Abstract
Background
Potentially traumatic events (PTEs) are common in the population, yet, the impact of total burden and specific types of PTEs on physical health has not been systematically investigated.
Methods
Data were drawn from the Detroit Neighborhood Health Study, a community sample of predominately African Americans living in Detroit, Michigan, interviewed in 2008–2009 (N = 1,547) and in 2009–2010 (N = 1,054). Kaplan–Meier and Cox proportional hazards models were used.
Results
Respondents with the highest levels of PTE exposure (8+ events) had an average age of adverse physical health condition diagnosis that was 15 years earlier than respondents with no exposure. There was a monotonic relation between number of PTEs and arthritis risk. Compared to those who reported no lifetime events, respondents with 1–2, 3–4, 5–7, and 8+ traumatic events had 1.06, 1.12, 1.73, and 2.44 times the hazard of arthritis. Assaultive violence (HR = 1.7; 95% CI 1.2–2.3) and other threats to physical integrity (HR = 1.5, 95% CI 1.1–2.1) were particularly strong risk factors for arthritis.
Conclusions
These results provide novel evidence linking PTEs, particularly those involving violence and threat to life, to elevated risk for arthritic conditions. Efforts to prevent or mitigate traumatic event exposures may have a broad range of benefits for health.
Keywords: trauma, traumatic events, chronic disease, arthritis, physical health, African Americans
Introduction
Violence, injury, and disaster are common events in the general population and are often traumatic. A majority of adults report experiencing at least one of these event experiences in their lifetime.[1–3] The impact of such events on short- and long-term mental health has been well documented.[1–8] Further, there is now robust evidence that individuals with posttraumatic stress disorder (PTSD) are at higher risk for adverse health outcomes, with substantial evidence for a link between PTSD and cardiovascular disease.[9–18] Prevention and treatment of PTSD and related sequelae are thus important public health goals.
Of growing interest, however, is whether exposure to stressful or potentially traumatic events (PTEs) can influence health in the absence of PTSD. There is evidence from both animal and human studies that the experience of stress and trauma, especially when chronic, can activate dysregulation in a number of systems including neuroendocrine,[19, 20] immune,[21–30] metabolic,[31, 32] and cardiovascular[14, 16,33–35] even in the absence of observable psychological consequences. Although these associations are mediated, in part, by health behaviors, such as smoking, alcohol use, and physical inactivity,[36–38] and by subsequent psychopathology, such as depression and generalized anxiety disorder (GAD),[39, 40] it has been suggested that exposure to traumatic events also induces changes in a number of physiological regulatory systems that may directly contribute to elevated risk of adverse physical health[41–43]. For example, dysregulation in the hypothalamic–pituitary–adrenal (HPA) axis and autonomic nervous system have been reported consistently among persons exposed to traumatic events.[44–50] Yet, population-based examinations of cumulative potentially traumatic stressors on physical health outcomes remain understudied.
Further, previous research has not systematically examined the potential differential effects of various types of traumatic experiences on disease outcomes across a comprehensive range of traumatic events in a population representative sample. Different types of traumatic events are likely to have different consequences for health. For example, the conditional risk of PTSD varies widely across traumatic event types, such that events involving interpersonal or assaultive violence are the most likely to lead to PTSD.[1,2] Whether the conditional risk of physical health problems differs by trauma type has not been examined. Delineating the degree to which these various types of traumatic events are associated with adverse physical health may shed light on the pathways through which the social environment influences health and provide important information for targeting treatment and prevention efforts.
In summary, exposure to stressful and potentially traumatic experiences, especially repeated exposures, may serve as a catalyst to dysregulation of physiological processes that accelerate the onset of chronic health problems. Whether these changes could possibly occur in the absence of PTSD is underinvestigated, but an important empirical question for prevention science.
The present study examined the association between exposure to PTEs, PTSD, and adverse physical health within a population-based community sample in Detroit, Michigan. Pervasive exposure to traumatic events has been previously documented in this sample, with 87.2% of respondents reporting at least one negative life event that is potentially traumatic (heretofore referred to as PTEs) event and 51.0% reporting exposure to violent assault such as being shot, stabbed, or raped.[51] First, we examine whether lifetime experience of PTSD is associated with adverse health conditions, including cardiovascular disease, arthritis, diabetes, atrial fibrillation, and respiratory disease. Second, controlling for lifetime experience of PTSD, we examine whether the amount of PTEs experienced predicts these adverse health conditions. Finally, again controlling for lifetime experiences of PTSD, we consider whether specific types of events differentially predict health outcomes.
Methods
Sample
Data were drawn from the Detroit Neighborhood Health Study (DNHS), a longitudinal cohort of predominately African-American adults (18+) living in Detroit, Michigan. Wave 1 was conducted between 2008 and 2009. Participants were selected using a dual-frame probability design, using telephone numbers obtained from the U.S. Postal Service Delivery Sequence Files as well as a list-assisted random-digit-dial frame. A total of 1,547 individuals participated in Wave 1, with an overall participation rate among eligible persons of 53%. Further information regarding baseline sampling can be found elsewhere.[28,51,52] Wave 2 was conducted 1 year following Wave 1; a total of 1,054 individuals were reinterviewed (68% of the baseline sample). Those who did not respond were younger (χ2 = 8.8, P < .01), less educated (χ2 = 19.0, P < .01), more likely to be unemployed (χ2 = 9.3, P = .01), and single (χ2 = 31.6, P < .01); sample weights were incorporated to account for attrition between Waves 1 and 2.
