Abstract
Patients with osteoarthritis (OA) primarily seek treatment due to pain and disability, yet the primary endpoints for rodent OA models tend to be histological measures of joint destruction. The discrepancy between clinical and preclinical evaluations is problematic, given that radiographic evidence of OA in humans does not always correlate to the severity of patient-reported symptoms. Recent advances in behavioral analyses have provided new methods to evaluate disease sequelae in rodents. Of particular relevance to rodent OA models are methods to assess rodent gait. While obvious differences exist between quadrupedal and bipedal gait sequences, the gait abnormalities seen in humans and in rodent OA models reflect similar compensatory behaviors that protect an injured limb from loading. The purpose of this review is to describe these compensations and current methods used to assess rodent gait characteristics, while detailing important considerations for the selection of gait analysis methods in rodent OA models.
Keywords: Osteoarthritis, Rodent Preclinical Models, Behavioral Analysis, Gait Analysis, Pain
Introduction
The primary reason that patients seek treatment for osteoarthritis (OA) is pain [1]; yet the primary endpoints in rodent models designed to mimic human OA tend to be histological measures of joint remodeling [2, 3]. The discrepancy between clinical evaluations of OA and scientific analyses in preclinical OA models is problematic, given that radiographic evidence of OA does not always correlate to patient reports of pain and disability [4]. Moreover, the most common endpoint for preclinical studies of new OA therapeutics is prevention of loss of cartilage based on joint histology, but this endpoint may be unrealistic in the clinical treatment of OA. In patients with OA, severe cartilage loss may occur well before the onset of symptoms, or symptoms may be present without significant radiographic changes, making it difficult to confirm OA as the source. The treating OA pain vs. treating OA-related degeneration debate is longstanding and without clear resolution (see 2013 OARSI Debate [5]). Clearly, OA pathogenesis and joint-related pain are not unique events, but the relationship between joint degeneration and OA symptoms is more complicated than simple cause and effect. The unknown links between joint degeneration and OA symptoms inhibits our ability to develop effective drugs for treating OA, and much work is needed to improve our understanding of the connections between OA progression and joint pain.
OA pain in humans can be studied through questionnaires and physical examinations [6–11], but detecting symptoms in preclinical OA models adds significant complexity [12]. Here, researchers rely on behavioral assays to elucidate pain-related behaviors [12–14]. Since joint pain is often associated with motion, gait analyses have recently been used to detect pain-related behaviors in rodent OA models, including genetic, chemical, and surgical models of OA [15, 16••, 17••, 18, 19, 20••, 21, 22]. In recent years, multiple gait analysis approaches have been developed for rodents [17••, 23, 24•, 25, 26•, 21, 27], but differences among approaches have introduced some inconsistency in gait parameters reported in the literature. The purpose of this review is to describe common rodent gait compensations associated with joint injuries as well as important considerations for gait analysis in rodent OA models. A synopsis of quadrupedal gait characteristics is first provided for a non-engineering audience, with descriptions of how these variables have been applied to rodent OA models. Then the nuances of rodent gait analysis are discussed, including common pitfalls. To be clear, it is not the intention of this review to recommend a specific gait analysis platform or methodology – each method has inherent strengths and weaknesses. Instead, the goal of this review is to inform the reader on key aspects of gait analysis in rodent OA models.
Characteristics of Rodent Gait
Gait Modifications as a Proxy Measures for OA Disease Sequelae
Patients with OA typically report that pain initially occurs as acute episodes associated with movement, then progresses to chronic dull aches with short periods of intense pain at later stages of disease. However, it is not yet clear which gait compensations are associated with ‘fear of movement’ and which are associated with physical dysfunction or mechanical impairment of the joint. Since movement-evoked pain is an early characteristic of OA, a person or animal may modify their gait pattern to protect an injured limb from loading and motion. If a protective pattern is repeated over time, or if normal daily activities are altered as a consequence, muscles surrounding the joint will atrophy. In addition, loss of articular cartilage and the formation of osteophytes as OA progresses could alter the internal mechanics of an articulating joint. Thus, the relationship between pain and joint dysfunction is closely intertwined in OA and difficult to separate. While this intersection of biology and mechanics can complicate the study of some OA disease mechanisms, it also highlights the importance of gait measures in the assessment of OA models.
Spatiotemporal Characteristics
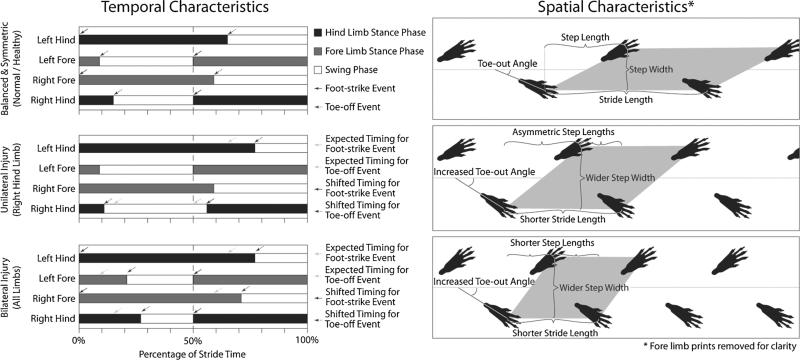
Spatial gait data describe the geometric characteristics of a footprint pattern, while temporal data describe the timing and synchronicity of foot-strike and toe-off events. Spatial variables include stride length, step length, step width, foot splay, and characterization of the paw print (Fig. 1). Note that stride length describes the distance between two foot strikes of the same limb. This should not be confused with ‘step length,’ which describes a limb's forward distance along the direction of travel relative to the contralateral limb. Also, some gait analysis platforms report different forms of the footprint pattern, such as distance and angle between the left and right paw [28]. It is important to note that these variables are only geometric transformations of stride length, step length, and step width, and not necessarily independent measures of the gait pattern. In addition to these spatial data, paw print characteristics may also indicate limb injury in rodent OA models, where the spacing of toes and paw print lengths can be used to form functionality indices for the sciatic, tibial, and peroneal nerves [29–32]. In addition, paw print area or intensity differences between limbs may indicate dynamic weight-bearing imbalances due to a musculoskeletal pathology [23, 26•, 33, 34].
Fig. 1.
Typical spatiotemporal gait sequences for walking rodents. Temporal variables describe the sequence of foot-strike and toe-off events during the gait cycle, where the gait cycle is defined by two sequential foot-trikes of the left hind limb. The time during which a limb is in contact with the ground is known as stance (black bars), with swing occurring as a limb translates forward (white bars). Stance begins at foot-strike (solid arrows), and swing begins with toe-off (dotted arrows). Spatial variables, including stride length, step length, step width, and toe-out angle, describe the geometric position of the paw prints during locomotion. Rodents tend to have symmetric gaits, where left and right limb foot-strikes (for either the fore or hind limbs) are spaced at approximately 50 % of the gait cycle in time and equidistant in space (step length is about 50 % of stride length). Similarly, rodent gaits tend to be balanced, where equal amounts of time are spent on the left and right limbs (for either the fore or hind limbs). Imbalanced and asymmetric gait patterns can occur with unilateral injury. Here, stance time on the injured limb (right hind in this example) decreases, the temporal gait pattern becomes asymmetric (right hind foot-strike occurs after 50 % of gait cycle), step widths widen, stride lengths shorten, toe-out angles increase in the injured limb, and the right and left limb step lengths become asymmetric. For bilateral injury, the gait pattern may remain symmetric and balanced. However, stance times increase for both the left and right limb (for the fore and hind limbs), stride lengths shorten, step widths widen, and toe-out angles increase for both limbs.
