Abstract
BACKGROUND
The authors evaluated the utility of immunofluorescence staining with an antipromyelocytic leukemia (anti-PML) antibody for patients with a suspected diagnosis of new or relapsed acute promyelocytic leukemia (APL) and correlated the findings with the results of other established diagnostic modalities.
METHODS
Bone marrow (BM) and/or peripheral blood (PB) smears from 349 patients in whom the diagnosis of APL was considered were assessed with the anti-PML antibody using immunofluorescence. The study group included 199 patients with confirmed APL and 150 with other conditions. The results of conventional cytogenetics, reverse transcription polymerase chain reaction (RT-PCR), and fluorescence in situ hybridization (FISH) performed on these patients were correlated with the PML results.
RESULTS
Among patients with confirmed APL, anti-PML antibody was positive in 182 of 184 BM and 32 of 33 PB smears. Conventional cytogenetics demonstrated t(15;17)(q22;q12) in 166 of 182 (91%) patients; 10 had a normal karyotype, 4 had insufficient mitoses to grow in culture, 1 was inconclusive, and 1 was 48, XX, +8, +8. Anti-PML staining was positive in 9 of 10 with a normal karyotype and in all 4 cases with insufficient mitoses. RT-PCR and FISH were positive for PML–retinoic acid receptor-α in 169 of 172 (98%) and 90 of 94 (96%) cases, respectively. Among the patients without APL, 148 of 150 (98.6%) were negative with anti-PML antibody. The sensitivity and specificity of the test were 98.9% and 98.7%, respectively.
CONCLUSIONS
PML immunofluorescence staining is a rapid (<4 hours turnaround time) and reliable frontline diagnostic approach that can facilitate initiation of targeted therapy, particularly in clinical settings where cytogenetic and molecular testing are not readily available.
Keywords: acute promyelocytic leukemia, rapid diagnosis, promyelocytic leukemia oncogenic domain, immunofluorescence
Acute promyelocytic leukemia (APL) is characterized by fusion of the PML (promyelocytic leukemia) and RARα (retinoic acid receptor-α) genes, leading to expression of PML-RARα protein. This abnormality is fundamentally important in the pathogenesis of the disease.1,2 The availability of highly effective therapy, capable of preventing the development of lethal hemorrhagic complications when instituted promptly, makes rendering an accurate and timely diagnosis of APL essential.3 However, morphologic diagnosis of APL can be problematic, as there can be substantial variation in the morphologic appearance of the promyelocytes in this disease. Thus, ancillary testing is required to confirm the diagnosis.
Immunophenotypic analysis by flow cytometry can validate the initial morphologic impression. However, preset analysis gates can be misleading, because of the finding that the variable size and granularity of the neoplastic promyelocytes make their light scatter characteristics different from those of blasts in other types of acute myeloid leukemia (AML). Although the neoplastic promyelocytes commonly lack expression of CD34 and human leukocyte antigen-DR (HLA-DR), this is not the case for the microgranular variant (M3v), and this immunophenotypic heterogeneity can lead to diagnostic difficulties.1,4
Conventional cytogenetic analysis is an excellent method for detecting the t(15;17)(q22;q12) in APL; however, this approach has a longer turnaround time than is necessary to make rapid treatment decisions. Fluorescence in situ hybridization (FISH) and reverse transcription polymerase chain reaction (RT-PCR) are 2 popular ancillary methods to demonstrate PML-RARα with high sensitivity and specificity and with turnaround time shorter than that of conventional cytogenetics.1,4 However, performance and interpretation of these tests are done in specialized laboratories by highly skilled personnel, and they are not commonly available in the community setting for the initial workup of patients with suspected APL. In addition, both false-positive and false-negative RT-PCR results are known to occur because of contamination and suboptimal RNA quality.
