RUC-2 (Figure 5A). Validation of the sampling of the configuration of water molecules within the binding pocket was achieved by comparison of the MD-derived water density maps with converged positions of the hydrating water molecules determined by independent grand-canonical Monte Carlo simulations, which showed similar water distribution in the binding pockets of RUC-2 and RUC-4 during simulations (Figure SII, panels A–C).
Figure 5.
Representative structures taken from the last snapshot of the 50 ns MD simulations of RUC-2 (A) and RUC-4 (B) bound to the IIb3 headpiece. Proteins are shown as cartoon, ligands and surrounding protein residues within 4 Å of the ligands are shown as sticks, and water molecules are shown as red spheres. Polar protein-ligand interactions are highlighted with dotted lines.
Pharmacokinetic and Pharmacodynamic Studies of RUC-2 and RUC-4 in Mice
We previously reported that RUC-2 at 1 µM does not inhibit murine αIIbβ333 and RUC-4 shares this property (data not shown). To assess the antiplatelet effects of RUC-2 and RUC-4, we therefore employed the mice developed by Poncz’s group that express human αIIb in combination with murine β3 (hαIIb/mβ3) since the αIIb subunit primarily determines the binding specificity of RUC-2 and RUC-4.38 These mice express 58±8% (mean±SD) of the amount of platelet αIIbβ3 expressed by WT mice (n=6; data not shown) and have mild to moderate thrombocytopenia [635±112 × 103 platelet/µl in the mice we studied in the reported experiments (n=26) compared to 1,257±179 × 103 platelet/µl in a group of WT C57Bl/6 mice (n=21)]. Our goal was to identify a dose of each agent that could completely inhibit platelet aggregation induced by 20 µM ADP within 15 minutes of administration while allowing for at least partial return of platelet aggregation within 2–4 hours. RUC-2 administered at 0.39 mg/kg (0.1 ml) IP produced complete inhibition of platelet aggregation induced by 20 µM ADP within 15 minutes, with return of the aggregation response beginning at 45 minutes (Figure 6A). Since the duration of inhibition was less than the 2–4 hours we hoped to achieve, we treated another group of mice with RUC-2 at 3.85 mg/kg (0.3 ml) IP. Platelet aggregation in these mice was completely inhibited within 15 minutes, and the high-grade inhibition lasted for approximately two hours, at which time the platelet aggregation response returned toward normal (Figure 6B). Since RUC-4 was more soluble than RUC-2, it could be administered IM in a smaller volume (0.05 ml). At 1.2 mg/kg, RUC-4 produced complete inhibition of platelet aggregation at 5 minutes, with partial return of aggregation at 4 hours (Figure 6C). A series of 7 mice that received saline instead of RUC-2 or RUC-4 showed variable partial reductions in the initial slope of platelet aggregation (47 ± 26%) at different time points, but there was no temporal pattern and none of them showed the complete inhibition of platelet aggregation consistently observed after receiving RUC-2 or RUC-4.
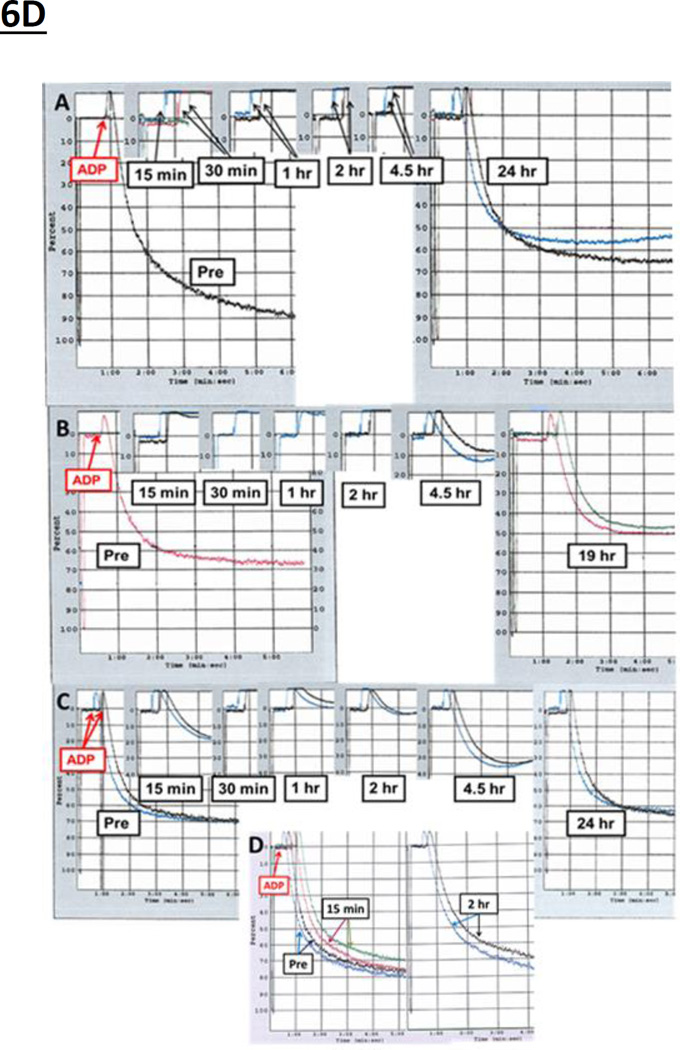
Figure 6. A–C. Effect of IP RUC-2 and IM RUC-4 on ADP-induced platelet aggregation in mice expressing hαIIbβ3 on their platelets.
