Abstract
Many organisms can produce alternative phenotypes in direct response to different environmental conditions, a phenomenon known as phenotypic plasticity. The environmentally sensitive gene regulatory networks (GRNs) that mediate such developmental flexibility are largely unknown. Yet, characterizing these GRNs is important not only for elucidating plasticity’s molecular basis, but also for shedding light onto whether and how plasticity might impact evolution. In this issue of Molecular Ecology, Schneider et al.) describe one of the first efforts to determine the GRN underlying a plastic trait. They focus on diet-induced plasticity in the cichlid fish, Astatoreochromis alluaudi. Depending on whether soft food (e.g. insects) or hard food (e.g. molluscs) is consumed, this species forms a lower pharyngeal jaw (LPJ) with many fine teeth or with fewer molar-like teeth, respectively (Fig. 1). The authors previously identified genes that are differentially expressed between LPJ morphs during early development. In the present study, they examine the expression of 19 of these genes across development and diet. By analysing these transcriptional data in combination with information on putative transcription factor binding sites, they construct a GRN that explains observed gene expression patterns and is likely to control LPJ morphology. This work advances our understanding of how plasticity can arise as a consequence of environmentally sensitive GRNs and promises to help illuminate how changes in such GRNs could facilitate evolution.
Keywords: adaptation, fish, gene regulatory networks, macroevolution, molecular evolution, phenotypic plasticity
The flexible organism
Among evolutionary biology’s central challenges is to explain why there are so many different species and where their distinctive traits come from. Phenotypic plasticity is increasingly viewed as playing a crucial role in both diversification and innovation (West-Eberhard 2003). When selection acts on quantitative genetic variation regulating the expression of an environmentally induced trait, it can promote the evolution of either increased or decreased plasticity through an evolutionary process known as ‘genetic accommodation’ (sensu West-Eberhard 2003). If the affected trait evolves decreased plasticity to the point of becoming constitutively expressed, ‘genetic assimilation’ occurs (sensu Waddington 1953). Through this process – in which a novel, complex trait evolves from a trait that was originally induced by the environment – new traits and possibly even new species can emerge (Pfennig et al. 2010).
Yet, little is known about whether and how genetic accommodation/assimilation shape ecologically and evolutionarily relevant traits in natural populations (Pfennig et al. 2010). East African cichlids are ideal for studying genetic accommodation/assimilation. This group is renowned both for its spectacular species richness (there are over 500 species in Lake Victoria alone) and for its stunning array of highly specialized feeding morphologies (Seehausen 2006). Indeed, this impressive diversity is thought to stem (in part) from the cichlid’s ability to rapidly evolve specializations for utilizing diverse feeding niches: these fish possess a versatile pharyngeal jaw, which has been modified repeatedly to exploit different resource (Liem 1974).
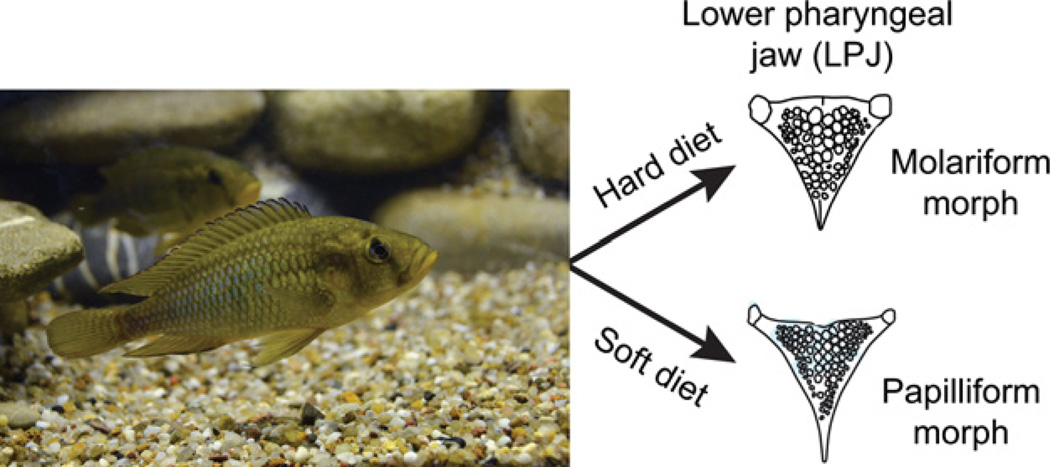
One recent hypothesis holds that the cichlid adaptive radiation arose when ecological circumstances favoured diversification in ancestral lineages that possessed a phenotypically plastic pharyngeal jaw; later, this flexible jaw repeatedly underwent genetic assimilation to produce the present-day diversity among species in feeding morphologies (West-Eberhard 2003). Astatoreochromis alluaudi is well suited for testing this hypothesis. This species is a dietary generalist, ancestral to modern East African cichlids. Moreover, it exhibits diet-induced phenotypic plasticity in its lower pharyngeal jaw (LPJ). If its preferred diet of soft food (e.g. insects) is present, A. alluaudi develops a slender ‘papilliform’ LPJ, bearing numerous fine teeth. However, if soft food is scarce, individuals feed on hard-shelled molluscs, which induces formation of a robust ‘molariform’ LPJ, bearing fewer, molar-like teeth (Fig. 1). Thus, LPJ plasticity in A. alluaudi represents a valuable model for examining how phenotypic plasticity might have contributed to the diversity of jaw morphologies among East African cichlids.
