Abstract
BACKGROUD
With the introduction of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), the management of acute promyelocytic leukemia (APL) has changed dramatically. We performed a population-based study of APL in the United States to determine its incidence and relative survival (RS) during a 34-year period.
METHODS
We identified 1397 patients diagnosed with APL between 1975 and 2008 in the Surveillance, Epidemiology, and End Results (SEER) database. Patients were categorized into four age groups and three calendar periods. As a comparison, we also reviewed the outcome of APL patients treated at our institution during approximately the same time interval.
RESULTS
The incidences of APL increased with the time period and patient age. Short- and long-term RS improved with each calendar period, with the greatest improvement occurring between 1991 and 1999; 5-year RS rates were 0.18 for patients diagnosed in 1975–1990, 0.52 in 1991–1999, and 0.64 in 2000–2008. Age was an important predictor of survival. For example, the 5-year RS rate in patients diagnosed in 2000–2008 was 0.38 for patients aged ≥60 years and 0.73 and 0.75 for patients aged <20 years and 20–39 years, respectively. Similar treads of improvements in the survival were observed in APL patients treated at our institution.
CONCLUSIONS
The incidence of APL has increased, especially in the last decade. Clinical outcome improved remarkably in patients with APL diagnosed from 1991 to 1999, mainly because of the increased use of ATRA.
Keywords: APL, incidence, survival, ATRA, ATO
INTRODUCTION
Acute promyelocytic leukemia (APL) accounts for 10%–15% of adult acute myeloid leukemias,1 with an incidence of 600–800 cases each year in the United States.2 APL is an important model for cancer therapy because it was the first neoplasm effectively treated with a molecularly targeted drug, representing a change of paradigm in the treatment of cancer.3–7
For many years, APL had a dismal prognosis with a high rate of early mortality due to hemorrhage from coagulopathy, which often worsened during chemotherapy. In the 1970’s, anthracycline-based therapies produced complete remission in 65%–80% of patients with APL and long-term survival in 15%–25%.8–10 The introduction of all-trans retinoic acid (ATRA) in 1985,11 the first successful agent specifically targeting the PML-RARA oncogene, dramatically changed the management of APL. The clinical discovery of arsenic trioxide (ATO) in 1992 has been viewed as a second milestone in the treatment of APL.12–15
In the United States, anthracyclines were introduced for APL treatment in the 1970’s and were the standard therapy during the 1980’s. Treatment of APL with ATRA was first introduced to the United States in June 1990,16, 17 and ATO treatment was introduced in October 1997.1, 18 The United States Food and Drug Administration (FDA) approved ATRA for the treatment of patients with APL in November 199519 and ATO for relapsed and refractory APL in September 2000.20 To evaluate the potential impact of introduction of ATRA and ATO on relative survival (RS), we used the years of 1991 and 2000 as cutoff points. The aim of this study was to examine trends of short- and long-term survival of patients with APL before and after the introduction of molecularly targeted therapy and its incidence in the United States. We also examined the outcome of the patients treated in a tertiary referral center over approximately the same time interval to further confirm the potential impact of ATRA and ATO on survival in APL observed on SEER population-based study.
PATIENTS AND METHODS
Patient Databases
Data from nine Surveillance, Epidemiology, and End Results (SEER) registries, covering about 10% of the US population, were reviewed to identify patients diagnosed with APL between 1975–2008, including Atlanta (Georgia), Connecticut, Detroit (Michigan), Hawaii, Iowa, New Mexico, San Francisco-Oakland (California), Seattle-Puget Sound (Washington), and Utah. To assess any possible impact of stem cell transplantation (SCT) on survival in APL, data from the Center for International Blood and Marrow Transplant Research (CIBMTR) were used to identify prescription patterns of SCT performed in patients with APL in United States during the study period.21 SCT was not performed in patients with APL in the United States before 1980. From 1980 to 2008, a total of 1062 transplants were reported to CIBMTR: 392 autologous, 670 allogeneic (455 from sibling donor, and 215 from unrelated donor). The numbers of allogeneic SCTs for APL in calendar periods 1975–1990, 1991–1999 and 2000–2008 in the United States were 120, 320, and 230, respectively.
The SEER database included 1397 patients with a diagnosis of APL as the first cancer between 1975 and 2008 who were followed for status until the end of 2008. We used the third edition of the International Classification of Disease for Oncology histology code 9866 to identify all patients with APL. For incidence rate calculations, all patients with APL were included. In 150 patients, APL was not the first cancer, 12 patients were reported by autopsy or death certificate, and 8 cases were without active follow-up. The remaining 1227 patients were included in our survival analysis.
APL Treatment at the University of Texas MD Anderson Cancer Center from 1980 to 2008
To further confirm the potential impact of ATRA and ATO on survival in APL observed on SEER population-based study, we examined the data on patients treated at our institution during approximately the same time period as the SEER group. Two hundred forty-two patients with newly diagnosed APL were treated at MD Anderson between 1980 and 2008. Their median age was 42 years (range, 13 to 81 years). During 1980 to 1990, 70 APL patients received treatment on frontline protocols, usually consisting of a combination of an anthracycline and cytarabine. The clinical studies with ATRA began in September 1991, and between 1991 and 2001, 87 patients with newly diagnosed APL were enrolled in clinical trials with ATRA and idarubicin-based regimens including 21 patients who were treated with ATRA and gemtuzumab ozogamicin. Thereafter, from 2002 to 2008, a combination of ATRA and ATO (± GO) was evaluated in 85 patients with APL to explore the potential for replacing traditional cytotoxic chemotherapy for the disease.
