Abstract
Mechanism is at the heart of understanding, and this chapter addresses underlying brain mechanisms and pathways of cognition and the impact of sleep on these processes, especially those serving learning and memory. This chapter reviews the current understanding of the relationship between sleep/waking states and cognition from the perspective afforded by basic neurophysiological investigations. The extensive overlap between sleep mechanisms and the neurophysiology of learning and memory processes provide a foundation for theories of a functional link between the sleep and learning systems. Each of the sleep states, with its attendant alterations in neurophysiology, is associated with facilitation of important functional learning and memory processes. For rapid eye movement (REM) sleep, salient features such as PGO waves, theta synchrony, increased acetylcholine, reduced levels of monoamines and, within the neuron, increased transcription of plasticity-related genes, cumulatively allow for freely occurring bidirectional plasticity (long-term potentiation (LTP) and its reversal, depotentiation). Thus, REM sleep provides a novel neural environment in which the synaptic remodeling essential to learning and cognition can occur, at least within the hippocampal complex. During nonREM sleep Stage 2 spindles, the cessation and subsequent strong bursting of noradrenergic cells and coincident reactivation of hippocampal and cortical targets would also increase synaptic plasticity, allowing targeted bidirectional plasticity in the neocortex as well. In delta nonREM sleep, orderly neuronal reactivation events in phase with slow wave delta activity, together with high protein synthesis levels, would facilitate the events that convert early LTP to long lasting LTP. Conversely, delta sleep does not activate immediate early genes associated with de novo LTP. This nonREM sleep-unique genetic environment combined with low acetylcholine levels may serve to reduce the strength of cortical circuits that activate in the ~50% of delta-coincident reactivation events that do not appear in their waking firing sequence. The chapter reviews the results of manipulation studies, typically total sleep or REM sleep deprivation, that serve to underscore the functional significance of the phenomenological associations. Finally, the implications of sleep neurophysiology for learning and memory will be considered from a larger perspective in which the association of specific sleep states with both potentiation or depotentiation is integrated into mechanistic models of cognition.
Keywords: Bidirectional plasticity, Long Term Potentiation (LTP), depotentiation, Spindles, Theta, Slow Waves, Acetylcholine (ACh), Norepinephrine (NE), Serotonin (5HT), Memory Consolidation
In the last twenty years, evidence linking sleep, specific sleep states, and the cognitive processes underlying learning and memory has been developed in several research domains. Other chapters in this volume describe aspects of the relationship between sleep and cognition using behavioral paradigms and/or neuroimaging data in humans to elucidate the functional anatomy underlying sleep and cognition. The focus of this chapter is on research, conducted almost exclusively in animal models, that has provided an outline of the underlying mechanisms whereby sleep affects learning and memory. Two broad areas will be covered: 1) physiological traits of REM sleep, nonREM sleep and the Stage 2 transition to REM state that affect the substrate of learning and memory, i.e. synaptic plasticity; and 2) revelations from sleep deprivation studies that demonstrate a functional role for sleep in the normal expression of learning and memory. The chapter concludes with a summary of unresolved controversies and future directions.
Cognitive processes involve neural oscillations and synchronization among brain regions that are active during attention, perception, working memory, short and long term memory acquisition, retention and recall, imagination and thought (Crick and Koch, 1998; Llinas and Ribary, 1993; Axmacher et al., 2006). Dynamic cognitive networks that become engaged in a thought or task, work through temporary bands of activity that synchronize multiple brain areas into a functional unit. For example, Jones and Wilson(2005) showed that the hippocampus and prefrontal cortex oscillate together when rats perform a spatial memory task requiring both areas. Just as periods of synchronization occur while performing cognitive tasks during waking, e.g. (Brown and McCormack, 2006), periods of synchronization also appear in all other states in various degrees, frequencies and extent of brain areas involved. Cheng et al. (2008) propose that sleep states themselves are a less useful categorization schema in which to examine what occurs offline for cognition. Instead, predominant electroencephalographic frequencies, which often coincide with particular sleep states, should be examined to uncover the role of sleep for cognition. Specific sleep traits including dominant frequencies, neurochemical milieu, and neuronal activation patterns, can be dissociated from their normal state under even normal conditions. Here we examine the role of each state with particular emphasis on their traits and the role of each trait for cognition, with particular regard for learning and memory.
Spontaneous physiological processes during natural sleep to support cognition
REM sleep
Two books that nicely explain the role of REM sleep for memory were published 22 years apart (Winson, 1985; Walter, 2007). Walter reviews most of the literature and consensus findings in “REM Illumination: Memory Consolidation”, providing a framework to understand current ongoing research. Theoretical perspectives in “Brain and Psyche: the Biology of the Unconscious” by Jonathan Winson in 1985 presented many of the concepts of REM sleep involvement in memory consolidation that are now found in most publications concerning REM sleep and learning. Winson and Pavlides (Pavlides et al., 1988; Pavlides and Winson, 1989) were the first to record hippocampal unit activity during activity and subsequent sleep, revealing startling activity patterns during sleep. This stimulated many others to measure the physiological processes at work supporting cognition during sleep on the cellular level, as discussed further below.
Not long after Pavlides and Winson joined the handful of those actively engaged in sleep and memory research (e.g. Hennvin, Guerrin, LeConte and Smith), two studies were published back to back that highlighted the sleep and learning field for a broader scientific audience. The first (Karni, 1994), replicated and expanded in 2002 (Mednick et al., 2002), showed that REM sleep effects on memory consolidation were circuit specific. A visual perceptual learning task that causes focal synaptic changes to occur in the visual cortex, needed REM sleep for strong, lasting improvements. The REM sleep associated synaptic gains were specific to the circuits trained and did not generally transfer to all other visual processing fields. This elegant study kindled new excitement in the puzzle of REM sleep and memory and revitalized the sleep and memory field in general. The other landmark Science paper ((Wilson and McNaughton, 1994), which was met with equal enthusiasm, amplified the Pavlides and Winson (1988, 1989) finding of orderly hippocampal reactivation during sleep and will be discussed in greater detail under nonREM sleep, below.
