Abstract
Sensory systems provide organisms from bacteria to human with the ability to interact with the world. Numerous senses have evolved that allow animals to detect and decode cues from sources in both their external and internal environments. Recent advances in understanding the central mechanisms by which the brains of simple organisms evaluate different cues and initiate behavioral decisions, coupled with observations that sensory manipulations are capable of altering organism lifespan, have opened the door for powerful new research into aging. While direct links between sensory perception and aging have been established only recently, here we discuss these initial discoveries and evaluate the potential for different forms of sensory processing to modulate lifespan across taxa. Harnessing the neurobiology of simple model systems to study the biological impact of sensory experiences will yield insights into the broad influence of sensory perception in mammals and may help uncover new mechanisms of healthy aging.
Keywords: Longevity, chemosensory processing, Drosophila melanogaster, Caenorhabditis elegans, obesity, odorant receptors, gustatory receptors
By a ‘sense’ is meant what has the power of receiving into itself the sensible forms of things without the matter.
–Aristotle
Introduction
Democritus explained sensation by the friction of atoms, and thought all senses were a variant of the sense of touch (Serres 1985). Aristotle distinguished four senses, each linked with one of the four elements – vision with water, sound with air, smell with fire, and touch with earth (Aristotle 350 B.C., Serres 1985). Scientists have since enumerated additional senses -- of heath, position, or pain, for example— supporting Socrates’ view that, “in addition to the recognized senses, such as sight, hearing, and smelling, there are others besides, a great number which have names, an infinite number which have not” (Serres 1985). As windows to the soul, the human senses have long been proclaimed by philosophers as powerful determinants of who we are and what we will become. In this review, we argue that modern science is coming to espouse this view, as research across a range of scientific disciplines has revealed that sensory perception alone is capable of modulating physiology, health, and even aging.
An appreciation of the relationship between sensory input and aging has been spearheaded by research in simple model systems. The landmark work of Apfeld and Kenyon in 1999 using the Caenorhabditis elegans nematode model established that suppression of sensory input could extend lifespan (Apfeld & Kenyon 1999). Subsequent work indicated that the answer might be more complicated; certain neurons enhance and others suppress longevity (Alcedo & Kenyon 2004). Our laboratory confirmed that the sensory-lifespan link was evolutionarily conserved in a second model system, Drosophila melanogaster (Libert et al 2007), and specific olfactory cues have been identified that influence lifespan in both systems (Libert et al 2007, Poon et al 2010, Smith et al 2008). Even the well-known relationship between body temperature and lifespan may have a sensory component (Lee & Kenyon 2009). These recent advances have generated new areas of interest in sensory circuits, which may provide a fast track for environmental cues to provoke whole-organism longevity responses. Sensory systems exist in order to inform the organism about the state of its environment and to allow for prediction of future states. The output of such assessment has been commonly viewed in terms of rapid behavioral changes (ie. aversive or appetitive responses to a presented substance). It now seems clear that sensory information is used to determine biology at a much deeper level – to regulate long-lasting changes in physiology.
The scope of this review is necessarily broad in concept and narrow in mechanism. At present we have only a rudimentary understanding of precisely how sensory perception modulates health and lifespan. Nevertheless, recent discoveries in fields outside of aging per se have led to new ideas about how sensory systems process environmental information to generate changes in physiology and metabolism and about how they integrate metabolic and environmental context to influence behavioral outputs. These discoveries lead-off our discussion of sensory processing in important aging models and help distinguish the more general “public” vs. “private,” i.e., organism-specific, mechanisms of aging (Martin 2002). The different types of information that organisms commonly perceive serve as a framework for exploring the links between sensory perception and aging, and details about how different species detect, decode, and respond to specific cues are presented in context. In rare instances, specifics about the neurobiology or particular downstream signaling events following sensory inputs are known, and inference about the molecular mechanisms required for appropriate responses can be made. Most often, however, clues and correlative evidence only serve to highlight the great potential for research in this area. We believe that exploiting this potential using new experimental techniques and paradigms in simple model systems that are amenable to aging studies will allow researchers to integrate modern neuroscience with metabolism to illuminate the role of sensory information in controlling human health and aging.
Sensory Processing and Aging Model Systems
The last decade has seen tremendous advances in our understanding of the mechanisms of sensory perception. Arguably, the greatest progress has been in the understanding the molecular nature of smell and taste, where the identification of a large of family of mouse odorant receptors was awarded with a Nobel Prize in 2004 (Buck & Axel 1991). To appreciate how this new research has impacted the study of aging, we look to the invertebrate model systems C. elegans and D. melanogaster, which are where sensory signaling has been established as an influential modulator of organism lifespan. Here we touch on a few important concepts of sensory biology that are particularly related to chemosensation, because of its link with longevity. A detailed discussion of the recent advances in other sensory modalities, such as vision or pain reception, sensory signal transduction, anatomy, neurophysiology, or learning/memory can be found in a number of excellent reviews (C. elegans (Bargmann 2006, Bergamasco & Bazzicalupo 2006), D. melanogaster (Gerber et al 2009, Masse et al 2009, Vosshall & Stocker 2007)).
The worm and the fly
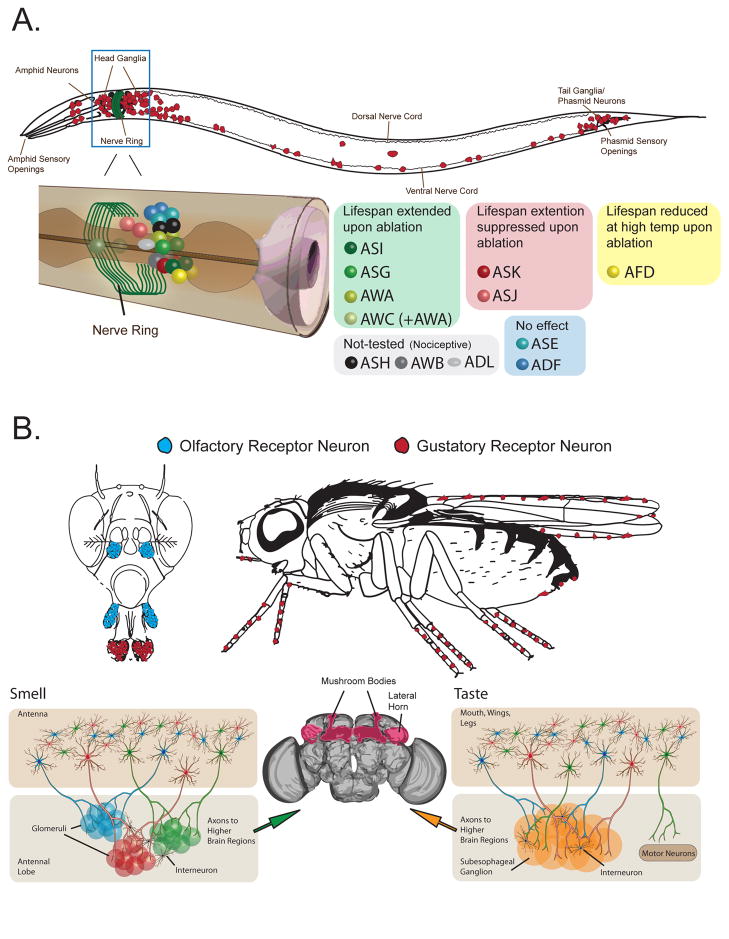
The adult C. elegans hermaphrodite consists of 959 cells, of which 302 are neurons. This includes at least 60 that are exposed to the environment as chemosensory neurons, similar numbers of mechanosensory neurons, and a small number of light-sensitive cells (Ward et al 2008). The cell bodies of most sensory neurons in C. elegans reside in ganglia around the nerve ring and in the tail; their dendrites are terminated by microtubule-rich cilia and housed in the amphid chemosensory organs in the nose of the worm (Figure 1A) (Ward et al 1975, Ware et al 1975). Neuron processes connect to other neurons in point synapses and extend into the nerve ring where they form synapses and release secretory granules into the pseudocoelomic space.
Figure 1. General schematic of sensory systems in Caenorhabditis elegans and Drosophila melanogaster.
A) General organization of the C. elegans adult hermaphrodite nervous system, which consists of 302 neurons. The nerve ring is the main central nervous system neuropil in the worm, and it receives sensory input from the amphids at the anterior tip of the animal. Many sensory neurons terminate their primary axons in the nerve ring, and there is potential for sensory integration. Inset: Close up of select sensory neurons that may be relevant to aging. Cell bodies are shown in their approximate actual position and are organized by color according to function and/or effects on aging. B) General organization of the adult female Drosophila chemosensory system. As described in text, olfactory input is integrated in a geographically organized antennal lobe where distinct glomeruli receive inputs from olfactory neurons that express the same receptor (indicated by red, blue, or green neurons). Such organization is not apparent in the subesophageal ganglion (SOG), which receives taste inputs from a wide variety of taste receptor neurons. Motor neurons have been observed in the SOG but not the antennal lobe, which suggests the existence of local gustatory circuits that can drive behaviors independent of input from higher brain centers. Sensory signals are carried by second order projection neuron axons from both the SOG and antennal love to different regions of the brain, such as the mushroom bodies and lateral horn, where they are presumably integrated with metabolic and other information to influence changes in physiology and lifespan. Worm drawing were inspired and adapted from information in Worm Atlas (http://www.wormatlas.com). Fly line drawings are based directly on (Matsunami & Amrein 2004), while information and guidance for the Drosophila brain illustration was provided by FlyCircuit (http://www.flycircuit.tw).
Estimates of the number of distinct sensory receptors in each animal are on the order of 500, as evidenced by neurons that function in taste reception (Bargmann & Horvitz 1991a), olfaction (Bargmann et al 1993), mechanosensation (Kaplan & Horvitz 1993), thermal perception (Mori & Ohshima 1995), and pheromone detection (Bargmann & Horvitz 1991b). Sensory receptors in C. elegans are thought to be metabotropic G-protein-coupled receptors (GPCRs) that each utilize one or more of different heterotrimeric G-proteins that lead to opening or closing of TRP or cGMP-gated channels (Jansen et al 1999). Several-to-many sensory receptors are expressed in each neuron. Lineage analysis, as well as genetic and functional studies, has resulted in a detailed anatomical map of individual chemosensory neurons together with their putative functions, ligands, and genes/proteins that are required for proper function (Bargmann 2006).
