Abstract
Japanese quail is very popular research animal model. Its continued characterization for various norms is highly desirable for obtaining accurate and reliable results. This study was designed to assess various physiological parameters which are associated with body growth and development. Among various physiological parameters, blood constituents and hormones are commonly used as diagnostic tools in both physiological and pathological evaluations of humans and animals. Japanese quail hatchlings were housed in the temperature controlled brooders up to 3 weeks of age and then shifted to hanging cages in air conditioned room at ~74 F under 14L:10D lighting system and free access to feed and fresh water. Starting d8, a group of birds of uniform size and weight were selected randomly and euthanized at 4-day intervals up to d52 of age. The birds were weighed and blood sampled from the brachial vein for measuring Blood Glucose (BGL), Total Plasma Proteins (PP) and Packed Cell Volume (PCV). It was found that starting d36 all the three physiological parameters altered with approaching sexual maturity (d48–52): BGL decreased (252 vrs. 182 mg/dl, p<0.05), PCV% increased (43.6 vrs. 49.6%, p<0.05) and PP also increased (2.7 vrs. 3.2 gm/dl, p>0.05). Accordingly, BGL, PCV and PP values demonstrated significant potential to predict approaching sexual maturity in male Japanese quail.
Keywords: Japanese quail, body growth, sexual maturity
INTRODUCTION
Various anatomical and physiological parameters are regularly being used in clinical evaluations from birth to adult age. Additional indicators are being identified for assessing dynamics of growth and associated physiological functions for normal and anomalous developments in birds (Druyan et al., 2009). Alteration in physical development, retarded growth rate, rapid growth periods, nutrition-related deformities, causes of poor weight gain, onset of puberty and other age-related variations and disruptions are important phases to identify progression towards adulthood and adult life (Spencer et al., 1968; Arora, 2010). Growth rate, a key morphological characteristic, is correlated with robustness in birds, therefore, ascertaining growth rate and developmental stages could provide valuable information on their physiology and behavior (Gebhardt-Henrich et al., 1998; Starck and Ricklefs, 1998). Changes in physiological parameters could become very important markers in identifying growth patterns thereby can be very useful tools for predicting both physiological and pathological consequences (Tilgar et al., 2008). Models for growth rate evaluations developed in two strains of chicken (Gavin et al., 1998) and Gull-billed Tern chicks (Albano et al., 2011), were very useful tools for comparative growth studies. Anatomy and physiology are intertwined and various physiological characteristics, undoubtedly, reflect on the capability and performance of anatomical structures in health and in disease such as the function of plasma proteins (PP), the building blocks of body tissues, in production of hormones and antibodies, carriers of numerous blood constituents, maintenance of osmotic pressure, controlling acid-base balance of the blood and production of series of enzymes associated with performance and maintenance of body activities (Harper et al., 1993; Druyan et al., 2007; Kiani et al., 2011). Furthermore, PCV has a vital role in blood viscosity and transportation of oxygen to the tissues and that of blood glucose in the production and regulation of energy in the tissues. Therefore, further delineation of growth and physiological norms would add valuable diagnostic tools for use in the Japanese quail for studying growth, physiology, reproduction, nutrition, pathology and toxicological studies. This study had two objectives: 1. To characterize relationships between growth dynamics and physiological values and 2. To assess interrelationship among various physiological values and their use as predictors of approaching sexual maturity in male Japanese quail.