Blood was obtained from the left ventricle under ultrasound guidance before (Pre bleed) and at the indicated times after the IP administration of (A) 0.1 ml of 96 µg/ml RUC-2 in water for the 0.39 mg/kg dose and (B) 0.3 ml of 321 µg/ml RUC-2 in water for the 3.85 mg/kg dose, and the IM administration (C) of 0.025 ml of RUC-4 dissolved in saline (600 µg/ml) in the caudal thigh muscles (semitendinosus-semimembranosus) in each leg. Each figure reflects composite data from different mice since each mouse was sampled only once. The 45 minute time points for the RUC-2 3.85 mg/kg dose and the RUC-4 1.2 mg/kg dose showed complete inhibition of platelet aggregation in all animals and so they were omitted to simplify the presentation. D. Effect of IM RUC-4 on ADP (20 µM)-induced platelet aggregation at times after administration to M. fascicularis. Panel A contains data on animals receiving 3.86 mg/kg; Panel B contains data on animals receiving 1.93 mg/kg; Panel C contains data on animals receiving 1.0 mg/kg; and Panel D contains data on animals receiving vehicle control (0.45% saline). Blood was collected at the indicated times and anticoagulated with 3.2% sodium citrate. Platelet-rich plasma (PRP) was prepared and aggregation was evaluated in an aggregometer. 2 animals were treated at each dose. At some time points, PRP could only be obtained from one of the animals. Otherwise the two adjacent tracings at each time point reflect data from two different animals.
The plasma concentrations of RUC-2 in the same samples used for the platelet aggregation studies, along with the primary slopes of platelet aggregation, are provided in Supplementary Table SI. With the exception of an outlier value in each series, the time to maximum plasma concentration was 15 minutes with RUC-2 and 5 minutes with RUC-4, perhaps reflecting more rapid absorption after IM than IP administration. The plasma levels of each agent dropped rapidly thereafter. The correlations between platelet aggregation and plasma concentrations comport well with RUC-4’s IC50 for hαIIb/mβ3 platelets (~0.01 µM; data not shown, n=3).
Pharmacokinetic and Pharmacodynamic Studies of RUC-4 in M. Fascicularis
RUC-4 was administered IM in volumes ranging from 0.26–0.47 ml to three cynomolgus monkeys at doses of 3.86 mg/kg, 1.93 mg/kg, and 1.0 mg/kg (Figure 6D and Supplementary Table SII). At the highest dose, aggregation was completely inhibited within 15 minutes and the high-grade inhibition lasted for more than 4.5 hours, but less than 24 hours; at the intermediate dose, inhibition was complete within 15 minutes and the aggregation response began to return to normal by 4.5 hours; at the lowest dose, inhibition of aggregation was partial at 15 minutes and complete at 30 minutes, with return toward normal aggregation evident at 2 hours. The injection of the vehicle control (0.45% NaCl) did not inhibit platelet aggregation of samples obtained at multiple time points (Figure 6D). The platelet counts in all three animals remained stable throughout the 24 hour period (Table SIII).