With roughly 100,000 neurons and over 2500 olfactory neurons alone, the Drosophila sensory system is considerably more complex than its C. elegans counterpart and shares important aspects of its organization with vertebrates (Vosshall & Stocker 2007). Fly odorant receptors (ORs) and their associated odorant receptor neurons (ORNs) are concentrated in sensillar hairs on the third segment of the antennae and on the maxillary palp, both of which are located on the head (Figure 1B). Taste receptors, on the other hand, are distributed around the body periphery and concentrated in regions of the mouthparts. Most known Drosophila olfactory receptor proteins contain seven transmembrane domains but apparently function as heterodimeric odor-gated ion channels rather than as GPCRs. They are therefore mechanistically distinct from vertebrate and nematode ORs (Benton et al 2006, Sato et al 2008, Vosshall et al 1999), although G-protein and cAMP signaling likely play a modulatory role (Wicher et al 2008). Generally speaking, one ligand-binding OR is expressed in each ORN along with the gene Or83b, which is likely not involved in ligand binding and is necessary for membrane targeting and function of co-expressed canonical OR (Larsson et al 2004). In contrast, gustatory receptors (GR) are putative GPCR, and co-expression of two or more ligand-binding GR in the same GRN is common (Gerber et al 2009, Weiss et al 2011). It is thought that most fly taste receptors function as heterodimers (Gerber et al 2009, Vosshall & Stocker 2007).
Sensory systems: “Public” or “private” mechanisms of aging?
Nearly all of the sensory-related manipulations that have been shown to influence lifespan involve functional modification of peripheral sensory neurons through knock-out or overexpression of specific chemosensory receptors, ablation of the neurons that house those receptors, or alteration of external chemosensory structures. While such studies provide important proof of principle linking sensory perception and lifespan, an important question is whether we might expect specific manipulations of this type in one organism to directly translate into mechanisms of aging in another. For example, loss of function of Gr63a—one component of a CO2 sensor—extends Drosophila lifespan (Poon et al 2010). Do we expect that the mammalian receptor most closely related to Gr63a, or its CO2-sensing functional analog (if one exists), will modulate human lifespan similarly to the way it does in flies? The answer is surely no. At the peripheral level, important sensory inputs and the receptors for those inputs are likely unique to the ecological challenges faced by the organism through evolution. Thus, specific cues, receptors, or individual sensory neurons that influence lifespan may be “private” mechanisms of aging (Martin 2002).
How then might sensory manipulations in simple model organisms help us understand human health and longevity? One way would be to inform us about how sensory information is integrated into specific types of perceptual experiences. Many insects are attracted to CO2; it can serve as a cue indicating a nearby food source, whether that food is rotting fruit or human blood. In such cases CO2 would be perceived as ‘food,’ and physiological responses would be initiated to prepare the animal for a meal. This may include production of digestive enzymes, secretion of specific hormones, or increased behavioral activity. In humans, the cephalic phase response has similar effects (e.g., the production of insulins and specific digestive enzymes in response to sensory cues that predict proximity to a food source), but CO2 is not the triggering sensory stimulus, which instead may be the smell of hot apple pie (Teff 2000). Nevertheless, all organisms share similar basic drives: the need for food and mates and the desire to avoid danger. Each organism has developed circuitry to achieve these goals, and we propose that it is the underlying biological responses to the perception of environmental cues that are likely evolutionarily conserved and important for modulating lifespan across taxa. These would reflect “public” mechanisms of aging.
Targeting sensory perception
Sensory integration can occur at a number of different levels to specify perception-dependent changes in aging or physiology. In C. elegans, the sensory neurons execute many aspects of perception that are reserved for higher brain centers in more complex systems. Unlike flies and mice, most nematode sensory neurons express multiple sensory receptors, which together provide simple integrative capabilities to the cell, and appropriate cell-nonautonomous responses in peripheral tissues can be directed by the neurons themselves rather than integrated through a central brain. Numerous secreted peptides of the TGF beta family, the insulin family, and neuropeptide families are produced by these neurons and they are known to directly influence organism-wide metabolic responses to oxidative stress, heat stress, and diet (Bishop & Guarente 2007, Li et al 2003).
Sensory modulation of aging in Drosophila involves more elaborate mechanisms of sensory integration and interpretation. In such organisms, signals from sensory neurons routinely undergo multiple levels of processing before physiological or behavioral responses are enacted. General properties of signal processing in the Drosophila olfactory system, for example, is well-defined by its spatial organization, which closely resembles that seen in mammals. ORNs that express the same olfactory receptor converge on glomeruli in the antennal lobe (the olfactory bulb in mammals). Odorant recognition results in a characteristic glomerular pattern of activity, termed an odor map (Laissue & Vosshall 2008). Here there is presynaptic peptidergic modulation of ORNs as well as excitatory and inhibitory regulation through local interglomerular and intraglomerular neurons (Figure 1B) (Ignell et al 2009, Olsen & Wilson 2008). Much less is known about the integration of the taste inputs in flies. GRNs converge on the suboesophageal ganglion (SOG), which is ventral to the brain. Unlike the antennal lobe, the SOG also contains motor neurons, suggesting that taste inputs may be more likely to induce responses that rely only on local circuitry and are independent of higher brain centers (Figure 1B) (Gerber et al 2009, Vosshall & Stocker 2007). Nevertheless, the general result of early smell and taste processing is a more finely tuned sensory response in a much smaller number of second-order projection neurons.
Information from projection neurons is passed to higher regions of the brain, such as the mushroom body and lateral horn in Drosophila, which are important for learning and memory and certain innate behavioral responses, respectively (Figure 1B) (Davis 2005, Gerber et al 2009). It is here that sensory signals are integrated with other types of information, which may include current metabolic state and previous experience, to provide context and to formulate a more integrated perceptive experience (Figure 2). Olfactory projection neuron input into the lateral horn is spatially well-defined, suggesting the possibility that certain regions respond to specific cues or groups of related cues, while the mushroom body contains a large number of narrowly tuned neurons that respond to specific patterns of projection neuron input (Gerber et al 2009, Masse et al 2009). The number and function of output neurons from the mushroom body and lateral horn is unknown, although some estimates for the mushroom body suggest less than 50 (Tanaka et al 2008). These higher brain areas, and their mammalian counterparts, represent attractive targets for linking perceptual experience with aging (Figure 2).
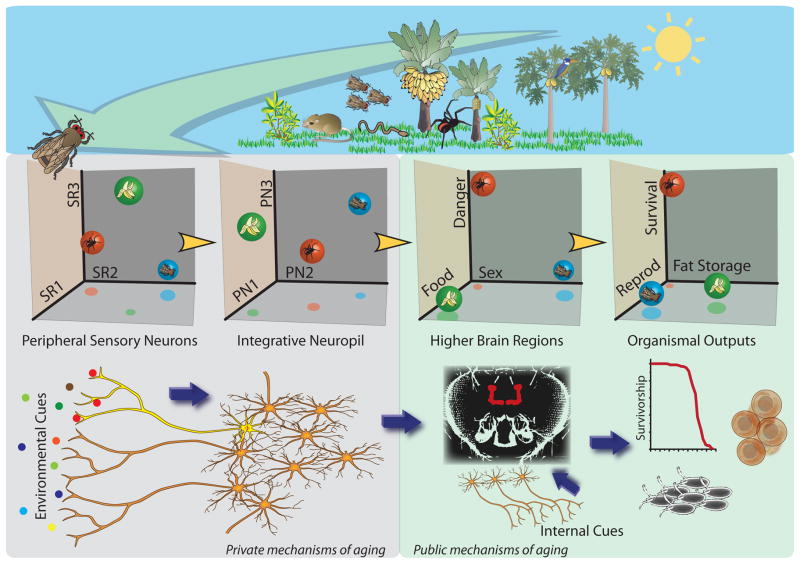
Figure 2. Detecting, decoding and interpreting sensory input to modulate aging and physiology.
Organisms are exposed to a wide-range of sensory stimuli from the environment. These inputs are detected by one or more peripheral sensory receptors that are tuned to specific ligands and that generate a characteristic set of neural responses in peripheral sensory neurons. Here, using the fly to illustrate phenomena that we believe are more general, we depict the processing of three hypothetical odorants (spider, fly, and banana) following their activation of three arbitrary sensory receptors (SR1, SR2, and SR3) to different degrees. The actual dimension of these spaces is much larger; flies, for example, express over sixty odorant receptors, while mice express over one-thousand. Neural signals from peripheral sensory neurons are processed in various types of integrative neuropil (e.g., the antennal lobe in flies, ring gland in worms, and olfactory bulb in mammals) to improve signal to noise ratio, maximize sensitivity, and enhance signal discrimination. Second-order neurons then pass the refined signals to higher regions of the brain (e.g., the mushroom bodies in flies and hypothalamus in mammals) where they are interpreted and perceived as ecologically relevant cues. From here, the cues may be integrated further with previous experience or with information about the nutritional and metabolic status of the organism, perhaps through internal sensory receptors. Very similar to behavior, the result may be to generate rapid and long-lasting effects on various aspects of organism physiology, including those that promote survival, reproductive output, and/or fat storage. We postulate that the mechanisms that are recruited to respond to perceived cues are evolutionary conserved “public” mechanisms, while those that are highly tuned to process specific signals are “private” and unique to each organism. Three-dimensional odor space diagrams are based on and expanded from ideas presented in Masse, Turner, and Jefferis (Masse et al 2009).
Nutritional Cues
Sensory systems play a critical role in fulfilling one of the most basic needs of all organisms—to distinguish nutritious from noxious, and in so doing to identify food from poison. The appearance and the smell of a substance are often the first cues detected, and they represent a primary mode for assessing the nutritional potential of the environment. Many things happen as a result. Visualization can activate regions of the brain, such as the dopaminergic midbrain in humans, which are normally associated with tasting (O’Doherty et al 2002). Pleasant and unpleasant food-related odors activate distinctly different regions of the olfactory cortex and induce different physiological reactions (Zatorre et al 1992). Gustation triggers changes that promote digestion or resistance to toxins. Accurate assessment of the suitability of a substance for consumption, however, usually involves an integration of various stimuli; highly nutritious substances often smell and taste sweet, while poisonous substances are often bitter and smell noxious (Chandrashekar et al 2006). These assessments form the basis for subsequent behavioral, metabolic, and physiologic decisions that alter whole-organism health and lifespan.