Data Analysis
Patient characteristics were summarized using descriptive statistics, and categorical variables were compared using the χ2 test for continuous variables. Age-adjusted incidence rate was expressed per 100,000 persons per year.
RS is defined as the ratio of observed survival in a population of cancer patients to the expected or background survival in a comparable set of cancer free individuals.22 Expected survival was obtained from the expected survival table by matching the cohort cases by age, sex, race, and date at which the age was coded. RS is a robust method of cause-specific survival analysis typically used in the analysis of cancer registry data. The advantage of using RS is that the cause of death need not be accurate. RS thus provides a way to accurately measure the survival rate associated with the cancer in question, regardless of whether the excess mortality (that is, the higher number of deaths or rate of death in the cancer population) is due to cancer or non-cancer deaths. As a result, our RS calculations captured excess mortality resulting from, for example, infection or other events related to APL. One-, 5- and 10-year RS were expressed as ratios of patients with APL who survived it at 1, 5, and 10 years, respectively. Overall survival and event-free survival were defined according to International Working Group criteria.23 We analyzed RS, OS and EFS using the Kaplan-Meier method.24, 25 SEER*Stat 7.0.4 statistical software and SPSS 16.0 software were used for data analysis.
RESULTS
Patient Characteristics and APL Incidence
We identified 1397 patients with APL from SEER, including 697 male (50%) and 700 female with a median age of 48 years (range, 0 to 98 years; Table 1). One hundred forty-nine patients (11%) were younger than 20 years, 449 patients (32%) were 60 years or older. Most patients (83%) were reported to be Caucasian.