MARTERIALS AND METHODS
For assessing growth and physiological norms, male Japanese quails from d8 to d52 were used. A breeding colony of Japanese quail having an overall fertility of ~90% provided the material. The parent birds, ~70 days of age, were housed in suspended cages, one male and three females to a cage, in a temperature controlled room at ~73°F under 14L:10D lighting system with a free access to feed and water. The eggs were collected between 3:00 to 6:00 PM daily and refrigerated overnight. Before transferring eggs into the incubator on the following day, the eggs were allowed to sit at room temperature for about 3 h. The eggs were incubated in auto-turner incubator at a temperature of ~99.5°F and ~65–70% relative humidity. The eggs were hatched in four sequential hatches for obtaining sufficient males for this study. All hatches were handled in an identical fashion. After hatching, the hatchlings were weighed and transferred to temperature controlled brooders for 21 days under a continuous light with free access to feed and water (Arora, 1979). The baby chicks were identified with small pieces of numbered adhesive tape applied on the underside of the right wing and finally tagged with metal wing bands on d8. Starting d8, a group of birds of uniform size and weight were euthanized at 4-day intervals up to d52. The gender of the birds was determined at necropsy because of the fact that the baby chicks do not exhibit plumage-based sexual dimorphism until around d20. The birds were weighed to the nearest 0.1 g and approximately 0.2 mL of blood was collected from the brachial vein using a lancet into EDTA-coated vials for determining Packed Cell Volume (PCV), Blood Glucose Level (BGL) and Total Plasma Proteins (PP). PCV was determined with microhematocrit tubes at 12,000 RPM for five minutes (UNICCO C-MH30) and total plasma proteins using clinical refractometer (T2-Ne Atago Co). Blood glucose was determined with glucometer (Elite XL) while collecting blood. This procedure continued at 4-day intervals until the birds reached d52. The data collected on the growth of body mass (weight) and blood constituents were analyzed for comparison between 4-day age intervals with descriptive and MANOVA analyses using SPSS version 19.0 and MS Office Excel 2007 statistical tools. The data is presented as means ± standard deviation (and minimum-maximum ranges) at a significance level of 0.05 and higher.
RESULTS AND DISCUSSION
Growth of body weight
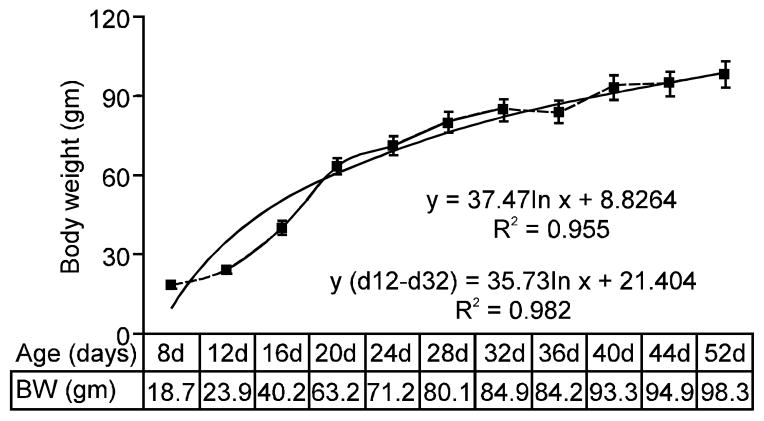
An overall growth of body weight (BW) in the male Japanese quails from d8 to d52 is presented in Fig. 1 and Fig. 2. The growth was almost linear from d12 to d32, leveled off at d36 and then grew more rapidly from d40 onwards to maturity. In terms of growth schedule the birds weighed averagely 23.9 (21–24) gm on d12, 63.2 (60–64) gm on d20, 80.1 (78–83) gm on d28, 84.2 (81–86) gm at d36, 94.9 (90–102) gm at d44 and 98.3 (96–108) gm on d52. The spread in body weights appeared larger as the birds reached sexual maturity. Body weight and age of the bird were significantly correlated (r = 0.933) and so were their percent deviations from the baseline d8 (r = 0.931; p<0.001).
Fig. 1.
BW growth from d8 to d52. Solid line represents the linear portion of the growth.
Fig. 2.
Tabulated values of physiological parameters as function of age. PP are depicted on the right-side axis.
Similarly, body weight percent deviation from the baseline (d8) as well as from the prior timelines showed very close association with age (r = 0.973 and r = −0.837, respectively; p<0.05). Percentage deviations in relation to prior values were considerably larger up to d20 and leveled off at d36–40. Two sizeable body growth spikes were observed during d24–28 and d36–40 age intervals with a mean growth of 75.65 and 88.75 gm and deviation of 12.5% and 10.8%, respectively (Fig. 3). As regards to body growth, Leptin, a polypeptide hormone encoded by the obese gene and secreted by adipose tissue, is known to enhance body mass, onset of puberty and sexual maturation by regulating feed in-take and utilization of nutrients (Kiess and Reich, 1999; Lamosova et al., 2003).