Temporal data are classically described by a Hildebrand plot of the gait cycle [35]; here, the gait cycle is reduced to a single repeatable sequence normalized by stride time. While some gait analysis systems report only raw forms of temporal data (such as stance time, swing time, and stride time), these data can be used to construct the Hildebrand plot (Fig. 1). This plot reduces temporal data to a few critical variables: limb duty factor, temporal symmetry, and limb phase. Limb duty factor, also known as limb percentage stance time [36, 20••], and seen below in (Eq. 1), represents the percentage of time a limb is in ground contact.
| (1) |
Temporal symmetry, sometimes referred to as gait symmetry [20••], and seen below in Eq. 2, describes the synchronicity of the left-right-left foot-strike sequence.
| (2) |
Similarly, limb phase, described below in Eq. 3, describes the synchronicity between the forelimb and hindlimb pairs.
| (3) |
Together, the limb duty factors for each limb, the temporal symmetry of the fore and hind limb sets, and limb phase describe the sequence of foot-strikes and toe-off events in the quadrupedal gait sequence.
Kinematic and Dynamic Characteristics
Kinematic variables describe limb position and movement in time and space, while dynamic variables describe forces related to locomotion. In human locomotion, 3-D kinematic data and ground reaction forces are often combined in biomechanical models to predict internal joint forces/torques [37–40]. While these same models can theoretically be applied to rodents, technologies to accurately assess 3-D kinematics in rodents are still in development (see Considerations section). As such, current kinematic measures of rodent gait are largely related to foot and ankle orientation [41–43], and current dynamic measures of rodent gait are largely limited to ground reaction forces [17••, 44, 21, 45, 46, 22, 47].
Kinematic measures of foot and ankle orientation, which are sensitive to peripheral nerve injuries, can be tracked using videographic methods [41, 48]. These measures include toeoff angle (also known as propulsion angle), which describes the foot/ankle angle during toe-off relative to the foot/ankle angle at midstance. Decreased toe-off angles can indicate unwillingness or inability to push-off with a given limb, an indirect measure of propulsive force (see below). Similarly, toe clearance and foot clearance angle describe the limb position during swing, and are often altered due to lameness [49]. Finally, swing velocities often change due to limb injury. However, since swing velocity is equal to the stride length divided by the swing time (or velocity divided by one minus the limb duty factor), swing velocity is not an independent measure relative to most spatiotemporal measures [50].
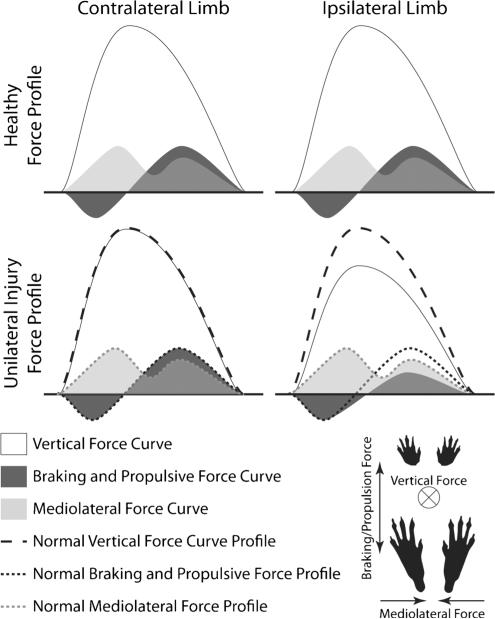
Dynamic measures assess forces and torques associated with locomotion; however, due to large errors associated with skin marker tracking in rodents [51•], dynamic measures are largely limited to ground reaction forces. Ground reaction forces can be separated into three components: vertical (out of the floor), braking/propulsion (direction of travel), and mediolateral stability (directed toward midline, Fig. 2) [52]. The vertical component is the largest, and represents the weight borne by a limb during locomotion (Fig. 2). Braking forces occur opposite the direction of travel and briefly slow the forward translation of the center of mass when a foot initially hits the ground. Following braking, the limb generates propulsive forces to continue to translate the center of mass forward. Mediolateral forces help stabilize the gait through two phases of forces, both directed at an animal's midline. First, as weight is initially shifted from the left to right limb, a stabilizing force directed toward the animal's midline must slow this weight shift shortly after the right limb hits the ground. Then, when weight is shifted back to the left limb from the right limb, a second mediolateral force directed toward the animal's midline must be generated to propel weight back to the contralateral limb. While each ground reaction force has a unique shape (Fig. 2), ground reaction forces are often generalized as peak force and/or impulse (area underneath the vertical force-time curve). Some examples of the use of ground reaction forces to describe the consequences of painful knee OA include work by Jay et al. [53••], where asymmetric vertical forces were observed in a rat anterior cruciate ligament transection (ACLT) model of knee OA and a treatment effect for synoviocyte-derived lubricin was demonstrated relative to phosphate buffered saline. Our group has also described changes in three-component ground reaction forces in rodents, including reduction in the injured limb peak vertical force and vertical impulse and modifications in the braking and propulsive forces of both limbs for a rat medical meniscus transection (MMT) model of OA [17••] and a rat model of intervertebral disc herniation [44].
Fig. 2.
Typical ground reaction forces for walking rodents. Ground reaction forces consist of three orthogonal components: vertical, braking/propulsive, and mediolateral. The vertical component carries the majority of weight, with the positive component directing out of the ground (+z). The vertical force curve for rodents tends to be semi-parabolic, with only one peak, in contrast to that of bipedal humans, which tends to have two peaks. The braking/propulsive curve aligns with the direction of travel and is composed of the negative braking component (180° from the direction of travel, −x) and the positive propulsive component (in the direction of travel, +x). While not shown, propulsive forces tend to be larger than braking forces for the hind limbs of rodents. However, this is flipped for the fore limbs, where braking forces tend to be larger than propulsive forces. The mediolateral curve is directed toward the animal's midline, typically defined as positive (+y). This convention is adapted for some models, as ground reaction forces for a left foot would not be defined by a right-handed coordinate system. The mediolateral curve consists of two positive peaks, where the first peak tends to be higher than the second. For unilateral medial meniscus tear in rats, we have observed decreased vertical force and propulsive forces on the injured limb [17••]
Gait Analysis Methods for Rodents
The goal of this review is to inform the reader on key aspects of gait analysis in rodent OA models. This may be done through commercial systems or via custom-built gait analysis arenas. The commercial systems to assess rodent gait include, but are not limited to:
The CatWalk by Noldus – overground walkway where rodent paws prints are illuminated by internally reflected light within the glass floor
DigiGait by MouseSpecifics – treadmill system that detects rodent paw position via color-based image registration
TreadScan and GaitScan by CleverSys – treadmill and overground systems to detect the rodent paw position via color-based image registration
Pressure-Sensing Walkway by TekScan – walkway that detects vertical ground reaction forces and spatiotemporal data based upon pressure-sensitive sensors within the floor.
Dynamic Weight Bearing Test by Bioseb – walkway that detects imbalance of weight distribution during locomotion via sensors within the walkway floor.