Table 1.
Patient Demographics and Incidence of Acute Promyelocytic Leukemia in1975–2008
| Characteristic | No. of patients | Incidence per 100,000 (95% CI) |
|---|---|---|
| Median age in yrs, (Range) | 48, (0–98) | |
| <20 | 149 | 0.06 (0.05–0.07) |
| 20–39 | 372 | 0.14 (0.13–0.16) |
| 40–59 | 427 | 0.22 (0.20–0.24) |
| ≥60 | 449 | 0.36 (0.32–0.39) |
| Total | 1397 | 0.18 (0.17–0.19) |
| Sex | ||
| Male | 697 | 0.19 (0.17–0.20) |
| Female | 700 | 0.17 (0.15–0.18) |
| Race | ||
| White | 1158 | 0.18 (0.17–0.19) |
| African American | 121 | 0.14 (0.12–0.17) |
| American Indians/Alaska Native and Asians/Pacific Islanders | 115 | 0.16 (0.13–0.19) |
| Unknown | 3 | |
| Calendar year of diagnosis | ||
| 1975–1990 | 352 | 0.11 (0.10–0.12) |
| 1991–1999 | 372 | 0.17 (0.15–0.18) |
| 2000–2008 | 673 | 0.27 (0.25–0.29) |
The incidence of APL increased significantly with each calendar period from 0.11 per 100,000 persons in 1975–1990 to 0.27 in 2000 to 2008 (Table 1; P<.05). A statistically significant linear increase in incidence was observed across all 3 study calendar periods (P<.05). The increase of APL incidence was similar in different age groups (data not shown). The overall annual age-adjusted incidence of APL during 1975–2008 was 0.18 per 100,000 (incidence was 0.17 per 100,000 in women and 0.19 in men). The incidence was 0.06 per 100,000 among those aged ≤20 years, but increased to 0.36 among those aged ≥60 years. Among racial groups, the incidence of APL was 0.18 per 100,000 in whites, 0.16 in other races (American Indians/Alaska Native and Asians/Pacific Islanders) and 0.14 in blacks (Table 1). Patients of Hispanic origin were not coded separately in the registry.