Another approach for diagnosing APL is immunofluorescence staining for PML protein.5,6 PML is involved in regulation of cell growth and apoptosis, and a role for PML as an antioncogene in APL has been suggested.7 The PML protein is crucial for the formation of PML nuclear bodies, also known as PML oncogenic domains, where it interacts with proteins, such as p53, Daxx, Sp100, and pRb.8,9 By using the PML stain, PML oncogenic domains can be observed as 5 to 30 intranuclear particles in normal cells.10 By contrast, in APL PML-RARα forms heterodimers with the wild-type PML and prevents the formation of the PML oncogenic domains. This aberrancy in APL promyelocytes can be observed with anti-PML antibodies as a microgranular nuclear pattern of staining—a finding that is easily detectable microscopically and can be used as a surrogate for genetic testing.3,11,12
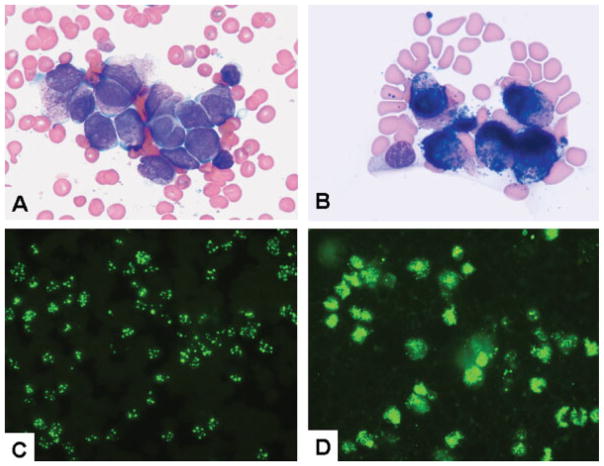
Rapid initiation of therapy with all-trans-retinoic acid (ATRA) is crucial to the successful management of APL, and therefore a rapid and reliable diagnostic method is desirable. Several investigators have suggested routine use of an anti-PML stain, and small studies using this approach are available.1,5,13,14 However, a large comprehensive study including the complete spectrum of APL cases and their mimics has not been performed. The goal of this study was to determine the utility of PML immunofluorescence staining in a large group of patients with APL (Fig. 1A and B) and other leukemias that are part of the differential diagnosis. In addition, we evaluated the utility of a noncommercially available anti-PML antibody, developed by Kun-Sang Chan at our institution. Our results show that PML staining compares favorably with other diagnostic approaches and has the advantages of speed and convenience. PML staining can be particularly advantageous in clinical settings, such as community hospitals, where other methods for detection of PML-RARα are not readily available.
Figure 1.
(A) A bone marrow aspirate smear shows many promyelocytes. Compared with the adjacent normal small lymphocytes, promyelocytes are of medium to large size with irregularly shaped to convoluted to bilobed nuclei, open chromatin, visible to prominent nucleoli, and a moderate amount of basophilic cytoplasm with abundant granularity. (B) Cytochemical stain for myeloperoxidase is shown. Compared with the negative small lymphocyte, the promyelocytes are strongly positive, with numerous granules covering the outlines of the nuclei. (C) Negative (macrogranular) immunofluorescent stain for promyelocytic leukemia (PML) is shown. The PML oncogenic domains are observed as several distinct particles in each nucleus. (D) Positive (microgranular) immunofluorescent stain for PML is shown. Numerous (too many to count) fine dusty granules are present in each nucleus.
MATERIALS AND METHODS
Patients
This study spans the time interval between January 1996 and November 2008 and includes 349 cases accessioned in the Department of Hematopathology at The University of Texas M. D. Anderson Cancer Center, in which the diagnosis APL was considered. All human subjects were included in accordance with the Helsinki Declaration, and this study was approved by the institutional review board of our institution. All cases were classified using the criteria of the World Health Organization and French-American-British classifications.
Study Group
The study group included 199 patients with APL and 150 patients with other diagnoses. The latter group included 67 patients with AML with maturation (M2); 24 with AML without maturation (M1); 18 with AML, not otherwise specified; 11 with acute myelomonocytic leukemia (M4); 11 with AML with t(8;21)(q22;q22); 2 with AML with inv(16)(p13.1q22); 2 with AML and multilineage dysplasia; 2 with therapy-related AML; 2 with chronic myelomonocytic leukemia (1 of which was in transformation to AML); 2 with chronic myelogenous leukemia (1 chronic phase and 1 blast phase); 1 with acute monoblastic leukemia (M5); 1 with acute erythroleukemia (M6); 1 with myelodysplastic syndrome (refractory anemia with excess blasts-1); 1 with precursor B-cell lymphoblastic leukemia, 1 with chronic lymphocytic leukemia, and 4 normal bone marrow (BM) smears.