This list is likely not exhaustive, and it is not the intention of this review to recommend a specific gait analysis platform or methodology – each method has inherent strengths and weaknesses. Many research groups, including our group, have engineered custom systems to assess rodent gait, largely driven by the complexity of rodent gait analysis as described in the Considerations section of this review. Nonetheless, commercial systems to assess the walking characteristics of rodents have been significantly improved over the past several years, and as a result, gait analysis is being more widely applied in the behavioral analysis of rodent preclinical models.
Detecting Limb Injuries Using Rodent Gait Analysis
Spatiotemporal Indicators of Unilateral Limb Injury
Unilateral gait compensations can be thought of as limps that protect an affected limb from loading by shifting the weight to the contralateral limb. Rodents tend to walk with balanced symmetric gaits, and deviations from this spatiotemporal pattern can help identify unilateral limb injuries that may be associated with pain [19]. As an example of temporal balance, left and right duty factors should be similar in the forelimb or hindlimb set; however, if the right limb is injured, the right limb duty factor will likely decrease, and the uninjured left limb duty factor will likely increase. Using duty factor imbalance (simply, left limb duty factor minus right limb duty factor), these unilateral compensations can be identified, where duty factor imbalances statistically greater than zero indicate right limb injury and duty factor imbalances statistically less than zero indicate left limb injury [19]. Shifts in the temporal gait sequence have been reported for several unilateral pain injury models, including but not limited to peripheral carrageenan or adjuvant injection [54, 33, 55, 24•, 25, 26•] and sciatic nerve constriction [23]. Similar temporal shifts have been reported for a collagen type II antibody-induced model of inflammatory arthritis [28], intra-articular over-expression of interleukin-1 [18], monoiodoacetate (MIA) model of knee OA [16••, 56, 57], ACLT model of knee OA [16••], and for the MMT model of knee OA [17••].
Rodents also tend to walk with symmetric gait patterns, meaning the foot-strike sequence is equally distributed in time and space. Envision a marching band, where the left-right-left foot-strike sequence is even and on the beat. Here, a right foot-strike occurs halfway between two left foot-strikes, both in time (temporal symmetry) and space (stride length symmetry). For unilateral injuries, gait patterns often become asymmetric, where a person or animal may be hesitant to shift weight to an injured limb. For unilateral right limb injuries, the left-to-right timing is delayed (hesitant to apply weight) while the right-to-left timing is more rapid (rapid removal of applied weight). This can be represented by temporal symmetry measures significantly greater than 0.5. Similarly, left limb injuries tend to cause temporal symmetry measures significantly less than 0.5. Like temporal asymmetry, stride length symmetry can describe unilateral compensations that may occur due to pain, but through the geometric space of the paw prints. For stride length symmetry (left step length divided by stride length), a stride length symmetry less than 0.5 tends to indicate left limb injury (left step length is less than 50 % of the stride length) and greater than 0.5 tends to indicate right limb injury. Like temporal imbalance, abnormalities in spatial gait parameters have been reported for a number of rodent models of knee OA [56, 16••, 18, 17••, 58, 57].
Stance time imbalance and temporal symmetry represent our group's preferred spatiotemporal gait descriptors for detecting unilateral gait compensations [19]. While these methods are consistent with the classic Hildebrand plot [35], other mathematical normalizations exist. For example, swing time ratios between the left and right limbs can be used to indicate unilateral compensations in rodents [59]. Swing time ratios are only an algebraic conversion of duty factor imbalance, as both variables indicate a preferential loading of one limb relative to its contralateral limb.
Kinematic and Dynamic Indicators of Unilateral Limb Injury
Differences in paw print intensity and area have also been used to indicate unilateral injury, where the pixel brightness or paw area of the injured foot-print will decrease relative to the contralateral limb. Paw print intensity is measured by assessing paw pixel brightness in systems like the CatWalk and is an indirect measure of pressure exerted by the paw on the floor [23]. Given the relationships between paw print intensity and limb loading, these unilateral compensations may be indicative of changes in limb loading that may occur due to movement-evoked pain. Though limb loading can be assessed more directly (see “Dynamic” section), changes in paw print intensity have been seen in a carrageenan-induced monoarthritis model [54, 33], nerve injury models [23, 60], and an ACLT model of OA [61••].
Spatiotemporal Indications of Bilateral Limb Injury
In bilateral injury, both limbs in the forelimb or hindlimb set are injured; thus the contralateral limb cannot completely compensate for an injury. As such, imbalance and asymmetries may not occur for bilateral injury; instead, bilateral injuries may be detected through altered gait patterns at a given velocity. These bilateral gait compensations are common in transgenic OA models [20••, 62] and in rheumatoid arthritis models [63, 28, 64].
Most gait parameters have moderate to strong correlations to velocity, and failure to accurately account for the velocity covariate significantly reduces the ability to detect bilateral compensations (see “Velocity Covariate” section). As an example, walking velocities are a function of stride length and stride frequency; in other words, a specific velocity can be achieved with long, less frequent strides or shorter, rapid strides. For bilateral injuries, shorter, more frequent steps are often observed. This ‘shuffle stepping’ gait reduces the period of time a limb must bear weight without contralateral support. In bipedal humans, this compensation reduces the ‘single-limb support’ phase for both limbs, and is common for bilateral injuries such as low back pain [65, 66]. Conceptually, the same is achieved in bilateral injuries in quadrupeds, though ‘double/single-limb support’ terminology is not commonly applied to quadrupedal gait sequences.
In bilateral injuries, stride length will typically decrease at a given velocity, corresponding to an increase in stride frequency at the same velocity. Note that these changes are not independent, since velocity is approximately equal to stride length multiplied by stride frequency. In addition, limb duty factors increase at a given velocity. By increasing the percentage of time spent on both hind limbs, the percentage of time a single hind limb must bear weight without contralateral support is reduced. These bilateral compensations have been reported in collagen IX knockout mice [20••].
Changes in Stability and Dynamic Limb Loading
A more stable gait provides an animal greater opportunity to protect an injured limb from painful motions. Spatiotemporal gait data can indirectly measure some aspects of gait stability, where wider step widths and larger toe-out angles generally indicate a compensatory gait pattern used to overcome instability [67–69]. Our group has observed comparable changes in a collagen IX knockout mouse with ubiquitous cartilage degeneration in its knees and lumbar spine [20••]. In bilateral injury, decreased peak vertical forces and decreased propulsive forces would be anticipated for both hind limbs. However, to our knowledge, ground reaction forces have not been investigated in a rodent model of bilateral joint injury.
Considerations for Rodent Gait Characterization
As can be seen from the parameters described above, the quantity of data generated from gait analysis can be burdensome, given the interactions between different gait parameters. Clearly, most spatiotemporal, kinematic, and dynamic gait data are closely intertwined, whereby a shift in one variable inherently causes shifts in other variables. Thus, a single compensatory gait sequence will likely be reflected by changes in multiple gait variables. Distilling gait variables to the most salient findings is an important process in streamlining the analysis of an experiment. This alone can be challenging for investigators unfamiliar with gait analysis. Also challenging is the lack of uniformity among different gait analysis platforms. As such, for comparisons between relevant studies, a solid conceptual understanding of the relationships between different gait variables is important for a thorough and accurate analysis of gait compensations in a rodent OA model. Beyond distilling gait variables to the most salient findings, several additional factors should be taken into consideration when selecting methods to assess rodent gait.