Measures
Potentially Traumatic Events
At Wave 1, respondents reported whether they had ever experienced any of 19 separate events that are potentially traumatic (see Goldmann et al., 2011, for more detail). Events that occurred between Waves 1 and 2 were not considered, as few of these events occurred prior to the onset of the physical health diagnosis. Five groupings were chosen a priori based on methods previously used in population-based samples[53] as follows: (1) assaultive violence included rape, other sexual assault, being shot or stabbed, held captive, tortured, kidnapped, mugged, held up, threatened with a weapon, or badly beaten up (52% experienced at least one of these, ranging from 5.2% for held captive/tortured/kidnapped to 33.8% for mugged, held up, or threatened); (2) other threats to physical integrity included serious motor vehicle crash, any other kind of serious accident or injury, fire, flood, earthquake or other natural disaster (42.1% experienced at least one of these, ranging from 15.2% for “other” serious accident or injury to 26.1% for serious motor vehicle crash); (3) PTEs in the social network included learning of a PTE that occurred to a loved one (rape, sexual assault, physical assault, serious injury in a motor vehicle crash, and serious injury in another accident), child diagnosed with a life threatening illness, witnessed someone being killed or seriously injured, or unexpectedly discovering a dead body (62.6% experienced at least one of these, ranging from 13.7% unexpectedly discovering a dead body to 43.7% leaning that a loved one was injured in a motor vehicle crash); (4) sudden unexpected death of a close friend or relative (72%); and (5) other extraordinarily stressful event was assessed based on asking the respondent whether they were exposed to “any other extraordinarily stressful situation or event” (25%). These categories were not mutually exclusive and there was substantial overlap in experiences across categories. Age at which the event occurred was not assessed; lifetime experiences of events were analyzed as binary yes/no variables.
Posttraumatic Stress Disorder
PTSD diagnosis was assessed via telephone using a structured diagnostic interview following DSM-IV criteria. PTSD symptoms (DSM-IV criteria B, C, and D) were measured using the PCL-C[54] referencing two traumatic events (criterion A1): one that the participant regarded as the worst and one randomly selected event from the remaining PTEs a respondent may have experienced. DSM-IV PTSD diagnoses were made by additionally querying whether the event caused helplessness, fear, or horror (criterion A2), as well as the duration (criterion E) and functional significance (criterion F) of the symptoms.
Physical Health
Physical health indicators were retrospectively assessed at Wave 2 (2009–2010). Respondents were asked about 22 health conditions, beginning with the stem “Has a doctor ever told you that you have any of the following health problems?” Then a list of conditions and the year of diagnosis were self-reported. This approach is standard practice in large-scale surveys, and self-reported medical diagnoses have documented fair to good reliability[55–57] and adequate validity.[58] Health outcomes were grouped into six categories: (1) cardiovascular disease included high blood pressure or hypertension (38.2%), myocardial infarction or stroke (7.9%), chest pain (3.0%), and congestive heart failure (3.8%), mean years since diagnosis 7.5 (SE = 0.6); (2) arthritis (31.1%), mean years since diagnosis 9.4 (SE = 0.9); (3) diabetes (13.6%), mean years since diagnosis 10.6 (SE = 1.0); (4) atrial fibrillation (6.3%), mean years since diagnosis 9.7 (SE = 2.0); and (5) respiratory disease included chronic obstructive pulmonary disease (COPD, 3.1%) and emphysema (1.3%), mean years since diagnosis 6.7 (SE = 1.4); and (6) gastrointestinal health included liver disease (hepatitis or cirrhosis; 2.5%), ulcer (either stomach, duodenal, or peptic; 5.7%), and gallbladder problems (2.8%), mean years since diagnosis 15.5 (SE =2.0). Several physical health conditions were also assessed but did not have sufficient power to be analyzed, including cancer, Parkinson's disease, Alzheimer's disease, and tuberculosis. Only disorders with reported adult onset were included presently.
Control Variables
Adjusted models controlled for demographic variables of sex, race/ethnicity, and Wave 1 age, education, employment, and marital status.
Traumatic personal experiences are associated with psychopathology, such as depression and GAD[39,40] and these disorders are also associated with adverse health outcomes.[59–61] Therefore, we also controlled for depression and GAD in final models to determine whether there is an addition between traumatic personal experiences and health that is unrelated to additional psychopathology. Lifetime major depression was assessed with DSM-IV criteria using a modified version of the Patient Health Questionnaire (PHQ-9).[62] GAD was assessed with a modified version of the GAD 7-item scale (GAD-7).[63] Clinical reappraisal using clinician interviews in this sample found good concordance between the measures of PTSD and depression and psychiatric diagnoses in a clinical reappraisal sample using the Structured Clinical Interview for DSM-IV (SCID) and the Clinician-Administered PTSD Scale (CAPS).[64]
Binge drinking was defined as five or more drinks on a single drinking occasion in the past month for men, four or more for women (lifetime binge drinking was not assessed). Smoking was defined as any current use of cigarettes, former use of cigarettes, or lifetime abstention.
Statistical Analysis
Kaplan–Meier analysis was used to examine the bivariate associations between lifetime traumatic event exposure at Wave 1 and age at diagnosis for each medical outcome that was reported in Wave 2.
Cox proportional hazards models were estimated. First, we estimate the association between lifetime experience of PTSD and onset of physical health outcomes. Second, we estimate associations between number and type of PTE and physical health outcome both unadjusted and adjusted for PTSD as well as age, gender, race, education, employment, lifetime history of major depression, and GAD, and past month binge drinking at baseline and current and former smoking.
To examine the effect of PTE exposure on number of physical health conditions, Poisson regression was used with the number of physical health conditions reported (0–20) as the outcome, and traumatic event categories as well as number of PTEs experienced as predictors.
Results
Table 1 provides the distributions of all relevant study variables in these data. Sudden unexpected death of a loved one was the most prevalent PTE, with 69.7% of the sample reporting exposure at some point in the life course. Approximately 15% of the sample reported no PTEs, and 20.2% of respondents reported eight or more PTEs in their lifetime. Common physical health conditions in the sample included cardiovascular disease (44.5%) and arthritis (31.1%). Respiratory conditions were the least common physical health condition included in analysis (3.9%). The average age of the sample was 44.3 (SE = 1.0).