Clinical evaluation of the animals revealed that RUC-4 was well tolerated, with little or no purpura at the sites of administration or blood drawing. Transient gum bleeding was noted in the animal receiving the 3.85 mg/kg dose and a slight amount of blood was found on the rectal thermal probe when removed from the animal receiving the 1.93 mg/kg dose on one occasion. All animals were judged by the veterinary staff to be clinically normal before being released back to the test facility.
Antithrombotic Effects of RUC-2 and RUC-4: FeCl3 Murine Carotid Artery Model
The antithrombotic effects of RUC-2 and RUC-4 were assessed in hαIIb/mβ3 mice using the FeCl3 carotid artery model. Mice treated with saline had platelet counts similar to those of mice treated with either RUC-2 (587±82 vs 677±132 × 103 platelets per µl, respectively) or RUC-4 (707±204 vs 674±176 × 103 platelets per µl, respectively). αIIbβ3 surface expression, judged by the binding of the mAb 10E5, was also similar on saline-treated mice compared to those treated with RUC-2 (231±53 vs 249±10 arbitrary fluorescence units, respectively) or RUC-4 (141±43 vs 137±35 arbitrary units, respectively). RUC-2 at 3.85 mg/kg IP and RUC-4 at 1.2 mg/kg IM protected mice from vaso-occlusion; the protection was complete with RUC-2 (8/8) and incomplete with RUC-4, with 2/11 mice developing occlusion during the experiment (Figure 7).
Figure 7. RUC-2 and RUC-4 protect against carotid artery thrombosis induced by FeCl3.
hαIIb/mβ3 mice were treated IP with saline (n=7) or RUC-2 (n=8; 3.85 mg/kg; top panel) or IM with saline (n=12) or RUC-4 (n=11; 1.2 mg/kg; bottom panel) after exposure of a carotid artery. 20 minutes later 20% FeCl3 was applied to the artery for 3 minutes and then the time to carotid artery occlusion was monitored with a flow probe.
Antithrombotic Effects of RUC-2 and RUC-4: Transgenic vWF Mouse with Infused Human Platelets
To assess the antithrombotic effect of RUC-4 on human platelets in a physiological relevant setting, we employed a genetically modified murine model in which substituting His for Arg at position 1326 in the vWF A1 domain results in a decrease in the ability of murine platelets to form thrombi in response to laser injury in the cremasteric circulation, while dramatically increasing in the ability of transfused human platelets to form thrombi.39 Intravital microscopy demonstrated that IV RUC-4 at 1.5 mg/kg resulted in a marked decrease in thrombus formation (>80%), comparable to the decrease found with the αIIbβ3 antagonist abciximab (p=0.15) (Figure 8 and Video SI and Table SIV).
Figure 8. Effect of RUC-4 on arterial thrombosis induced by laser injury.
Comparison of maximal thrombus size (µm2) in laser-injured arterioles of vWFR1326H mice infused with human platelets in the absence or presence of RUC-4 or the αIIbβ3 antagonist abciximab. Each symbol represents the area of a thrombus in 1 arteriole of a mouse (n=5 mice per drug, 5 arterioles per animal). B indicates bolus; I, infusion.
Discussion
Despite universal agreement on the benefits of early treatment of MI,40,41 administering an effective agent in the pre-hospital setting poses a number of challenges. The first is the ability of emergency medical service personnel to diagnose ST segment-elevation MI (STEMI) in the field based on clinical and electrocardiographic criteria. In the IMMEDIATE trial, improvements in training and the algorithms used to assess the field electrocardiograms resulted in a relatively low rate of misdiagnosis, with 88.7% of the patients with ST segment elevation in the field electrocardiogram later demonstrating evidence of myocardial infarction.42
The second is the impact of cardiovascular instability on the patient’s ability to absorb oral medications. Studies of P2 Y12 antagonists provide strong evidence that impaired gastrointestinal absorption during acute coronary syndromes results in marked, and variable, delays in the onset of the antiplatelet effect.29–31
The third is the need for a rapid and convenient method of administration. The current αIIbβ3 antagonists all require IV administration with ongoing infusion, which can be difficult to achieve under emergency conditions. For example, in a study comparing IV lorazepam with an IM midazolam administered by autoinjector by emergency service personnel for the treatment of status epilepticus, 42 of 445 (9.4%) of patients did not receive the lorazepam because the personnel could not obtain IV access. In contrast only 5 of 448 patients (1.1%) of patients did not receive the IM midazolam and in each case it was due to autoinjector failure. The median time to drug administration was also shorter for the midazolam group (~1 vs ~5 minutes). In the IMMEDIATE trial of patients with acute coronary syndromes the emergency medical service personnel could not obtain IV access in 51/1483 (3.4%) and in those with IV access, subsequent infusion pump failure occurred in 16/1087 (1.5%) of patients.42
The fourth is having the proper pharmacokinetics, such that the onset of action is rapid, but the effect wears off in several hours as a safety measure and so that the physicians at the receiving hospital can introduce the therapy they think best, including surgery. Thus, an ideal antiplatelet agent for pre-hospital therapy of STEMI should: 1. Have the potency of an αIIbβ3 antagonist, the most potent of currently available agents, 2. Be rapidly absorbed and achieve high-grade inhibition of platelet aggregation within minutes when administered IM, 3. Have its antiplatelet effects begin to wear off within several hours. 4. Possess sufficient solubility so that it can be administered in ~1.5 ml, the practical limit for autoinjector.