Velocity Covariate
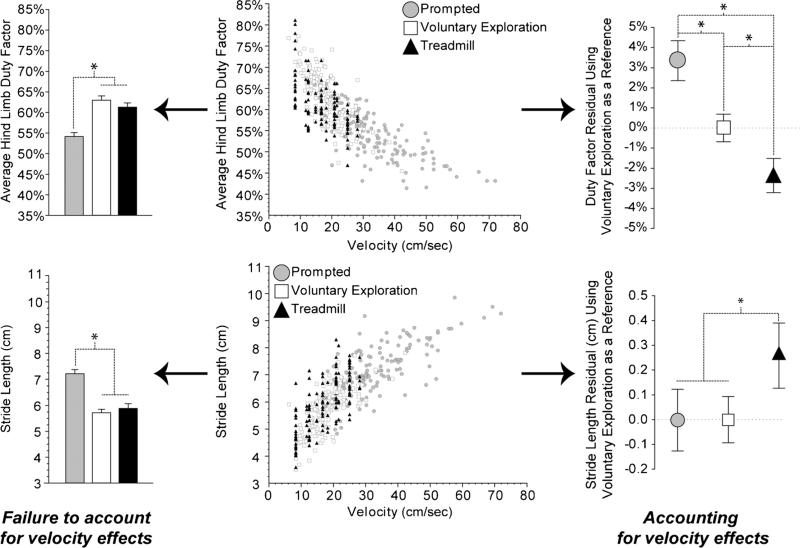
The vast majority of gait variables correlate with velocity (Fig. 3). Even spatial and temporal symmetry, which are relatively consistent over walking speeds, shift to asymmetric sequences as a quadrupedal animal approaches running/bounding velocities [70, 35]. Inability to accurately measure velocity increases the variability observed in many spatial measures. Even within a trial, gait parameters in an animal can shift due to acceleration or deceleration. As an example, while some spatial variables can be assessed using inkpad-based foot-printing methods [13], assessment of velocity with these methods tends to be relatively imprecise. In our hands, we were unable to detect velocity-influenced gait abnormalities in collagen IX knockout mice using an inkpad, but were able to detect gait abnormalities in the same mice using high-speed videography [20••]. This was largely attributed to the advantage of high-speed videographic techniques, where walking velocity can be easily and accurately assessed in conjunction with spatiotemporal parameters. Even with an accurate assessment of velocity, the velocity covariate must be accounted for in statistical models by normalizing for velocity effects or through statistical techniques like analysis of covariance (ANCOVA) or generalized linear regression models (GLRM). Alternatively, walking velocity can be controlled via a treadmill, reducing the variability of rodent walking speed seen during voluntary exploration [26•]. Selection of overground vs. treadmill gait characterization is an important consideration in its own right [71], and is covered in the “Gait Environment” subsection; nonetheless, high-speed videography does provide the ability to account for the velocity correlate in subsequent statistical analyses.
Fig. 3.
The effect of velocity on the spatiotemporal gait characteristics of rodents. The vast majority of gait variables correlate to velocity, and failure to account for velocity effects can significantly affect the statistical analysis of data. Shown are the average hind limb duty factor and stride length for naïve, healthy C57Bl/6 mice (n=25, 5 trials per animal per gait environment). Data were collected at Duke University in 2008–2009 on IACUC-approved protocols with techniques adhering to AAALAC ethical guidelines . The center graphs indicate that both duty factor and stride length have strong correlations to velocity. Statistical analyses ignoring this covariate are shown on the left, whereas statistical analyses that account for velocity are shown on the right. As can be seen from this demonstration, prompted gaits would appear to be very different from those of voluntary exploration or the treadmill if the velocity covariate was ignored, but this difference is largely due to the fact that prompted gaits occurred at higher velocities. If velocity effects are accounted for in the analysis, the stride length is very similar between prompted gait and voluntary exploration, but tends to be slightly longer on treadmills (≈0.3 cm longer at a given velocity). Similarly, duty factor differences among all three groups occur if velocity effects are considered. Here, duty factor tended to be shorter on the treadmill and longer for prompted gaits relative to voluntary exploration. Closer inspection of the raw data reveals a deviation from a linear correlate near duty factors of 50 %. Since a duty factor of less than 50 % would be defined as running, this implies that a transition between walking and running gaits is likely the driver of duty factor differences between voluntary exploration and prompted gaits (walking-to-running transition)
Size and Age
In addition to velocity, the size and age of the animal can affect gait characteristics . Therefore, when gait analysis is performed across cohorts, naïve age- and weight-matched subjects can help account for general differences in gait characteristics due to size and age. For many transgenic OA models, however, age- and weight-matched controls may not be available due to the effects of the genetic mutation. Consequently, it can be challenging to decipher the effects of a genetic mutation from the side effects of a change in animal size. Appropriate controls will ultimately depend on the transgenic model and hypotheses being tested, but understanding the expected effects of age and size on rodent gait parameters can assist researchers in the evaluation of their experimental design.
Gait Environment
The variance between overground- and treadmill-based gaits can be significant. Figure 3 shows the gait characteristics of 3-month-old wild-type C57Bl/6 mice for voluntary overground exploration, prompted overground movements, and treadmill trials, with the same animals recorded in each environment. At the same velocity, the spatio-temporal gait pattern varies significantly. As such, studies using treadmill systems may not directly compare to studies using overground systems. However, it is not yet clear whether one environment is preferable to the other, and this will likely depend on the OA model. Treadmills simplify the statistical analysis by reducing the effects of the velocity covariate. However, they also eliminate the ability to “self-select” walking velocity, a potentially powerful measure of gait compensations [36, 20••, 33, 22].
In addition to the differences between treadmill and overground gait sequences, interactions with the researcher can also affect rodent behaviors [72, 73]. During voluntary overground exploration, animals freely explore an open arena with minimal researcher interaction, allowing the animal to select its walking velocity and gait sequence. Prompted trials occur when the researcher brushes the animal's hind quarters with a swab or stick to trigger a rapid scurry across the gait arena. These two types of trials can vary significantly, where prompted gaits tend to have more rapid velocities, often approaching running (Fig. 3). More importantly, prompting movements may trigger ‘flight instincts’ in the animal, reducing self-selected gait compensations seen during voluntary exploration [20••]. Noxious light may also be used to prompt movement [74], though its effects on gait selection in rodents are currently unknown. Similarly, the effects of a positive cue on gait sequences, such as food reward or return to a home cage [75], are largely unknown, and it is not yet clear whether positive cues will alter the sensitivity of gait compensations relative to voluntary exploration.
Stride Length, Step Length, and Stride Length Symmetry
The distinction between stride length and step length is important, and unfortunately, a common pitfall in the analysis of spatio-temporal data is the comparison of left and right limb stride lengths [76]. This comparison may seem instinctive, but left and right limb stride lengths must be approximately equal if the animal is walking in a straight line. If the left and right stride lengths are consistently different, the animal will turn in a circle or the gait sequence will not be repeatable (a lack of synchronicity will ultimately result in a period of time where neither left nor right limb can support the body). While stride length cannot vary between limbs, step lengths can [77], as shown in Fig. 1. As discussed above, this can be described through stride length symmetry or other permutations of step length differences.