Table 1. Characteristics of the Detroit Neighborhood Health Study participants, 2009–2010 (N = 1,054).
| N | Percent | (SE) | |
|---|---|---|---|
| Lifetime traumatic event experiences reported at baseline | |||
| Assaultive violence | 507 | 48.7% | (2.5) |
| Other threats to physical integrity | 444 | 41.3% | (2.5) |
| Traumatic events in social network | 711 | 68.0% | (2.4) |
| Sudden unexpected death of loved one | 761 | 69.7% | (2.4) |
| Other extraordinary event | 265 | 21.9% | (2.0) |
| Number of traumatic events experience in lifetime: | |||
| 0 | 136 | 14.5% | (1.9) |
| 1–2 | 267 | 23.9% | (2.1) |
| 3–4 | 207 | 17.8% | (1.9) |
| 5–7 | 249 | 23.7% | (2.1) |
| 8–19 | 195 | 20.2% | (2.1) |
| Demographic characteristics at baseline | |||
| Male | 426 | 47.2% | (2.6) |
| Female | 628 | 52.8% | (2.6) |
| Married/living with someone as if married | 281 | 28.9% | (2.1) |
| Widowed/separated/divorced | 413 | 26.2% | (2.0) |
| Never married | 360 | 44.9% | (2.6) |
| African American | 897 | 91.7% | (1.3) |
| Other race | 116 | 8.3% | (1.3) |
| Looking for work and unemployed | 168 | 25.9% | (2.5) |
| Other employment situation (e.g., full time, part time, student, retired) | 886 | 74.1% | (2.5) |
| Less than high school education | 133 | 15.3% | (1.9) |
| High school degree | 301 | 42.9% | (2.6) |
| More education than high school completed | 620 | 41.8% | (2.4) |
| Psychiatric morbidity and health behaviors at baseline | |||
| Lifetime DSM-IV posttraumatic stress disorder | 146 | 13.8% | (1.7) |
| Lifetime DSM-IV major depression | 188 | 18.5% | (2.0) |
| Lifetime DSM-IV generalized anxiety disorder | |||
| Past-month episode of 5+ drinks in a single sitting | 41 | 4.7% | (1.1) |
| Used cigarettes in the last 30 days | 327 | 36.2% | (2.5) |
| Ever been a smoker but not currently using cigarettes | 287 | 21.0% | (1.9) |
| Never smoker | 432 | 42.9% | (2.5) |
| Physical health conditions in adulthood reported at follow-up | |||
| Cardiovascular disease | 580 | 42.0% | (2.4) |
| Arthritis | 452 | 31.1% | (2.0) |
| Diabetes | 192 | 13.6% | (1.5) |
| Gastrointestinal health | 138 | 9.5% | (1.3) |
| Atrial fibrillation | 102 | 6.3% | (0.6) |
| Respiratory conditions | 53 | 3.9% | (0.9) |
| Number of physical health conditions in adulthood: | |||
| 0 | 469 | 44.5% | (2.6) |
| 1–2 | 351 | 33.3% | (2.3) |
| 3–4 | 185 | 17.6% | (1.7) |
| 5–11 | 49 | 4.7% | (0.8) |
Respondents who experienced a PTE were at significantly increased risk for an earlier diagnosis of a physical health condition as shown in Fig. 1 (Log-rank test P-value = .003). For example, the median age of onset of a physical health condition was 55 for respondents with no PTE exposure and 50 for respondents who experienced a PTE. Risk associated with any physical health condition by the number of events was also examined (Fig. 2). Respondents with the highest levels of PTE (8+ events) had an average age of physical condition onset that was 15 years earlier than respondents with no exposure.
Figure 1.
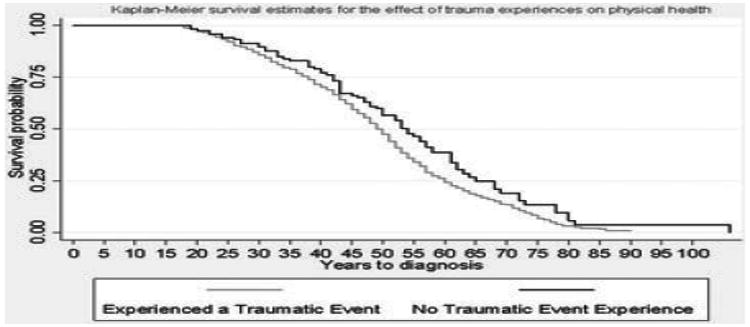
Kaplan–Meier curve for the association between potentially traumatic events (PTEs) and physical health condition onset in the Detroit Neighborhood Health Study participants, 2009–2010 (N = 1,054).
Figure 2.
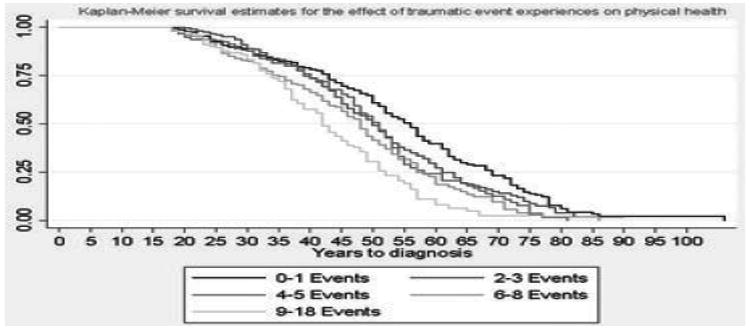
Kaplan–Meier curve for number of potentially traumatic events (PTEs) and physical health condition onset in the Detroit Neighborhood Health Study participants, 2009–2010 (N = 1,054).
Table 2 shows associations between lifetime experience of PTSD and hazard of each physical health condition. In unadjusted models, PTSD was associated with the hazard of arthritis (HR = 1.95, 95% CI 1.29–2.95) and respiratory disease (HR = 3.22, 95% CI 1.17–8.89). When adjusted for covariates, PTSD was not statistically significantly associated with hazard of any physical health outcomes.
Table 2. Association between lifetime posttraumatic stress disorder (N = 146) and hazard of physical health conditions: evidence from a population-based sample in Detroit (N = 1,054).
| Unadjusted | Adjusteda | ||
|---|---|---|---|
| N | HR (95% CI) | HR (95% CI) | |
| Cardiovascular disease | 580 | 1.40 (0.97–2.01) | 1.08 (0.78–1.51) |
| Arthritis | 452 | 1.95 (1.29–2.95)** | 1.44 (0.93–2.23) |
| Diabetes | 192 | 1.61 (0.87–2.97) | 1.33 (0.71–2.48) |
| Atrial fibrillation | 102 | 1.61 (0.69–3.76) | 1.08 (0.37–3.18) |
| Respiratory disease | 53 | 3.22 (1.17–8.89)* | 2.33 (0.53–10.19) |
| Gastrointestinal health | 138 | 1.71 (0.88–3.33) | 1.20 (0.43–3.37) |
P < .05;
P < .01.