Fig. 3.

Age-dependent percentage deviations in the body weight, PCV, plasma proteins and blood glucose in relation to baseline (d8)
Physiological parameters
Packed cell volume
The PCV values during d8 to d52 of age are depicted in Fig. 4. PCV values between d8 to d16 were 38.4±0.6%, somewhat lower between d20 and d32 (38.1±0.4%; p>0.05). PCV increased steadily from d36 (40.0±03%) onwards reaching a peak level on d52 (48.5±1.2%; p<0.05). At this age, the males are expected to be at a stage of spermatogenesis and secretion of testosterone hormone (Huss et al., 2008). Overall, there was an increase of 23.2% and 26.7% of PCV values at d44 and d52, respectively, as compared to baseline values at d8. Furthermore, at this age, PCV exhibited highly significant correlation with age (r = 0.810; p<0.01). Earlier Van Wyk et al. (1998) utilized multiple blood variables included BGL, PP and PCV for morphological comparative studies in free-living African White-backed vulture nestlings. In Japanese quail, Atwal et al. (1964) reported a continued rise in PCV with age until maturity; the values were: 34±1.10% on d8, 36±0.35% on d22 and 46±0.86% on d36. From d36, there was a rapid increase in PCV reaching 42 to 46% at d50 and was closely associated with rapid body growth, on-set of sexual maturity and increase in plasma proteins values. The authors also reported some drop in hemoglobin values between d10 and d20, which was quite similar to the drop in PCV observed in our study. This was probably due to various physiological adjustments taking place at this age including rapid formation of feathers. Nirmalan and Robinson (1972) reported PCV values as 41.1±0.1% in 2-week old Japanese quail chick and as 53.1±0.8% in 10-week adult males. These values were somewhat higher than our observations. The authors suggested that increase in PCV resulted from enhanced erythropoises in the bone marrow, however, the role of testosterone in stimulating erythropoises is well established (Mirand et al., 1965).
Fig. 4.

PCV values as a function of body weight from d8 to d52. Note: PCV surge after 85g body weight and d36 of age. BW = Body weight (gm)
Total plasma proteins
Plasma proteins, the building blocks of body, steadily increased from d8 to d52. Following d12 (2.5 gm/dL), the plasma proteins increased steadily to d36 (2.7 gm/dL). However, after d36 the plasma proteins rose sharply to 3.2 gm/dL reflecting an increase of 12%, 20% and 28% at d40, d44 and d52, respectively, as compared to d8 level. Atwal et al. (1964) reported PP values as 2.9 gm/dL in d2 male baby Japanese quail, 3.1 gm at d26 and 3.8 in the adult males at d50, whereas, Nirmalan and Robinson (1972) reported plasma proteins values as 2.7±0.11 gm/dL in 2-week old male Japanese quail chicks and 3.1±0.1 gm/dL in 10-week adult males. This increase in plasma proteins was attributed to its increased production in the liver (Brandt et al., 1951) for utilization in the formation of reproductive organs and other tissues.
Blood glucose level
Blood Glucose (BGL) values observed during the growth periods of the male Japanese quail are given in Fig. 2 and Fig. 6. BG level was somewhat stable up to d40 except a little dip at d16. Following d40, BGL declined sharply by d52 (33.8% as compared to d8 values), the time period of rapid growth and approaching sexual maturity (Fig. 6). BGL has very important role to play in the regulation of glucose in the tissues and production of energy for use in the metabolic processes (Kiani et al., 2011). Timeline BGL values showed significant association with their percentage deviations from the baseline values at d8 (r = 0.912 at p<0.01). Downward slope of BGL from d40 to d52 reflected a negative relationship with age approaching sexual maturity and this can be expressed as = −31.46 log N (number of days) + 212.76, with R2 = 0.908.
Fig. 6.

BGL values as a function of BW and age. Note sharp fall in BGL after d36.