Recording Speed
The gait sequence of rodents is very rapid, with a foot-strike event occurring about every 0.4 seconds for both mice and rats. At a recording speed of 100 frames per second, which is the most common speed for currently-available commercial systems, this average gait cycle would be represented by approximately 40 frames. The stance phase of the gait cycle will be approximately two-thirds of total gait cycle, or 26–27 video frames; thus, a shift in stance time of one frame would represent about a 3.8 % increase or decrease in limb duty factor. While this may seem to be a fairly low number, it is important to note that this is not a percentage change in limb duty factor, but the raw shift. For OA patients, limb duty factor shifts are often only 1–2 %. Since duty factors group tightly between 50–80 % for the rodent gait cycle (see Fig. 3), this is a major limitation to a gait platform's sensitivity. Moreover, detection of a 1 frame shift is an ideal situation for automated digitization; automation errors only further reduce the sensitivity of a technique. This nuance is very important in rodent OA models, where behavioral changes are often very subtle. Using recording speeds of 200 frames per second and by-hand digitization, we were able to detect ~2 % shifts in a medial meniscus tear model of knee OA in the rat [17••], but this subtle abnormality was unlikely to be detected at a lower speed. Even at 200 frames per second, the ability to detect a drug effect is limited by the recording speed. Similar sensitivity issues can occur with recording resolution, though resolution will ultimately depend on the distance between the camera and arena and the distortion caused by the camera lens. In short, while technologies for rodent gait analysis have greatly advanced, significant technological issues can still affect the sensitivity and resolution of the techniques.
Skin Markers
As in humans, skin markers have been used to describe the 3-D motion of a rat [75, 78]. These markers are used to approximate kinematic variables such as joint position and joint angles. However, copious loose skin on rodents, combined with large leg muscles such as the gluteus superficialis and biceps femoris, complicate the consistent placement of markers on the skin. Even with good marker placement, work by Bauman and Chang [51•] demonstrated that skin markers poorly represented the actual position of bones. As such, a few groups have recently advanced fluoroscopic techniques to track rodent limb positions [51•, 79, 80•, 81••, 82]. However, the recording speeds necessary to track rodent limbs generally exceed the recording speeds of most commercial fluoroscopy systems (≈30 frames per second). Therefore, accurate assessment of joint angles in time currently requires sophisticated, custom-built fluoroscopic systems.
Time Course for the Development of Chronic Pain
One motivation for using rodent models to study OA is the accelerated progression of the disease in rodents relative to humans. Often, OA studies in rodents are limited to 4–8 weeks, where significant cartilage lesion formations are seen by this time. However, this time frame may not represent the development of chronic pain. Recent studies have shown significant changes in pain-related behaviors far beyond the traditional endpoints for rodent OA models, lengthening the scale to 4– 6 months [61••, 83, 84]. Thus, it may be prudent to commit to carrying out rodent OA studies to these later time points, especially in the context of chronic pain and disability.
Conclusions
The assessment of pain and disability is essential for the clinical management of OA, yet in preclinical rodent models, behavioral measures of pain and disability have only recently become popular in the assessment of the consequences of OA. Gait analysis, in particular, has seen more widespread use in rodent preclinical models over the last decade and has demonstrated utility for the behavioral characterization of rodent OA models. As described in this review, a number of gait parameters can be generated through gait analysis, but the majority of these data are associated or correlated in some fashion. A fundamental understanding of the relationships between different gait parameters can assist researchers in identifying gait abnormalities that are indicative of unilateral or bilateral compensation. Moreover, understanding the limitations of current techniques is critical for the continued advancement of the field. While the novel techniques developed recently represent significant technological improvements over inkpad-based methods, these advances still have significant limitations that can affect the sensitivity and specificity of the generated gait parameters. Nonetheless, assessing changes in disease sequelae in rodent models of OA will help to develop a more fundamental understanding of the mechanisms and links between OA pathogenesis and OA-related pain and disability.
Acknowledgments
This publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health under award numbers K99/ R00AR057426. Data presented in Fig. 3 were collected during fellowship support for KDA in 2008-09 (F32AR056190). These data have not been previously published and were collected under IACUC-approved protocols at Duke University. Besides providing funds, the funding sources did not participate in collection, analysis, or interpretation of these data.
Footnotes
Author Contributions KDA is primarily responsible for the discussion points within this review. BYJ conducted the literature review and drafted the manuscript with KDA. HEK created the figures with the assistance of KDA. All authors have approved the final version.
Compliance with Ethics Guidelines
Conflict of Interest Dr. Brittany Y. Jacobs, Dr. Heidi E. Kloefkorn-Adams, and Dr. Kyle D. Allen report grants from National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and are currently developing gait analysis technologies for rodent preclinical models, including a device for characterization of dynamic characteristics and a software package for spatiotemporal characteristics. At the time of this publication, there are no commercial plans for these technologies.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors for the purposes of this review article.
Contributor Information
Brittany Y. Jacobs, J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, PO Box 116131, Gainesville, FL 32611, USA
Heidi E. Kloefkorn, J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, PO Box 116131, Gainesville, FL 32611, USA
Kyle D. Allen, J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, PO Box 116131, Gainesville, FL 32611, USA Institute for Cell Engineering and Regenerative Medicine, University of Florida, PO Box 116131, Gainesville, FL 32611, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Creamer P. Osteoarthritis pain and its treatment. Curr Opin Rheumatol. 2000;12(5):450–5. doi: 10.1097/00002281-200009000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Bendele AM. Animal models of osteoarthritis in an era of molecular biology. J Musculoskelet Neuronal Interact. 2002;2(6):501–3. [PubMed] [Google Scholar]