Adjusted for sex, marital status, race, employment, education, lifetime major depressive disorder (MDD) and generalized anxiety disorder (GAD), current and former smoking, and current binge drinking.
Table 3 shows the association between the number of events experienced and the hazard of each physical health condition. In unadjusted models, the hazard associated with cardiovascular disease was elevated for the highest exposure group (HR = 1.74, 95% CI 1.06–2.84) but the magnitude of the result diminished upon adjustment and the hazard ratio was no longer statistically significant (HR = 1.40, 95% CI 0.84–2.34).
Table 3. Number of traumatic events and physical health conditions: evidence from a population-based sample in Detroit (N = 1,054).
| Cardiovascular diseasea (N = 580) | Arthritis (N = 452) | Diabetes (N = 192) | Atrial fibrillation (N = 102) | Respiratory diseaseb (N = 53) | Gastrointestinal health (N = 138) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
||||||||
| N | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Model 1: Unadjusted | |||||||||||||
| 0 | 136 | – | – | – | – | – | – | – | – | – | – | – | – |
| 1–2 | 267 | 1.26 | 0.79–2.02) | 0.76 | (0.47–1.24) | 0.64 | (0.30–1.36) | 0.36 | (0.13–1.02) | 0.11 | (0.02–0.64)** | 0.39 | (0.14–1.04) |
| 3–4 | 207 | 1.18 | (0.75–1.84) | 1.17 | (0.71–1.94) | 0.97 | (0.47–1.98) | 0.91 | (0.31–2.64) | 0.61 | (0.10–3.50) | 0.50 | (0.19–1.29) |
| 5–7 | 249 | 1.36 | (0.87–2.13) | 1.26 | (0.76–2.10) | 1.47 | (0.72–3.00) | 0.86 | (0.29–2.54) | 1.92 | (0.34–10.77) | 0.56 | (0.23–1.38) |
| 8–19 | 195 | 1.74 | (1.06–2.84)* | 2.71 | (1.59–4.60)* | 1.28 | (0.57–2.86) | 1.55 | (0.42–5.77) | 1.95 | (0.34–11.14) | 1.29 | (0.54–3.11) |
| Model 2: Adjusted for demographics, lifetime PTSD, GAD, and MDD, current and former smoking, and current binge drinking | |||||||||||||
| 0 | 136 | – | – | – | – | – | – | – | – | – | – | – | – |
| 1–2 | 267 | 1.28 | (0.81–2.04) | 1.06 | (0.63–1.79) | 0.59 | (0.27–1.28) | 0.34 | (0.11–1.09) | 0.08 | (0.01–0.49)** | 0.30 | (0.11–0.81)** |
| 3–4 | 207 | 1.05 | (0.67–1.63) | 1.12 | (0.67–1.88) | 0.86 | (0.40–1.84) | 0.61 | (0.19–1.94) | 0.37 | (0.07–2.07) | 0.32 | (0.13–1.08) |
| 5–7 | 249 | 1.14 | (0.73–1.76) | 1.73 | (1.04–2.22)* | 1.32 | (0.64–2.76) | 0.62 | (0.20–1.94) | 1.20 | (0.25–5.87) | 0.42 | (0.18–1.31) |
| 8–19 | 195 | 1.40 | (0.84–2.34) | 2.44 | (1.39–4.27)** | 1.11 | (0.50–2.43) | 0.93 | (0.30–2.93) | 1.11 | (0.21–5.76) | 0.87 | (0.37–2.05) |
Cardiovascular disease included high blood pressure or hypertension, myocardial infarction or stroke, chest pain, and congestive heart failure.
Respiratory disease included chronic obstructive pulmonary disease (COPD) and emphysema.
P < .05;
P < .01.
GAD, generalized anxiety disorder; PTSD, posttraumatic stress disorder.
For arthritis, in unadjusted models, the hazard for the highest exposure group was elevated (HR = 2.71, 95% CI 1.59–4.60); elevated hazard persisted after control for covariates (HR = 2.44, 95% CI 1.39–4.27). In adjusted models, the hazard of arthritis associated with five to seven PTEs was also elevated (HR = 1.73, 95% CI 1.04– 2.22). The increase in hazard upon adjustment reflects the confounding effects of gender imbalance; men are more likely to have five to seven PTEs compared women, but have a lower risk of arthritis. The log linear trend between number of events and arthritis hazard indicated evidence of a dose response between PTEs and hazard of arthritis; each increase in PTE categories was associated with 1.3 times increased hazard of arthritis (HR = 1.30, 95% CI 1.12–1.50).
Respiratory illness had a lower hazard in those who experienced one to two events compared to those who experienced no PTEs, in both unadjusted (HR=0.11, 95% CI 0.02–0.64) and adjusted models (HR = 0.08, 95% CI 0.01–0.49). For gastrointestinal illness, the unadjusted model associated with hazard based on one to two PTEs was not significant (HR = 0.39, 95% CI 0.14–1.04) but became significant upon adjustment (HR = 0.30, 95% CI 0.11–0.81), indicating a lower hazard of respiratory illness based on experience of one to two PTEs versus none. The increase in hazard upon adjustment reflects the confounding effects of race; Blacks in this sample are more likely than Whites to experience one to two PTEs, but have 3.05 higher hazard of gastrointestinal illness compared to Whites (95% CI 1.29–7.23).
Different types of cardiovascular disease were also analyzed separately. Hazard ratios for high blood pressure, intermittent claudication, chest pain, and congestive heart failure were in the same direction with the overall hazard ratios reported in Table 2, but results were not statistically significant. For example, for myocardial infarction, the hazard ratios for those experiencing 1–2, 3–4, 5–7, and 8+ events was 3.07 (95% CI 0.92–10.24), 2.45 (95% CI 0.72–8.34), 2.77 (95% CI 0.81–9.42), and 3.31 (95% CI 0.88–12.49) compared to those with no traumatic events, respectively, in fully adjusted models.
Table 4 shows the association between each type of PTE and each physical health condition.