The APL group included 109 men and 90 women ranging in age from 1 to 81 years. Among them, 21 patients (12 men and 9 women) had therapy-related APL. For the purpose of the study, conventional cytogenetic analysis, FISH, or RT-PCR, showing evidence of the t(15;17)(q22;q12) or PML-RARα fusion, were required for the diagnosis of APL. One hundred seventy-three patients had classical macrogranular morphology (M3) (Fig. 1A and B), and 26 patients had the microgranular variant (M3v) (Table 1). The absolute promyelocyte counts in peripheral blood (PB) ranged from 0 to 179,000/mL. In BM aspirate smears, the percentage of blasts and promyelocytes ranged from 21% to 97%.
Table 1.
Characteristics of Patients With APL at Diagnosis
| Characteristic | Value |
|---|---|
| No. | |
| Total No. | 199 |
| Age, median y [range] | 46.2 [1–81] |
| Sex, male:female | 109:90 |
| Promyelocyte counts | |
| Absolute counts in PB, ×109/L | 0–179 |
| Percentage in BM aspirates | 21–97 |
| FAB morphology, M3:M3v | 173:26 |
APL indicates acute promyelocytic leukemia; PB, peripheral blood; BM, bone marrow; FAB, French-American-British.
Conventional Cytogenetic Analysis
Conventional G-band karyotype analysis was performed on BM aspirate specimens of 182 APL cases as described previously.15 Cells were placed in 10 mL of Ham F10 medium with 20% fetal serum at a concentration of 2 to 4 × 106 nucleated cells per milliliter. The culture was incubated at 37°C for approximately 24 hours. Standard harvesting procedures were used. Demecolcine (Colcemid) (0.1 mL/10 mL) was added to the culture for 30 minutes at room temperature. For hypotonic treatment, 0.075 mol/L KCl was used for 30 minutes at room temperature. The fixation procedure consisted of 3 changes of methanol/glacial acetic acid (3:1) with a 10-minute interval between each change. A drying chamber (Thermaton Industries, Holland, Mich) was used for slide preparation. Slides were placed in a 60°C oven overnight, followed by GTG banding. The karyotype reports were written using the International System for Human Cytogenetic Nomenclature 1995.16
RT-PCR
Real-time quantitative RT-PCR was performed to detect PML/RARα transcripts, as previously described.17 Total RNA was extracted from either PB or BM aspirate specimens, and cDNA was synthesized using 1–2 μg total RNA, random hexamer primers, and 300 U Superscript II (Invitrogen, Houston, Tex). RT-PCR was performed using the primers P3 (5″-ACCGATGGCTTCGAC GAGTTC-3′) and R4a (5″-AGCCCTTGCAGCCCT CACAG-3′).18 The values were normalized to ABL transcript levels and expressed as a percentage of PML-RARα to ABL product. The sensitivity of the test is approximately 1 in 100,000.
Fluorescence In Situ Hybridization
Fluorescence in situ hybridization was performed on interphase nuclei of BM aspirate samples as previously reported.19,20 Assessment for the t(15;17)(q22;q21) was performed using the commercially available Vysis LSI PML/RARα dual-color, dual-fusion translocation probe (Vysis, Abbott Laboratories, Abbott Park, Ill). Slides were treated with 2× sodium saline citrate for 30 minutes at 37°C, dehydrated in 70%, 85%, and 100% ethanol solutions for 2 minutes each, and immediately transferred serially into cold 70%, 85%, and 100% ethanol solutions for 2 minutes each and air dried. Probes were denatured and hybridized according to the manufacturer’s instructions. Slides were examined using a fluorescent microscope (Carl Zeiss, Thornwood, NY) with appropriate filters. A minimum of 200 interphases were analyzed in each case.
Immunofluorescent Staining
We used a novel anti-PML antibody generated at our institution by Dr. K. Chang, as previously described.21 Briefly, this is a polyclonal antibody raised in rabbit and directed against the GST-PML fusion protein. The fusion protein consists of the full length of the PML IV sequence, present on both wild-type 90-kD PML protein and the 110-kD PML/RARα fusion protein.22,23
Depending on the clinical presentation and the feasibility of performing BM aspiration and biopsy, either BM smears, PB smears, or both were used for PML staining. A chunky, macrogranular pattern, demonstrating 5 to 30 particles per nucleus, was considered to be a negative (normal) result, not supporting the diagnosis of APL (Fig. 1C). A fine microgranular or dusty pattern with numerous minute particles (often too numerous to count) was considered a positive (abnormal) result supporting the diagnosis of APL (Fig. 1D).