- 3.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil / OARS Osteoarthr Res Soc. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. doi:10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Bedson J, Croft PR. The discordance between clinical and radio-graphic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. doi:10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochberg MC. We Should Treat Pain! Osteoarthr Cartil. 2013;21:S6. [Google Scholar]
- 6.Conrad BP, Shokat MS, Abbasi AZ, Vincent HK, Seay A, Kennedy DJ. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38(4):987–92. doi: 10.1016/j.gaitpost.2013.05.010. doi:10.1016/j.gaitpost.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 8.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kean WF, Kean R, Buchanan WW. Osteoarthritis: symptoms, signs and source of pain. Inflammopharmacology. 2004;12(1):3–31. doi: 10.1163/156856004773121347. doi: 10.1163/156856004773121347. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT. The sources of pain in knee osteoarthritis. Curr Opin Rheumatol. 2005;17(5):624–8. doi: 10.1097/01.bor.0000172800.49120.97. [DOI] [PubMed] [Google Scholar]
- 11.Fairbank JCT, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940–52. doi: 10.1097/00007632-200011150-00017. doi:10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 12.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151(1):12–7. doi: 10.1016/j.pain.2010.07.015. doi:10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Crawley JN. What's wrong with my mouse? : behavioral pheno-typing of transgenic and knockout mice. 2nd ed. Wiley-Interscience; Hoboken: 2007. [Google Scholar]
- 14.Whishaw IQ, Kolb B. The behavior of the laboratory rat : a handbook with tests. Oxford University Press; Oxford. New York: 2005. [Google Scholar]
- 15.Combe R, Bramwell S, Field MJ. Neurosci Lett. 2–3. Vol. 370. 236–40: 2004. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? doi:10.1016/j.neulet.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 16••.Ferland CE, Laverty S, Beaudry F, Vachon P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol Biochem Behav. 2011;97(3):603–10. doi: 10.1016/j.pbb.2010.11.003. doi:10.1016/j.pbb.2010.11.003. [This paper describes gait abnormalities in the rat due to ACL transection with medial menisectomy as well as monoiodoacetate injection. In addition, the work shows that pain-relieving therapeutics can reverse some gait abnormalities in rodent preclinical models.] [DOI] [PubMed] [Google Scholar]
- 17••.Allen KD, Mata BA, Gabr MA, Huebner JL, Adams SB, Jr, Kraus VB, et al. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res Ther. 2012;14(2):R78. doi: 10.1186/ar3801. doi:10.1186/ar3801. [This paper describes both spatiotemporal and 3-component ground reaction force modifications due to surgical simulation of a radial meniscus tear in the rat.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen KD, Adams SB, Jr, Mata BA, Shamji MF, Gouze E, Jing L, et al. Gait and behavior in an IL1beta-mediated model of rat knee arthritis and effects of an IL1 antagonist. J Orthop Res Off Publ Orthop Res Soc. 2011;29(5):694–703. doi: 10.1002/jor.21309. doi:10.1002/jor.21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen KD, Adams SB, Setton LA. Evaluating intra-articular drug delivery for the treatment of osteoarthritis in a rat model. Tissue Eng B Rev. 2010;16(1):81–92. doi: 10.1089/ten.teb.2009.0447. doi:10.1089/ten.teb.2009.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Allen KD, Griffin TM, Rodriguiz RM, Wetsel WC, Kraus VB, Huebner JL, et al. Decreased physical function and increased pain sensitivity in mice deficient for type IX collagen. Arthritis Rheum. 2009;60(9):2684–93. doi: 10.1002/art.24783. doi:10.1002/art.24783. [This work describes bilateral compensations for collagen IX knockout mice. Moreover, it demonstrates the importance of carefully describing gait analysis methods, as differences were found between inkpad and high-speed gait analysis methods.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60(3):840–7. doi: 10.1002/art.24304. doi:10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 22.Clarke KA, Heitmeyer SA, Smith AG, Taiwo YO. Gait analysis in a rat model of osteoarthrosis. Physiol Behav. 1997;62(5):951–4. doi: 10.1016/s0031-9384(97)00022-x. [DOI] [PubMed] [Google Scholar]
- 23.Vrinten DH, Hamers FF. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain. 2003;102(1–2):203–9. doi: 10.1016/s0304-3959(02)00382-2. [DOI] [PubMed] [Google Scholar]
- 24•.Gabriel AF, Marcus MA, Walenkamp GH, Joosten EA. The CatWalk method: assessment of mechanical allodynia in experimental chronic pain. Behav Brain Res. 2009;198(2):477–80. doi: 10.1016/j.bbr.2008.12.018. doi: 10.1016/j.bbr.2008.12.018. [This recent work represents the continued use of the CatWalk method to assess rodent gait characteristics as a surrogate measure of pain and disability.] [DOI] [PubMed] [Google Scholar]
- 25.Gabriel AF, Marcus MA, Honig WM, Walenkamp GH, Joosten EA. The CatWalk method: a detailed analysis of behavioral changes after acute inflammatory pain in the rat. J Neurosci Methods. 2007;163(1):9–16. doi: 10.1016/j.jneumeth.2007.02.003. doi:10.1016/j.jneumeth.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 26•.Berryman ER, Harris RL, Moalli M, Bagi CM. Digigait quantitation of gait dynamics in rat rheumatoid arthritis model. J Musculoskelet Neuronal Interact. 2009;9(2):89–98. [While this work describes a rheumatoid arthritis model rather than an osteoarthritis model, it describes gait modifications using a treadmill-based gait analysis system.] [PubMed] [Google Scholar]
- 27.Min SS, Han JS, Kim YI, Na HS, Yoon YW, Hong SK, et al. A novel method for convenient assessment of arthritic pain in voluntarily walking rats. Neurosci Lett. 2001;308(2):95–8. doi: 10.1016/s0304-3940(01)01983-8. doi:10.1016/S0304-3940(01)01983-8. [DOI] [PubMed] [Google Scholar]
- 28.Vincelette J, Xu Y, Zhang LN, Schaefer CJ, Vergona R, Sullivan ME, et al. Gait analysis in a murine model of collagen-induced arthritis. Arthritis Res Ther. 2007;9(6) doi: 10.1186/ar2331. doi:10.1186/Ar2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deumens R, Jaken RJP, Marcus MAE, Joosten EAJ. The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. J Neurosci Methods. 2007;164(1):120–30. doi: 10.1016/j.jneumeth.2007.04.009. doi:10.1016/j.jneumeth.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Khullar SM, Brodin P, Messelt EB, Haanaes HR. The Effects of Low-Level Laser Treatment on Recovery of Nerve-Conduction and Motor Function after Compression Injury in the Rat Sciatic-Nerve. Eur J Oral Sci. 1995;103(5):299–305. doi: 10.1111/j.1600-0722.1995.tb00030.x. doi:10.1111/j.1600-0722.1995.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 31.Hare GM, Evans PJ, Mackinnon SE, Best TJ, Midha R, Szalai JP, et al. Walking track analysis: utilization of individual footprint parameters. Ann Plast Surg. 1993;30(2):147–53. doi: 10.1097/00000637-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Brown CJ, Evans PJ, Mackinnon SE, Bain JR, Makino AP, Hunter DA, et al. Inter- and intraobserver reliability of walking-track analysis used to assess sciatic nerve function in rats. Microsurgery. 1991;12(2):76–9. doi: 10.1002/micr.1920120204. [DOI] [PubMed] [Google Scholar]