Table 4. Specific traumatic events and risk for physical health conditions: evidence from a population-based sample in Detroit (N = 1,054).
| Cardiovascular diseasea (N = 580) | Arthritis (N =452) | Diabetes (N =192) | Atrial fibrillation (N =102) | Respiratory diseaseb (N =53) | Gastrointestinal health (N = 138) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
||||||||
| N | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Model 1: Unadjusted | |||||||||||||
| Assaultive violence | 507 | 1.00 | (0.76–1.31) | 1.71 | (1.25–2.32)** | 1.33 | (0.85–2.10) | 1.24 | (0.57–2.68) | 2.65 | (1.01–6.95)* | 1.32 | (0.74–2.35) |
| Other threats to physical integrity | 444 | 1.27 | (0.97–1.66) | 1.59 | (1.16–2.19)** | 1.38 | (0.88–2.15) | 1.11 | (0.52–2.38) | 3.04 | (1.11–8.35)** | 1.35 | (0.76–2.39) |
| Traumatic events in social network | 711 | 1.15 | (0.87–1.52) | 1.56 | (1.14–2.14)* | 1.58 | (1.01–2.52)* | 1.36 | (0.63–2.93) | 2.69 | (0.64–11.28) | 1.13 | (0.60–2.12) |
| Sudden unexpected death of loved one | 761 | 1.48 | (1.11–1.97)** | 1.37 | (0.98–1.93) | 1.50 | (0.90–2.51) | 1.05 | (0.49–2.27) | 1.50 | (0.43–5.26) | 0.83 | (0.45–1.56) |
| Other extraordinary event | 265 | 1.43 | (1.06–1.93)* | 1.83 | (1.29–2.62)* | 1.00 | (0.58–1.73) | 2.59 | (1.11–6.0)** | 1.37 | (0.51–3.72) | 1.58 | (0.84–2.97) |
| Model 2: Adjusted for demographics, lifetime PTSD, GAD, and MDD, current and former smoking, and current binge drinking | |||||||||||||
| Assaultive violence | 507 | 0.99 | (0.66–1.22) | 1.68 | (1.24–2.27)* | 1.32 | (0.79–2.20) | 0.83 | (0.40–1.70) | 1.82 | (0.78–4.25) | 1.02 | (0.58–1.80) |
| Other threats to physical integrity | 444 | 1.20 | (0.92–1.57) | 1.54 | (1.11–2.14)* | 1.29 | (0.81–2.05) | 0.95 | (0.50–1.81) | 2.21 | (0.78–6.28) | 1.10 | (0.65–1.86) |
| Traumatic events in social network | 711 | 1.01 | (0.75–1.36) | 1.37 | (0.98–1.91) | 1.51 | (0.91–2.49) | 0.95 | (0.45–2.01) | 1.86 | (0.47–7.35) | 0.87 | (0.47–1.62) |
| Sudden unexpected death of loved one | 761 | 1.32 | (0.99–1.76) | 1.24 | (0.88–1.77) | 1.39 | (0.82–2.37) | 0.78 | (0.34–1.77) | 1.24 | (0.35–4.37) | 0.71 | (0.40–1.26) |
| Other extraordinary event | 265 | 1.26 | (0.93–1.72) | 1.49 | (0.96–2.33) | 0.77 | (0.45–1.30) | 1.96 | (0.98–3.93) | 0.93 | (0.20–3.49) | 1.29 | (0.63–2.66) |
Cardiovascular disease included high blood pressure or hypertension, myocardial infarction or stroke, chest pain, and congestive heart failure.
Respiratory disease included chronic obstructive pulmonary disease (COPD) and emphysema.
P < .05;
P < .01.
GAD, generalized anxiety disorder; PTSD, posttraumatic stress disorder.
For cardiovascular disease, sudden unexpected death of a loved one was associated with hazard onset in unadjusted models (HR = 1.48, 95% CI 1.11–1.97), but the result was no longer statistically significant upon control for covariates.
For arthritis, assaultive violence (HR = 1.71, 95% CI 1.25–2.32), other threats to physical integrity (HR = 1.59, 95% CI 1.16–2.19), traumatic events in social network (HR = 1.56, 95% CI 1.16–2.19), and other extraordinary events (HR = 1.83, 95% CI 1.29–2.62) were significantly associated with hazard in unadjusted models. When adjusted for covariates, assaultive violence and other threats to physical integrity with arthritis remained significantly associated with arthritis (HRs 1.68 and 1.54, respectively).
For atrial fibrillation, other extraordinary events were associated with hazard of onset (HR = 2.59, 95% CI 1.11–6.00), but the result was no longer statistically significant after adjustment.
For respiratory disease, assaultive violence (HR = 2.65, 95% CI 1.01–6.95) and other threats to physical integrity (HR = 3.04, 95% CI 1.11–8.35) were associated with hazard of onset, but results were no longer statistically significant after adjustment.
Finally, the effect of PTEs on the number of physical health conditions was examined. There were no significant associations between any PTE category and number of physical health conditions.
Discussion
Using data from a representative population-based sample, the present study demonstrates that exposure to PTEs, especially violent assault and other threats to physical integrity, are potentially important risk factors for the subsequent onset of arthritis. These findings indicate a dose–response relation between the number of events experienced across the life course and arthritis risk, controlling for psychiatric morbidity and health behaviors. Further, we document that the experience of PTEs has an effect above and beyond individuals diagnosed with PTSD; in fact, the relation between PTSD and arthritis is explained by covariates in the present sample. Thus, these results add to a growing literature suggesting that trauma exposure, independent of PTSD, has deleterious consequences not only for mental health, but also for physical health.[12,65–68] The present study extend this literature by examining associations of a comprehensive range of PTEs with diverse physical health conditions, and identify a potentially novel risk factor for arthritis in urban populations. The present study also reports that potentially traumatic experiences are related neither to comorbidity of physical health conditions nor to conditions, such as diabetes, suggesting specificity to particular health outcomes and reducing concerns of residual confounding.