Learning increases REM sleep
Spontaneous increases in REM sleep follow training and precede large increases in performance during learning (Lucero, 1970; Hennevin et al., 1974; Fishbein et al., 1974; Smith et al., 1980; Portell-Cortes et al., 1989; Smith and Wong, 1991; Bramham et al., 1994; Smith and Rose, 1996) associated with acquiring the task (Hennevin et al., 1995; Datta, 2000). REM sleep increases in humans just after intensive learning trials as well (De Koninck et al., 1989; Mandai et al., 1989; Smith and Lapp, 1991; Smith, 1995). Learning can either prolong and/or intensify REM sleep (Smith et al., 1980; Dujardin et al., 1990; Guerrien et al., 1989; Mandai et al., 1989). While it is not the case that smarter animals have more REM sleep (Siegel, 2001), studies of increased REM sleep during times of learning have never been made across species. It appears from the majority of studies, that the total time in REM sleep is not as important as the REM sleep augmentation that is accomplished during times of high learning demands.
PGO waves increase with learning
Waves of excitation originating from the brainstem, called Ponto-geniculo-occipital (PGO) waves, or P-waves in the rat, occur only during REM sleep and the transition to REM sleep (TR) (Jouvet, 1962). PGO waves are coincident with rapid eye movements and middle ear muscle activity as well as occasional myoclonic jerks of postural musculature (Lai and Siegel, 1991). Both auditory and somatosensory stimuli influence PGO wave activity (for review, see (Callaway et al., 1987)). The dorsal part of the nucleus subcoeruleus of the pons in the rat is the most effective area for inducing PGO waves and probably initiates their propagation through the medial reticular formation (MRF) to the forebrain. This area projects to the hippocampus and amygdala, among other structures (Datta et al., 1998). During intensive learning PGO waves increase in intensity and density during REM sleep and TR, and their increase is directly correlated with task retention (Datta, 2000). PGO waves are hypothesized by Datta (2004) to be a potent regulator of synaptic plasticity in both the hippocampus and amygdala, as they are comprised of large, synchronous excitatory waves of glutamate that terminate directly on forebrain targets.
Hippocampus for associative learning and memory consolidation
The rapid encoding structure of the hippocampus is where the building block for memory encoding, long term potentiation (LTP), was discovered (Bliss and Lomo, 1973). Memory consolidation is the transfer of this hippocampal plasticity to more permanent long term storage sites distributed throughout the neocortex. LTP requires postsynaptic membrane depolarization coincident with presynaptic action potentials to allow the intracellular cascade of molecular events that effect long term increases in synaptic strength. LTP is easily induced during wakefulness and during REM sleep in the hippocampus (Bramham and Srebro, 1989).
REM sleep neuronal reactivation
Pavlides and Winson (1989) were the first to show increased firing of hippocampal neurons in REM sleep that had been active in prior waking. Many others have repeated and enhanced that work. Hippocampal pyramidal cells can act as place field encoders, or place cells, firing rapidly in a particular area of the environment as the animal navigates through, then falling silent elsewhere. Studies have shown that place cells that have overlapping fields on the maze and therefore fire in an ordered, overlapping fashion, show increased co-activation during “off-line” hippocampal large irregular activity (LIA), which occurs during nonREM sleep and quiet waking (QW) ((Wilson and McNaughton, 1994). Very structured replay of the waking running sequences were seen also during REM sleep (Louie and Wilson, 2001). Our study (Poe et al., 2000) showed that spontaneous reactivation of hippocampal neurons during REM sleep occurs in a theta-specific pattern concordant with the induction of both LTP and with its reversal, depotentiation, during the REM sleep state.
Theta phase specificity
Place cells fire in a specific relationship to the ongoing electroencephalographic (EEG) theta rhythm activity during active waking. Most spikes occur at the peaks (corresponding to the maximum depolarization of the cell membrane) of theta, as the cell discharges through the place field (Buzsaki et al., 1983; Fox et al., 1986). Pavlides (1988) found that stimulation on the positive phase of the hippocampal theta rhythm induced LTP; stimulation at the opposite phase, the theta trough, induced a decrease in synaptic efficacy (depotentiation). Huerta and Lisman (1995) found that LTP and depotentiation could be induced at the theta peak and trough, respectively, with a burst of only four 200 Hz stimuli lasting 20 ms and applied to the Schaeffer collaterals. Others have shown this result in the intact animal (Hyman et al., 2003; Orr et al., 2001). These experiments showed that LTP could be induced with physiologically feasible stimuli, if timed to the naturally occurring theta activity.
Acetylcholine(ACh) inputs allow the hippocampus to display theta activity during active waking and REM sleep. Givens (1996) found a stimulus-evoked resetting of the dentate theta rhythm during working memory tasks in rats. Acetylcholine is important to the induction of LTP in the intact animal and to learning in the hippocampus (Winson, 1978; Mizumori et al., 1990; Hasselmo and Bower, 1993; Givens, 1996; Rashidy-Pour et al., 1996). Similarly, disruptions of hippocampal theta impair learning (see (Vertes and Kocsis, 1997)). Hasslemo and Bower (1993) suggest that cholinergic activity may be modulated by ongoing memory demands in the system.