Our data demonstrate that RUC-4 fulfills these criteria, being a potent inhibitor of platelet aggregation and thrombus formation in several different models that correlate with the efficacy of known antiplatelet agents, including a non-human primate model and a transgenic mouse model that employs human platelets. Moreover, RUC-4, like RUC-2 has a unique mechanism of action that does not trigger the conformational changes in the receptor induced by other αIIbβ3 antagonists based on the R(K)GD sequence that prime the receptor to bind fibrinogen and perhaps expose neoepitopes that lead to thrombocytopenia.
Pre-hospital therapy with a potent antiplatelet agent raises important safety concerns, especially since the use of αIIbβ3 antagonists employed during percutaneous coronary artery interventions are associated with increased risk of bleeding and bleeding is associated with adverse outcomes. Some of the bleeding associated with these agents can be ameliorated by proper dosing, especially in women43 and by employing radial rather than femoral access.44 Fortunately, αIIbβ3 antagonists have been associated with a much lower frequency of intracranial hemorrhage relative to thrombolytic therapy, with data from 367,294 patients treated with αIIbβ3 antagonists in the U.S. between 2000–2002 showing a rate of 0.13%, some of which may have been unrelated to the therapy.45 Moreover, the reported bleeding complications associated with αIIbβ3 antagonists comes from studies in which patients received aspirin and an anticoagulant, whereas in the pre-hospital setting, patients will likely only receive aspirin and the αIIbβ3 antagonist. In addition, a sizable fraction of the bleeding associated with αIIbβ3 antagonists occurs at the PCI arterial access site, and since arterial access only occurs after hospitalization, it will not contribute to pre-hospital hemorrhagic risk. None-the-less, the possibility of increased bleeding associated with therapeutic doses of RUC-4 remains to be evaluated.
Finally, in addition to the potential short term benefits of early potent antiplatelet therapy,6–14 there is emerging evidence that it may also decrease the longer term risk of congestive heart failure (CHF).7,10,46 This is important because in 2010 14.2% of Medicare patients with MI were hospitalized for CHF within 1 year and among those hospitalized, 45.5% died within the next year.47 In a recent review, Goel et al. concluded that every 1-hour delay in time to reperfusion is associated with an approximately 4–12% increased risk of new-onset CHF.48 It is important therefore to assess whether the rapid increase in cardiac blood flow associated with αIIbβ3 antagonist treatment of STEMI49 will translate into decreased morbidity and mortality from CHF.
Supplementary Material
Acknowledgments
We wish to thank Dr. Roman Osman for insightful discussions, Dr. Mihaly Mezei for technical assistance with the cavity-biased MC method, and Mr. George David III for technical assistance with the human platelet studies. Simulations were supported through the computational resources and staff expertise provided by the Scientific Computing Facility at the Icahn School of Medicine at Mount Sinai, and by the National Science Foundation through TeraGrid advanced computing resources provided by Texas Advanced Computing Center under TG-MCB080109N.
Sources of Funding
Supported, in part, by NIH grant NHLBI 19278 and NCATS CTSA UL1TR000043, as well as funds from Rockefeller University (Bridges to Better Medicine Award) and Stony Brook University.