Survival
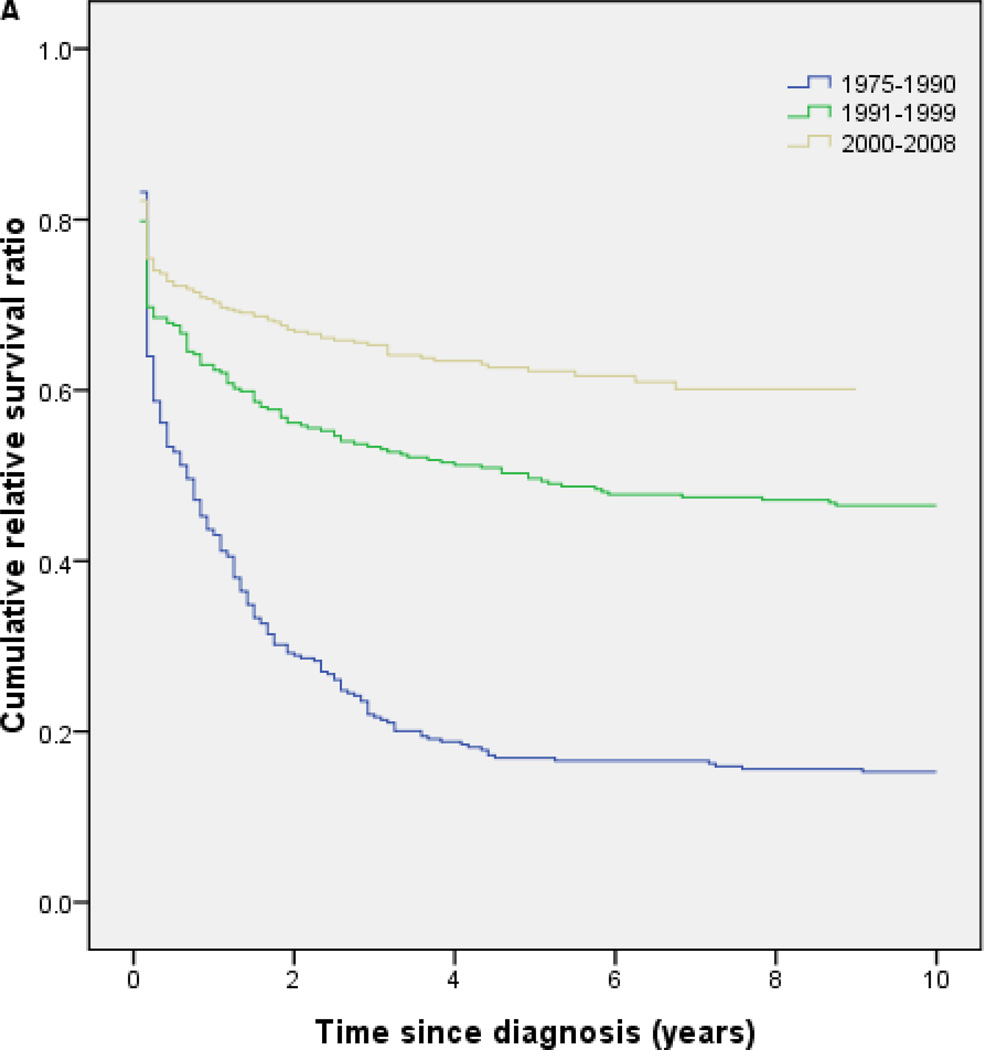
RS for the entire cohort of patients with APL increased significantly with each calendar period during the study period, with the major improvement in survival occurring among patients diagnosed in 1991–1999 (Fig 1A). Five-year RS was 0.18 in the calendar period 1975 to 1990, and 0.52 and 0.64 in the calendar periods 1991–1999, and 2000–2008, respectively (Fig 1A; Table 2); that is, excess mortality due to APL within 5 years of diagnosis was as high as 82% in 1975–1990 but fell as low as 36% in 2000–2008 compared to cancer free individuals.
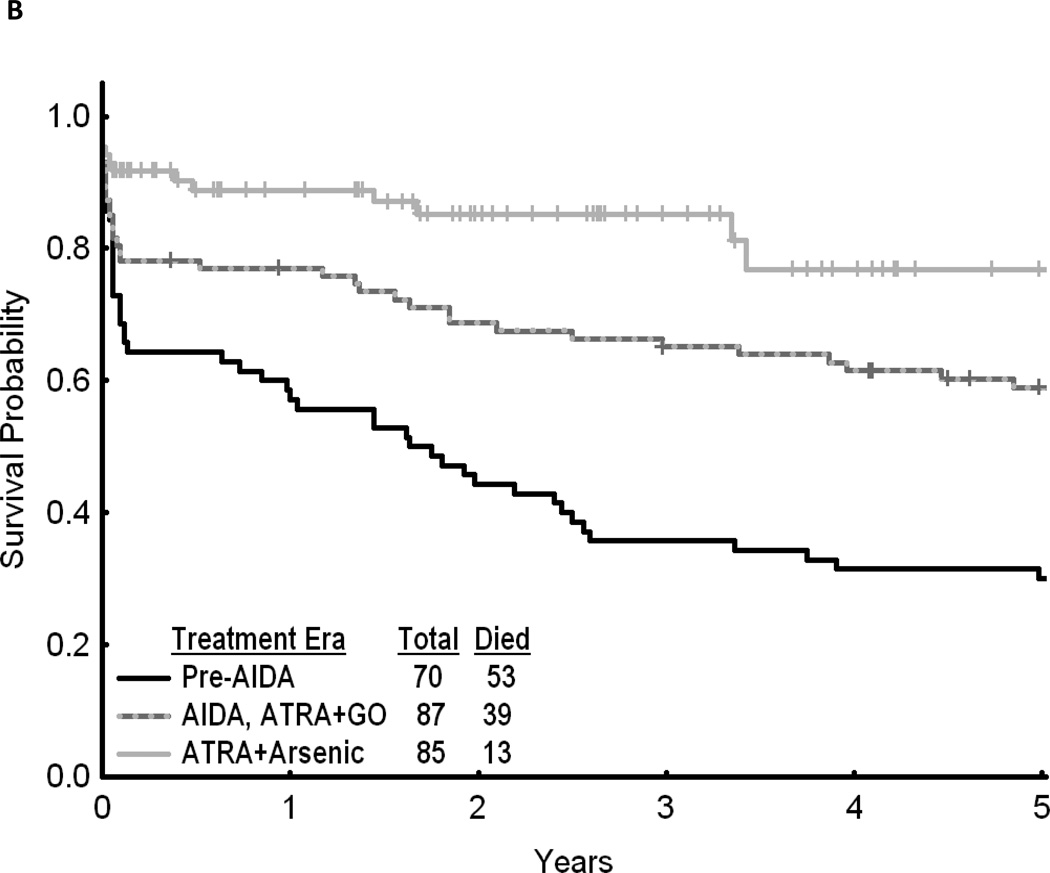
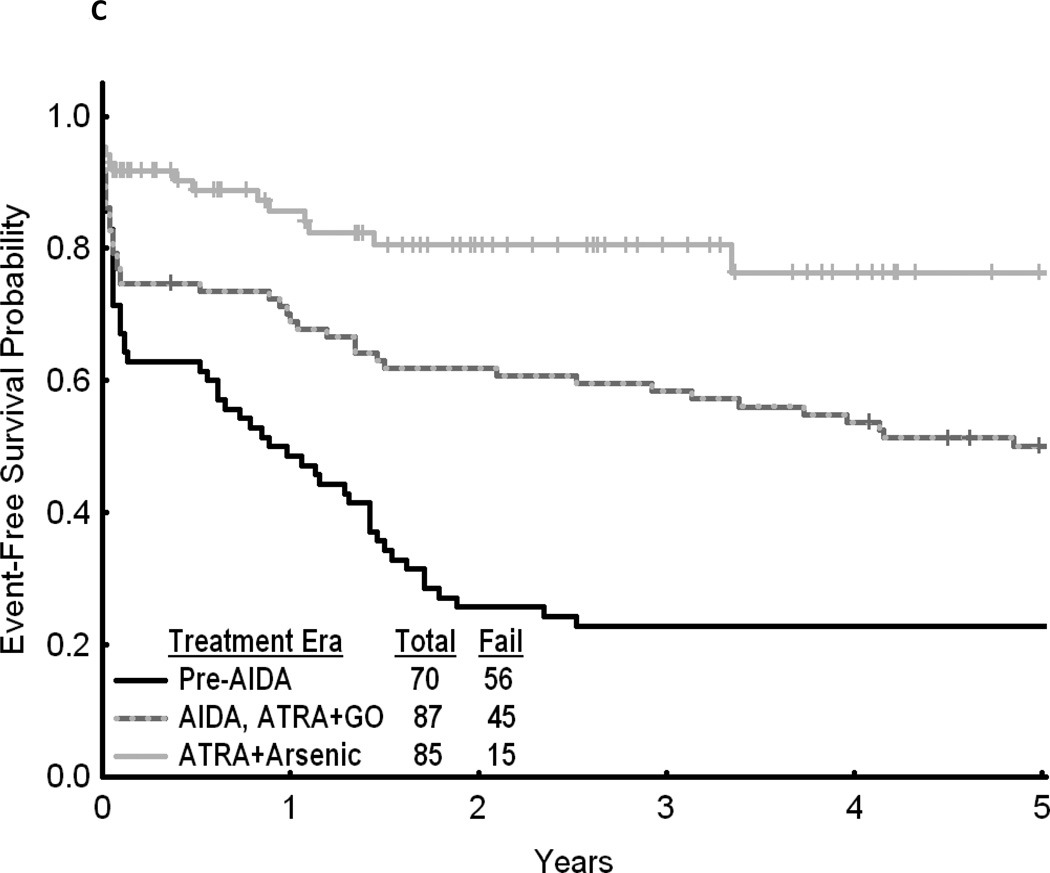
Fig 1.
(A) Cumulative relative survival of all patients with acute promyelocytic leukemia by calendar period of diagnosis (SEER). (B) Overall survival of patients with acute promyelocytic leukemia by treatment era (MD Anderson Cancer Center). (C) Event-free survival of patients with acute promyelocytic leukemia by treatment era (MD Anderson Cancer Center). AIDA, all-trans retinoic acid plus idarubicin; ATRA, all-trans retinoic acid; GO, gemtuzumab ozogamicin.
Table 2.
Cumulative Relative Survival in Patients with Acute Promyelocytic Leukemia from 1975–2008 by Age, Sex, Race, or Calendar Year
| characteristic | No. of patients | Relative Survival | |
|---|---|---|---|
| 1-year survival (95%CI) | 5-year survival (95%CI) | ||
| Age, years | |||
| <20 | 147 | 0.79 (0.71–0.85) | 0.52 (0.43–0.60) |
| 20–39 | 358 | 0.71 (0.66–0.76) | 0.57 (0.51–0.62) |
| 40–59 | 375 | 0.67 (0.62–0.71) | 0.57 (0.51–0.62) |
| ≥60 | 347 | 0.38 (0.32–0.43) | 0.24 (0.19–0.29) |
| Sex | |||