Sensory modulation of aging is revealed
The first clear demonstration of sensory modulation of lifespan emerged from work using nematode worms with mutations that disrupt ciliary structure or general sensory signal transduction in the two amphid organs that contact the external environment near the mouth (Apfeld & Kenyon 1999). Such mutants are deficient in chemotaxis in response to a range of stimuli, including their bacterial food source (Perkins et al 1986, Ryu et al 2008), and Apfeld and Kenyon showed that they are significantly long-lived (Apfeld & Kenyon 1999). Beyond sensory function, mutant animals are generally normal—they exhibit normal feeding rate, reproductive rate, and development time, suggesting that changes in lifespan are a direct result of altered sensory perception (Apfeld & Kenyon 1999). Ablation of individual sensory neuron pairs also alters nematode lifespan, revealing that partial loss of sensory perception is sufficient to affect aging (Alcedo & Kenyon 2004). Furthermore, loss of different neurons produces qualitatively different effects. Ablation of ASI or ASG gustatory neurons extends lifespan, as does loss of AWA or AWC olfactory neurons. Loss of other neurons, such as the ASK gustatory neurons, shortens lifespan (Figure 1A) (Alcedo & Kenyon 2004).
Direct evidence for the modulation of aging by perception of food cues was first shown in Drosophila using dietary manipulations. Dietary restriction is a reduction in nutrient availability that increases lifespan in many different organisms (Fontana et al 2010). In flies and worms, it produces a rapid change in patterns of aging (Kaeberlein et al 2006, Mair et al 2003), which in flies is accompanied by changes in the expression of genes involved in olfaction (Libert et al 2007). Exposure of flies to the odor of an important food source (live yeast) as well as knock-out of a critical co-factor for normal olfactory function (Or83b) established that: i) the perception of food cues is sufficient to partially reverse diet-restriction longevity extension, ii) proper olfactory function is required for a normal diet restriction response, iii) similar to C. elegans, general loss of olfaction in Drosophila results in increased lifespan, and iv) sensory modulation of aging is evolutionarily conserved, at least from nematodes to fruit flies (Libert et al 2007). Loss of Or83b increases lifespan without changing food intake, activity, respiration, or early-life reproduction, again suggesting a direct effect of sensory perception. Lifespan extension by dietary restriction in C. elegans is also reduced by exposing worms to a diffusible cue from the food source (Smith et al 2008). Food odors have no effect on aging in fully fed animals, suggesting that perceptual context, represented by the integration of olfactory information with other ecological or physiological cues, is important in determining the extent and magnitude of longevity effects (Pletcher 2009).
In Drosophila, only one specific receptor has been tested for its effects on lifespan, olfactory receptor Gr63a. The Gr63 and Gr21 receptors heterodimerize independent of Or83b to mediate a specific olfactory response to CO2. Mutants lacking Gr63a are slow to chemotax toward live yeast, are long lived, and are resistant to the lifespan-shortening effects of live yeast odor (Poon et al). Interestingly, suppression of Gr63a and Or83b both increase fat storage in female flies and confer resistance to starvation (Libert et al 2007, Poon et al). It is compelling to speculate that an imbalance between sensory and ingestion-associated cues may drive the organism to prepare for starvation despite food availability. A more complete description of the sensory-lifespan connectivity in model organisms will be important for understanding how perceptual cues may integrate with nutritional cues to modulate both metabolism and longevity.
Lifespan may also be modulated by sensing properties of food that are distinct from their nutritional value. Nematodes with loss of function in nmur-1 (a homolog of neuromedin U receptors in mammals) exhibit lifespan extension only on certain strains of E. coli that share a particular type of lipopolysaccharide structure (Maier et al 2010). This effect depends on osm-3, which is required for the proper function of a majority of sensory cilia, and it is likely independent of nutrient level, as reproductive rate and other correlates of diet-restriction were not observed (Maier et al 2010). The ability to sense multiple properties of a food source and initiate different internal responses highlights the potential for a complex interplay between the sensory system and metabolic state even in a relatively simple organism. For instance, all C. elegans food sources are pathogenic to some extent (Reddy et al 2009, Styer et al 2008), and they may stimulate an interplay between immune response and nutrient response that can lead to unexpected results. It is possible that mammalian food sources may also be detected through multiple sensory modalities and modulate metabolism independent of the primary sensory modalities of taste and smell.
Candidate molecular mechanisms
What are the molecular mechanisms that underlie how nutritional cues impact longevity? Within seconds of receipt of nutritional stimuli, humans and other mammals undergo changes in hormone signaling (Fischer et al 1972, Horio 2000, Nicolaidis 1969, Pavlov 1902). One common effect involves changes in insulin/IGF-like signaling (IIS), which is also one of only a handful of pathways that is known to affect aging across species (Tatar et al 2003). Long-lived worms, flies, mice and humans often exhibit reduced levels of IIS molecules (Fontana et al 2010, Tatar et al 2003). In C. elegans, partial loss of the daf-2 insulin-like receptor dramatically extends lifespan, and its effects are completely dependent on the forkhead transcription factor daf-16/foxo (Lin et al 1997, Ogg et al 1997). Lifespan extension via mutation in the sensory genes daf-10, daf-19, osm-3, osm-5, and tax-4 is partially abrogated by loss of daf-16 (Apfeld & Kenyon 1999), suggesting that this pathway is a primary target of sensory signaling in peripheral tissues. daf-16 is also required for longevity following specific ablation of olfactory (partial dependence) and gustatory neurons (complete dependence) (Alcedo & Kenyon 2004). This is also true for extended lifespan via modulation of a range of G-proteins (Lans & Jansen 2007). In Drosophila, long-lived Or83b mutant flies have reduced levels of at least one insulin-like peptide (dILP3), and Or83b and Gr63a mutants exhibit phenotypes that resemble animals with reduced IIS, including enhanced stress resistance and increased fat storage (Libert et al 2007, Poon et al). However, extended lifespan persists in Or83b mutants that carry a severe loss of function in dFoxo (P. Poon and S. Pletcher, unpublished data). Thus, while several aspects of sensory modulation of aging interact strongly with insulin-like signaling, this is not the whole story. Olfactory modulation of lifespan, for example, is at least partially independent of insulin signaling.
It is therefore likely that multiple downstream signaling pathways emanate from sensory neurons to mediate longevity extension. In C. elegans, sensory neurons interact with the germline to modulate lifespan (Alcedo & Kenyon 2004, Apfeld & Kenyon 1999). The somatic gonad is thought to produce signals that promote lifespan, and one set of olfactory neurons (the AWA neurons) is required for this affect. Thus, part of the somatic gonad effect resides in olfactory neurons. Germline longevity relies extensively on the nuclear hormone receptor daf-12, and it will be interesting to know whether sensory signaling is also daf-12 dependent. nmur-1 acts at least partly independently of daf-16/foxo through unknown mechanisms (Maier et al 2010). Two sensory neurons (the ASI neurons) in the head of C. elegans are required for dietary restriction (which is also independent of daf-16/foxo) to increase worm life span through a mechanism that involves activation of mitochondria or increased metabolic efficiency (Bishop & Guarente 2007). The two receptors that modulate lifespan in flies (Or83b and Gr63a) are expressed in distinctly different sensory neurons, Gr63a alone exhibits sex-specific effects, and only Or83b seems to interact with dietary restriction (Poon et al 2010), suggesting that there are at least two sensory-longevity pathways in Drosophila. Elucidating the conditions under which multiple downstream pathways trigger effects on lifespan can both clarify our understanding of sensory-lifespan connectivity and provide new targets for modulating metabolism and longevity.
Other neurotransmitters and neuropeptides regulate homeostatic functions such as appetite, growth, and metabolism, and these may play an important role in integrating and transducing sensory input. Pharmacogical inhibition of serotonin affects fat storage and longevity in C. elegans, albeit with some conflicting results (Petrascheck et al 2007, Zarse & Ristow 2008). In Drosophila, serotoninergic neurons control levels of insulin-like peptides to regulate growth in response to food availability (Kaplan et al 2008), perhaps by synapsing directly onto insulin-producing cells (Kaplan et al 2008). Drosophila neuropeptide f (dNPF) a homologue of mammalian neuropeptide Y, regulates fly growth, circulation of insulin-like peptides, and longevity (Lee et al 2008). The fly homologue of nmur-1, Hugin, is expressed in neurons that are innervated by axons of gustatory neurons and pharyngeal chemosensory neurons (Melcher et al 2006). Hugin-expressing neurons control feeding behavior and project to major neuroendocrine regions of the brain and higher brain centers (Melcher et al 2006). As we gain a clearer understanding of the wiring associated with neuronal regulation of metabolic homeostasis, it will be important to evaluate the role of these and other signaling molecules as potential effectors of lifespan downstream of sensory processing.
It remains to be determined whether other (in addition to IIS) known aging pathways are involved in sensory-lifespan regulation. The Target of rapamycin (TOR) and AMP Kinase (AMPK) intracellular signaling cascades are strong candidates because of their prominent roles controlling energy metabolism, reproductive signals, homeostatic regulation of food intake, and aging in response to nutrient availability (Greer et al 2007, Kapahi et al 2004, Selman et al 2009, Zoncu et al 2010). Elevation of neuronal S6 kinase signaling in Drosophila leads to increased serotonin levels (Vargas et al), shifts food-type preference from carbohydrates to yeast (Vargas et al 2010), and causes an abrogation of starvation-induced hunger response in Drosophila larvae (Wu et al 2005). It also alters food preference in response to mating through signaling in pickpocket-expressing sensory neurons in the reproductive tract (Ribeiro & Dickson 2010). The connection of these neurons or the TOR/AMPK pathway to sensory-dependent lifespan is still unknown.
Daily/Seasonal Cues
One important function of the sensory system is to track daily and seasonal cycles of the environment. Because the pattern of these cycles is largely predictable, organisms have developed a cell-autonomous oscillator comprised of positive and negative transcriptional feedback loops that cycles with a roughly 24 hour (circadian) endogenous period and has been reviewed extensively elsewhere (Nitabach & Taghert 2008, Reppert & Weaver 2002). The presence of a circadian clock allows organisms to model their environment as a cyclic oscillator with a daily rhythm and to predict future environmental conditions based on prior events with minimal sensory input. However, the circadian clock relies on sensory input for daily entrainment and adjustment to new environmental patterns. Outputs of the circadian clock include regulation of behavioral patterns, hormone release, nutrient metabolism, and feedback regulation of sensory acuity. Thus, the complete function of the circadian clock requires an intact network of sensory and circadian cells in order for environmental cues to integrate with endogenous rhythms.