- 33.Coulthard P, Simjee SU, Pleuvry BJ. Gait analysis as a correlate of pain induced by carrageenan intraplantar injection. J Neurosci Methods. 2003;128(1–2):95–102. doi: 10.1016/s0165-0270(03)00154-7. doi:10.1016/S0165-0270(03)00154-7. [DOI] [PubMed] [Google Scholar]
- 34.Gregersen LS, Røsland T, Arendt-Nielsen L, Whiteside G, Hummel M. Unrestricted Weight Bearing as a Method for Assessment of Nociceptive Behavior in a Model of Tibiofemoral Osteoarthritis in Rats. J Behav Brain Sci. 2013;03(03):306–14. [Google Scholar]
- 35.Hildebrand M. The Quadrupedal Gaits of Vertebrates. Bioscience. 1989;39(11):766–75. doi:10.2307/1311182. [Google Scholar]
- 36.Parvathy SS, Masocha W. Gait analysis of C57BL/6 mice with complete Freund's adjuvant-induced arthritis using the CatWalk system. BMC Musculoskelet Disord. 2013;14:14. doi: 10.1186/1471-2474-14-14. doi:10.1186/1471-2474-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koopman B, Grootenboer HJ, Dejongh HJ. An Inverse Dynamics Model for the Analysis, Reconstruction and Prediction of Bipedal Walking. J Biomech. 1995;28(11):1369–76. doi: 10.1016/0021-9290(94)00185-7. doi:10.1016/0021-9290(94)00185-7. [DOI] [PubMed] [Google Scholar]
- 38.Alkjaer T, Simonsen EB, Dyhre-Poulsen F. Comparison of inverse dynamics calculated by two- and three-dimensional models during walking. Gait Posture. 2001;13(2):73–7. doi: 10.1016/s0966-6362(00)00099-0. doi:10.1016/S0966-6362(00)00099-0. [DOI] [PubMed] [Google Scholar]
- 39.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radio-graphic disease severity, toe out angle and pain. J Orthop Res Off Publ Orthop Res Soc. 2002;20(1):101–7. doi: 10.1016/S0736-0266(01)00081-X. doi:10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 40.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41(7):1233–40. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. doi:10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 41.Varejao AS, Cabrita AM, Meek MF, Bulas-Cruz J, Filipe VM, Gabriel RC, et al. Ankle kinematics to evaluate functional recovery in crushed rat sciatic nerve. Muscle Nerve. 2003;27(6):706–14. doi: 10.1002/mus.10374. doi: 10.1002/mus.10374. [DOI] [PubMed] [Google Scholar]
- 42.Liang JI, Chen MY, Hsieh TH, Liu CY, Lam CF, Chen JJ, et al. Video-based gait analysis for functional evaluation of healing achilles tendon in rats. Ann Biomed Eng. 2012;40(12):2532–40. doi: 10.1007/s10439-012-0619-z. doi:10.1007/s10439-012-0619-z. [DOI] [PubMed] [Google Scholar]
- 43.Lin FM, Pan YC, Hom C, Sabbahi M, Shenaq S. Ankle stance angle: a functional index for the evaluation of sciatic nerve recovery after complete transection. J Reconstr Microsurg. 1996;12(3):173–7. doi: 10.1055/s-2007-1006472. doi:10.1055/s-2007-1006472. [DOI] [PubMed] [Google Scholar]
- 44.Allen KD, Shamji MF, Mata BA, Gabr MA, Sinclair SM, Schmitt DO, et al. Kinematic and dynamic gait compensations in a rat model of lumbar radiculopathy and the effects of tumor necrosis factor-alpha antagonism. Arthritis Res Ther. 2011;13(4):R137. doi: 10.1186/ar3451. doi: 10.1186/ar3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch JM, Wade JA, Hillberry BM, Weaver CM. Force platform for rats measures fore and hind forces concurrently. J Biomech. 2009;42(16):2734–8. doi: 10.1016/j.jbiomech.2009.08.002. doi:10.1016/j.jbiomech.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Clarke KA, Still J. Gait analysis in the mouse. Physiol Behav. 1999;66(5):723–9. doi: 10.1016/s0031-9384(98)00343-6. [DOI] [PubMed] [Google Scholar]
- 47.Parker AJ, Clarke KA. Gait topography in rat locomotion. Physiol Behav. 1990;48(1):41–7. doi: 10.1016/0031-9384(90)90258-6. [DOI] [PubMed] [Google Scholar]
- 48.Varejao AS, Cabrita AM, Meek MF, Bulas-Cruz J, Gabriel RC, Filipe VM, et al. Motion of the foot and ankle during the stance phase in rats. Muscle Nerve. 2002;26(5):630–5. doi: 10.1002/mus.10242. doi:10.1002/mus.10242. [DOI] [PubMed] [Google Scholar]
- 49.Alluin O, Karimi-Abdolrezaee S, Delivet-Mongrain H, Leblond H, Fehlings MG, Rossignol S. Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. J Neurotrauma. 2011;28(9):1963–81. doi: 10.1089/neu.2011.1840. doi:10.1089/neu.2011.1840. [DOI] [PubMed] [Google Scholar]
- 50.Clarke KA. Swing time changes contribute to stride time adjustment in the walking rat. Physiol Behav. 1991;50(6):1261–2. doi: 10.1016/0031-9384(91)90593-d. [DOI] [PubMed] [Google Scholar]
- 51•.Bauman JM, Chang YH. High-speed X-ray video demonstrates significant skin movement errors with standard optical kinematics during rat locomotion. J Neurosci Methods. 2010;186(1):18–24. doi: 10.1016/j.jneumeth.2009.10.017. doi:10.1016/j.jneumeth.2009.10.017. [This work eloquently describes the challenges with skin-based marker systems in rats.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howard CS, Blakeney DC, Medige J, Moy OJ, Peimer CA. Functional assessment in the rat by ground reaction forces. J Biomech. 2000;33(6):751–7. doi: 10.1016/s0021-9290(00)00023-3. [DOI] [PubMed] [Google Scholar]
- 53••.Jay GD, Elsaid KA, Kelly KA, Anderson SC, Zhang L, Teeple E, et al. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2012;64(4):1162–71. doi: 10.1002/art.33461. doi: 10.1002/art.33461. [This work describes the use of a pressure-mapping system to investigate weight-bearing imbalances during locomotion in the rat. Moreover, this behavioral system is used to evaluate a treatment effect for tribosupplementation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angeby-Moller K, Berge OG, Hamers FP. Using the CatWalk method to assess weight-bearing and pain behaviour in walking rats with ankle joint monoarthritis induced by carrageenan: effects of morphine and rofecoxib. J Neurosci Methods. 2008;174(1):1–9. doi: 10.1016/j.jneumeth.2008.06.017. doi:10.1016/j.jneumeth.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Piesla MJ, Leventhal L, Strassle BW, Harrison JE, Cummons TA, Lu P, et al. Abnormal gait, due to inflammation but not nerve injury, reflects enhanced nociception in preclinical pain models. Brain Res. 2009;1295:89–98. doi: 10.1016/j.brainres.2009.07.091. doi:10.1016/j.brainres.2009.07.091. [DOI] [PubMed] [Google Scholar]
- 56.Ferland CE, Beaudry F, Vachon P. Antinociceptive effects of eugenol evaluated in a monoiodoacetate-induced osteoarthritis rat model. Phytother Res PTR. 2012;26(9):1278–85. doi: 10.1002/ptr.3725. doi:10.1002/ptr.3725. [DOI] [PubMed] [Google Scholar]
- 57.Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, et al. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett. 2011;493(3):72–5. doi: 10.1016/j.neulet.2011.01.027. doi:10.1016/j.neulet.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62(8):2382–91. doi: 10.1002/art.27550. doi:10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orito K, Kurozumi S, Ishii I, Tanaka A, Sawada J, Matsuda H. A sensitive gait parameter for quantification of arthritis in rats. J Pharmacol Sci. 2007;103(1):113–6. doi: 10.1254/jphs.sc0060156. doi:10.1254/jphs.SC0060156. [DOI] [PubMed] [Google Scholar]