Hippocampal measures of acetylcholine release during REM sleep exceed those measured during waking, though they are probably equal to levels present during active waking behaviors (Marrosu et al., 1995). Stimulation of the MRF, the same area that propagates PGO waves during waking startle and REM sleep, induces theta rhythm activity in the hippocampus (Vertes, 1982). The evidence suggests that the timing of incoming stimuli relative to the cell’s membrane polarity, which normally oscillates with theta, influences the induction of LTP and depotentiation; dampened theta amplitudes are less conducive to learning. When the reticular formation is stimulated in REM sleep, increasing hippocampal theta, rats improve significantly on memory retention tasks over those given the same stimulation in slow wave sleep (e.g.(Hennevin et al., 1989)). Reticular stimulation can reverse the mnemonic impairments imposed by REM sleep deprivation (Hars and Hennevin, 1983). Reticular neurons exert their influence on hippocampal theta through projections to the supramammillary nucleus, which in turn projects to the septal nuclei that provide timed cholinergic and GABAergic inputs to CA1 pyramidal cells and interneurons (Vertes and Kocsis, 1997).
We found that neurons active in novel places discharge at the peaks of local theta (5-10 Hz) oscillations in both waking and REM sleep (Poe et al., 2000). Such peak theta phase firing is consistent with establishing LTP, thought to underlie memory formation. Once the place becomes familiar to the animal, neurons that were active in these places, called place cells, reverse phase at which they fire with respect to local theta oscillations during REM sleep. That is, although they fired at theta peaks in REM sleep when the environment was novel, they fire at theta troughs in REM sleep once the environment is familiar. Cells active only in familiar areas of the environment in waking, fire at theta troughs in REM sleep. Although the mechanism of such reversed phase firing during REM sleep is unclear, such phase-reversed firing during theta is consistent with patterns that induce the depotentiation of previously potentiated synapses.
The importance of depotentiation
Depotentiation is important to theories of learning and memory that incorporate a temporary associative memory structure such as the hippocampus. The hippocampus is a temporary storage facility for memory; hippocampal synapses are more limited in number as compared to the entire neocortical mantle, where long term memories are probably stored in a parallel, distributed fashion. Thus, if the hippocampus serves to form associative memories and temporarily store them until they are consolidated to the neocortex, it is logical that the hippocampal network of weighted synapses involved with consolidated memories should be recycled for future differential weighting of associative memories in other functional assemblies. Depotentiation may serve that synaptic recycling function in the CA1 region of the hippocampus where there is no adult neurogenesis.
A growing literature suggests that depotentiation is also critical to cognitive function (Braunewell and Manahan-Vaughan, 2001; Manahan-Vaughan and Braunewell, 1999; Nakao et al., 2002). Depotentiation seems to be associated with the presence of new stimuli in a contextual frame (Kemp and Manahan-Vaughan, 2004). Biphasic changes in synaptic strength could separate the acquisition of different types of information. Novel spatial exploration induces depotentiation of previously induced but irrelevant LTP (Xu et al., 1998). After two exposures to an environment with the same objects always located in the same spatial locations, depotentiation was expressed after a third exposure merely by changing the configuration of the objects. The re-association of objects within a particular spatial context triggers depotentiation, probably due to the necessity of rewiring the memory network to accommodate the new change in formation for efficient storage.
Requirements of depotentiation met in REM sleep: NE and 5HT absence
Depotentiation may be more reliably induced in the absence of norepinephrine (NE), which pushes the net effect of all activity toward LTP. NE working at both beta and alpha1 receptors blocks depotentiation in the hippocampus and enhances LTP (Thomas, 1996; Katsuki et al., 1997; Yang et al., 2002). Either stimulation of the noradrenergic cells of the locus coeruleus, or direct intracerebroventricular application of norepinephrine enhances and prolongs LTP (Almaguer-Melian et al., 2005).
REM sleep is a time when noradrenergic inputs are suppressed (Aston-Jones and Bloom, 1981; Nitz and Siegel, 1997). Noradrenergic neurons are tonically active in all states except REM sleep (Aston-Jones and Bloom, 1981), and in brief periods before each spindle wave during nonREM sleep. Therefore we hypothesize that REM sleep and possibly the TR are the only times when theta trough activity in the CA1 region has the opportunity to depotentiate synapses from spontaneous neural activity in the intact animal.
Serotonin is present in the hippocampus in all states but is vastly diminished in REM sleep (Park et al., 1999), when dorsal raphe serotonergic neurons are inactive (McGinty and Harper, 1976). We hypothesize that the absence of serotonin in the hippocampus during REM sleep allows that state to serve a depotentiation function supporting the reworking of networks for maximally efficient cognitive function.
Both depotentiation and habituation to an environment are inhibited by serotonin agonist application (Kemp and Manahan-Vaughan, 2004). Serotonin agonist application also improved acquisition but impaired memory consolidation (Meneses and Hong, 1997). The pretraining stimulation of serotonergic type 4 (5-HT4) receptors enhanced the acquisition of a conditioned response, while post-training activation of postsynaptic 5-HT4 receptors impaired the consolidation of learning. Thus, it may be that 5-HT4 receptor activation is beneficial for certain types of information acquisition that depend on LTP and detrimental for other types that depend on depotentiation. Thus the available studies show that, through the 5-HT4 receptor, serotonin depresses depotentiation and impairs memory consolidation.
These two neurotransmitter systems, noradrenaline and serotonin, influence synaptic excitability and plasticity and fall uniquely silent during REM sleep (McGinty and Harper, 1976; Aston-Jones and Bloom, 1981), allowing the hippocampus to both strengthen and refine the memories it encodes. Thus the neurochemical environment of REM sleep and specific reactivation patterns relative to the theta rhythm are consistent with a unique opportunity to depotentiate, or weaken, those synapses encoding memories already consolidated to structures outside the hippocampus. Such depotentiation would be especially important during intensive learning periods such as during development and for integrating old knowledge with changing novel information.