Significant insight into mammalian circadian biology has come from work in Drosophila, and circadian rhythm of gene expression was recently reported in C. elegans (van der Linden et al 2010). Both invertebrates and mammals rely on a central pacemaker, which consists of ~20,000 clustered circadian clock cells in the suprachiasmatic nucleus (SCN) of the hypothalamus in mammals (Reppert & Weaver 2002) and is distributed across roughly 150 cells of the brain in Drosophila (Nitabach & Taghert 2008). Peripheral circadian clock cells are also found in other sites of the body including olfactory and gustatory organs (Chatterjee et al 2010, Krishnan et al 2009). Olfactory and gustatory clocks are necessary and sufficient for circadian rhythm of sensory perception in Drosophila (Chatterjee et al 2010, Krishnan et al 2009), although they are dependent on input from the SCN in mammals. Current evidence indicates that environmental entrainment of circadian clocks can occur in 3 major ways: 1) via sensory receptors within clock cells, 2) via neuronal communication from distinct sensory organs, and 3) via metabolic cues. Multiple sensory modalities can influence entrainment, though light is the most powerful entrainment cue in both mammals and Drosophila.
The fact that most organisms display a ~24 hour endogenous period in their circadian clockwork indicates that there is a selective advantage to maintaining synchrony with the external environment (although it is notable that nearly all organisms display an endogenous period that is not exactly 24 hours). Laboratory selection experiments with cyanobacteria confirm this suspicion (Ouyang et al 1998), and there is evidence of reproductive resonance with the circadian clock in outbred populations of the mosquito Wyeomyia smithii (Emerson et al 2008). Is there a cost of sensory-circadian desynchrony in terms of longevity? In Drosophila melanogaster and the blowfly Phormia terranovae, experimental manipulation of the environmental period shortens lifespan (Pittendrigh & Minis 1972, von Saint Paul & Aschoff 1978). However, other experiments have indicated that there is no effect of environmental period on longevity (Emerson et al 2008, Klarsfeld & Rouyer 1998). Reduced rhythmicity has also been associated with shortened lifespan in wild-type Drosophila (Kumar et al 2005). Finally, repeated switching between multiple light-dark schedules shortens lifespan in Drosophila and accelerated death in a mouse tumor model (Li & Xu 1997). In general, it seems clear that forcing an organism to adjust the circadian clock to accommodate changes in environmental light cycles can have a detrimental effect on lifespan.
Disruption of genes in the well-described circadian core clockwork can also provide us insight into the relationship between the circadian sensory system and regulation of lifespan. Mutants of the circadian genes cycle and period are associated with shortened lifespan in Drosophila (Hendricks et al 2003, Klarsfeld & Rouyer 1998) and increased susceptibility to mid-life oxidative stress (Krishnan et al 2009) (though the effects of cycle were notably male-specific). In mouse, disruption of the core clock proteins CLOCK and BMAL1 leads to shortened lifespan, and signs of premature aging (Dubrovsky et al 2010, Kondratov et al 2006). In these examples, the rhythm of sensory acuity is disrupted along with other rhythmic biological processes. It is not clear whether dysregulation of the sensory system plays a direct role in the lifespan-shortening effects of circadian disruption, but further experiments involving sensory-specific knockdown of circadian proteins are likely to provide insight.
Social Cues
Social interactions are known correlates of health and longevity in several species. Higher social rank and stronger social bonds are associated with increased health and longevity in non-human primates (Sapolsky 2005, Silk et al 2010). Demographic studies indicate that the type of social interactions and the extent of social networks are significantly associated with human lifespan (Giles et al 2005, Holt-Lunstad et al 2010, House et al 1988, Rodriguez-Laso et al 2007). The importance of social cues is manifest through a myriad of specialized signals that have developed for intraspecific communication. These provide information about the relevant aspects of an individual’s social environment including the presence of conspecifics, reproductive opportunity, social order, and competition.
Conspecifics and population density
In C. elegans, dauer-inducing pheromone allows worms to respond to the presence of conspecifics, and at sufficient concentration it diverts developing worms at the second of four larval stages to an alternative form, called the dauer, which is long-lived and stress resistant (Golden & Riddle 1982, Hu 2007). Dauer pheromone is constitutively released by wild-type worms, and detection requires the amphid neurons (Perkins et al 1986). The pheromone is a mixture of glycosides, called ascarosides, that act synergistically and in a concentration-dependent manner (Butcher et al 2007, Edison 2009, Jeong et al 2005, Srinivasan et al 2008). Two G protein-coupled receptors, SRBC-64 and SRBC-66, in ASK neurons were the first identified dauer pheromone receptors (Kim et al 2009); ASK neurons also modulate worm lifespan (Alcedo & Kenyon 2004). Other sensory neurons that are involved in dauer pheromone sensing or the regulation of dauer phenotypes are known to affect lifespan, including ASI, ASG, ASJ, AWC, and AWA (Figure 1A) (Alcedo & Kenyon 2004, Schackwitz et al 1996). Sensory pathways that influence dauer formation also regulate general physiology, including fat storage and body size in mature animals (Ashrafi et al 2003, Ogg et al 1997, Sze et al 2000). While the dauer form is long-lived, there is a question about whether dauer pheromone influences lifespan in normal, adult worms. Crude pheromone extracts have been reported to extend adult lifespan in an insulin-dependent manner (Kawano et al 2005), but pheromone itself seems to have little or no effect (Alcedo & Kenyon 2004) -- toxicity of the dauer pheromone has even been suggested (Gallo & Riddle 2009). It is likely that the detection of conspecifics through pheromone sensing involves multiple pathways which may have opposing effects on lifespan regulation. C. elegans also detects conspecifics for social feeding behaviors through the activation of the nociceptive ASH and ADL neurons (de Bono et al 2002), although the role of these neurons in lifespan regulation is unknown.
In Drosophila, the detection of conspecifics can be beneficial. Loss of function in the antioxidant enzyme Cu/Zn superoxide dismutase (SOD) shortens lifespan, but this reduction is partially rescued when mutant animals are cohoused with normal, young flies (Ruan & Wu 2008). Stress resistance and motor ability are also improved. Cohousing with aged flies or physically-impaired flies does not provide similar benefits. Notably, the ability of young flies to improve Sod mutant lifespan was limited if the assay was carried out in the dark, or if the mutant flies were also defective in olfaction or mechanosensation, suggesting that sensory perception is playing an important role here (Ruan & Wu 2008).
Reproductive opportunity
Reproductive pheromones are some of the most potent compounds known for eliciting behavioral and physiological responses from conspecifics (Karlson & Luscher 1959), and similar to feeding, the expectation and execution of sex has tremendous biological impact. Just a whiff of female tears in humans alters testosterone levels and sexual arousal in human males (Gelstein et al 2011). Many elaborate sensory structures have evolved the sole function of detecting the opposite sex (Crawford 1991, Warren et al 2009). Male Drosophila release a volatile pheromone, cis-vaccenyl acetate (cVA), that stimulates female receptivity, suppresses male courtship (Kurtovic et al 2007), and limits female re-mating (Ejima et al 2007). The first identified olfactory receptor for cVA is Or67d, which functions with Sensory Neuron Membrane Protein (SNMP) and odor binding protein OBP76a (also called LUSH) (Benton et al 2007, Jin et al 2008, Xu et al 2005). A second receptor, Or65a, may be responsible for the aversive effects of cVA (Ejima et al 2007). In female flies, cuticular hydrocarbons act as pheromones to induce male courtship at short distance (Ferveur 2005). A gustatory receptor, Gr68a has been identified as a receptor for female pheromones (Bray & Amrein 2003), and inactivation of Gr68a-expressing neurons or knockdown of Gr68a mRNA significantly inhibits the male’s ability to find and effectively court females. Other sensory molecules that may have pheromone-related functions include bitter taste receptor Gr66a, whose neurons in males respond to male inhibitory cuticular pheromone (Lacaille et al 2007), and gustatory receptors Gr32a or Gr33a, which also inhibit male-male courtship (Miyamoto & Amrein 2008, Moon et al 2009). Female flies live shorter when exposed to males who are incapable of mating, which suggests an effect of sensory perception on fly longevity that is independent of mating behavior (Partridge & Fowler 1990). It will be of high priority to determine whether inhibition or activation of any of these pheromone receptors or their associated neurons is sufficient to modulate fly lifespan or physiology.
In C. elegans, hermaphrodites release mating cues to attract males. Male responses to these chemical cues are mediated by a TRPV (transient receptor potential vanilloid) channel encoded by osm-9, ocr-1 and ocr-2(White et al 2007); ocr-2 also modulates lifespan (Lee & Ashrafi 2008). Three classes of neurons are necessary for sexual attraction: the AWA and AWC olfactory neurons and the male-specific CEM (cephalic companion) neurons (White et al 2007). Ablation of AWA and AWC neurons extends lifespan, suggesting that perception of mates may shorten lifespan in C. elegans as well. Components of the mating pheromone include dauer-inducing ascarosides #2, 3, 4 and 8 (Srinivasan et al 2008). At the very low concentrations, these compounds act as a male attractant, whereas at the concentrations required for dauer formation the chemicals become repulsive to hermaphrodites and no longer attractive to males (Srinivasan et al 2008). The ascaroside system is therefore an intriguing candidate for linking reproduction, development, sensory perception, and aging.
Social Order
Eusocial species, such as bees, ants and termites, provide an opportunity to study the impact of social structure on longevity. In honey bees (Apis mellifera), queen bees are exposed to higher nutrition and are highly reproductive, but surprisingly they exhibit up to a 100-fold longer lifespan than genetically identical sterile workers (Page & Peng 2001). This may be driven by enhanced IIS levels in workers, which is usually associated with reduced lifespan in other species (Corona et al 2007). In the queen bee, higher nutrition paradoxically leads to low levels of IIS and juvenile hormone, which induces expression of the antioxidant vitellogenin (Vg) and has a positive influence on longevity, immunity, and stress resistance (Corona et al 2007).
Evidence for pheromonal modulation of aging is found in the worker bees, where lifespan is highly plastic and is affected by social context. Nurse bees execute within-nest tasks, including feeding, cleaning and warming, for days to weeks before they switch to foragers, who then die between four and eight weeks of age (Rueppell et al 2007). The transition from nurse to forager can be accelerated, delayed, or even reversed depending on social interactions and hive conditions (Robinson 1992). Queen mandibular pheromone (QMP), which affects lipid storage, starvation resistance, and inhibits ovarian development, can influence workers’ longevity by slowing this behavioral transition (Fischer & Grozinger 2008, Robinson et al 1998). Chemical cues from foragers (ethyl oleate) and larvae (brood pheromone) can also increase workers’ lifespan by delaying the foraging ontogeny (Leoncini et al 2004, Pankiw 2004). More important, the decline in Vg content and colony survivorship, which reflects worker longevity, can result solely from exposure to synthetic brood pheromone independent of behavioral transition (Smedal et al 2009).