RESULTS
PML Immunofluorescence Staining
Because of our interest in the test as a rapid means of confirming a suspected diagnosis, the antibody was developed for use on fresh samples (BM and PB smears), and we did not evaluate formalin-fixed, paraffin-embedded, or archived material. This study design therefore allows for the assessment of the diagnostic utility of PML staining as a frontline test for rapid confirmation of suspected APL. The test was not intended to be used for evaluating possible minimal residual disease.
For the 199 patients with confirmed APL, 184 BM and 33 PB smears were stained with the anti-PML antibody. Concurrent BM and PB were assessed in 18 patients, and PB only was examined in 15. A positive anti-PML result was observed in 32 of 33 PB and 182 of 184 BM smears (Table 2). One PB smear was inconclusive, 1 BM was negative, and 1 BM had too few cells to be evaluated (Table 3). In the subgroup with concurrent BM and PB, 17 patients had concordant results, and 1 patient with a microgranular variant of APL (M3v) had an inconclusive PB and positive BM smear. All 15 patients with only PB examined were positive.
Table 2.
PML Antibody Results Correlated With Other Testing Modalities
| PML | FISH | Cytogenetics | RT-PCR | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |
| 182 BM; 32 PB | 90 | 4 | 117 simple; 49 complex | 5 insufficient/inconclusive; 10 diploid; 1 48, XX, +8, +8 | 169 | 1 negative; 2 technical problems |
PML indicates promyelocytic leukemia; FISH, fluorescent in situ hybridization; RT-PCR, reverse transcription polymerase chain reaction; BM, bone marrow aspirate; PB, peripheral blood.
Table 3.
Summary of Results in 3 APL Patients With Negative PML Stain
| PML | FISH | Cytogenetics | RT-PCR | |
|---|---|---|---|---|
| Patient A | 1 PB, inconclusive | Not done | Complex | Positive |
| Patient B | 1 BM, negative | Negative | Normal | Positive |
| Patient C | 1 BM, few cells | Positive | Typical | Positive |
APL indicates acute promyelocytic leukemia; PML, promyelocytic leukemia; FISH, fluorescence in situ hybridization; RT-PCR, reverse transcription polymerase chain reaction; PB, peripheral blood; BM, bone marrow.
The patient with inconclusive anti-PML staining on a PB smear (heretofore referred to as Patient A) had a complex karyotype including t(15;17)(q22;q12). PML-RARα was detected by RT-PCR, and FISH was not performed. The patient with a negative anti-PML stain on a BM smear (designated as Patient B) also tested negative by FISH. The sample demonstrated normal karyotype, but the diagnosis of relapsed APL was confirmed by RT-PCR detection of a low-level PML-RARα. Morphological evaluation in this patient showed few neoplastic cells in BM aspirate smear, a negative BM core biopsy specimen, and unequivocal involvement of the BM aspirate clot, raising the possibility of patchy involvement of the BM as a probable explanation for the discrepancy in test results. The patient with a BM smear with too few cells for evaluation by PML staining (designated as Patient C) was positive by cytogenetics, FISH, and RT-PCR.
Among the 21 patients with therapy-related APL, 21 BM and 2 PB smears were evaluated, and the results were uniformly positive. BM smears from 23 patients with suspected relapsed APL were studied, and the results were positive in 22, the negative case being Patient B (described above). M3v was identified in 26 patients; anti-PML stain was performed on 15 BM, 5 PB, and both BM and PB smears in 6 patients. Anti-PML staining was positive in all, although in 1 patient with a positive BM an inconclusive PB staining was observed (Patient A).