- 60.Bozkurt A, Deumens R, Scheffel J, O'Dey DM, Weis J, Joosten EA, et al. CatWalk gait analysis in assessment of functional recovery after sciatic nerve injury. J Neurosci Methods. 2008;173(1):91–8. doi: 10.1016/j.jneumeth.2008.05.020. doi:10.1016/j.jneumeth.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 61••.Fu SC, Cheuk YC, Hung LK, Chan KM. Limb Idleness Index (LII): a novel measurement of pain in a rat model of osteoarthritis. Osteoarthr Cartil / OARS Osteoarthr Res Soc. 2012;20(11):1409–16. doi: 10.1016/j.joca.2012.08.006. doi:10.1016/j.joca.2012.08.006. [This work describes gait abnormalities out to 6 months after ACLT in the rat, representing a potential shift to chronic pain and disability.] [DOI] [PubMed] [Google Scholar]
- 62.Poulet B, de Souza R, Knights C, Gentry C, Wilson A, Bevan S, et al. Modifications in gait as predictors of natural osteoarthritis progression in Str/ort mice. Arthritis Rheumatol. 2014 doi: 10.1002/art.38616. doi: 10.1002/art.38616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffmann MH, Hopf R, Niederreiter B, Redl H, Smolen JS, Steiner G. Gait changes precede overt arthritis and strongly correlate with symptoms and histopathological events in pristane-induced arthritis. Arthritis Res Ther. 2010;12(2):R41. doi: 10.1186/ar2950. doi:10.1186/ar2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hartog A, Hulsman J, Garssen J. Locomotion and muscle mass measures in a murine model of collagen-induced arthritis. BMC Musculoskelet Disord. 2009;10:59. doi: 10.1186/1471-2474-10-59. doi:10.1186/1471-2474-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vincent HK, Seay AN, Montero C, Conrad BP, Hurley RW, Vincent KR. Functional Pain Severity and Mobility in Overweight Older Men and Women with Chronic Low-Back Pain-Part I. Am J Phys Med Rehabil. 2013;92(5):430–8. doi: 10.1097/PHM.0b013e31828763a0. doi:10.1097/Phm.0b013e31828763a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elbaz A, Mirovsky Y, Mor A, Enosh S, Debbi E, Segal G, et al. A Novel Biomechanical Device Improves Gait Pattern in Patient With Chronic Nonspecific Low Back Pain. Spine. 2009;34(15):E507–12. doi: 10.1097/BRS.0b013e3181a98d3f. doi:10.1097/Brs.0b013e3181a98d3f. [DOI] [PubMed] [Google Scholar]
- 67.Young PMM, Dingwell JB. Voluntarily changing step length or step width affects dynamic stability of human walking. Gait Posture. 2012;35(3):472–7. doi: 10.1016/j.gaitpost.2011.11.010. doi:10.1016/j.gaitpost.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jenkyn TR, Hunt MA, Jones IC, Giffin JR, Birmingham TB. Toeout gait in patients with knee osteoarthritis partially transforms external knee adduction moment into flexion moment during early stance phase of gait: A tri-planar kinetic mechanism. J Biomech. 2008;41(2):276–83. doi: 10.1016/j.jbiomech.2007.09.015. doi:10.1016/j.jbiomech.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 69.Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech. 2000;33(11):1433–40. doi: 10.1016/s0021-9290(00)00101-9. doi:10.1016/S0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 70.Nanua P, Waldron KJ. Energy comparison between trot, bound, and gallop using a simple model. J Biomech Eng-TAsme. 1995;117(4):466–73. doi: 10.1115/1.2794209. doi:10.1115/1.2794209. [DOI] [PubMed] [Google Scholar]
- 71.Pereira JE, Cabrita AM, Filipe VM, Bulas-Cruz J, Couto PA, Melo-Pinto P, et al. A comparison analysis of hindlimb kinematics during overground and treadmill locomotion in rats. Behav Brain Res. 2006;172(2):212–8. doi: 10.1016/j.bbr.2006.04.027. doi:10.1016/j.bbr.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 72.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11(6):629–32. doi: 10.1038/nmeth.2935. doi:10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 73.Lau D, Harte SE, Morrow TJ, Wang SY, Mata M, Fink DJ. Herpes Simplex Virus Vector-Mediated Expression of Interleukin-10 Reduces Below-Level Central Neuropathic Pain After Spinal Cord Injury. Neurorehabil Neural Repair. 2012;26(7):889–97. doi: 10.1177/1545968312445637. doi: 10.1177/1545968312445637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlton S, Zhou S. A novel operant method to test acute heat hypersensitivity in mice using a modification of the Coy Operant Mechanical Conflict Avoidance System. J Pain. 2013;14(4):S43. [Google Scholar]
- 75.Webb AA, Kerr B, Neville T, Ngan S, Assem H. Kinematics and ground reaction force determination: a demonstration quantifying locomotor abilities of young adult, middle-aged, and geriatric rats. J Vis Exp JoVE. 2011;(48) doi: 10.3791/2138. doi:10.3791/ 2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olmarker K, Iwabuchi M, Larsson K, Rydevik B. Walking analysis of rats subjected to experimental disc herniation. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deformity Soc Eur Sect Cervical Spine Res Soc. 1998;7(5):394–9. doi: 10.1007/s005860050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roerdink M, Beek PJ. Understanding inconsistent step-length asymmetries across hemiplegic stroke patients: impairments and compensatory gait. Neurorehabil Neural Repair. 2011;25(3):253–8. doi: 10.1177/1545968310380687. doi:10.1177/1545968310380687. [DOI] [PubMed] [Google Scholar]
- 78.Filipe VM, Pereira JE, Costa LM, Mauricio AC, Couto PA, Melo-Pinto P, et al. Effect of skin movement on the analysis of hindlimb kinematics during treadmill locomotion in rats. J Neurosci Methods. 2006;153(1):55–61. doi: 10.1016/j.jneumeth.2005.10.006. doi:10.1016/j.jneumeth.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 79.Boettger MK, Leuchtweis J, Schaible HG, Schmidt M. Videoradiographic analysis of the range of motion in unilateral experimental knee joint arthritis in rats. Arthritis Res Ther. 2011;13(3) doi: 10.1186/ar3342. doi: 10.1186/Ar3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80•.Histing T, Kristen A, Roth C, Holstein JH, Garcia P, Matthys R, et al. In vivo gait analysis in a mouse femur fracture model. J Biomech. 2010;43(16):3240–3. doi: 10.1016/j.jbiomech.2010.07.019. doi:10.1016/j.jbiomech.2010.07.019. [This work describes new methods to detect joint kinematics in a mouse on a running wheel.] [DOI] [PubMed] [Google Scholar]
- 81••.Wehner T, Wolfram U, Henzler T, Niemeyer F, Claes L, Simon U. Internal forces and moments in the femur of the rat during gait. J Biomech. 2010;43(13):2473–9. doi: 10.1016/j.jbiomech.2010.05.028. doi:10.1016/j.jbiomech.2010.05. 028. [This work demonstrates the potential to investigate internal forces and moments in rats using advanced biomechanical models.] [DOI] [PubMed] [Google Scholar]
- 82.Gravel P, Tremblay M, Leblond H, Rossignol S, de Guise JA. A semi-automated software tool to study treadmill locomotion in the rat: From experiment videos to statistical gait analysis. J Neurosci Methods. 2010;190(2):279–88. doi: 10.1016/j.jneumeth.2010.05.006. doi:10.1016/j.jneumeth.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 83.Miller RE, Tran PB, Ishihara S, Larkin J, Malfait AM. Therapeutic Efficacy of Anti-Adamts5 Antibody in the Dmm Model. Osteoarthr Cartil. 2014;22:S35–6. [Google Scholar]
- 84.Miller RE, Zaki S, Ishihara S, Tran PB, Little CB, Malfait AM. Establishing a Method for Measuring Primary Knee Hyperalgesia in the Murine Dmm Model of Osteoarthritis. Osteoarthr Cartil. 2014;22:S419. [Google Scholar]