There are several candidate mechanisms for pheromonal modulation of aging in honey bees. One bona fide pheromone receptor has been identified, odorant receptor 11 (AmOr11) (Wanner et al 2007). AmOr11 responds to 9-oxo-2-decenoic acid (9-ODA), a main component in QMP and QRP (queen retinue pheromone). QMP also activates the D2-like dopamine receptor AmDOP3 (Beggs & Mercer 2009). Studies focusing on the antennal-specific proteins (ASPs) for pheromone perception have identified three different classes of odorant binding proteins: ASP1, ASP2, and ASP3. Whereas ASP2 was assigned to be a general odorant binding protein (Danty et al 1997), ASP1 has been shown to be associated with detection of queen pheromone (Danty et al 1999). In contrast, ASP3 was shown to bind specifically to large fatty acids and ester derivatives, which are brood pheromone components (Briand et al 2002). While the biochemistry of these molecules is promising, more work is needed to establish a role in longevity.
The relationship between social order and longevity extends to eusocial mammals. The naked mole rat can live more than 28 years (Buffenstein 2005), a striking contrast to the similarly-sized Mus musculus which lives ~3 years. The role of social perception on the extreme longevity in these eusocial animals will be important to elucidate.
Competition
Aggressive behaviors originate in response to competition for resources, and they are often reduced by social grouping and increased by social isolation (Dierick 2007, Wang et al 2008). In Drosophila, cuticular pheromones have been identified as one of several cues to trigger male fighting (Fernandez Mde et al 2010). The role of acoustic signals has also been suggested (Jonsson et al 2010). The first gene identified as having an effect on fly aggression through sensory perception is Cyp6a20, whose expression is decreased in flies selected for increased aggression (Dierick & Greenspan 2006). Flies carrying a loss of function mutation in Cyp6a20 are also highly aggressive, but only when reared socially (Wang et al 2008), suggesting an interaction between aggression and social cues. Cyp6a20 is co-expressed in non-neuron support cells with the odorant binding protein Lush, which is involved in the detection of male specific pheromone cVA (Xu et al 2005). Male aggression is increased by cVA, and its binding to olfactory receptor Or67d (Wang & Anderson 2010). cVA may also be an aggregation pheromone, which synergizes with food odors to attract both males and females (Xu et al 2005). Its dual effects on both aggregation and aggression (which promotes dispersal) suggests a role for cVA in homeostatic control of male fly population density (Wang & Anderson 2010), which also affects fly lifespan (Graves & Mueller 1993). In summary, the perception of cVA plays a major role in fly social behaviors, which may influence animal longevity directly or indirectly.
Overall we find that social cues have a compelling link with longevity in model systems. Some social cues, such as the presence of conspecifics, may extend lifespan whereas others, such as the presence of potential mates, may shorten lifespan. We are beginning to learn the molecular identity of the receptors and cues that mediate each of these social functions, providing a growing list of specific candidates for investigating the effect of social perception on lifespan and metabolism.
Internal Cues
Although chemosensory receptors have been best characterized for the key role they play in specialized sensory organs, emerging evidence from mammalian model systems has begun to illuminate a potential non-canonical role of chemosensory receptors in internal tissues where they may be important for controlling physiology and longevity. Initial interest in non-canonical olfaction developed from a potential role for olfactory receptors in sperm chemosensation and guidance (Spehr et al 2003). More recently, microarray analysis of human and mouse odorant receptor transcripts has revealed widespread expression outside of the olfactory epithelium in tissues such as the testis, lung, kidney, liver, and heart (Feldmesser et al 2006, Zhang et al 2007). While a gene signature alone cannot demonstrate functional significance, at least one receptor (mouse OR23) was recently described to regulate cell adhesion and migration in muscle regeneration in response to a soluble ligand (Griffin et al 2009). This finding opens up the questions of whether olfaction can play a role in tissue regeneration and repair during aging and whether internal chemoperception through canonical odorant receptors may be a novel avenue for regulation of lifespan.
A second connection between internal chemoperception and the regulation of known longevity factors involves expression of gustatory receptors outside the mammalian tongue where they regulate hormone levels and nutrient absorption. Glucose-dependent secretion of glucagon-like peptide 1 (GLP1) and glucose dependent insulinotropic polypeptide (GIP) from duodenal enteroendocrine cells depend on the presence of duodenal sweet (T1R2/T1R3) taste receptors and the gustatory G-protein a-gustducin (Jang et al 2007, Kokrashvili et al 2009, Margolskee et al 2007). GLP-1 and GIP are both incretins that stimulate insulin release. Given the role for IIS pathways in longevity regulation, manipulating sensory receptors in specific tissues will be of great interest.
A final connection between sensory perception of the internal environment and regulation of longevity-associated signals involves the transient receptor potential (TRP) channel family. This ancient multifunctional ion channel superfamily was initially characterized from a Drosophila mutant that exhibited an unexpectedly transient rhabdomere calcium response following a light stimulus (Cosens & Manning 1969). TRP channels are found in nearly all cell types and can respond to extracellular or intracellular activators. The TRP gene family can be classified into seven subtypes: 1.) TRPC (Classic), which controls cation release following phospholipase C activation, 2.) TRPV (Vanilloid), which responds to chemical, physical and warm to hot thermal stimuli, 3.) TRPM (Melastatin), involved in sensory perception (e.g., the perception of taste), and detection of cool thermal stimuli 4.) TRPA (Ankyrin), cold thermal stimuli and hearing, 5.) TRPN (NOMPC, no mechanopotential C), mechanosensation in C. elegans, Drosophila, and Danio rerio (although no TRPN has been identified in mammals), 6.) TRPP (Polycystin) and 7.) TRPML (Mucolipin), both of which are more distantly related to the other TRPs, but both of which have been linked to human diseases. In Drosophila, 13 TRP channels mediate behavioral responses to light, temperature, noxious stimuli, and osmolarity. While TRP channels function in the specialized sensory organs, their near-ubiquitous localization and links to wide-ranging diseases such as polycystic kidney disease, deafness, and cancer indicate that these channels may also detect internal cues (Montell 2005). The link to longevity remains largely unexplored. Mutation of the ocr-2 gene, which encodes one of the 17 C. elegans TRP channels and is involved in pheromone detection, extends lifespanin a daf-16/foxo-dependent manner (Lee & Ashrafi 2008). Investigation of potential roles for other TRP channels in aging is likely to yield many insights into how the perception of internal and external environments together inform the organism about its current state.
Sensory Structure/Organization and Lifespan
It is clear that interpretation of important environmental and internal cues by sensory receptors may play a role in lifespan regulation. However, there is evidence to suggest that both the structure of sensory cells and the integration of multiple sensory inputs can modulate lifespan as well.
In simple and complex organisms, sensory cells monitor their environment by presenting sensory receptors localized on ciliated structures. There is some evidence that the ciliary structure itself is crucial for modulating the effects of sensory perception. The initial characterization of long-lived C. elegans sensory mutants by Apfeld and Kenyon included genes that regulate the formation and intraflagellar transport of cilia (Apfeld & Kenyon 1999) and result in morphological disruption of the ciliary structure along with lifespan extension. However, ciliary structural proteins can play a role in lifespan regulation independent of morphological defects. Loss of the nephrocystins nph-1 and nph-4, or the ciliary transport protein IFTA-2, disrupts chemotaxis and extends lifespan in C. elegans despite no apparent morphological defects (Schafer et al 2006, Winkelbauer et al 2005). A similar lifespan extension was seen for double mutants affecting the C. elegans Meckel Syndrome-like ciliary proteins MKS-1/MKRS-1 or MKS-1/MKRS-2 (Bialas et al 2009). These findings demonstrate that the combined effect of sensory receptors and their structural environment is crucial for modulating sensory function, and they open the door to new targets for modulating aging without disrupting sensory receptors.
In addition to their structure, the integration of information from multiple sensory cells also plays a role in modulating lifespan regulation. The longevity-extending effects of ASI neuron ablation is lost when combined with ablation of another gustatory neuron pair (ASJ and/or ASK), one of which (ASJ) has no effect when ablated alone (Figure 1A) (Alcedo & Kenyon 2004). Additionally, loss of the AIY sensory interneurons limits longevity, presumably due to the loss of ability to integrate sensory cues (Shen et al). A survey of mutant G-proteins expressed in worm sensory neurons found that loss of function in the Gα subunits odr-3, gpa-1 and gpa-9 and the Gγ subunit gpc-1 increases worm lifespan (Lans & Jansen 2007). A dramatic increase in lifespan can be achieved by combining certain combinations of G-protein manipulations. Worms with overexpression of gpa-11 in combination with odr-3 loss of function live over 100 days, a roughly 4-fold increase in maximum lifespan (Lans et al 2009, Lans & Jansen 2007). Despite the relatively simple C. elegans nervous system, therefore, an important degree of sensory integration must occur in these animals, and such integration can work to synergize competing inputs and generate large changes in lifespan. Sensory integration in Drosophila and mammals occurs in dedicated olfactory and gustatory processing centers which then innervate central brain structures. The role of sensory integration on lifespan in these organisms is likely to be an active area of future investigation.
Human/Mammal Translational Link
While the bulk of evidence for a link between sensory processing and longevity results from research in invertebrate systems, there is some evidence to suggest that the human sensory system can modulate physiology and aging in ways that we do not yet understand. Following Pavlov’s work over 100 years ago that demonstrated a gastric secretion response from the perception of food in dogs, we now know that the pre-prandial response to food cues can alter insulin release (Teff 2000), appetite, and even exercise performance (Chambers et al 2009) in humans. The potential role of social interaction in the progression of aging and disease is probably the most exciting area of active research, which may provide valuable insight into currently intractable problems in health decline and general morbidity. The striking finding that obesity clusters in social networks (Christakis & Fowler 2007) suggests that sensory cues from the social world can be a key target for intervention. There is also emerging evidence that social interaction can slow the decline in cognitive function and onset of dementia in older populations (Ertel et al 2008, Laura et al 2004). Do these sensory cues influence physiology directly or indirectly through altering behavioral patterns? Can the perception of these sensory cues be directly targeted to promote healthy aging?
Outlook
It seems clear that manipulation of sensory information, particularly suppression of sensory input, is a potent mechanism for modulating lifespan in model organisms. We are left to reflect on sensory perception as an evolutionary trade-off that compromises longevity in natural populations. Because the force of natural selection declines with age, adaptations that enhance early-life fitness at the expense of late-life survival are subject to positive selection (Williams 1957). This phenomenon, known as antagonistic pleiotropy, has been invoked to explain other evolutionary trade-offs that affect lifespan, such as reproductive capacity. Might an organism gain a fitness advantage from enhanced abilities to detect food, danger, or potential mates and to adapt to new sensory environments only to suffer physiological consequences? For instance, hypersensitivity to the presence of dangerous or toxic environmental factors may confer a fitness advantage, but an overreaction may increase stress and tissue damage through activation of defense response pathways. Other behavioral adaptations such as food-seeking or mate-seeking behavior following perception may lead to unknown physiological trade-offs. As we develop a greater understanding of the specific sensory inputs that modulate lifespan, it will be important to consider whether sensory tuning can maximize the short-term benefits of sensory acuity while minimizing long-term damage.