| male | 613 | 0.60 (0.56–0.64) | 0.45 (0.40–0.49) |
| female | 614 | 0.63 (0.60–0.67) | 0.50 (0.45–0.54) |
| Race | |||
| white | 999 | 0.60 (0.57–0.63) | 0.47 (0.43–0.50) |
| Black | 113 | 0.68 (0.58–0.76) | 0.48 (0.38–0.58) |
| American Indians/Alaska Native and Asians/Pacific Islanders | 114 | 0.63 (0.53–0.71) | 0.47 (0.37–0.56) |
| unknown | 1 | ||
| Calendar year of diagnosis | |||
| 1975–1990 | 322 | 0.44 (0.38–0.49) | 0.18 (0.14–0.22) |
| 1991–1999 | 327 | 0.65 (0.61–0.69) | 0.52 (0.46–0.57) |
| 2000–2008 | 578 | 0.71 (0.68–0.73) | 0.64 (0.59–0.68) |
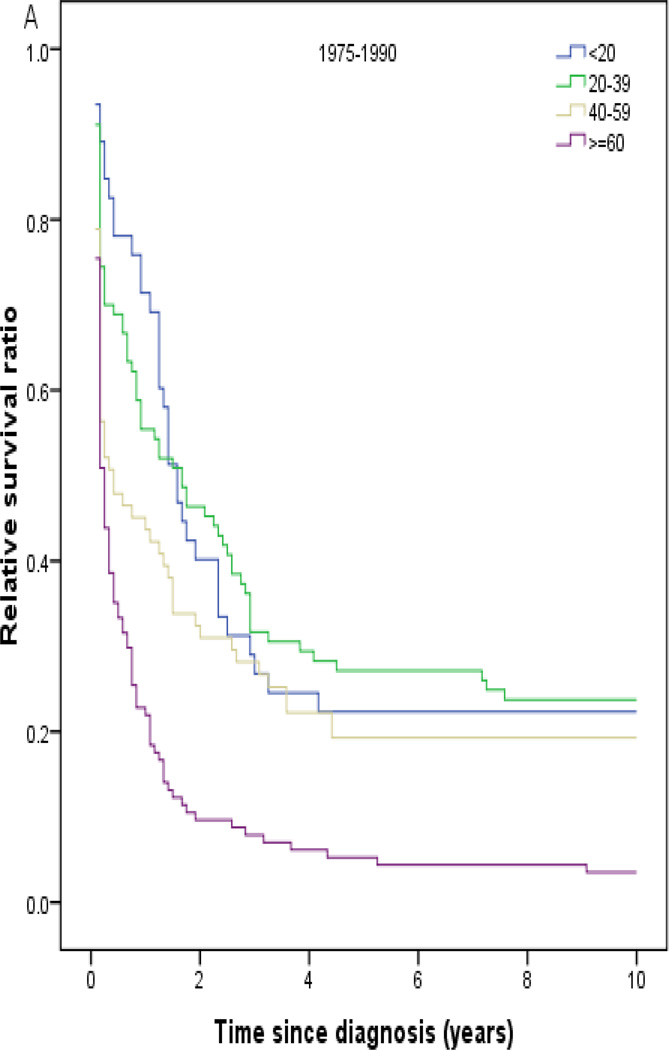
Throughout all calendar periods, age was a strong predictor of long-term survival, with 1- and 5-year RS decreasing sharply for patients aged ≥ 60 years (Table 2). RS increased with calendar period in all age groups (Fig 2). Patients diagnosed in the last calendar period had the highest rates of RS among all age groups. Among the patients treated at MD Anderson, 5-year OS was 0.59 (95% CI, 0.48 to 0.70; n=87) in 1991–2001 and 0.77 (95% CI, 0.68 to 0.86; n=85) in 2002–2008 compared to 0.30 (95% CI, 0.19 to 0.41; n=70) in 1980–1990 (P<.001) (Fig 1B). A similar pattern was noted when analyzing EFS (Fig 1C).
Fig 2.
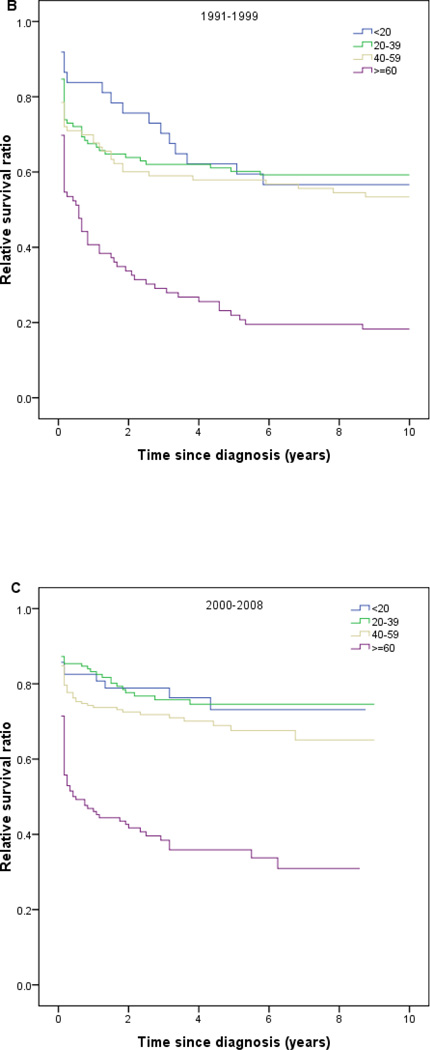
Relative survival in patients with acute promyelocytic leukemia by age and calendar period of diagnosis (SEER).
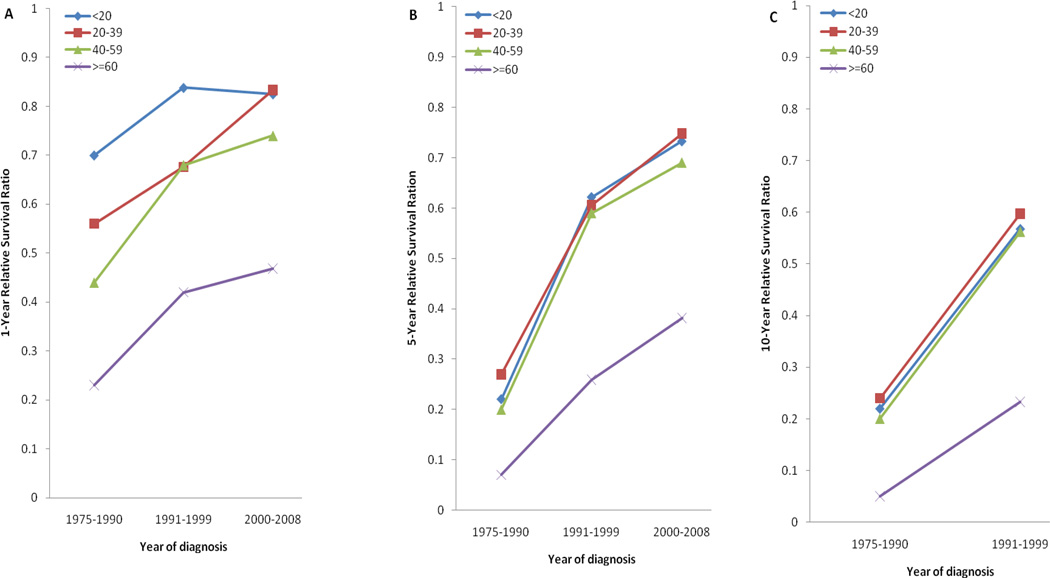
Between the first and last calendar periods under study (Fig 3; Table 3), 5-year RS increased from 0.20 to 0.73 for patients aged ≤20 years, from 0.27 to 0.75 for patients aged 20–39 years, from 0.20 to 0.69 for patients aged 40–59 years, and from 0.07 to 0.38 for patients aged ≥60 years. One- and 10-year RS results showed similar trends (Table 3; Fig 3).