More evidence for synaptic plasticity in REM sleep: gene studies
It could also be true that neural reactivation in any stage of sleep or wakefulness serves no purpose for learning and memory. This is unlikely to be the case, especially in REM sleep when acetycholine levels are high, theta is present in the hippocampus, the forebrain is activated by PGO waves, and LTP is readily induced as mentioned above (Bramham and Srebro, 1989). Furthermore, Ribeiro and Pavlides (1999; 2002) have shown that zif-268, an immediate early gene involved in LTP, is upregulated in the hippocampus and associated neocortical structures during REM sleep following waking learning and LTP. Further, the zif-268 activation occurs in the hippocampus and entorhinal cortex in the first REM sleep period after learning, then in primary and secondary associated structures in ensuing REM sleep periods, as though consolidation of the memory from the hippocampus to the neocortex were being accomplished in one sleep session.
Multiple cellular tests of LTP and depotentiation are now available in the freely behaving animal. Thus the question of whether reactivation results in synaptic plasticity and learning will be directly addressed in future research.
NonREM sleep
Learning amplifies slow waves (Huber et al., 2004; Wamsley et al.). Slow waves, shown in a rat recording in Figure 1, allow a reactivation of neurons that were involved in learning or encoding during theta states on a much accelerated (~300x), condensed time scale (Pavlides and Winson, 1989; Wilson and McNaughton, 1994; Kudrimoti et al., 1999).
Fig. 1.
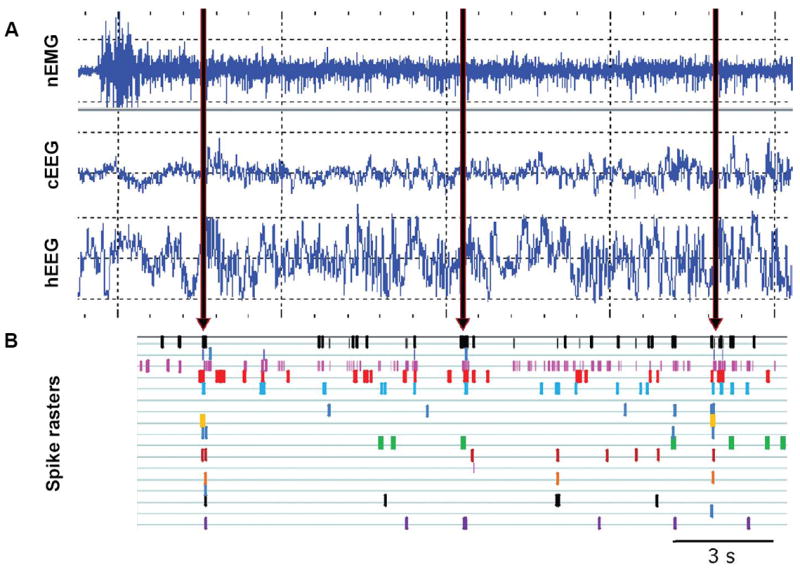
Electroencephalographic (EEG) and electromyographic (EMG) signals across 20 s of recording time. (A) Vertical grid spacing is 200 mV. Cortical EEG was taken as the differential signal from screw electrodes placed over the frontal cortices. Hippocampal EEG (hEEG) was recorded from a tetrode (4 individual 12 μm nichrome recording wires twisted together such that the distance between wires is ~15 μm) placed at the hilus and referenced to a tetrode placed in the corpus callosum. These data were taken from a rat implanted with a tetrode assembly (12 tetrodes) recorded from a resting pot after a maze run during a period of wakefulness (high variable EMG) without voluntary movement, allowing slow waves rather than theta to appear in the hEEG trace. Slow waves appear in the hippocampus both during non-REM sleep and during quiet wakefulness. (B) Times of action potentials from 17 simultaneously recorded hippocampal neurons (1 spike raster row for each cell) show bursts of coincident reactivation at the depolarized peaks of the hippocampal slow waves (arrows) during this period of quiet wakefulness.
Part B of Figure 1 shows an example of multiple single units recorded in our laboratory during hippocampal slow waves. Many labs have documented a rise in slow wave activity during slow wave sleep associated with increased waking task requirements demanded from the same area neocortex (Borbely et al., 1981; Ferrara et al., 2008; Franken et al., 1991; Krueger and Obal, 1993; Vyazovskiy et al., 2000; Borbely and Achermann, 2005; Esser et al., 2007; Riedner et al., 2007; Vyazovskiy et al., 2007). Such reactivation events occur during these delta or sharp waves (e.g. (Kudrimoti et al., 1999; Ribeiro and Nicolelis, 2004; Wilson and McNaughton, 1994)), which we will henceforth call slow waves.
NonREM sleep reactivation for memory interleaving and consolidation
The function of such coordinated offline reactivation has been proposed to be for memory consolidation. A host of multiple single unit publications hypothesize that slow wave-dependent activity would serve to strengthen memory circuits even though most of them have shown that cross correlations strong at the start of slow wave activity are weakened within 10-20 minutes of such activity (Kudrimoti et al., 1999; Wilson and McNaughton, 1994). LTP requires postsynaptic membrane depolarization coincident with presynaptic action potentials to allow the intracellular cascade of molecular events that effect long term increases in synaptic strength. The conditions of LTP may be well satisfied during hippocampal slow wave oscillations and reactivations (Tsukamoto-Yasui et al., 2007). Cells are synchronously depolarized allowing voltage-gated Ca2+ channels to open and the unblocking of NMDA channels associated with plasticity changes, and connected neurons that synchronously fire together allow spike timing dependent plasticity (STDP) (Bi and Poo, 1998) to occur at their synapses.