There is still much work to be done identifying specific sensory receptors, neurons, and neural circuits that modulate lifespan, particularly in flies. A summary of current the known interventions modulating lifespan can be found in Table 1. Of more than 60 olfactory receptors, similar numbers of gustatory receptors, TRP channels, and other recently described sensory receptors in Drosophila (Abuin et al 2011, Benton et al 2009, Cameron et al 2010), we know of only one related to ligand binding (Gr63a) that affects lifespan. Do all types of sensory input affect aging or only some? How are diverse sensory signals integrated across neural circuits that influence lifespan? A comprehensive characterization that touches on these questions is within reach; particularly with the development of new genetic and cell biological tools allow the creation or elimination of sensory experiences at will. For example, the modified mammalian ion channel VR1E600K, pheromone-sensitive odorant receptor bmorOR1, or light-activated channelrhodopsin variants, can be expressed in particular sensory neurons. The effects of neuron activation can then be studied in genetically identical individuals by exposing experimental flies to either capsaicin, Bombyx pheromone, or light, respectively, and using unexposed flies (of the same genotype) as controls. Heterologous systems such as these are particularly powerful for studying aging because they eliminate problems associated with variability of genetic background, which often confound longevity measures (Partridge & Gems 2007).
Table 1.
Effect of sensory interventions on lifespan in invertebrate model systems.
| Intervention type | Genes | Direction of change | Reference | Other comments |
|---|---|---|---|---|
| Caenorhabditis elegans | ||||
| Sensory cilia mutants | che-2, che-13, osm-1, osm-5, osm-6, che-3, che-11, daf-19, daf-10, daf-6, mec-8, mec-1, osm-3 | extend | (Apfeld & Kenyon 1999), daf-10 & osm-3 (Maier et al 2010), che-11 (Bialas et al 2009), osm-3 & osm-5 (Lee & Kenyon 2009) |
daf-10 and osm-5 are required by somatic gonad signaling daf-10, daf-19, osm-3 and osm-5 are partially daf-16 dependent |
| mec-1 | no effect | (Apfeld & Kenyon 1999) | weak defect in amphid cilia fasciculation | |
| Amphid sheath cell ablation | extend | (Apfeld & Kenyon 1999) | ||
| Sensory signal mutants | tax-4 | extend | (Apfeld & Kenyon 1999) | tax-4 is daf-16 dependent |
| tax-2 | no effect | (Apfeld & Kenyon 1999) | ||
| Gustatory neurons ablations | ASI, ASG, | extend | (Alcedo & Kenyon 2004) | Full (ASI, ASG) or partial (AWA, AWC) daf-16 and daf-2 dependent |
| ASJ, ASK, ASI/ASJ, ASI/ASK, ADF, | no effect | |||
| Olfactory neurons ablations | AWA, AWA/AWC | extend | (Alcedo & Kenyon 2004) | Partially daf16 dependent |
| ASE, AWC | no effect | |||
| Olfactory mutants | odr-7, odr-2, odr-3, str-2 | extend | (Alcedo & Kenyon 2004) | |
| odr-1, odr-10 | no effect | |||
| G-protein mutants | odr-3, gpa-1, gpa-9, gpc-1, gpa-11 | extend | (Alcedo & Kenyon 2004, Lans et al 2009, Lans & Jansen 2007) | gpa-1, gpa-11, odr-3 and gpc-1 are daf-16 dependent |
| A diffusible bacterial product exposure | shorten | (Smith et al 2008) | The exposure suppresses lifespan extension from DR | |
| Thermosensory neurons (AFD/AIY) neurons (AFD/AIY) | AFD (ablation), ttx-1, ttx-3, tax-2, tax-4 | shorten | (Lee & Kenyon 2009) | At warm temperature (25°C). daf-9 and daf-12 dependent |
| Different food type exposure | nmur-1 | both | (Maier et al) | nmur-1 effect is dependent on the bacterial lipopolysaccharide structure |
| Dauer pheromone exposure | no effect | (Alcedo & Kenyon 2004) | ||
| extend | (Kawano et al 2005) | daf-16 dependent | ||
| TRP channel mutant | ocr-2 | extend | (Lee & Ashrafi 2008) | daf-16 dependent |
| Mutants in ciliary functions | mks-1/mksr-1, mks-1/mksr-2, mksr-1/mksr-2, nph-1, nph-4, ifta-2 | extend | (Bialas et al 2009, Schafer et al 2006, Winkelbauer et al 2005) |
nph-1 and nph-4 are daf-19 dependent ifta-2, mks-1/mksr-1/mksr-2 are part of the daf-2 pathway and depends on daf-16 |
| Drosophila melanogaster | ||||
| Food-derived odor exposure | shorten | (Libert et al 2007, Poon et al 2010) | Gr63a-dependent | |
| Olfactory mutant CO2 sensor mutant Abnormal light/dark period |
Or83b Gr63a |
extend extend shorten |
(Libert et al 2007) (Poon et al 2010) (Pittendrigh & Minis 1972) |
|
| Social cues from conspecifics | extend | (Ruan & Wu 2008) | In short-lived Sod mutant background | |
| Phormia terraenovae | ||||
| Abnormal light/dark period | shorten | (von Saint Paul & Aschoff 1978) | ||
| Apis mellifera | ||||
| Brood pheromone exposure | shorten | (Smedal et al 2009) | ||
It is important to establish whether sensory perception modulates aging in mice. There are far more sensory receptors and neurons in mice than in flies or worms, making gene-by-gene or neuron-by-neuron approaches less attractive. Nevertheless, various techniques are available that reduce or eliminate major aspects of peripheral sensory function (Brunet et al 1996, Harding et al 1978); these are a reasonable first step. Such manipulations would be similar to Or83b mutation in flies or disruption of chemosensory structures in worms, both of which increase lifespan. A second option might be to target taste, which compared to olfaction exhibits greatly reduced dimensionality in both flies and mammals, where behaviors are determined by simple integration of sweet and bitter inputs in flies and only five taste modalities in human (sweet, salty, bitter, sour and umami) (Masek & Scott 2010, Yarmolinsky et al 2009).
How important is context, learning and memory, and metabolic state? Is the Drosophila mushroom body required for sensory perception mediated lifespan; what about the lateral horn? In humans, does healthy aging boil down to a state of mind? These questions are just now being informed by experiments in behavioral neurobiology. Appetitive conditioning, for example, is a phenomenon whereby Drosophila learn to associate a specific odor with a sugar reward (Krashes & Waddell 2008). However, flies only retrieve memories and approach a conditioned odor stimulus when they are hungry. Dopaminergic control of neuropeptide dNPF is a key player in this response, and stimulation of dNPF-expressing neurons simulates a state of food deprivation and permits memory retrieval in satiated flies (Krashes et al 2009). Similalry, the smell of food only influences lifespan in nematodes and flies when the animals are diet-restricted (Libert et al 2007, Smith et al 2008), which suggests that an animal’s metabolic and cognitive states may be important for sensory modulation of aging. Future experiments such as these are likely to provide fascinating glimpses into how sensory perception interacts with satiety, motivation, and desire to influence aging and aging-related disease across taxa.
Summary Points.
Suppression of chemoattractant sensory perception in both C. elegans and Drosophila is sufficient to extend lifespan.
The perception of food without consumption is sufficient to shorten lifespan in both C. elegans and Drosophila.
Determining the importance of integration of sensory information from external and internal sources in higher brain areas is imperative.
Molecular mechanisms that are important for enacting sensory modulation of aging include contributions from well-known longevity pathways, such as insulin/IGF-1 like signaling, as well as currently unknown processes.
Acknowledgments
The authors wish to thank B. Chung, C. Gendron, O. Shafer, and H. Dierick for critical comments on the manuscript. We also wish to apologize to those colleagues whose work we were unable to discuss due to space limitations. This work was supported by funding from the Ellison Medical Foundation (S.P.), NIH K01AG031917 (N.L.), NIH R01AG030593 (S.P.), and NIH F31AG033981 (T.C.)