Fig 3.
One-, 5-, and 10-year relative survival in patients with acute promyelocytic leukemia by age group and calendar period of diagnosis (SEER).
Table 3.
1-, 5-, and 10-year Relative Survival in Patients with Acute Promyelocytic Leukemia by Age and Calendar Period of Diagnosis
| 1-year relative survival (95% CI) | |||
| Age, years | 1975–1990 | 1991–1999 | 2000–2008 |
| <20 | 0.70(0.54–0.81) | 0.84(0.67–0.92) | 0.83(0.71–0.90) |
| 20–39 | 0.56(0.45–0.65) | 0.68(0.58–0.75) | 0.83(0.76–0.88) |
| 40–59 | 0.44(0.32–0.55) | 0.68(0.57–0.77) | 0.74(0.67–0.79) |
| ≥60 | 0.23(0.16–0.31) | 0.42(0.31–0.52) | 0.47(0.38–0.55) |
| 5-year relative survival (95% CI) | |||
| Age, years | 1975–1990 | 1991–1999 | 2000–2008 |
| <20 | 0.22(0.11–0.35) | 0.62(0.45–0.76) | 0.73(0.59–0.83) |
| 20–39 | 0.27(0.19–0.37) | 0.61(0.51–0.69) | 0.75(0.67–0.81) |
| 40–59 | 0.20(0.11–0.30) | 0.59(0.48–0.68) | 0.69(0.61–0.76) |
| ≥60 | 0.07(0.03–0.13) | 0.26(0.16–0.37) | 0.38(0.29–0.47) |
| 10-year relative survival (95% CI) | |||
| Age, years | 1975–1990 | 1991–1999 | 2000–2008 |
| <20 | 0.22(0.16–0.33) | 0.57(0.39–0.71) | |
| 20–39 | 0.24(0.16–0.33) | 0.60(0.50–0.68) | |
| 40–59 | 0.20(0.11–0.30) | 0.56(0.45–0.66) | |
| ≥60 | 0.05(0.02–0.12) | 0.23(0.14–0.34) | |
DISCUSSION
In this study, we noted a significant improvement in RS starting in 1990 (Fig 1 and Table 2). Several factors may have contributed to the observed improved clinical outcome in the latter calendar periods of study. These include better supportive care and increased use of anthracycline-based regimens but most importantly the discovery and use of ATRA and ATO. Although the number of patients who underwent allogeneic SCT increased during this calendar period, this is unlikely to have a major role in the improvement as the number of patients with APL who received allogeneic SCT was very small. It is also widely accepted that allogeneic SCT has limited impact on the outcome of patients with acute myelogenous leukemia and favorable-risk cytogenetic in first remission, including those with APL.26
A dramatic improvement in survival for all age groups was observed in the calendar period 1991–1999. The 5-and 10-year RS rates for patients aged ≥ 40 years at diagnosis more than doubled in comparison to those in the previous calendar period (Figs 1 and 3; Table 3). Although the observed improvement could be partially due to better supportive care, this dramatic change in outcome can be clearly attributed to the increased use of ATRA. In the United States, treatment of APL with ATRA was first introduced in June 1990.16, 17 Subsequently, the US Intergroup study, a multicenter clinical trial in 350 patients with newly diagnosed APL, was conducted during 1992–1995. Between June 1991 and November 1995, more than 1,500 patients with APL received ATRA on a compassionate-use basis.19 That is, at least approximately 43% of the total number of patients with newly diagnosed APL in the US was treated with ATRA during this period. The high response rates reported in the clinical trials of ATRA in patients with APL led to its approval for the treatment of APL by the FDA.19 Subsequently, ATRA plus chemotherapy with daunorubicin (or idarubicin) and cytarabine became the standard induction regimen for this disease.9, 27, 28 Following the successful treatment of APL with ATO in China, a US pilot study was conducted in 12 patients with relapsed APL in October 1997,1, 18 and the first US multicenter trial was conducted in 40 patients with relapsed APL in 1998–1999.29 Soon after, the FDA approved ATO for relapsed and refractory APL in September, 2000,20 so its impact on survival during the calendar period prior to the year 2000 cannot be excluded but is expected to be limited.