Footnotes
Disclosures
In accord with Federal law and the policies of the Research Foundation of the State University of New York, Mount Sinai School of Medicine, and Rockefeller University, respectively, B.S. C. has royalty interests in abciximab (Centocor) and the VerifyNow assays (Accumetrics). B. S. C, M. F., and C. J. T. have royalty interests in RUC compounds.
Significance
RUC-4 is a novel and potent antiplatelet agent that was specifically designed to meet the unmet need for an easy to administer agent to improve the pre-hospital of myocardial infarction.
References
- 1.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch X, Marrugat J, Sanchis J. Platelet glycoprotein IIb/IIIa blockers during percutaneous coronary intervention and as the initial medical treatment of non-ST segment elevation acute coronary syndromes. Cochrane Database Syst Rev. 2010;8:CD002130. doi: 10.1002/14651858.CD002130.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Bledzka K, Smyth SS, Plow EF. Integrin alphaIIbbeta3: from discovery to efficacious therapeutic target. Circ Res. 2013;112:1189–1200. doi: 10.1161/CIRCRESAHA.112.300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao T, Takagi J, Coller BS, Wang J, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aster RH, Curtis BR, Bougie DW, Dunkley S, Greinacher A, Warkentin TE, Chong BH. Thrombocytopenia associated with the use of GPIIb/IIIa inhibitors: position paper of the ISTH working group on thrombocytopenia and GPIIb/IIIa inhibitors. J Thromb Haemost. 2006;4:678–679. doi: 10.1111/j.1538-7836.2006.01829.x. [DOI] [PubMed] [Google Scholar]
- 6.Hassan AK, Jukema JW, van der Laarse A, Hasan-Ali H, Wolterbeek R, van der Kley F, Spano F, Atsma DE, Schalij MJ. Incidence, patient characteristics and predictors of aborted myocardial infarction in patients undergoing primary PCI: prospective study comparing pre- and in-hospital abciximab pretreatment. EuroIntervention. 2009;4:662–668. doi: 10.4244/eijv4i5a110. [DOI] [PubMed] [Google Scholar]
- 7.Hassan AK, Liem SS, van der Kley F, Bergheanu SC, Wolterbeek R, Bosch J, Bootsma M, Zeppenfeld K, van der Laarse A, Atsma DE, Jukema JW, Schalij MJ. In-ambulance abciximab administration in STEMI patients prior to primary PCI is associated with smaller infarct size, improved LV function and lower incidence of heart failure: results from the Leiden MISSION! acute myocardial infarction treatment optimization program. Catheter Cardiovasc Interv. 2009;74:335–343. doi: 10.1002/ccd.21980. [DOI] [PubMed] [Google Scholar]
- 8.De Luca G, Gibson CM, Bellandi F, et al. Early glycoprotein IIb-IIIa inhibitors in primary angioplasty (EGYPT) cooperation: an individual patient data meta-analysis. Heart. 2008;94:1548–1558. doi: 10.1136/hrt.2008.141648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakowski T, Siudak Z, Dziewierz A, Birkemeyer R, Legutko J, Mielecki W, Depukat R, Janzon M, Stefaniak J, Zmudka K, Dubiel JS, Partyka L, Dudek D. Early abciximab administration before transfer for primary percutaneous coronary interventions for ST-elevation myocardial infarction reduces 1-year mortality in patients with high-risk profile. Results from EUROTRANSFER registry. Am Heart J. 2009;158:569–575. doi: 10.1016/j.ahj.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Xu Q, Yin J, Si LY. Efficacy and safety of early versus late glycoprotein IIb/IIIa inhibitors for PCI. Int J Cardiol. 2012;162:210–219. doi: 10.1016/j.ijcard.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 11.De Luca G, Bellandi F, Huber K, Noc M, Petronio AS, Arntz HR, Maioli M, Gabriel HM, Zorman S, DE CM, Rakowski T, Gyongyosi M, Dudek D. Early glycoprotein IIb-IIIa inhibitors in primary angioplasty-abciximab long-term results (EGYPT-ALT) cooperation: individual patient's data meta-analysis. J Thromb Haemost. 2011;9:2361–2370. doi: 10.1111/j.1538-7836.2011.04513.x. [DOI] [PubMed] [Google Scholar]