Abbreviations/ Acronyms
- IIS
Insulin/Insulin-like Signaling
- daf-16/foxo
C. elegans forkhead transcription factor required for many effects of daf-2
- daf-2
C. elegans insulin-like receptor
- OR
odorant receptor
- ORN
odorant receptor neuron
- GR
gustatory receptor
- GRN
gustatory receptor neuron
- TRP
transient receptor potential
- GPCR
G-protein coupled receptor
- cVA
cis-vaccenyl acetate, a volatile Drosophila pheromone
Key terms / Definitions
- Amphids
a pair of sensillia on the C. elegans head which contain dendrites for 11 chemosensory neurons and one thermosensory neuron
- Mushroom body
Higher integrative brain center in Drosophila and other insects involved in learning and memory, sleep control and other complicated behaviors
- Lateral horn
Higher integrative brain center in Drosophila and other insects that is putatively involved in naïve odor responses and understudied
- Public mechanism of aging
Molecular mechanisms of aging that are presumed to have evolved in a common ancestor and been maintained in multiple lineages
- Private mechanism of aging
Molecular mechanisms of aging that are idiosyncratic, in that they are characteristic of a particular population or species
- Dauer
An alternative to the third larval stage of C. elegans development which is induced under unfavorable conditions
- Pheromone
a chemical signal released by an organism in order to trigger a social response
- Dietary restriction
limiting food availability without malnutrition promotes lifespan extension in multiple model organisms
- Sensory cilia
Microtubule-based projections extending from the dendrites of sensory cells which typically contain the sensory receptors
- Circadian clock
Cell-autonomous oscillatory network of transcriptional feedback loops which regulates daily cycles in behavioral and metabolic rhythms
References
- Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69:44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41:45–55. doi: 10.1016/s0896-6273(03)00816-x. Reports lifespan of C. elegans following ablation of chemotaxis amphid neurons individually and in combinations. [DOI] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–9. doi: 10.1038/45544. The first report of a direct connection between sensory input and lifespan regulation. [DOI] [PubMed] [Google Scholar]
- Aristotle De Anima (On the soul) 350 B C [Google Scholar]
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–72. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Chemosensation in C. elegans. WormBook. 2006:1–29. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–27. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991a;7:729–42. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991b;251:1243–6. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- Beggs KT, Mercer AR. Dopamine receptor activation by honey bee queen pheromone. Curr Biol. 2009;19:1206–9. doi: 10.1016/j.cub.2009.05.051. [DOI] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical Membrane Topology and Heteromeric Function of Drosophila Odorant Receptors In Vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–62. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007;450:289–93. doi: 10.1038/nature06328. [DOI] [PubMed] [Google Scholar]
- Bergamasco C, Bazzicalupo P. Chemical sensitivity in Caenorhabditis elegans. Cell Mol Life Sci. 2006;63:1510–22. doi: 10.1007/s00018-006-6114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialas NJ, Inglis PN, Li C, Robinson JF, Parker JD, et al. Functional interactions between the ciliopathy-associated Meckel syndrome 1 (MKS1) protein and two novel MKS1-related (MKSR) proteins. J Cell Sci. 2009;122:611–24. doi: 10.1242/jcs.028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–9. doi: 10.1038/nature05904. Demontrates that longevity by dietary restriction requires expression of transcription factor skn-1 in two sensory neurons. [DOI] [PubMed] [Google Scholar]
- Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–29. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- Briand L, Swasdipan N, Nespoulous C, Bezirard V, Blon F, et al. Characterization of a chemosensory protein (ASP3c) from honeybee (Apis mellifera L.) as a brood pheromone carrier. Eur J Biochem. 2002;269:4586–96. doi: 10.1046/j.1432-1033.2002.03156.x. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–93. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–87. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–77. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–2. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–5. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ES, Bridge MW, Jones DA. Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. J Physiol. 2009;587:1779–94. doi: 10.1113/jphysiol.2008.164285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–94. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Tanoue S, Houl JH, Hardin PE. Regulation of gustatory physiology and appetitive behavior by the Drosophila circadian clock. Curr Biol. 2010;20:300–9. doi: 10.1016/j.cub.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–9. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, et al. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc Natl Acad Sci U S A. 2007;104:7128–33. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–7. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Crawford JD. Sex recognition by electric cues in a sound-producing mormyrid fish, Pollimyrus isidori. Brain Behav Evol. 1991;38:20–38. doi: 10.1159/000114377. [DOI] [PubMed] [Google Scholar]
- Danty E, Briand L, Michard-Vanhee C, Perez V, Arnold G, et al. Cloning and expression of a queen pheromone-binding protein in the honeybee: an olfactory-specific, developmentally regulated protein. J Neurosci. 1999;19:7468–75. doi: 10.1523/JNEUROSCI.19-17-07468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danty E, Michard-Vanhee C, Huet JC, Genecque E, Pernollet JC, Masson C. Biochemical characterization, molecular cloning and localization of a putative odorant-binding protein in the honey bee Apis mellifera L. (Hymenoptera : Apidea) FEBS Lett. 1997;414:595–8. doi: 10.1016/s0014-5793(97)01048-x. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Dierick HA. A method for quantifying aggression in male Drosophila melanogaster. Nat Protoc. 2007;2:2712–8. doi: 10.1038/nprot.2007.404. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat Genet. 2006;38:1023–31. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2010 doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison AS. Caenorhabditis elegans pheromones regulate multiple complex behaviors. Curr Opin Neurobiol. 2009;19:378–88. doi: 10.1016/j.conb.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima A, Smith BP, Lucas C, van der Goes van Naters W, Miller CJ, et al. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson KJ, Bradshaw WE, Holzapfel CM. CONCORDANCE OF THE CIRCADIAN CLOCK WITH THE ENVIRONMENT IS NECESSARY TO MAXIMIZE FITNESS IN NATURAL POPULATIONS. Evolution. 2008;62:979–83. doi: 10.1111/j.1558-5646.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel KA, Glymour MM, Berkman LF. Effects of Social Integration on Preserving Memory Function in a Nationally Representative US Elderly Population. Am J Public Health. 2008;98:1215–20. doi: 10.2105/AJPH.2007.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser E, Olender T, Khen M, Yanai I, Ophir R, Lancet D. Widespread ectopic expression of olfactory receptor genes. BMC Genomics. 2006;7:121. doi: 10.1186/1471-2164-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fernandez ML, Chan YB, Yew JY, Billeter JC, Dreisewerd K, et al. Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol. 2010;8:e1000541. doi: 10.1371/journal.pbio.1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur JF. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35:279–95. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- Fischer P, Grozinger CM. Pheromonal regulation of starvation resistance in honey bee workers (Apis mellifera) Naturwissenschaften. 2008;95:723–9. doi: 10.1007/s00114-008-0378-8. [DOI] [PubMed] [Google Scholar]
- Fischer U, Hommel H, Ziegler M, EJ The Mechanism of Insulin Secretion After Oral Glucose Stimulation, III. Investigations of the Mechanism of a Reflectoric Insulin Mobilization After Oral Stimulation. Diabetologia. 1972;8:385–90. doi: 10.1007/BF01212164. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo M, Riddle DL. Effects of a Caenorhabditis elegans dauer pheromone ascaroside on physiology and signal transduction pathways. J Chem Ecol. 2009;35:272–9. doi: 10.1007/s10886-009-9599-3. [DOI] [PubMed] [Google Scholar]
- Gelstein S, Yeshurun Y, Rozenkrantz L, Shushan S, Frumin I, et al. Human tears contain a chemosignal. Science. 2011;331:226–30. doi: 10.1126/science.1198331. [DOI] [PubMed] [Google Scholar]
- Gerber B, Stocker RF, Tanimura T, Thum AS. Smelling, tasting, learning: Drosophila as a study case. Results Probl Cell Differ. 2009;47:139–85. doi: 10.1007/400_2008_9. [DOI] [PubMed] [Google Scholar]
- Giles LC, Glonek GF, Luszcz MA, Andrews GR. Effect of social networks on 10 year survival in very old Australians: the Australian longitudinal study of aging. J Epidemiol Community Health. 2005;59:574–9. doi: 10.1136/jech.2004.025429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–80. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- Graves JL, Jr, Mueller LD. Population density effects on longevity. Genetica. 1993;91:99–109. doi: 10.1007/BF01435991. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–56. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin CA, Kafadar KA, Pavlath GK. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell. 2009;17:649–61. doi: 10.1016/j.devcel.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JW, Getchell TV, Margolis FL. Denervation of the primary olfactory pathway in mice. V. Long-term effect of intranasal ZnSO4 irrigation on behavior, biochemistry and morphology. Brain Res. 1978;140:271–85. doi: 10.1016/0006-8993(78)90460-2. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Lu S, Kume K, Yin JC, Yang Z, Sehgal A. Gender dimorphism in the role of cycle (BMAL1) in rest, rest regulation, and longevity in Drosophila melanogaster. J Biol Rhythms. 2003;18:12–25. doi: 10.1177/0748730402239673. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T. Effects of Various Taste Stimuli on Heart Rate in Humans. Chemical Senses. 2000;25:149–53. doi: 10.1093/chemse/25.2.149. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–5. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Hu PJ. Dauer. WormBook. 2007:1–19. doi: 10.1895/wormbook.1.144.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignell R, Root CM, Birse RT, Wang JW, Nassel DR, Winther AM. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc Natl Acad Sci U S A. 2009;106:13070–5. doi: 10.1073/pnas.0813004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–74. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RH. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet. 1999;21:414–9. doi: 10.1038/7753. [DOI] [PubMed] [Google Scholar]
- Jeong PY, Jung M, Yim YH, Kim H, Park M, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–5. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- Jin X, Ha TS, Smith DP. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci U S A. 2008;105:10996–1001. doi: 10.1073/pnas.0803309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Kravitz EA, Heinrich R. Sound production during agonistic behavior of male Drosophila melanogaster. Fly (Austin) 2010;5 doi: 10.4161/fly.5.1.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, et al. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5:487–94. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–90. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DD, Zimmermann G, Suyama K, Meyer T, Scott MP. A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size. Genes & Development. 2008;22:1877–93. doi: 10.1101/gad.1670508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, Horvitz HR. A Dual Mechanosensory and Chemosensory Neuron in Caenorhabditis-Elegans. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2227–31. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson P, Luscher M. Pheromones’: a new term for a class of biologically active substances. Nature. 1959;183:55–6. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- Kawano T, Kataoka N, Abe S, Ohtani M, Honda Y, et al. Lifespan extending activity of substances secreted by the nematode Caenorhabditis elegans that include the dauer-inducing pheromone. Biosci Biotechnol Biochem. 2005;69:2479–81. doi: 10.1271/bbb.69.2479. [DOI] [PubMed] [Google Scholar]
- Kim K, Sato K, Shibuya M, Zeiger DM, Butcher RA, et al. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 2009;326:994–8. doi: 10.1126/science.1176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A, Rouyer F. Effects of circadian mutations and LD periodicity on the life span of Drosophila melanogaster. J Biol Rhythms. 1998;13:471–8. doi: 10.1177/074873098129000309. [DOI] [PubMed] [Google Scholar]
- Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr. 2009;90:822S–5S. doi: 10.3945/ajcn.2009.27462T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–73. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–27. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–13. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Kretzschmar D, Rakshit K, Chow E, Giebultowicz JM. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging (Albany NY) 2009;1:937–48. doi: 10.18632/aging.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Mohan A, Sharma VK. Circadian dysfunction reduces lifespan in Drosophila melanogaster. Chronobiol Int. 2005;22:641–53. doi: 10.1080/07420520500179423. [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–6. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Lacaille F, Hiroi M, Twele R, Inoshita T, Umemoto D, et al. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS One. 2007;2:e661. doi: 10.1371/journal.pone.0000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laissue PP, Vosshall LB. The olfactory sensory map in Drosophila. Adv Exp Med Biol. 2008;628:102–14. doi: 10.1007/978-0-387-78261-4_7. [DOI] [PubMed] [Google Scholar]