PML results were negative in 148 of 150 non-APL patients. One patient presenting with weight loss, anorexia, coagulopathy, and an absolute blast count in the PB of 83 × 109/L had a positive anti-PML result. BM smears showed 98% blasts with morphology consistent with M3v. FISH showed the PML/RARα fusion gene in 14% of the 300 cells counted (cutoff 8% ± 2%), and flow cytometry demonstrated positivity for CD13 and CD33 and negativity for CD34, HLA-DR, and lymphoid markers. The patient was initially treated with ATRA and chemotherapy. However, RT-PCR did not confirm the suspected diagnosis of APL, cytogenetic studies revealed a normal karyotype, and therapy was changed appropriately. The patient achieved a complete remission and underwent an allogenic stem cell transplant. Another positive PML stain was observed in a patient with AML associated with t(1;15)(p35;q22) and without evidence of t(15;17)(q22;q12). Using the above data, the sensitivity of the test was 98.9%, and the specificity was 98.7%.
Comparison With Conventional Cytogenetics
Conventional karyotyping was attempted in 182 patients. We defined the presence of an isolated t(15;17)(q22;q21) as a simple APL karyotype, and the presence of t(15;17)(q22;q21) along with additional cytogenetically detectable numerical or structural aberrations in 2 or more metaphases as a complex karyotype. A simple APL karyotype was detected in 117 patients, 49 had complex chromosomal abnormalities, 10 demonstrated a normal karyotype, 4 had insufficient mitotic cells for analysis, 1 was inconclusive, and 1 demonstrated 48, XX, +8, +8.
PML staining was positive in 9 (1 PB and 8 BM) of the 10 patients with a normal karyotype and was negative in the case with patchy involvement by relapsed APL (Patient B). RT-PCR was positive in all cytogenetically normal APL patients, whereas FISH was positive in 5 of 8 tested patients.
Comparison With RT-PCR Results
Positive RT-PCR, defined as the presence of either short or long PML-RARα transcripts, was documented in 169 of 172 patients. Negative results were reported in 1 patient, and technical problems precluded extraction or amplification of RNA in 2 patients. The anti-PML antibody was positive in BM smears from the 3 patients with negative or inadequate RT-PCR results, and the diagnosis of APL was also confirmed by cytogenetic or FISH analysis (or both).
Comparison With Fluorescence In Situ Hybridization
Ninety of 94 patients analyzed demonstrated positive fusion signals, confirming the presence of PML-RARα. The results were negative in 4 patients, 3 de novo APL and 1 relapsed APL. The anti-PML stain was positive in BM smears of the de novo patients, whereas it was negative in the relapsed APL patient (Patient B).
Conventional cytogenetic studies were negative for t(15;17)(q22;q12) in all 4 patients negative by FISH; 3 had normal karyotypes, and 1 had 48, XX, +8, +8. RT-PCR detected the PML-RARα fusion product in all cases.
DISCUSSION
We evaluated the clinical utility of using a new anti-PML antibody staining as a rapid initial test in patients with suspected APL. The results were correlated with other established confirmatory diagnostic tools with longer turnaround times, such as conventional cytogenetics, RT-PCR, and FISH. The presence of an aberrant microgranular immunofluorescence pattern of staining in leukemic promyelocytes was relatively easy to detect, with excellent interobserver interpretation consistency. The test had high sensitivity (98.9%) and specificity (98.7%). The results were available in <4 hours, a turnaround time significantly shorter than that for RT-PCR or FISH studies (usually 24 hours).
Our study is comprehensive in that it included a large number of PB and BM samples from APL patients with both typical and microgranular morphology, with isolated t(15;17) as well as complex karyotypes, and involved cases with different lengths of fusion transcripts (short and long). Our results indicate that anti-PML immunofluorescence staining can be used as a fast and reliable first-line confirmatory test when the diagnosis of APL is suspected clinically or morphologically, thus expediting the initiation of highly effective therapy for prevention of fatal hemorrhagic complications. The test is used routinely in all cases with suspected diagnosis of APL at our institution, and the results correlate favorably with subsequent confirmatory FISH, cytogenetic, and molecular testing.
Eighty percent to 90% of APL patients present with a hemorrhagic syndrome, and up to 20% of them die from intracranial bleeding.1 The implementation of specific therapy with retinoid acid derivatives has led to a marked improvement of the prognosis, with a complete remission rate of 90% to 95%.14,24 Recent modifications of the regimen, incorporating arsenic trioxide into front-line therapy, have been successful in achieving a high and sustained response.25 A major obstacle and a management priority is the occurrence of early hemorrhagic complications that may be potentially averted by rapid diagnosis and initiation of the appropriate therapy.3 The availability of a rapid screening test that is readily available and evaluated without the need for specialized laboratories may be a means of achieving this end, particularly in settings in which there is limited access to specialized laboratories that can perform FISH or RT-PCR testing.