The findings in the SEER population-based study are in line with those for MD Anderson study group. During the 1991–2001 period, 87 patients with newly diagnosed APL were treated with ATRA plus idarubicin at MD Anderson. OS was significantly increased over that in the 70 patients with APL treated without ATRA in 1980–1990 (Fig 1B; P<.001).Therefore, the introduction of ATRA clearly contributed to the observed overall RS of 0.65 at 1 year and 0.52 at 5 years for patients treated during the same period (1991–1999) in the SEER population-based study.
Recent studies suggest that ATO is more effective than ATRA in eradicating leukemic stem cells.30 Clinical trials have demonstrated that single agent ATO can induce durable remissions in patients with previously untreated APL,31, 32 and the addition of ATO to standard induction and consolidation therapies may improve clinical outcomes in adults with newly diagnosed APL.27, 33, 34 In the present study, we observed improvement in RS in the last calendar period; overall RS increased from 0.52 in 1991–1999 to 0.64 in 2000–2008 (Table 2). Our findings in population-based study are in accordance with results of North American Leukemia Intergroup Study C9710, a randomized trial of 481 patients with previously untreated APL from 1999 to 2005, in which it was reported that 3-year OS was better for patients assigned to receive a ATO-containing regimen than for those assigned to a regimen of ATRA in combination with daunorubicin for consolidation (86% compared to 81%; P=0.06).27 The observed improvement in RS in this latest calendar period is also likely to be due to a more widespread use of ATO for relapsed APL resulting in the improved RS in relapsed patients. However, other potential factors that may have contributed to this improvement include improvement of supportive care measures as well as increased knowledge and experience of teams who care for patients with APL, and more widespread use of ATRA for maintenance therapy. Early detection of relapse through monitoring for the fusion transcript PML-RARA using polymerase chain reaction, and prompt initiation of therapy with ATO for relapsed patients also have some impact. Furthermore, our observations in the SEER population-based study are consistent with the results from an MD Anderson trial of ATO in combination with ATRA (± GO) in 85 newly diagnosed patients with APL treated between 2002 and 2008, suggesting that such a combination may improve OS compared to ATRA plus idarubicin or GO (n=87) during the period 1991–2001 (Fig 1B; P<.001),34 although part of this improvement must be attributed to better overall care of the patients.
All patients with available long-term follow-up data for our population-based study were obtained from SEER registries with no selection biases. Limitations of the SEER database include the lack of treatment information for individual patients. It is impossible to know whether or not the patients received chemotherapy and/or other agents; therefore, the proportion of patients who actually received various treatments, and more specifically ATRA and ATO, is unknown. Clearly, the conclusions of this study are based on the assumption of changing patterns of treatment with the FDA approval of ATRA and ATO in the United States, and data from related publications. However, the inclusion of the single-institution data serves to confirm that these patterns may well reflect the actual practice and that the improved outcomes are clearly related to the changed practice.
We also observed that the incidence of APL has increased in the United States over the past several decades. The incidence was 0.27 per 100,000 in 2000–2008 compared to 0.11 in 1975–1990, and 0.06 among population aged ≤20 years compared to 0.36 per 100,000 among those aged ≥ 60 years in the latter period (Table 2). Similar incidence rates of 0.16 per 100,000 in 1992–1995, 0.22 in 1996–2001 and 0.28 in 2002–2007 were reported in another recent report.35 The reasons behind this apparent increase are unclear. Although the use of better diagnostic tools, such as molecular techniques, may have increased the diagnostic accuracy over the years, this is unlikely to be the sole explanation for the increased incidence because APL can be diagnosed reliably by the presence of typical presenting features, distinctive bone marrow morphology, and chromosome abnormality.
In summary, in this large population-based study of 1397 patients diagnosed with APL between 1975 to 2008, we found that the incidence of APL increased over time, especially in the last decade; the RS of patients with APL increased, with the most improvement occurring in 1991–1999 for all age groups, presumably because of increasing use of the molecularly targeted agent ATRA. Future studies with longer observation periods are needed to evaluate the impact of the introduction of ATO and its inclusion earlier in the course of treatment on outcomes in APL. The combination of ATRA and ATO has shown the potential to eliminate standard cytotoxic chemotherapy for APL.36, 37 ATO alone or in combination with ATRA is an excellent option for older patients who are often not able to tolerate anthracycline-based therapy,31–33, 38–40 and it will also be interesting to see the impact of ATO on the survival of this particular population in the future.
Acknowledgements
None.
Financial disclosures: Farhad Ravandi has received honoraria and has participated in advisory meetings for Cephalon (Teva Pharmaceuticals).
FUNDING STATEMENT:
This article funded by National Institute of Health (P30 CA016672).
Footnotes
Previous presentations: None.