Fig. 1.
The cichlid fish, Astatoreochromis alluaudi, exhibits diet-induced plasticity in the morphology of its lower pharyngeal jaw.
Changes in transcription across development and diet
Although the molecular mechanisms underlying phenotypic plasticity, genetic accommodation and genetic assimilation are not well understood, changes in gene expression are likely crucial (Renn & Schumer 2013). In A. alluaudi, mechanical strain from chewing affects the expression of genes that influence jaw development. Previous work using RNA-seq on individuals reared on hard versus soft diets identified 187 transcripts that are differentially expressed between adult LPJ morphs (Gunter et al. 2013). However, this earlier study did not examine gene expression changes at different points in development. Such investigations are necessary because the changes in gene expression that underlie plastic traits may occur at specific times during development (Aubin-Horth & Renn 2009). Further, components of the regulatory cascades that control development function in a sequential manner can only be seen analysing gene expression across a time-course (Aubin-Horth & Renn 2009).
To better characterize how changes in gene expression contribute to plasticity in LPJ morphology, Schneider et al. (2014) measured the expression of 19 previously identified candidate genes for LPJ morphology over 8-months. 17 of the 19 genes initially showed higher expression among individuals reared on soft vs. hard diet, but most of these genes showed higher expression in fish fed on hard diet after 3 months of treatment. The genes were classified into six functional categories related to bone and muscle formation (a seventh group had unknown functions). Several of these functional categories showed time pointspecific expression differences between individuals grown on soft versus hard diet. These results show that dynamic expression patterns underlie environmentally responsive development.
Constructing GRNs that underlie phenotypic plasticity and genetic accommodation
Complex traits, including those that show phenotypic plasticity, are specified by gene regulatory networks involving many components (Nuzhdin et al. 2012). To analyse their system within a GRN framework,Schneider et al. (2014) first examined the data for modules of genes that showed correlated expression. Through a combination of principal components analysis and hierarchical clustering, they identified three such modules. They then tried to determine the mechanism underlying coregulation of genes in these modules using an analysis of transcription factor binding sites. Through this analysis, they identified transcription factors that likely regulate all of the 19 genes, as well as transcription factors that influence the expression of specific functional categories of genes or expression modules. By integrating their results, they were able to formulate a GRN model that can explain how different LPJ morphologies are induced by diet.
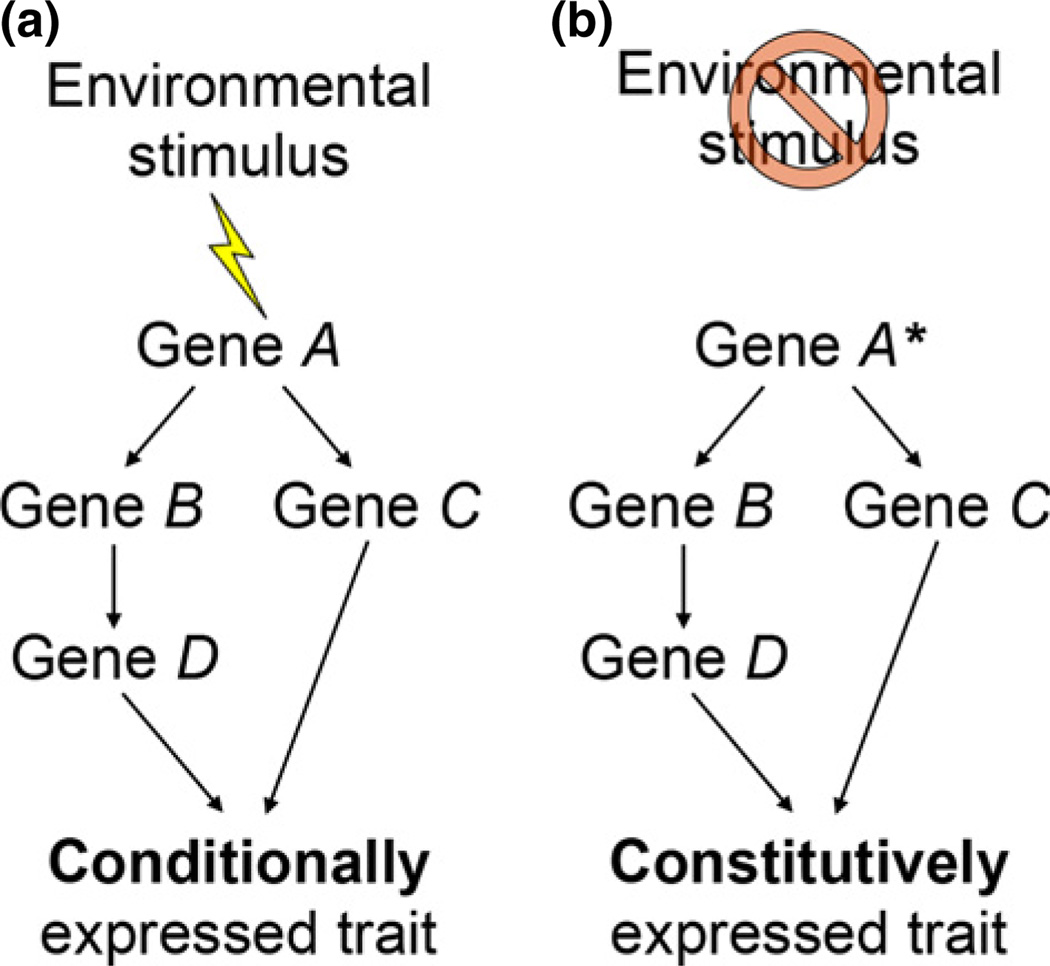