Neurochemistry not right for LTP in nonREM sleep
However, direct evidence of a synaptic plasticity function of spontaneous offline hippocampal reactivation, whether during waking or sleep, is lacking. Indeed, synchronous firing may not be enough. Acetylcholine, so influential for synaptic plasticity as mentioned in the REM sleep section above, is at a minimum in the forebrain during nonREM sleep. Indeed, the addition of ACh eliminates slow waves (Lapierre et al., 2007; Vanderwolf and Stewart, 1986). In fact, ACh is not present in the slow wave producing hemisphere during unihemispheric sleep (Lapierre et al., 2007).
Genes not available for LTP in nonREM sleep
Further complicating the hypothesis that reactivation during nonREM sleep serves synaptic plasticity is the problem that no one has successfully induced LTP in vivo in the absence of ACh, during slow wave sleep (Bramham and Srebro, 1989; Leonard et al., 1987). More evidence against slow wave reactivation as the state to enhance LTP includes the finding that calcium-dependent gene expression related to synaptic plasticity is absent during nonREM sleep (Pompeiano et al., 1994; Ribeiro et al., 1999; Ribeiro et al., 2002). In fact there is a rise in depotentiation-related genes and drop in LTP-related genes during sleep (Pompeiano et al., 1994; Pompeiano et al., 1995; Pompeiano et al., 1997; Basheer et al., 1997; Cirelli and Tononi, 2000b; Cirelli and Tononi, 2000a).
NonREM sleep reduces synaptic strength?
The best two pieces of physiological evidence to date that slow wave dependent processing affects synaptic weights are 1) a rise in the amplitude of slow waves after extended waking experience that becomes attenuated again after a period of slow wave activity ensues (Vyazovskiy et al., 2000; Steriade, 2004; Hanlon et al., 2009), and 2) the decline in evoked potentials across sleep (Esser et al., 2006). These results support a role of slow wave processing in reducing the strength of synapses, rather than increasing them, which runs counter to most hypotheses of the role of slow wave processing for learning and memory.
Mechanisms for nonREM sleep downscaling
One mechanism whereby slow wave coincident reactivation could lead to synaptic downscaling or depotentiation rather than LTP is the finding that slow waves activate voltage gated Ca2+ channels to allow in a little calcium, and that small amount of intracellular Ca2+ is not augmented by simultaneous pre and postsynaptic depolarization in the ~ 50% of slow waves that do not include ordered replay (Karlsson and Frank, 2009; Foster and Wilson, 2006). A disorderly replay would cause heterosynaptic depotentiation, caused by the asynchronous activation of post-then-presynaptic elements according to the rules of STDP. If postsynaptic activity is also not amplified by muscarinic acetylcholine receptor activation during nonREM sleep, then the intracellular cascade and absent strong activation of beta adrenergic receptors is set up to produce long term depression (LTD) instead of LTP. Small rises in intracellular Ca2+ set into motion a cascade of events that lead to LTD or depotentiation, rather than LTP (for review, see (Blitzer et al., 2005)).
Synaptic stabilization and embossing
Instead of de novo synaptic strengthening, nonREM sleep may be a time for the secondary and tertiary changes required to convert short term potentiation (early LTP, lasting minutes to a few hours) into long lasting LTP (late LTP, measured for months). Protein synthesis, which is required for long term LTP (late-LTP) (Krug et al., 1984; Frey et al., 1988; Otani et al., 1989), is extensively increased during the nonREM sleep (Ramm and Smith, 1990). Without protein synthesis, early LTP, which is protein synthesis-independent, would not turn into late-LTP and would last only last 4-6 hours (Abraham and Bear, 1996; Abraham, 2003; Krug et al., 1984; Frey et al., 1988; Reymann and Frey, 2007), which is, perhaps not by mere coincidence, the same critical window for sleep dependent consolidation of novel learning (see Sleep Deprivation section, below). Thus, nonREM sleep reactivation may serve to stabilize synapses by completing the necessary protein synthesis for those synapses that underwent sufficient LTP during prior wakefulness to induce such protein synthesis.
At the same time, nonREM sleep could serve to downscale synapses that were only weakly potentiated. If a neuron is used in an established cognitive circuit during wakefulness, then its synapses may be enhanced and ‘tagged’ (see below), but may not be sufficiently strengthened, e.g. by the extra reverberatory firing that novelty imparts or without the push toward LTP that neuromodulators like cortisol and noradrenalin induced by novelty would give. Such weak synaptic enhancement during wakeful cognition would create a synaptic tag (exact mechanisms still unknown, Frey and Morris, 1997, 1998; Frey and Frey, 2008) that would degrade over a few hours without stabilization with plasticity related proteins, if strong stimuli at another synapse does not set into motion the production of such plasticity related protein synthesis.
We propose that the approximate length of time that a tagged synapse has to be boosted or degraded probably depends on how near the synapse is to the cell soma and thus how much interference it receives through backpropagation (Golding et al., 2001) and how active the cell is in other circuits. The closer to the soma and more active the cell is in other cognitive tasks, the more likely early LTP is to be degraded through heterosynaptic depotentiation mechanisms. This process may explain proactive interference. The length of time that a tagged synapse has to survive before being degraded or enhanced probably also defines the timing of the sleep-critical window (Smith, 1985). Further, the strength of the waking experience may dictate whether only nonREM sleep-related protein synthesis is needed in that window, or de novo synaptic strengthening that can be induced by REM sleep reactivation. NonREM sleep-related synaptic interference could downscale weakly potentiated synapses whose ‘tag’ was not picked up. Protein synthesis in nonREM sleep could stabilize strongly strengthened synapses and their weakly tagged neighbors. But, it is probably only during REM sleep that synaptic remodeling (de novo LTP and targeted depotentiation) occurs, thereby embossing the memory system, downscaling some synapses and strengthening others, for all the reasons outlined in the REM sleep section, above.
If downscaling of reactivated familiar memories occurs during nonREM sleep (noise elimination), then the hypothesis that REM sleep is for forgetting purported by Francis Crick and Mitcheson in 1983(1983) would also apply to the state of nonREM sleep.