Anti-PML antibodies have been used previously for the detection of disrupted PML oncogenic domains in small numbers of patients by others. Dyck et al, using a polyclonal anti-PML antibody, showed positive results in 16 of 17 cases of active APL and in 1 patient with complete remission who subsequently relapsed.26 This study demonstrated the utility of the test in patients with either short or long PML-RARα transcripts. Falini et al assessed 14 APL patients using the monoclonal PG-M3 anti-PML antibody, and demonstrated diagnostic utility in 3 patients with M3v misdiagnosed as AML M4 or M5, as well as in 6 patients misdiagnosed as APL based on morphological and clinical findings.5 A perfect correlation between the results of PML staining and molecular tests was established, confirming the utility of the test in cases with different PML breakpoints (bcr1, bcr2, and bcr3). In another study, Villamor and colleagues reported abnormal staining in 25 of 26 patients with APL.27 To date, only 1 large study using the PG-M3 antibody has been published, reporting an abnormal staining in BM and/or PB blasts in 108 of 110 APL patients with false-negative results because of a paucity of cells.28 In all earlier studies, the anti-PML staining results were not completely correlated with blast morphology, PML-RARα transcript length, or karyotypic and FISH findings, as we have done.
The presence of 3 breakpoints in the PML gene results in PML-RARα fusion proteins of different size.2,14 These proteins do not result in clinical, morphological, or survival differences and are treated similarly. Similar to the previous monoclonal antibody (PG-M3), our polyclonal anti-PML antibody was equally effective in detecting all variants of the chimeric protein without interference from breakpoint/fusion differences. In contrast to PG-M3, which is directed against an epitope in the amino-terminal of PML, the antibody we used targets an amino acid sequence located very close to the coiled-coil domain of the PML protein. This fragment is present in all forms of PML-RARα fusion protein, even in rare forms of APL with deletion of exon 7, making the antibody a reliable tool. The antibody is not commercially available, and other groups will likely want to confirm our results. However, the large number of patients included in our study undoubtedly demonstrates the utility of the test.
Rarely (in <2% of APL cases), the classical t(15;17) is not detected by conventional cytogenetic or FISH studies, and PML-RARα fusion can only be demonstrated on molecular level.24,29,30 In such cryptic translocations, the formation of the nuclear domains (PML oncogenic domain) is still disrupted, making the PML immunostaining pattern abnormal. Of note, we observed a microspeckled (positive) pattern of PML staining in an isolated patient with AML with a complex karyotype that included t(1;15)(p35;q22) and was not associated with t(15;17)(q22;q22). Considering the finding that the PML-coding region (15q22) was translocated in this patient, these cytogenetic findings may represent a new variant of APL, and the positivity of the PML oncogenic domain test is not surprising; however, further workup was not undertaken at the time of presentation of this patient. Pathobiologic mechanisms, interfering with the organization of nuclear PML oncogenic domains, similar to the ones involved in APL with t(15;17), might have been implicated in this case.
Conversely, if genes other than PML are involved in APL variants, disruption of the nuclear PML oncogenic domain is unlikely to occur, and the PML stain would not be expected to be positive.5 These translocations are very rare, and there were no such cases included in our study. Because patients with t(11;17) do not respond to ATRA, the significance of rapid identification of these alternative APL translocations is questionable.14,24,29,30
In summary, PML immunofluorescence staining is a rapid and sensitive method that can facilitate the timely diagnosis of APL and expedite the initiation of targeted therapy. It can be performed with equal reliability in typical and microgranular variants of APL, on PB and BM smears, and we believe it is an excellent diagnostic tool in the initial workup of patients with APL, justifying prompt initiation of specific therapy while confirmation with cytogenetic and molecular testing is awaited. In our study the anti-PML stain outperformed conventional cytogenetics (positive in 9 of 10 patients with normal karyotype) and FISH studies. The utility of staining on PB smears allows diagnosis in the absence of a BM specimen in patients in whom BM biopsy is contraindicated. We believe that earlier therapy, allowed by confirmation of the diagnosis by PML staining, could further improve the outcome of patients with APL by reducing the potential for early hemorrhagic complications because of delayed diagnosis, particularly in the settings where access to conventional cytogenetics, FISH, or RT-PCR testing is limited or delayed.