This study indicates a link between traumatic event exposure and arthritis risk. Rheumatoid arthritis (RA) has long been hypothesized to be potentiated by stressful experiences[69] and population-based studies documenting an association between stressful life events and post-traumatic stress with onset or worsening of symptoms has been reported.[70, 71] Given that osteoarthritis (OA) has a much higher prevalence than RA (approximately 14% versus 1%, respectively, based on population-based data[72, 73]), however, it is reasonable to hypothesize that many of the arthritis cases in the present sample are OA. Several recently published studies have suggested that anxiety and posttraumatic stress symptoms predict OA suggesting that both OA and RA may have a connection to trauma.[15, 42,74]
Although the pathway through which traumatic events may increase risk of OA remains unclear, there are several plausible mechanisms deserving attention in future research in this area. First, chronic exposure to traumatic events could result in injury to a joint where arthritis subsequently develops. The plausibility of this pathway is heightened given our data that traumas involving assault and other violent events are more related to arthritis than other kinds of events. Second, it is possible that chronic inflammatory processes may play a key role in mediating this association. Repeatedly experiencing stressful experiences is hypothesized to exacerbate inflammatory responses throughout the nervous system,[44,47,48,75–77] and as such, may be implicated in the etiology of OA. Growing evidence indicates that individuals exposed to PTEs exhibit elevated levels of proinflammatory cytokines and other inflammatory markers such as Creactive protein and fibrinogen.[27,28,30] However, the plausibility of this pathway is decreased given that we did not find strong associations between PTE exposure and cardiovascular disease once models were adjusted for health behaviors and co-occurring disorders. Given that inflammation plays a key role in the pathway to adverse cardiovascular health and that previous studies have found an association between traumatic event exposure and cardiovascular health,[9–18] we would expect associations between traumatic event exposure and cardiovascular health should in this pathway be operative. Future research specifically focused on elucidating mechanisms, such as inflammation and injury in promoting the association between traumatic events and arthritis is needed.
We also document that low levels of PTE exposure was protective for respiratory and GI illness. Although this finding was unexpected, it is possible that exposure to low levels of trauma, compared to no exposure and high exposure, facilitates the development of coping skills to manage emotional and physiological responses to stress that might protect against the development of some adverse health outcomes. Indeed, some evidence suggests that low levels of stress are associated with lower rates of mental health problems than no exposure or high exposure to stress.[78] However, future research is needed to determine whether these protective associations are evident in other samples.
Contrary to a previous body of literature,[13–16,79] we did not find strong associations between PTSD and the onset of physical health conditions in the present sample. Sample composition could potentially explain some of these contradictory findings, as this sample comprised predominately African-American individuals in a large urban city with a high prevalence of physical health problems. The prevalence of arthritis in this sample is substantially higher than the prevalence of arthritis reported in national samples of African Americans (31.1% versus 19.0%, respectively)[80]; this sample is an urban population with a high degree of traumatic event exposure, which could potentially explain these differences. Previous research has demonstrated that the stressors experienced by African Americans are both qualitatively and quantitatively different than those experienced by racial majority groups.[53, 81] Further, African Americans differ in coping responses, adaptive capacity, community resources, and responses to stress compared to other ethnic groups,[82–84] thus, consequences of PTE exposures for this sample may differ from samples with more ethnic diversity.
The present study had several limitations. First, both PTE exposure and physical health outcomes were self-reported in these data. This may bias these results, as individuals with poor mental health may be more likely to misreport medical conditions as well as traumatic event exposures.[85, 86] However, studies using more objective measures of physical health have documented associations between mental and physical health of similar direction and magnitude as those using self-reported measures.[87, 88] It is, therefore, unlikely that self-report biases completely explain these results. Second, the present study has limited measures of health behaviors; alcohol use was assessed in the past month rather than lifetime, and dietary health, exercise, and BMI were not assessed. More comprehensive measures of physical health and health behaviors would be beneficial to understand to what extent these associations are mediated by factors, such as diet and substance use.
Third, the present study was not able to establish temporality between the event occurrence and the onset of the medical condition, as age of trauma exposure was not ascertained for every traumatic event. However, PTEs that could be secondary to the outcome (e.g., diagnosed with a life threatening illness as a traumatic event) were not included, thus, mitigating serious reverse causality threats to inference. Further, the most robust associations in the present study were found for assaultive violent events, which have a young mean age of occurrence and are unlikely to be a consequence of a medical condition. However, data establishing temporality are necessary in this literature. These findings therefore warrant replication in prospective studies with more comprehensive information on timing of trauma exposure and onset of physical health conditions.
These weaknesses are mitigated by the substantial strengths of the present study. Using a population-based community sample of adults' representative of a large urban city, the present study documents robust associations between potentially traumatic experiences, such as violence and injury with the development of arthritis. The potential role of traumatic events as common contributors to mental and physical health suggests that mental and physical health problems may be linked through a potentially more cohesive set of social and biological pathways than previously considered.
Acknowledgments
We thank Rebecca M. Coul-born for overseeing DNHS specimen collection, Janie Slayden for coordinating the overall DNHS project, and Amy Weckle and Richelo Soliven for handling the DNHS specimen processing and laboratory technical assistance; the many Detroit residents who chose to participate in the DNHS; and Allison Aiello, Jorge Delva, Larry Gant, Bob Marans, and Trivellore Raghunathan for contributing to the conceptual development of the DNHS. This study was supported by National Institutes of Health Grants DA022720, DA022720-S1, MH088283, MH078152, as well as MH082729 (to S.G.), MH070627 and MH078928 (to K.K.), and MH092526 (to K.M.).
Footnotes
The authors report no conflicts of interest.