A very notable exception to the idea that nonREM sleep allows the strongly potentiated synapses (and strong paired with weak) to be stabilized while weak synapses are allowed to fade, is the activity and neurochemical environment surrounding the occurrence of sleep spindles, which also occur during nonREM sleep, but normally during the ascending Stage 2 of nonREM sleep, also known as intermediate sleep or the transition to REM sleep.
TR/Stage 2 sleep spindles
Siapas and Wilson (1998) and Siapas et al., (2005) showed that strong synchronization between the hippocampus and prefrontal cortex occurred during a state characterized by the simultaneous appearance of cortical sleep spindles (during nonREM sleep) and hippocampal theta (characterizing REM sleep) in the normal learning rat. Rats trained on a spatial maze showed enhanced correlated firing between these areas during a working spatial memory task compared with a nonspatial task (Jones and Wilson, 2005). But Romcy-Pereira and Pavlides (2004) found that transmission between the medial prefrontal cortex and the hippocampus is strongest during this nonREM sleep state. We have observed such dissociated states in learning rats (Emrick et al., 2008).
One of the fascinating characteristics of sleep spindles is that they are generated by the thalamic reticular nucleus, and do not occur in the presence of NE. In fact, locus coeruleus neurons fall silent in the second preceding each spindle (Aston-Jones and Bloom, 1981), allowing the reticular neurons to hyperpolarize until they emit Ca2+ spikes in the spindle frequency and coordinate the cortical projection neurons to cause cortical cells to oscillate together in a characteristic spindle (11-16 Hz) fashion (Lee and McCormick, 1996). Serotonin and ACh in the thalamus also prevent spindling. Thus spindles occur in a neurochemical environment similar to that of REM sleep, but perhaps in the absence of ACh and only in brief (1-3 s) bursts during nonREM sleep.
In several studies there is a very good correlation between spindle occurrences during sleep and waking learning (Molle et al., 2006). And in the highly plastic visual cortex of the kitten, synaptic changes after monocular deprivation are well correlated with spindle density (Frank et al., 2001). Datta et al. (Datta, 2000; Mavanji and Datta, 2003; Datta et al., 2004) found that the TR state showed the highest increase (>300%) after intensive two-way active avoidance training in those animals that retained the task best between the training and testing period.
If neuronal activity between the hippocampal output CA1/subiculum region and prefrontal cortex are coordinated during spindles or theta/spindle complexes, while NE is absent and serotonin levels are low, then the hippocampus could cause the same kind of targeted bidirectional synaptic reorganization in the prefrontal cortex during nonREM sleep that it may cause within itself during REM sleep. Further, the strong reactivation of the locus coeruleus that initiates the termination of each spindle (Aston-Jones and Bloom, 1981) could allow the de novo synaptic strengthening that the circuit could need for memory consolidation. Such coordination between the hippocampus and prefrontal cortex may not occur during REM sleep, as it has been observed that the two areas do not fire together during REM sleep.
Waking “offline” processing
The hippocampus also exhibits slow wave activity during “offline” waking, here called quiet waking. A paper by Foster and Wilson showed that reactivation sequences occurring in the hippocampus during waking slow waves are backward in firing order compared to waking experience (Foster and Wilson, 2006). Spike timing dependent plasticity principles would dictate that reversed reactivation sequences may actually reverse the LTP gains on the maze run. The CA1 cells we record receive their major inputs from the CA3 region of the hippocampus, which is extensively recurrently connected. If postsynaptic target neurons fire before the presynaptic inputs arrive, as would be the case with backwards reactivation, then LTD or depotentiation should result. Such a result would be consistent with the observation that increased periods of wakefulness from the time of training to testing, without intervening sleep, serve to interfere with memory performance. Waking reactivation may serve an opposing plasticity function to waking learning and sleep reactivation, consistent with behavioral data of reduced performance across post-training wakefulness (Jenkins and Dallenbach, 1924; Wamsley et al.). This unraveling of memory during prolonged wakefulness may be why the traits of sleep are so important for memory preservation and consolidation. The theoretical need for reactivation to occur offline is to interleave the new information in with the old in the absence of sensory interference (Jenkins and Dallenbach, 1924; Melton and Irwin, 1940; Winson, 1990; McClelland et al., 1995; Dave and Margoliash, 2000). In songbirds learning their song, Deregnaucourt et al (2005) found that across waking experience the song becomes more complex and error filled, but the song simplifies with the preservation of correct learned elements across sleep. Both song and speech learning literature indicates a 40-50% decrease in dendritic spines in zebra finches and mynah birds, respectively (Wallhausserfranke et al., 1995; Rausch and Scheich, 1982). It would make sense that such pruning of unneeded synaptic elements would occur during a sleep state where depotentiation and synaptic pruning were allowed.
Dissociated states
The traits mentioned for each state listed above are largely dissociable from that state, even under normal conditions. Thus it may be less useful to ask what state serves what cognitive function, than what set of traits is necessary for each stage of preparing the brain for maximal waking effectiveness. Persons suffering under depression have altered brain neurochemistry. It could be that the noradrenergic and serotonergic environment and hippocampal patterned reactivation necessary for memory consolidation could occur, under certain conditions (e.g. prolonged sleep restriction and psychiatric illness), during the normally short state of intermediate sleep at the transition from nonREM sleep to REM sleep (Lairy et al., 1967; Mahowald and Schenck, 1992). Intermediate sleep is much increased under certain psychiatric conditions; whereas in normal persons transitions are scored at less than 10%, under psychiatric illness it can comprise up to 40% of total sleep time (Akindele et al., 1970; Nielsen, 2000). Features of REM sleep can appear in other states, a phenomenon Neilsen called “covert REM” (Nielsen, 2000). Data from Subimal Datta’s lab shows drastic increases in transitions to REM sleep during intensive learning (Datta, 2000; Mavanji and Datta, 2003; Datta et al., 2004). However, very little overall time in any state with the right traits present may be necessary to accomplish LTP or depotentiation: since single bursts of theta frequency stimuli can cause LTP and depotentiation (Huerta and Lisman, 1995), such effect could be obtained in only a few seconds of replay in the proper phase of the ongoing EEG.