Relationship between body mass and physiological parameters
This study demonstrated very important relationships between increase in body mass and some associated physiological parameters in the male Japanese quail. The growth of body weight was almost linear from d12 and d32. Three physiological parameters assessed simultaneously with growth of the bird were: PP, PCV and BGL. These physiological parameters increased steadily with mass up to d36. Thereafter, the PCV and plasma proteins levels increased significantly, whereas, the blood sugar level dropped sharply nearing sexual maturity. At this stage, BGL had negative relationship with body weight (r = −0.636), with plasma proteins (r = −0.827) and with PCV (r = −0.838; p<0.01). As the PCV values increased, the BGL level dropped with approaching sexual maturity. The PCV values were highly correlated with age (r = 0.810). and with plasma protein (r = 0.701; p<0.01). Plasma proteins exhibited significant positive relationship with body weight (r = 0.736; p<0.05). Similar strong associations among the physiological parameters have been reported in growing chicken and pigeons (Gavin et al., 1998; Gayathri et al., 2004) and between developmental and biochemical indices in reptiles and mammals (Roark et al., 2009; Mohri et al., 2007). Gain in body weight was considerably slow after the onset of maturity and no further growth spikes were identified. Similar observations in Athens-Canadian random bred chickens were reported by Barbalo (1992). In conclusion, there was an increase in plasma proteins and PCV with increase of body weight with age and rapid decline in blood sugar level nearing sexual maturity. The increase of plasma proteins could be attributed to increased production from liver due to increasing body mass and metabolic activities during sexual maturation (Harper et al., 1993; Druyan et al., 2007; Kiani et al., 2011). An increase of PCV probably followed enhanced erythropoiesis due to higher growth rate, metabolic activities and production of gonadotropins, sex and metabolic hormones. On the other hand, the decline in blood sugar level could be ascribed to changes in carbohydrate metabolism induced by metabolic hormones closely involved in glucose production, storage and metabolism. Furthermore, the increased production of Leptin hormone may play an important role in growth, on-set of puberty and sexual maturation. It is clear from the data that, in addition to age (45–50 days) and body weight (98–105 grams), all the three physiological parameters assessed in this study could be used as single or in combination as predictors of approaching sexual maturity in male Japanese quail.
Fig. 5.

Plasma proteins as a function of BW and age.
Acknowledgments
The authors are thankful to Tracy Marshall in the editing of this manuscript.
References
- Albano N, Masero JA, Villegas A, Abad-Gomez JM, Sanchez-Guzman JM. Plasma metabolite levels predict bird growth rates: A field test of model predictive ability. Comp Biochem Physio - A Mole Inte Physio. 2011;160:9–15. doi: 10.1016/j.cbpa.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Arora KL. Differences in hemoglobin and packed cell volume in blood collected from different sites in Japanese quail (Coturnix japonica) Int J Poult Sci. 2010;9:8–830. [Google Scholar]
- Arora KL. Blood sampling and intravenous injections in Japanese quail (Coturnix coturnix japonica) Lab Anim Sci. 1979;29:114–118. [PubMed] [Google Scholar]
- Atwal OS, McFarland LZ, Wilson WO. Hematology of Coturnix from birth to maturity. Poult Sci. 1964;43:1392–1401. [Google Scholar]
- Barbalo GF. Divergent selection for exponential growth rate at fourteen or forty-two days of age. Poult Sci. 1992;71:1985–1993. doi: 10.3382/ps.0711985. [DOI] [PubMed] [Google Scholar]
- Brandt LW, Clegg RC, Andrew AC. The effect of age and degree of maturity on the serum proteins of the chicken. J Biol Chem. 1951;191:105–111. [PubMed] [Google Scholar]