REFERENCES
- 1.Soignet SL, Maslak P, Wang ZG, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339(19):1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997–2002. Cancer Causes Control. 2008;19(4):379–390. doi: 10.1007/s10552-007-9097-2. [DOI] [PubMed] [Google Scholar]
- 3.de The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10(11):775–783. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- 4.Licht JD. Acute promyelocytic leukemia--weapons of mass differentiation. N Engl J Med. 2009;360(9):928–930. doi: 10.1056/NEJMcibr0810371. [DOI] [PubMed] [Google Scholar]
- 5.Sanz MA, Lo-Coco F. Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol. 2011;29(5):495–503. doi: 10.1200/JCO.2010.32.1067. [DOI] [PubMed] [Google Scholar]
- 6.Nowak D, Stewart D, Koeffler HP. Differentiation therapy of leukemia: 3 decades of development. Blood. 2009;113(16):3655–3665. doi: 10.1182/blood-2009-01-198911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ablain J, de The H. Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood. 2011;117(22):5795–5802. doi: 10.1182/blood-2011-02-329367. [DOI] [PubMed] [Google Scholar]
- 8.Tallman MS, Nabhan C, Feusner JH, Rowe JM. Acute promyelocytic leukemia: evolving therapeutic strategies. Blood. 2002;99(3):759–767. doi: 10.1182/blood.v99.3.759. [DOI] [PubMed] [Google Scholar]
- 9.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337(15):1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Keating MJ, Walters RS, et al. Acute promyelocytic leukemia. M.D. Anderson Hospital experience. Am J Med. 1986;80(5):789–797. doi: 10.1016/0002-9343(86)90617-0. [DOI] [PubMed] [Google Scholar]
- 11.Huang ME, Ye YC, Chen SR, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72(2):567–572. doi: 10.1182/blood-2016-11-750182. [DOI] [PubMed] [Google Scholar]
- 12.Sun HD, Ma L, Hu X-C. Ai-Ling 1 treated 32 cases of acute promyelocytic leukemia. Chin J Integrat Chin Western Med. 1992;12:170–172. [Google Scholar]
- 13.Chen GQ, Shi XG, Tang W, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89(9):3345–3353. [PubMed] [Google Scholar]
- 14.Shen ZX, Chen GQ, Ni JH, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89(9):3354–3360. [PubMed] [Google Scholar]
- 15.Zhang P, Wang SY, Hu XH. Arsenic trioxide treated 72 cases of acute promyelocytic leukemia. Chin J Hematol. 1996;17:58–62. [Google Scholar]
- 16.Warrell RP, Jr, Frankel SR, Miller WH, Jr, et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid) N Engl J Med. 1991;324(20):1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- 17.Frankel SR, Eardley A, Heller G, et al. All-trans retinoic acid for acute promyelocytic leukemia. Results of the New York Study. Ann Intern Med. 1994;120(4):278–286. doi: 10.7326/0003-4819-120-4-199402150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Camacho LH, Soignet SL, Chanel S, et al. Leukocytosis and the retinoic acid syndrome in patients with acute promyelocytic leukemia treated with arsenic trioxide. J Clin Oncol. 2000;18(13):2620–2625. doi: 10.1200/JCO.2000.18.13.2620. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services archive page. doi: 10.3109/15360288.2015.1037530. http://archive.hhs.gov/news/press/1995pres/951128a.html. [DOI] [PubMed]
- 20.Antman KH. Introduction: the history of arsenic trioxide in cancer therapy. Oncologist. 2001;6(Suppl 2):1–2. doi: 10.1634/theoncologist.6-suppl_2-1. [DOI] [PubMed] [Google Scholar]
- 21.Center for International Blood and Marrow Transplant Research (CIBMTR) homepage. http://www.cibmtr.org/pages/index.aspx. [Google Scholar]
- 22.the Surveillance, Epidemiology and End Results (SEER) Program of National Cancer Institute homepage. http://seer.cancer.gov/.
- 23.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Kristinsson SY, Landgren O, Dickman PW, Derolf AR, Bjorkholm M. Patterns of survival in multiple myeloma: a population-based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007;25(15):1993–1999. doi: 10.1200/JCO.2006.09.0100. [DOI] [PubMed] [Google Scholar]
- 25.Brenner H, Arndt V. Long-term survival rates of patients with prostate cancer in the prostate-specific antigen screening era: population-based estimates for the year 2000 by period analysis. J Clin Oncol. 2005;23(3):441–447. doi: 10.1200/JCO.2005.11.148. [DOI] [PubMed] [Google Scholar]
- 26.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751–3757. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100(13):4298–4302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- 29.Soignet SL, Frankel SR, Douer D, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19(18):3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 30.Zheng X, Seshire A, Ruster B, et al. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARalpha-positive leukemic stem cells. Haematologica. 2007;92(3):323–331. doi: 10.3324/haematol.10541. [DOI] [PubMed] [Google Scholar]
- 31.Mathews V, George B, Chendamarai E, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol. 2010;28(24):3866–3871. doi: 10.1200/JCO.2010.28.5031. [DOI] [PubMed] [Google Scholar]
- 32.Mathews V, George B, Lakshmi KM, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107(7):2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 33.Estey E, Garcia-Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107(9):3469–3473. doi: 10.1182/blood-2005-10-4006. [DOI] [PubMed] [Google Scholar]
- 34.Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27(4):504–510. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JH, Qiao B, Panageas KS, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118(5):1248–1254. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JH, Tallman MS. Treatment of acute promyelocytic leukemia without cytotoxic chemotherapy. Oncology (Williston Park) 2011;25(8):733–741. [PubMed] [Google Scholar]
- 37.Ravandi F. Acute promyelocytic leukemia can be treated successfully without cytotoxic chemotherapy. Oncology (Williston Park) 2011;25(8):741–743. [PubMed] [Google Scholar]
- 38.Hu J, Liu YF, Wu CF, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106(9):3342–3347. doi: 10.1073/pnas.0813280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–3324. [PubMed] [Google Scholar]
- 40.Ghavamzadeh A, Alimoghaddam K, Rostami S, et al. Phase II Study of Single-Agent Arsenic Trioxide for the Front-Line Therapy of Acute Promyelocytic Leukemia. J Clin Oncol. 2011;29(20):2753–2757. doi: 10.1200/JCO.2010.32.2107. [DOI] [PubMed] [Google Scholar]