Sleep deprivation: physiological ramifications
Controversies and consensus
There have been many informative sleep loss studies looking at what changes between those deprived of all or various stages of sleep and paired controls, to understand how sleep is important to cognition. There have been some mixed results in the sleep deprivation and learning field, however. An important early review by McGrath and Cohen (McGrath, 1978) gave logical methodological reasons for the discrepancies, but still, given the relative dearth of knowledge in both the sleep and the learning fields, interest in the crossover between them waned for more than a decade. Some generalities that can be pulled from the data are as follows:
For rats, tasks requiring little change in the animal’s behavioral repertoire are REM sleep independent, according to Seligman (1970). When training intensity is high, the need for REM sleep for memory consolidation is more immediate (Smith, 1995); if REM sleep is prevented in the first few hours after training, memory for the task will be impaired even if total time spent in REM sleep is not diminished over the sleep period (Smith and Butler, 1982; Smith and Rose, 1996). Animals allowed to enter REM sleep in the first few hours perform normally the next day, even when deprived of REM sleep in a later time window of the sleep cycle. Thus the idea of critical REM windows for memory consolidation was introduced (Smith and Butler, 1982; Smith, 1985).
There may also be critical windows for nonREM sleep-dependent reactivation. The critical window for the conversion of early LTP to late LTP that varies with the strength of LTP as well as other factors (discussed above) could be the physiological basis for these critical sleep windows.
LTP effects of sleep deprivation
More recent studies have delved into the cellular consequences of sleep deprivation. REM sleep deprivation inhibits LTP induction and maintenance (Campbell et al., 2002; Davis et al., 2003; McDermott et al., 2003; Romcy-Pereira and Pavlides, 2004; Marks and Wayner, 2005). Even basic cellular excitability metrics are depressed after the platform over water method of REM sleep deprivation (McDermott et al., 2003).
Hippocampal neurogenesis
Hairston (2005) found that even only 6 hours of gentle REM sleep restriction impaired place learning and abolished the learning-dependent rise in hippocampal dentate gyrus neurogenesis. Two years earlier Guzman-Marin et al. (2003) found that total sleep deprivation for 96 hours on an intermittently powered treadmill reduced neurogenesis in the dentate by 40% and specifically those cells with neuronal cell markers by over 50% (Guzman-Marin et al., 2005). Because adult neurogenesis is involved in learning and memory (Gould et al., 1999), it is likely that such sleep deprivation induced reductions in neurogenesis would impact future learning. One unanswered question is how long such depression in neurogenesis lasts after recovery from sleep deprivation, and how long the behavioral effects of prior suppression of neurogenesis lasts.
Neurogenesis occurs in the dentate and CA3 regions of the hippocampus (Tonchev and Yamashima, 2006), the novelty encoding network. These are structures of the hippocampus that do not fire primarily at theta troughs in REM sleep (Buzsaki, personal communication) and, also unlike the CA1 region, they do not receive direct temperoammonic inputs from the entorhinal cortex layer III to their distal dendrites. Therefore these areas may not be able to depotentiate and refresh their synapses for novel encoding, but instead, once their synaptic structure is saturated, they must be replaced with new neurons with fresh dendritic trees. The inability to replenish saturated dendritic trees with new neurons may be the origins of the sense of brain saturation and of feeling cognitively impaired after periods of sleep deprivation.
Other informative effects of sleep deprivation
Because other chapters in this book cover the human and imaging sleep and cognition data, including the effects of sleep deprivation, we will only list some of the other effects that could give clues as to the mechanisms of sleep impacting cognition here. Sean Drummond’s lab has shown (Drummond et al., 2001) that sleep deprivation causes the subjects to recruit more brain areas in the performance of the same cognitive task. Matt Walker showed that hippocampal function is drastically reduced in an fMRI study after one overnight total sleep deprivation (Yoo et al., 2007). One of the arguments against the relationship between sleep and cognition is that people treated for depression with monoamine oxidase inhibitors and other antidepressants obtain little to no REM sleep for long periods of time, yet have no obvious learning and memory deficits. However, learning and memory deficits have been documented for persons and animals on antidepressant medications (Burt et al., 1995; Burgos et al., 2005). Furthermore, while it is true that drugs like mono-amine oxidase inhibitors (MAOIs) significantly reduce REM sleep time, depending on the dose and individual, they often do not eliminate REM sleep altogether; on any given night the amount of REM sleep recorded from persons on MAOIs is from 0-75% of baseline (Akindele et al., 1970; Nicholson et al., 1989; Riemann et al., 2001). Learning needs may make homeostatic demands on the REM sleep generating mechanisms (e.g. the PGO wave generator and/or the theta generator) that either temporarily increase REM sleep levels in these people despite their medications, or cause the traits to dissociate into other states. Interestingly, reticular stimulation either electrically or with carbachol (an ACh agonist) can reverse the mnemonic impairments imposed by REM sleep deprivation (Hars and Hennevin, 1983; Datta and Siwek, 2002).