References
- 1.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Breslau N, Kessler RC, Chilcoat HD, et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 3.Roberts AL, Gilman SE, Breslau J, et al. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol Med. 2011;41:71–83. doi: 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenen KC, Lyons MJ, Goldberg J, et al. Co-twin control study of relationships among combat exposure, combat-related PTSD, and other mental disorders. J Trauma Stress. 2003;16(5):433–438. doi: 10.1023/A:1025786925483. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl. 5):4–12. [PubMed] [Google Scholar]
- 6.Shalev AY, Freedman S, Peri T, et al. Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry. 1998;155:630–637. doi: 10.1176/ajp.155.5.630. [DOI] [PubMed] [Google Scholar]
- 7.Fullerton CS, Ursano RJ, Wang L. Acute stress disorder, posttraumatic stress disorder, and depression in disaster or rescue workers. Am J Psychiatry. 2004;161:1370–1376. doi: 10.1176/appi.ajp.161.8.1370. [DOI] [PubMed] [Google Scholar]
- 8.Galea S, Ahern J, Resnick H, et al. Psychological sequelae of the September 11 terrorist attacks in New York city. N Engl J Med. 2002;346:982–987. doi: 10.1056/NEJMsa013404. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, et al. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108(1):29–33. doi: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- 10.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2006;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 11.Boscarino JA. Post-traumatic stress disorder and cardiovascular disease link: time to identify specific pathways and interventions. Am J Cardiol. 2011;108(7):1052–1053. doi: 10.1016/j.amjcard.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 13.Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med. 2001;63(4):585–594. doi: 10.1097/00006842-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Dirkzwager AJ, van der Velden PG, Grievink L, Yzermans CJ. Disaster-related posttraumatic stress disorder and physical health. Psychosom Med. 2007;69(5):435–440. doi: 10.1097/PSY.0b013e318052e20a. [DOI] [PubMed] [Google Scholar]
- 15.Schnurr PP, Spiro A, Paris AH. Physician-diagnosed medical disorders in relation to PTSD symptoms in older male military veterans. Health Psychol. 2000;19(1):91–97. doi: 10.1037//0278-6133.19.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Kubzansky LD, Koenen KC. Is posttraumatic stress disorder related to development of heart disease? An update. Cleve Clin J Med. 2009;76(Suppl 2):S60–S65. doi: 10.3949/ccjm.76.s2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Physical health conditions associated with posttraumatic stress disorder in U.S. older adults: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Am Geriatr Soc. 2012;60(2):296–303. doi: 10.1111/j.1532-5415.2011.03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubzansky LD, Koenen KC, Jones C, Eaton WW. A prospective study of post-traumatic stress disorder symptoms and coronary heart disease in women. Health Psychol. 2009;28(1):125–130. doi: 10.1037/0278-6133.28.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maestripieri D, Hoffman CL. Chronic stress, allostatic load, and aging in nonhuman primates. Dev Psychopathol. 2011;23(4):1187–1195. doi: 10.1017/S0954579411000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proietti R, Mapelli D, Volpe B, et al. Mental stress and ischemic heart disease: evolving awareness of a complex association. Future Cardiol. 2011;7(3):425–437. doi: 10.2217/fca.11.13. [DOI] [PubMed] [Google Scholar]
- 21.Mahbub ES, Haque N, Salma U, Ahmed A. Immune modulation in response to stress and relaxation. Pak J Biol Sci. 2011;14(6):363–374. doi: 10.3923/pjbs.2011.363.374. [DOI] [PubMed] [Google Scholar]
- 22.Bornstein SR, Rutkowski H. The adrenal hormone metabolism in the immune/inflammatory reaction. Endocr Res. 2002;28(4):719–728. doi: 10.1081/erc-120016992. [DOI] [PubMed] [Google Scholar]
- 23.Dhabhar FD, McEwen BS. Bidirectional effects of stress and glucocorticoid hormones on immune function: possible explanations for paradoxical observations. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. New York: Academic Press; 2001. pp. 301–338. [Google Scholar]
- 24.Dhabhar FS. Stress-induced augmentation of immune function— the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16(6):785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 25.Elenkov IJ, Chrousos GP. Stress, cytokine patterns and susceptibility to disease. Best Pract Res Clin Endocrinol Metabol. 1999;13(4):583–595. doi: 10.1053/beem.1999.0045. [DOI] [PubMed] [Google Scholar]
- 26.von Kanel R, Hepp U, Kraemer B, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Danese A, Pariante CM, Caspi A, et al. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uddin M, Koenen KC, Aiello AE, et al. Epigenetic and inflammatory marker profiles associated with depression in a community-based epidemiologic sample. Psychol Med. 2011;41(5):997–1007. doi: 10.1017/S0033291710001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med. 1993;55:364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type I. Proc Natl Acad Sci. 2009;106:2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Heredia FP, Gomez-Martinez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71(2):332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 32.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332(7540):521–525. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sareen J, Cox BJ, Stein MB, et al. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med. 2007;69(3):242–248. doi: 10.1097/PSY.0b013e31803146d8. [DOI] [PubMed] [Google Scholar]
- 34.Kubzansky LD, Park N, Peterson C, et al. Healthy psychological functioning and incident coronary heart disease: the importance of self-regulation. Arch Gen Psychiatry. 2011;68(4):400–408. doi: 10.1001/archgenpsychiatry.2011.23. [DOI] [PubMed] [Google Scholar]
- 35.Riley EH, Wright RJ, Jun HJ, et al. Hypertension in adult survivors of child abuse: observations from the Nurses' Health Study II. J Epidemiol Community Health. 2010;64(5):413–418. doi: 10.1136/jech.2009.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobie DJ, Kovlahan DR, Maynard C, et al. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Int Med. 2004;164:394–400. doi: 10.1001/archinte.164.4.394. [DOI] [PubMed] [Google Scholar]
- 37.Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- 38.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 39.Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: the posttraumatic stress disorder-major depression connection. Biol Psychiatry. 2000;48(9):902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- 40.Chantarujikapong SI, Scherrer JF, Xian H, et al. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Res. 2001;103(2–3):133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 41.Kubzansky LD, Adler GK. Aldosterone: a forgotten mediator of the relationship between psychological stress and heart disease. Neurosci Biobehav Rev. 2010;34(1):80–86. doi: 10.1016/j.neubiorev.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott KM, Von Korff M, Angermeyer MC, et al. Association of childhood adversities and early-onset mental disorders with adult-onset chronic physical conditions. Arch Gen Psychiatry. 2011;68(8):838–844. doi: 10.1001/archgenpsychiatry.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krause N, Shaw BA, Cairney J. A descriptive epidemiology of lifetime trauma and the physical health status of older adults. Psychol Aging. 2004;19(4):637–648. doi: 10.1037/0882-7974.19.4.637. [DOI] [PubMed] [Google Scholar]