- Lans H, Dekkers MP, Hukema RK, Bialas NJ, Leroux MR, Jansen G. Signaling proteins that regulate NaCl [corrected] chemotaxis responses modulate longevity in C. elegans. Ann N Y Acad Sci. 2009;1170:682–7. doi: 10.1111/j.1749-6632.2009.04362.x. [DOI] [PubMed] [Google Scholar]
- Lans H, Jansen G. Multiple sensory G proteins in the olfactory, gustatory and nociceptive neurons modulate longevity in Caenorhabditis elegans. Dev Biol. 2007;303:474–82. doi: 10.1016/j.ydbio.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–14. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Laura F, Stephanie P-B, Bengt W. An active and socially integrated lifestyle in late life might protect against dementia. The Lancet Neurology. 2004;3:343–53. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Lee BH, Ashrafi K. A TRPV channel modulates C. elegans neurosecretion, larval starvation survival, and adult lifespan. PLoS Genet. 2008;4:e1000213. doi: 10.1371/journal.pgen.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, et al. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol. 2008;10:468–75. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kenyon C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr Biol. 2009;19:715–22. doi: 10.1016/j.cub.2009.03.041. Reported the effect of thermosensory neurons on C. elegans lifespan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini I, Le Conte Y, Costagliola G, Plettner E, Toth AL, et al. Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. Proc Natl Acad Sci U S A. 2004;101:17559–64. doi: 10.1073/pnas.0407652101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JC, Xu F. Influences of light-dark shifting on the immune system, tumor growth and life span of rats, mice and fruit flies as well as on the counteraction of melatonin. Biol Signals. 1997;6:77–89. doi: 10.1159/000109112. [DOI] [PubMed] [Google Scholar]
- Li W, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 2003;17:844–58. doi: 10.1101/gad.1066503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–7. doi: 10.1126/science.1136610. First report of a role for olfactory suppression and food odors on lifespan in Drosophila. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–22. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Maier W, Adilov B, Regenass M, Alcedo J. A neuromedin U receptor acts with the sensory system to modulate food type-dependent effects on C. elegans lifespan. PLoS Biol. 8:e1000376. doi: 10.1371/journal.pbio.1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Adilov B, Regenass M, Alcedo J. A neuromedin U receptor acts with the sensory system to modulate food type-dependent effects on C. elegans lifespan. PLoS Biol. 2010;8:e1000376. doi: 10.1371/journal.pbio.1000376. Reports that sensory-dependent lifespan depends on the food source. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–3. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–80. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM. Keynote: mechanisms of senescence--complificationists versus simplificationists. Mech Ageing Dev. 2002;123:65–73. doi: 10.1016/s0047-6374(01)00335-9. \PDF\Indexed\Martin_Comp_vs_Simp.pdf. discussion 5–9. [DOI] [PubMed] [Google Scholar]
- Masek P, Scott K. Limited taste discrimination in Drosophila. Proc Natl Acad Sci U S A. 2010;107:14833–8. doi: 10.1073/pnas.1009318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19:R700–13. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Matsunami H, Amrein H. Taste perception: how to make a gourmet mouse. Curr Biol. 2004;14:R118–20. [PubMed] [Google Scholar]
- Melcher C, Bader R, Walther S, Simakov O, Pankratz MJ. Neuromedin U and its putative Drosophila homolog hugin. PLoS Biol. 2006;4:e68. doi: 10.1371/journal.pbio.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci. 2008;11:874–6. doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed]
- Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–7. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I, Ohshima Y. Neural Regulation of Thermotaxis in Caenorhabditis-Elegans. Nature. 1995;376:344–8. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- Nicolaidis S. Early systemic responses to orogastric stimulation in the regulation of food and water balance: functional and electrophysiological data. Ann N Y Acad Sci. 1969;157:1176–203. doi: 10.1111/j.1749-6632.1969.tb12942.x. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–9. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Cracking neural circuits in a tiny brain: new approaches for understanding the neural circuitry of Drosophila. Trends Neurosci. 2008;31:512–20. doi: 10.1016/j.tins.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci US A. 1998;95:8660–4. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RE, Jr, Peng CY. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp Gerontol. 2001;36:695–711. doi: 10.1016/s0531-5565(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Pankiw T. Worker honey bee pheromone regulation of foraging ontogeny. Naturwissenschaften. 2004;91:178–81. doi: 10.1007/s00114-004-0506-z. [DOI] [PubMed] [Google Scholar]
- Partridge L, Fowler K. Non-mating costs of exposure to males in female Drosophila melanogaster. Journal of Insect Physiology. 1990;36:419–25. [Google Scholar]
- Partridge L, Gems D. Benchmarks for ageing studies. Nature. 2007;450:165–7. doi: 10.1038/450165a. [DOI] [PubMed] [Google Scholar]
- Pavlov ItbWT. Work of the Digestive Glands. 2 1902. [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–87. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Petrascheck M, Ye X, Buck LB. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature. 2007;450:553–6. doi: 10.1038/nature05991. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Minis DH. Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1972;69:1537–9. doi: 10.1073/pnas.69.6.1537. Abnormal circadian environement shortens Drosophila lifespan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher SD. The modulation of lifespan by perceptual systems. Ann N Y Acad Sci. 2009;1170:693–7. doi: 10.1111/j.1749-6632.2009.04926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon PC, Kuo TH, Linford NJ, Roman G, Pletcher SD. Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. 8:e1000356. doi: 10.1371/journal.pbio.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon PC, Kuo TH, Linford NJ, Roman G, Pletcher SD. Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. 2010;8:e1000356. doi: 10.1371/journal.pbio.1000356. First report of a specific sensory receptor that modulates lifespan in Drosophila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KC, Andersen EC, Kruglyak L, Kim DH. A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science. 2009;323:382–4. doi: 10.1126/science.1166527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol. 2010;20:1000–5. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- Robinson GE. Regulation of division of labor in insect societies. Annu Rev Entomol. 1992;37:637–65. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Winston ML, Huang Z, Pankiw T. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J Insect Physiol. 1998;44:685–92. doi: 10.1016/s0022-1910(98)00040-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Laso A, Zunzunegui MV, Otero A. The effect of social relationships on survival in elderly residents of a Southern European community: a cohort study. BMC Geriatr. 2007;7:19. doi: 10.1186/1471-2318-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Wu CF. Social interaction-mediated lifespan extension of Drosophila Cu/Zn superoxide dismutase mutants. Proc Natl Acad Sci U S A. 2008;105:7506–10. doi: 10.1073/pnas.0711127105. Social interaction extends Sod mutant Drosophila lifespan through sensory perception. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Bachelier C, Fondrk MK, Page RE., Jr Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.) Exp Gerontol. 2007;42:1020–32. doi: 10.1016/j.exger.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–82. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–52. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–6. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–28. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Schafer JC, Winkelbauer ME, Williams CL, Haycraft CJ, Desmond RA, Yoder BK. IFTA-2 is a conserved cilia protein involved in pathways regulating longevity and dauer formation in Caenorhabditis elegans. J Cell Sci. 2006;119:4088–100. doi: 10.1242/jcs.03187. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–4. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres M. Les Cinq Sens. London: Continuum International Publishing Group; 1985. [Google Scholar]
- Shen L, Hu Y, Cai T, Lin X, Wang D. Regulation of longevity by genes required for the functions of AIY interneuron in nematode Caenorhabditis elegans. Mech Ageing Dev. 131:732–8. doi: 10.1016/j.mad.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, et al. Strong and consistent social bonds enhance the longevity of female baboons. Curr Biol. 2010;20:1359–61. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Smedal B, Brynem M, Kreibich CD, Amdam GV. Brood pheromone suppresses physiology of extreme longevity in honeybees (Apis mellifera) J Exp Biol. 2009;212:3795–801. doi: 10.1242/jeb.035063. [DOI] [PubMed] [Google Scholar]
- Smith ED, Kaeberlein TL, Lydum BT, Sager J, Welton KL, et al. Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC Dev Biol. 2008;8:49. doi: 10.1186/1471-213X-8-49. Extends Drosophila results by showing modulation of C. elegans lifespan by bacterial food odorants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–8. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, et al. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–8. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer KL, Singh V, Macosko E, Steele SE, Bargmann CI, Aballay A. Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science. 2008;322:460–4. doi: 10.1126/science.1163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–4. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–55. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–51. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000;34:206–13. doi: 10.1006/appe.1999.0282. [DOI] [PubMed] [Google Scholar]
- van der Linden AM, Beverly M, Kadener S, Rodriguez J, Wasserman S, et al. Genome-wide analysis of light- and temperature-entrained circadian transcripts in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000503. doi: 10.1371/journal.pbio.1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MA, Luo N, Yamaguchi A, Kapahi P. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr Biol. 20:1006–11. doi: 10.1016/j.cub.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MA, Luo N, Yamaguchi A, Kapahi P. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr Biol. 2010;20:1006–11. doi: 10.1016/j.cub.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Saint Paul U, Aschoff J. Longevity among blowfliePhormia terraenovaeR.D. kept in non-24-hour light-dark cycles. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1978;127:191–5. [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–36. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–33. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–31. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5657–63. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner KW, Nichols AS, Walden KK, Brockmann A, Luetje CW, Robertson HM. A honey bee odorant receptor for the queen substance 9-oxo-2-decenoic acid. Proc Natl Acad Sci U S A. 2007;104:14383–8. doi: 10.1073/pnas.0705459104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, Liu J, Feng Z, Xu XZ. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat Neurosci. 2008;11:916–22. doi: 10.1038/nn.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Thomspon N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–37. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- Ware RW, Clark D, Crossland K, Russell RL. The nerve ring of the nematode Caenorhabditis elegans: sensory input and motor output. J Comp Neurol. 1975;162:71–110. [Google Scholar]
- Warren B, Gibson G, Russell IJ. Sex Recognition through midflight mating duets in Culex mosquitoes is mediated by acoustic distortion. Curr Biol. 2009;19:485–91. doi: 10.1016/j.cub.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–72. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JQ, Nicholas TJ, Gritton J, Truong L, Davidson ER, Jorgensen EM. The sensory circuitry for sexual attraction in C. elegans males. Curr Biol. 2007;17:1847–57. doi: 10.1016/j.cub.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–11. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Winkelbauer ME, Schafer JC, Haycraft CJ, Swoboda P, Yoder BK. The C. elegans homologs of nephrocystin-1 and nephrocystin-4 are cilia transition zone proteins involved in chemosensory perception. J Cell Sci. 2005;118:5575–87. doi: 10.1242/jcs.02665. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci U S A. 2005;102:13289–94. doi: 10.1073/pnas.0501914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Atkinson R, Jones DN, Smith DP. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 2005;45:193–200. doi: 10.1016/j.neuron.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–44. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarse K, Ristow M. Antidepressants of the serotonin-antagonist type increase body fat and decrease lifespan of adult Caenorhabditis elegans. PLoS One. 2008;3:e4062. doi: 10.1371/journal.pone.0004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–40. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]
- Zhang X, De la Cruz O, Pinto JM, Nicolae D, Firestein S, Gilad Y. Characterizing the expression of the human olfactory receptor gene family using a novel DNA microarray. Genome Biol. 2007;8:R86. doi: 10.1186/gb-2007-8-5-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2010;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]