Acknowledgments
FUNDING STATEMENT:
ARTICLE ID : CNCR _24775
This article was supported by National Institute of Health (P30 CA016672).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Avvisati G, Lo Coco F, Mandelli F. Acute promyelocytic leukemia: clinical and morphologic features and prognostic factors. Semin Hematol. 2001;38:4–12. [PubMed] [Google Scholar]
- 2.Warrell RP, Jr, de The H, Wang ZY, Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 3.Sanz MA, Grimwade D, Tallman MS, et al. Guidelines on the management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–1891. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 4.Nagendra S, Meyerson H, Skallerud G, Rosenthal N. Leukemias resembling acute promyelocytic leukemia, microgranular variant. Am J Clin Pathol. 2002;117:651–657. doi: 10.1309/KD1G-NUR1-J75P-HQ28. [DOI] [PubMed] [Google Scholar]
- 5.Falini B, Flenghi L, Fagioli M, et al. Immunocytochemical diagnosis of acute promyelocytic leukemia (M3) with the monoclonal antibody PG-M3 (anti-PML) Blood. 1997;90:4046–4053. [PubMed] [Google Scholar]
- 6.Santamaria C, Chillon MC, Fernandez C, et al. Using quantification of the PML-RARalpha transcript to stratify the risk of relapse in patients with acute promyelocytic leukemia. Haematologica. 2007;92:315–322. doi: 10.3324/haematol.10734. [DOI] [PubMed] [Google Scholar]
- 7.Le XF, Yang P, Chang KS. Analysis of the growth and transformation suppressor domains of promyelocytic leukemia gene, PML. J Biol Chem. 1996;271:130–135. doi: 10.1074/jbc.271.1.130. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y, Lallemand-Breitenbach V, Zhu J, de The H. PML nuclear bodies and apoptosis. Oncogene. 2004;23:2819–2824. doi: 10.1038/sj.onc.1207533. [DOI] [PubMed] [Google Scholar]
- 9.Hodges M, Tissot C, Howe K, Grimwade D, Freemont PS. Structure, organization, and dynamics of promyelocytic leukemia protein nuclear bodies. Am J Hum Genet. 1998;63:297–304. doi: 10.1086/301991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskiw CH, Bazett-Jones DP. The promyelocytic leukemia nuclear body: sites of activity? Biochem Cell Biol. 2002;80:301–310. doi: 10.1139/o02-079. [DOI] [PubMed] [Google Scholar]
- 11.Dyck JA, Maul GG, Miller WH, Jr, Chen JD, Kakizuka A, Evans RM. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 12.Lo-Coco F, Ammatuna E. The biology of acute promyelocytic leukemia and its impact on diagnosis and treatment. Hematology Am Soc Hematol Educ Program. 2006;514:156–161. doi: 10.1182/asheducation-2006.1.156. [DOI] [PubMed] [Google Scholar]
- 13.Exner M, Thalhammer R, Kapiotis S, et al. The “typical” immunophenotype of acute promyelocytic leukemia (APL-M3): does it prove true for the M3-variant? Cytometry. 2000;42:106–109. [PubMed] [Google Scholar]
- 14.Mandelli F, Avvisati G, Lo Coco F. Advances in the understanding and management of acute promyelocytic leukemia. Rev Clin Exp Hematol. 2002;6:60–71. doi: 10.1046/j.1468-0734.2002.00061.x. discussion 86–87. [DOI] [PubMed] [Google Scholar]
- 15.Khoury JD, Sen F, Abruzzo LV, Hayes K, Glassman A, Medeiros LJ. Cytogenetic findings in blastoid mantle cell lymphoma. Hum Pathol. 2003;34:1022–1029. doi: 10.1053/s0046-8177(03)00412-x. [DOI] [PubMed] [Google Scholar]
- 16.Schreck RR, Disteche CM, Adler D. ISCN standard idiograms. Curr Protoc Hum Genet. 2001 doi: 10.1002/0471142905.hga04bs18. Appendix 4B. [DOI] [PubMed] [Google Scholar]