- 44.Carpenter LL, Carvalho JP, Tyrka AR, et al. Responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacMillan HL, Georgiades K, Duku EK, et al. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the Youth Mood Project. Biol Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Bellis MD, Chrousos GP, Dorn LD, et al. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metabol. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- 47.Heim C, Newport DJ, Wagner D, et al. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- 48.Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 49.Hart J, Gunnar MR, Cicchetti D. Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Dev Psychopathol. 1995;7:11–26. [Google Scholar]
- 50.Suglia SF, Staudenmayer J, Cohen S, et al. Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an urban cohort. Psychol Trauma. 2010;2(4):326–334. doi: 10.1037/a0018953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldmann E, Aiello A, Uddin M, et al. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American Urban Community: the Detroit Neighborhood Health Study. J Trauma Stress. 2011;24(6):747–751. doi: 10.1002/jts.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koenen KC, Uddin M, Chang SC, et al. SLC6A4 methylation modifies the effect of the number of traumatic events on risk for posttraumatic stress disorder. Depress Anxiety. 2011;28(8):639–647. doi: 10.1002/da.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts AL, Gilman SE, Breslau J, et al. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol Med. 2011;41(1):71–83. doi: 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weathers FW, Ford J. Psychometric review of PTSD Checklist (PCL-C, PCL-S, PCL-M, PCL-PR) In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Lutherville, MD: Sudran Press; 1996. pp. 250–251. [Google Scholar]
- 55.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 56.Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79(11):1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lampe FC, Walker M, Lennon LT, et al. Validity of a self-reported history of doctor-diagnosed angina. J Clin Epidemiol. 1999;52(1):73–81. doi: 10.1016/s0895-4356(98)00146-2. [DOI] [PubMed] [Google Scholar]
- 58.Smith B, Chu LK, Smith TC, et al. Challenges of self-reported medical conditions and electronic medical records among members of a large military cohort. BMC Med Res Methodol. 2008;8:37. doi: 10.1186/1471-2288-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulz R, Beach SR, Ives DG, et al. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160(12):1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 60.Carroll D, Phillips AC, Thomas GN, et al. Generalized anxiety disorder is associated with metabolic syndrome in the Vietnam experience study. Biol Psychiatry. 2009;66(1):91–93. doi: 10.1016/j.biopsych.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 61.Phillips AC, Batty GD, Gale CR, et al. Generalized anxiety disorder, major depressive disorder, and their comorbidity as predictors of all-cause and cardiovascular mortality: the Vietnam experience study. Psychosom Med. 2009;71(4):395–403. doi: 10.1097/PSY.0b013e31819e6706. [DOI] [PubMed] [Google Scholar]
- 62.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 64.Uddin M, Aiello AE, Wildman DE, et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci USA. 2010;107(20):9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boscarino JA. Diseases among men 20 years after exposure to severe stress: implications for clinical research and medical care. Psychosom Med. 1997;59(6):605–614. doi: 10.1097/00006842-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Boscarino JA, Chang J. Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: research and clinical implications. Psychosom Med. 1999;61(3):378–386. doi: 10.1097/00006842-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 67.Boscarino JA, Chang J. Electrocardiogram abnormalities among men with stress-related psychiatric disorders: implications for coronary heart disease and clinical research. Ann Behav Med. 1999;21(3):227–234. doi: 10.1007/BF02884839. [DOI] [PubMed] [Google Scholar]
- 68.Dong M, Giles WH, Felitti VJ, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 69.Fuller HW. On Rheumatism, Rheumatic Gout, and Sciatica. London: John Churchill; 1860. [Google Scholar]
- 70.Guillemin G, Krol B, Briancon S, et al. Stressful life events and disability in early rheumatoid arthritis. Eur J Public Health. 1995;5(3):163–168. [Google Scholar]
- 71.Boscarino JA, Forsberg CW, Goldberg J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosom Med. 2010;72(5):481–486. doi: 10.1097/PSY.0b013e3181d9a80c. [DOI] [PubMed] [Google Scholar]
- 72.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Myasoedova E, Crowson CS, Kremers HM, et al. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Gabalawy R, Mackenzie CS, Shooshtari S, Sareen J. Comorbid physical health conditions and anxiety disorders: a population-based exploration of prevalence and health outcomes among older adults. Gen Hosp Psychiatry. 2011;33(6):556–564. doi: 10.1016/j.genhosppsych.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52(1):1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 76.Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16(6):622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 77.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 78.Forehand R, Biggar H, Kotchick BA. Cumulative risk across family stressors: short- and long-term effects for adolescents. J Abnorm Child Psychol. 1998;26(2):119–128. doi: 10.1023/a:1022669805492. [DOI] [PubMed] [Google Scholar]
- 79.Boscarino JA. Diseases among men 20 years after exposure to severe stress: implications for clinical research and medical care. Psychosom Med. 1997;59(6):605–614. doi: 10.1097/00006842-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 80.Bolen J, Schieb L, Hootman JM, et al. Differences in the prevalence and impact of arthritis among racial/ethnic groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis. 2010;7(3):A64. Available at: http://www.cdc.gov/pcd/issues/2010/may/10_0035.htm. [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchell SJ, Ronzio CR. Violence and other stressful life events as triggers of depression and anxiety: what psychosocial resources protect African American mothers? Matern Child Health J. 2011;15(8):1272–1281. doi: 10.1007/s10995-010-0668-6. [DOI] [PubMed] [Google Scholar]
- 82.Crocker J, Major B. Social stigma and self-esteem: the self-protective properties of stigma. Psychol Rev. 1989;96(4):608–630. [Google Scholar]
- 83.Haley WE, Roth DL, Coleton MI, et al. Appraisal, coping, and social support as mediators of well-being in black and white family caregivers of patients with Alzheimer's disease. J Consult Clin Psychol. 1996;64(1):121–129. doi: 10.1037//0022-006x.64.1.121. [DOI] [PubMed] [Google Scholar]
- 84.Fuller-Rowell TE, Doan SN, Eccles JS. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology. 2012;37(1):107–118. doi: 10.1016/j.psyneuen.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blaney PH. Affect and memory: a review. Psychol Bull. 1986;99(2):229–246. [PubMed] [Google Scholar]
- 86.Bower GH. Mood and memory. Am Psychol. 1981;36(2):129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- 87.Martens EJ, de Jonge P, Na B, et al. Scared to death? Generalized anxiety disorder and cardiovascular events in patients with stable coronary heart disease: the Heart and Soul Study. Arch Gen Psychiatry. 2010;67(7):750–758. doi: 10.1001/archgenpsychiatry.2010.74. [DOI] [PubMed] [Google Scholar]
- 88.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]