- 12.Ortolani P, Marzocchi A, Marrozzini C, Palmerini T, Saia F, Taglieri N, Baldazzi F, Dall'Ara G, Nardini P, Gianstefani S, Guastaroba P, Grilli R, Branzi A. Long-term effectiveness of early administration of glycoprotein IIb/IIIa agents to real-world patients undergoing primary percutaneous interventions: results of a registry study in an ST-elevation myocardial infarction network. Eur Heart J. 2009;30:33–43. doi: 10.1093/eurheartj/ehn480. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann HC, Lu J, Brodie BR, Armstrong PW, Montalescot G, Betriu A, Neuman FJ, Effron MB, Barnathan ES, Topol EJ, Ellis SG. Benefit of facilitated percutaneous coronary intervention in high-risk ST-segment elevation myocardial infarction patients presenting to nonpercutaneous coronary intervention hospitals. JACC Cardiovasc Interv. 2009;2:917–924. doi: 10.1016/j.jcin.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Cox D. Oral GPIIb/IIIa antagonists: what went wrong? Curr Pharm Des. 2004;10:1587–1596. doi: 10.2174/1381612043384673. [DOI] [PubMed] [Google Scholar]
- 15.Chew DP, Bhatt DL, Topol EJ. Oral glycoprotein IIb/IIIa inhibitors: why don't they work? Am J Cardiovasc Drugs. 2001;1:421–428. doi: 10.2165/00129784-200101060-00002. [DOI] [PubMed] [Google Scholar]
- 16.Jennings LK, White MM. Expression of ligand-induced binding sites on glycoprotein IIb/IIIa complexes and the effect of various inhibitors. Am Heart J. 1998;135:S179–S183. doi: 10.1016/s0002-8703(98)70246-7. [DOI] [PubMed] [Google Scholar]
- 17.Honda S, Tomiyama Y, Aoki T, Shiraga M, Kurata Y, Seki J, Matsuzawa Y. Association between ligand-induced conformational changes of integrin IIbbeta3 and IIbbeta3-mediated intracellular Ca2+ signaling. Blood. 1998;92:3675–3683. [PubMed] [Google Scholar]
- 18.Dickfeld T, Ruf A, Pogatsa-Murray G, Muller I, Engelmann B, Taubitz W, Fischer J, Meier O, Gawaz M. Differential antiplatelet effects of various glycoprotein IIb-IIIa antagonists. Thromb Res. 2001;101:53–64. doi: 10.1016/s0049-3848(00)00385-6. [DOI] [PubMed] [Google Scholar]
- 19.Blue R, Murcia M, Karan C, Jirouskova M, Coller BS. Application of high throughput screening to identify a novel αIIb-specific small molecule inhibitor of αIIbβ3-mediated platelet Interaction with fibrinogen. Blood. 2008;111:1248–1256. doi: 10.1182/blood-2007-08-105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hantgan RR, Stahle MC. Integrin priming dynamics: mechanisms of integrin antagonist-promoted alphaIIbbeta3:PAC-1 molecular recognition. Biochemistry. 2009;48:8355–8365. doi: 10.1021/bi900475k. [DOI] [PubMed] [Google Scholar]
- 21.Bassler N, Loeffler C, Mangin P, Yuan Y, Schwarz M, Hagemeyer CE, Eisenhardt SU, Ahrens I, Bode C, Jackson SP, Peter K. A mechanistic model for paradoxical platelet activation by ligand-mimetic alphaIIb beta3 (GPIIb/IIIa) antagonists. Arterioscler Thromb Vasc Biol. 2007;27:e9–e15. doi: 10.1161/01.ATV.0000255307.65939.59. [DOI] [PubMed] [Google Scholar]
- 22.Du XP, Plow EF, Frelinger AL, III, O'Toole TE, Loftus JC, Ginsberg MH. Ligands "activate" integrin alpha IIb beta 3 (platelet GPIIb-IIIa) Cell. 1991;65:409–416. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- 23.Frelinger AL, III, Furman MI, Krueger LA, Barnard MR, Michelson AD. Dissociation of glycoprotein IIb/IIIa antagonists from platelets does not result in fibrinogen binding or platelet aggregation. Circulation. 2001;104:1374–1379. doi: 10.1161/hc3701.095950. [DOI] [PubMed] [Google Scholar]