- 17.Lin P, Hao S, Medeiros LJ, et al. Expression of CD2 in acute promyelocytic leukemia correlates with short form of PML-RARalpha transcripts and poorer prognosis. Am J Clin Pathol. 2004;121:402–407. doi: 10.1309/XC8P-9M8N-KQDT-38LB. [DOI] [PubMed] [Google Scholar]
- 18.Yin CC, Glassman AB, Lin P, et al. Morphologic, cytogenetic, and molecular abnormalities in therapy-related acute promyelocytic leukemia. Am J Clin Pathol. 2005;123:840–848. doi: 10.1309/TJFF-K819-RPCL-FKJ0. [DOI] [PubMed] [Google Scholar]
- 19.Merzianu M, Medeiros LJ, Cortes J, et al. inv(p13q22) in chronic myelogenous leukemia in blast phase: a clinicopathologic, cytogenetic, and molecular study of 5 cases. Am J Clin Pathol. 2005;124:807–814. doi: 10.1309/3HFE-16DK-MB1D-BFMN. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Morgan R, Stone JF, Sandberg AA. Identification of complex t(15;17) in APL by FISH. Cancer Genet Cytogenet. 1994;72:73–74. doi: 10.1016/0165-4608(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 21.Chang KS, Fan YH, Andreeff M, Liu J, Mu ZM. The PML gene encodes a phosphoprotein associated with the nuclear matrix. Blood. 1995;85:3646–3653. [PubMed] [Google Scholar]
- 22.Vallian S, Chin KV, Chang KS. The promyelocytic leukemia protein interacts with Sp1 and inhibits its transactivation of the epidermal growth factor receptor promoter. Mol Cell Biol. 1998;18:7147–7156. doi: 10.1128/mcb.18.12.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu ZX, Timanova-Atanasova A, Zhao RX, Chang KS. PML colocalizes with and stabilizes the DNA damage response protein TopBP1. Mol Cell Biol. 2003;23:4247–4256. doi: 10.1128/MCB.23.12.4247-4256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 25.Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504–510. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyck JA, Warrell RP, Jr, Evans RM, Miller WH., Jr Rapid diagnosis of acute promyelocytic leukemia by immunohistochemical localization of PML/RAR-alpha protein. Blood. 1995;86:862–867. [PubMed] [Google Scholar]
- 27.Villamor N, Costa D, Aymerich M, et al. Rapid diagnosis of acute promyelocytic leukemia by analyzing the immunocytochemical pattern of the PML protein with the monoclonal antibody PG-M3. Am J Clin Pathol. 2000;114:786–792. doi: 10.1309/J6PU-3XY6-R0C3-NW26. [DOI] [PubMed] [Google Scholar]
- 28.Gomis F, Sanz J, Sempere A, et al. Immunofluorescent analysis with the anti-PML monoclonal antibody PG-M3 for rapid and accurate genetic diagnosis of acute promyelocytic leukemia. Ann Hematol. 2004;83:687–690. doi: 10.1007/s00277-004-0902-7. [DOI] [PubMed] [Google Scholar]
- 29.Sainty D, Liso V, Cantu-Rajnoldi A, et al. A new morphologic classification system for acute promyelocytic leukemia distinguishes cases with underlying PLZF/RARA gene rearrangements. Group Francais de Cytogenetique Hematologique, UK Cancer Cytogenetics Group and BIOMED 1 European Community-Concerted Action “Molecular Cytogenetic Diagnosis in Haematological Malignancies”. Blood. 2000;96:1287–1296. [PubMed] [Google Scholar]
- 30.Grimwade D, Biondi A, Mozziconacci MJ, et al. Characterization of acute promyelocytic leukemia cases lacking the classic t(15;17): results of the European Working Party. Groupe Francais de Cytogenetique Hematologique, Groupe de Francais d’Hematologie Cellulaire, UK Cancer Cytogenetics Group and BIOMED 1 European Community-Concerted Action “Molecular Cytogenetic Diagnosis in Haematological Malignancies”. Blood. 2000;96:1297–1308. [PubMed] [Google Scholar]