As Schneider et al. (2014) suggest, recognition that complex GRNs often underlie plastic traits helps clarify the molecular mechanisms by which genetic variation might influence the responsiveness of development to the environment. Specific hypotheses can be generated about connections among genes within a GRN that can be broken, strengthened or created anew to alter environmental sensitivity, potentially resulting in genetic accommodation or genetic assimilation (Fig. 2). Further, extension of the GRN framework to genetically diverse individuals that differ in environmental sensitivity and plasticity may provide direct insights into the evolutionary process underlying genetic accommodation and genetic assimilation. Such research is not far off, and the work by Schneider et al. (2014) represents a meaningful step forward in evaluating the related problems of phenotypic plasticity, genetic accommodation and genetic assimilation from a GRN perspective. Ultimately, such studies promise to provide key insights into the role of phenotypic plasticity in promoting evolutionary innovation and diversification in such spectacularly diverse groups as East African cichlids.
Fig. 2.
Complex, environmentally induced traits are likely specified by gene regulatory networks (GRNs). Moreover, genetic assimilation – when selection causes a trait that was originally environmentally induced to become expressed constitutively – is likely to occur as a result of mutations in upstream elements of these GRNs For example, (a) in an ancestral lineage, an environmental stimulus might be necessary to activate a GRN and produce a particular trait. (b) In a derived lineage, however, a mutation in the upstream element, ‘Gene A’ (indicated here by an asterisk), might result in this trait being produced constitutively, that is, without the original environmental stimulus.
Acknowledgements
We thank K. Pfennig and one anonymous reviewer for comments on the manuscript. D.W.P. was supported by a grant from the NSF (DEB1019479). I.M.
Footnotes
D.W.P. and I.M.E. wrote this article together.
References
- Aubin-Horth N, Renn SCP. Genomic reaction norms: using integrative biology to understand molecular mechanisms of phenotypic plasticity. Molecular Ecology. 2009;18:3763–3780. doi: 10.1111/j.1365-294X.2009.04313.x. [DOI] [PubMed] [Google Scholar]
- Gunter HM, Fan SH, Xiong F, et al. Shaping development through mechanical strain: the transcriptional basis of dietinduced phenotypic plasticity in a cichlid fish. Molecular Ecology. 2013;22:4516–4531. doi: 10.1111/mec.12417. [DOI] [PubMed] [Google Scholar]
- Liem KF. Evolutionary strategies and morphological innovations: cichlid pharyngeal jaws. Systematic Zoology. 1974;22:425–441. [Google Scholar]
- Nuzhdin SV, Friesen ML, McIntyre LM. Genotype-phenotype mapping in a post-GWAS world. Trends in Genetics. 2012;28:421–426. doi: 10.1016/j.tig.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig DW, Wund MA, Snell-Rood EC, et al. Phenotypic plasticity’s impacts on diversification and speciation. Trends in Ecology and Evolution. 2010;25:459–467. doi: 10.1016/j.tree.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Renn SCP, Schumer ME. Genetic accommodation and behavioural evolution: insights from genomic studies. Animal Behaviour. 2013;85:1012–1022. [Google Scholar]
- Schneider RF, Li Y, Meyer A, Gunter HM. Regulatory gene networks that shape the development of adaptive phenotypic plasticity in a cichlid fish. Molecular Ecology. 2014;23:4511–4526. doi: 10.1111/mec.12851. [DOI] [PubMed] [Google Scholar]
- Seehausen O. African cichlid fish: a model system in adaptive radiation research. Proceedings of the Royal Society, Series B. 2006;273:1987–1998. doi: 10.1098/rspb.2006.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. Genetic assimilation of an acquired character. Evolution. 1953;7:118–126. [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford University Press; 2003. [Google Scholar]