- Druyan S, Shlosberg A, Cahaner A. Evaluation of growth rate, body weight, heart rate and blood parameters as potential indicators for selection against susceptibility to the Ascites syndrome in young broilers. Poult Sci. 2007;86:621–629. doi: 10.1093/ps/86.4.621. [DOI] [PubMed] [Google Scholar]
- Druyan S, Shinder D, Shlosberg A, Cahaner A, Yahav S. Physiological parameters in broiler lines divergently selected for the incidence of Ascites. Poult Sci. 2009;88:1984–1990. doi: 10.3382/ps.2009-00116. [DOI] [PubMed] [Google Scholar]
- Gavin A, Konarzewski M, Wallis I, McDevitt R. Relationship between metabolic rate and organ size in two strains of chicken. Br Poult Sci. 1998;39:S51–52. doi: 10.1080/00071669888368. [DOI] [PubMed] [Google Scholar]
- Gayathri KL, Shenoy KB, Hegde SN. Blood profile of pigeons (Columba livia) during growth and breeding. Comp Biochem Physiol Part A: Molecular Int Physiol. 2004;138:187–192. doi: 10.1016/j.cbpb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gebhardt-Henrich SG, Heeb P, Richner H, Tripet F. Does loss of mass during breeding correlate with reproductive success? A study on Blue Tits Parus caeruleus. Int J Avi Sci. 1998;140:210–213. [Google Scholar]
- Harper IS, Bond JM, Chacon E, Reece JM, Herman B, Lemasters JJ. Inhibition of Na+/H+ exchange preserves viability, restores mechanical function and prevents the pH paradox in reperfusion injury to rat neonatal myocytes. Basic Res Cardiol. 1993;88:430–42. doi: 10.1007/BF00795410. [DOI] [PubMed] [Google Scholar]
- Huss D, Poynter G, Lansford R. Japanese quail (Coturnix japonica) as a laboratory animal model. Lab Anim. 2008;37:513–519. doi: 10.1038/laban1108-513. [DOI] [PubMed] [Google Scholar]
- Kiani A, Nielsen MO, Tauson AH, Tygesen MP, Husted SM, Chwalibog A. Long-term effects of fetal under-nutrition on intermediary metabolism in growing lambs. Arch Anim Nutr. 2011;65:46–54. doi: 10.1080/1745039x.2010.533551. [DOI] [PubMed] [Google Scholar]
- Kiess W, Reich A. A role of leptinin sexual maturation and puberty. Hormone Res. 1999;51:55–63. doi: 10.1159/000053163. [DOI] [PubMed] [Google Scholar]
- Lamosova D, Macajova M, Zeman M, Mozes S, Jezova D. Effect of in novo leptin administration on the development of Japanese quail. Physiol Res (Prague) 2003;52:201–209. [PubMed] [Google Scholar]
- Mirand EA, Gordon AS, Wenig J. Mechanism of testosterone action in erythropoiesis. Nature, London. 1965;296:270–272. doi: 10.1038/206270a0. [DOI] [PubMed] [Google Scholar]
- Mohri M, Sharifi K, Eidi S. Hematology and serum biochemistry of Holstein dairy calves: Age-related changes and comparison with blood composition in adults. Res Vet Sci. 2007;83:30–39. doi: 10.1016/j.rvsc.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Nirmalan GP, Robinson GA. Hematology of the Japanese quail (Coturnix coturnix japonica) Br Poult. 1972;12:475–481. doi: 10.1080/00071667108415903. [DOI] [PubMed] [Google Scholar]
- Roark AM, Bjorndal KA, Bolten AB, Leeuwenburgh C. Biochemical indices as correlates of recent growth in juvenile green turtles (Chelonia mydas) J Exp Marine Biol Ecol. 2009;376:59–67. doi: 10.1016/j.jembe.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RP, Sagel SS, Garn SM. Age changes in five parameters of metacarpal growth. Invest Radiol. 1968;3:27–34. doi: 10.1097/00004424-196801000-00005. [DOI] [PubMed] [Google Scholar]
- Starck JM, Ricklefs RE. Variation, constraint and phylogeny. Comparative analysis of variation in growth. In: Strack JM, Ricklefs RE, editors. Avian Growth and Development. New York: Oxford University Press; 1998. pp. 247–265. [Google Scholar]
- Tilgar V, kilgas P, Viitak A, Reynolds SJ. The rate of bone mineralization in birds is directly related to alkaline phosphatase activity. Physiol Biochem Zool. 2008;81:106–111. doi: 10.1086/523305. [DOI] [PubMed] [Google Scholar]
- Van Wyk E, Van-der Bank H, Verdoorn GH. Dynamics of haematology and stry in free-living African whitebacked vulture (Pseudoyps africanus) nestlings. Comp Biochem Physiol - Part A: Molecular Int Physiol. 1998;120:495–508. [Google Scholar]