- 24.Hantgan RR, Stahle MC. Integrin priming dynamics: mechanisms of integrin antagonist-promoted alphaIIbbeta3:PAC-1 molecular recognition. Biochemistry. 2009;48:8355–8365. doi: 10.1021/bi900475k. [DOI] [PubMed] [Google Scholar]
- 25.Hantgan RR, Stahle MC, Connor JH, Connor RF, Mousa SA. AlphaIIbbeta3 priming and clustering by orally active and intravenous integrin antagonists. J Thromb Haemost. 2007;5:542–550. doi: 10.1111/j.1538-7836.2007.02351.x. [DOI] [PubMed] [Google Scholar]
- 26.Kouns WC, Kirchhofer D, Hadvary P, Edenhofer A, Weller T, Pfenninger G, Baumgartner HR, Jennings LK, Steiner B. Reversible conformational changes induced in glycoprotein IIb-IIIa by a potent and selective peptidomimetic inhibitor. Blood. 1992;80:2539–2547. [PubMed] [Google Scholar]
- 27.Ndrepepa G, Guerra E, Schulz S, Fusaro M, Cassese S, Kastrati A. Weight of the bleeding impact on early and late mortality after percutaneous coronary intervention. J Thromb Thrombolysis. 2014 doi: 10.1007/s11239-014-1084-3. [DOI] [PubMed] [Google Scholar]
- 28.Cannon CP. Oral platelet glycoprotein IIb/IIIa receptor inhibitors--part II. Clin Cardiol. 2003;26:401–406. doi: 10.1002/clc.4960260903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osmancik P, Jirmar R, Hulikova K, Peroutka Z, Pompachova A, Motovska Z, Widimsky P. A comparison of the VASP index between patients with hemodynamically complicated and uncomplicated acute myocardial infarction. Catheter Cardiovasc Interv. 2010;75:158–166. doi: 10.1002/ccd.22248. [DOI] [PubMed] [Google Scholar]
- 30.Heestermans AA, van Werkum JW, Taubert D, Seesing TH, von BN, Hackeng CM, Schomig E, Verheugt FW, ten Berg JM. Impaired bioavailability of clopidogrel in patients with a ST-segment elevation myocardial infarction. Thromb Res. 2008;122:776–781. doi: 10.1016/j.thromres.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, Koutsogiannis N, Damelou A, Tsigkas G, Davlouros P, Hahalis G. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2012;5:797–804. doi: 10.1161/CIRCINTERVENTIONS.112.972323. [DOI] [PubMed] [Google Scholar]
- 32.Asmussen S, Maybauer DM, Maybauer MO. Intramuscular versus intravenous benzodiazepines for status epilepticus. N Engl J Med. 2012;366:1943–1944. doi: 10.1056/NEJMc1203428. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Choi WS, McCoy J, et al. Structure-guided design of a high affinity platelet integrin αIIbβ3 receptor antagonist that disrupts Mg2+ binding to the MIDAS. Sci Transl Med. 2012;4:1–12. doi: 10.1126/scitranslmed.3003576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J, Negri A, Provasi D, Filizola M, Coller BS, Springer TA. Closed headpiece of integrin αIIbβ3 and its complex with an αIIbβ3-specific antagonist that does not induce opening. Blood. 2010;116:5050–5059. doi: 10.1182/blood-2010-04-281154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J, McCoy JG, Shen M, et al. A novel class of ion displacement ligands as antagonists of the αIIbβ3 receptor that limit conformational reorganization of the receptor. Bioorganic & Medicinal Chemistry Letters. 2014;24:1148–1153. doi: 10.1016/j.bmcl.2013.12.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips DR, Teng W, Arfsten A, Nannizzi-Alaimo L, White MM, Longhurst C, Shattil SJ, Randolph A, Jakubowski JA, Jennings LK, Scarborough RM. Effect of Ca2+ on GPIIb-IIIa interactions with integrilin: Enhanced GPIIb-IIIa binding and inhibition of platelet aggregation by reductions in the concentration of ionized calicum in plasma anticoagulated with citrate. Circulation. 1997;96:1488–1494. doi: 10.1161/01.cir.96.5.1488. [DOI] [PubMed] [Google Scholar]
- 37.Kereiakes DJ, Lorenz T, Young JJ, Kukielka G, Mueller MN, Nanniazzi-Alaimo L, Phillips DR. Differential effects of citrate versus PPACK anticoagulation on measured platelet inhibition by abciximab, eptifibatide and tirofiban. J Thromb Thrombolysis. 2001;12:123–127. doi: 10.1023/a:1012991303381. [DOI] [PubMed] [Google Scholar]
- 38.Blue R, Kowalska MA, Hirsch J, Murcia M, Janczak CA, Harrington A, Jirouskova M, Li J, Fuentes R, Thornton MA, Filizola M, Poncz M, Coller BS. Structural and therapeutic insights from the species specificity and in vivo antithrombotic activity of a novel αIIb-specific αIIbβ3 antagonist. Blood. 2009;114:195–201. doi: 10.1182/blood-2008-08-169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magallon J, Chen J, Rabbani L, Dangas G, Yang J, Bussel J, Diacovo T. Humanized mouse model of thrombosis is predictive of the clinical efficacy of antiplatelet agents. Circulation. 2011;123:319–326. doi: 10.1161/CIRCULATIONAHA.110.951970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verheugt FW, Gersh BJ, Armstrong PW. Aborted myocardial infarction: a new target for reperfusion therapy. Eur Heart J. 2006;27:901–904. doi: 10.1093/eurheartj/ehi829. [DOI] [PubMed] [Google Scholar]
- 41.Bates ER, Jacobs AK. Time to treatment in patients with STEMI. N Engl J Med. 2013;369:889–892. doi: 10.1056/NEJMp1308772. [DOI] [PubMed] [Google Scholar]
- 42.Selker HP, Beshansky JR, Sheehan PR, et al. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA. 2012;307:1925–1933. doi: 10.1001/jama.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander KP, Chen AY, Newby LK, Schwartz JB, Redberg RF, Hochman JS, Roe MT, Gibler WB, Ohman EM, Peterson ED. Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) initiative. Circulation. 2006;114:1380–1387. doi: 10.1161/CIRCULATIONAHA.106.620815. [DOI] [PubMed] [Google Scholar]
- 44.De Carlo M, Borelli G, Gistri R, Ciabatti N, Mazzoni A, Arena M, Petronio AS. Effectiveness of the transradial approach to reduce bleedings in patients undergoing urgent coronary angioplasty with GPIIb/IIIa inhibitors for acute coronary syndromes. Catheter Cardiovasc Interv. 2009;74:408–415. doi: 10.1002/ccd.22008. [DOI] [PubMed] [Google Scholar]
- 45.Qureshi AI, Hussain MS, Nasar A, Kirmani JF, Divani AA, Ahmed S, Suri MF. Intracranial hemorrhages associated with intravenous platelet glycoprotein IIB/IIIA receptor inhibitors in the United States. Cardiovasc Drugs Ther. 2005;19:371–373. doi: 10.1007/s10557-005-4390-3. [DOI] [PubMed] [Google Scholar]
- 46.Rakowski T, Zalewski J, Legutko J, Bartus S, Rzeszutko L, Dziewierz A, Sorysz D, Bryniarski L, Zmudka K, Kaluza GL, Dubiel JS, Dudek D. Early abciximab administration before primary percutaneous coronary intervention improves infarct-related artery patency and left ventricular function in high-risk patients with anterior wall myocardial infarction: a randomized study. Am Heart J. 2007;153:360–365. doi: 10.1016/j.ahj.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Hsieh AF, Dharmarajan K, Masoudi FA, Krumholz HM. National trends in heart failure hospitalization after acute myocardial infarction for medicare beneficiaries: 1998–2010. Circulation. 2013;128:2577–2584. doi: 10.1161/CIRCULATIONAHA.113.003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goel K, Pinto DS, Gibson CM. Association of time to reperfusion with left ventricular function and heart failure in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: a systematic review. Am Heart J. 2013;165:451–467. doi: 10.1016/j.ahj.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Gold HK, Garabedian HD, Dinsmore RL, Guerrero LJ, Cigarroa JE, Palacios IF, Leinbach RC. Restoration of coronary flow in myocardial infarction by intravenous chimeric 7E3 antibody without exogenous plasminogen activators: observations in animals and man. Circulation. 1997;95:1755–1759. doi: 10.1161/01.cir.95.7.1755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.