Some Unsolved Mysteries
The mysteries still outnumber the things known and hold pace with the unsolved questions in both the sleep and learning fields. The principles of working memory are only beginning to be understood among neuroscientists, and there are few studies describing the effects of sleep loss on working memory (Hagewoud et al., 2010; Smith et al., 1998). It is not yet known whether memory recall always requires reconsolidation, the cellular basis for reconsolidation, and whether there is a sleep function for reconsolidation. There are major implications of sleep dependent reconsolidation for those suffering from sleep disturbance. For example, those with Alzheimer’s disease have abnormal sleep and would thus, perhaps, not want to perform memory recall exercises if reconsolidation mechanisms are not in place; memories could be erased faster. Do dreams matter? If our brain is metaplastic during REM sleep, and we are dreaming, would the content of the dream, even if not consciously remembered, change our synaptic network structure and thus change our mind?
Future directions
This chapter presents phenomenological studies that outline mechanisms through which sleep could serve learning and memory and general synaptic circuit reorganization. A missing piece of the puzzle is direct evidence that the events occurring during sleep have lasting effects on brain circuitry. The best studies published thus far were done by Frank and colleagues (Frank et al., 2001; Aton et al., 2009), who showed that sleep deprivation following monocular deprivation did not allow the plasticity events that occurred during wakefulness to stabilize and be amplified. But there are many tests of synaptic plasticity in intact functional circuits that can and should be employed in the online sleeping and learning brain. Such tests will probably reveal a startling amount of neural plasticity over sleep and waking states.
Summary
This chapter describes neurophysiologic processes during sleep that impact cognition and those processes compromised by sleep loss. REM sleep traits like PGO waves, theta synchrony, high acetylcholine levels, the loss of norepinephrine and serotonin and the return of plasticity related gene transcription allow for freely occurring bidirectional plasticity: long term potentiation (LTP) or its reversal, depotentiation. This bidirectional plasticity during REM sleep affords synaptic remodeling in support of learning and cognition in the hippocampal complex. Traits of nonREM Stage 2 sleep, especially spindles and the neurochemical milieu that promotes them, are also probably highly conducive to synaptic plasticity – perhaps allowing targeted bidirectional plasticity in the neocortex. Neuronal reactivation of waking sequences at peaks of slow wave delta activity during nonREM sleep, together with high protein synthesis levels, could serve to convert early LTP to late LTP, but not engender new plasticity. Reactivation events on delta waves are sometimes disordered, even backward, which could depotentiate synaptic circuits by the principles of spike timing dependent plasticity, especially as delta sleep is characterized by low acetylcholine levels and by the activation of genes supporting depotentiation. Such depotentiation events at delta peaks could serve to downscale synapses of certain circuits during nonREM sleep and perhaps quiet wakefulness. Finally, sleep deprivation studies revealed deficits in neuronal excitability and LTP mechanisms that would affect future learning as well as memory consolidation and possibly reconsolidation. The evidence set forth points to an overall function of embossing of synaptic circuits to add definition to and integrate memories, distinguishing them from the noise introduced to synaptic weights throughout wakefulness. The activity patterns, neurochemical and gene environments of sleep thus serve to clear noise and strengthen weakened networks of neural circuits for efficient subsequent cognitive processing demands.
Acknowledgments
Research described herein in the Poe et al., studies were supported by MH60670, MH 76280, and the Department of Anesthesiology at the University of Michigan. Special thanks to Victoria Booth, Ph.D. and to Brett Riley for their contributions to the development of some of these concepts.
Abbreviations
- 5HT
Serotonin
- ACh
Acetylcholine
- LC
locus coeruleus
- LTP
Long Term Potentiation
- LTD
Long Term Depression
- MAOIs
mono amine oxidase inhibitors
- MRF
medial reticular formation
- NE
Norepinephrine
- nonREM sleep
non rapid eye movement sleep
- PGO waves
ponto-geniculo-occipital waves
- REM sleep
rapid eye movement sleep
- TA
temporo-ammonic pathway
- TR
transition to REM sleep
References
- Abraham WC. How long will long-term potentiation last? Philos Trans R Soc Lond B Biol Sci. 2003;358:735–44. doi: 10.1098/rstb.2002.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–30. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Akindele MO, Evans JI, Oswald I. Mono-amine oxidase inhibitors, sleep and mood. Electroencephalogr Clin Neurophysiol. 1970;29:47–56. doi: 10.1016/0013-4694(70)90078-7. [DOI] [PubMed] [Google Scholar]
- Almaguer-Melian W, Rojas-Reyes Y, Alvare A, Rosillo JC, Frey JU, Bergado JA. Long-term potentiation in the dentate gyrus in freely moving rats is reinforced by intraventricular application of norepinephrine, but not oxotremorine. Neurobiol Learn Mem. 2005;83:72–8. doi: 10.1016/j.nlm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–66. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernández G, Elger C, Fell J. Memory formation by neuronal synchronization. Brain Research Reviews. 2006;52:170–182. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Basheer R, Sherin JE, Saper CB, Morgan JI, Mccarley RW, Shiromani PJ. Effects of sleep on wake-induced c-fos expression. J Neurosci. 1997;17:9746–50. doi: 10.1523/JNEUROSCI.17-24-09746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–72. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Iyengar R, Landau EM. Postsynaptic signaling networks: cellular cogwheels underlying long-term plasticity. Biological psychiatry. 2005;57:113–9. doi: 10.1016/j.biopsych.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier Saunders; 2005. [Google Scholar]
- Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–95. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Maho C, Laroche S. Suppression of long-term potentiation induction during alert wakefulness but not during ‘enhanced’ REM sleep after avoidance learning. Neuroscience. 1994;59:501–9. doi: 10.1016/0306-4522(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Srebro B. Synaptic plasticity in the hippocampus is modulated by behavioral state. Brain Res. 1989;493:74–86. doi: 10.1016/0006-8993(89)91001-9. [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Manahan-Vaughan D. Long-term depression: a cellular basis for learning? Rev Neurosci. 2001;12:121–40. doi: 10.1515/revneuro.2001.12.2.121. [DOI] [PubMed] [Google Scholar]
- Brown G, Mccormack T. The role of time in human memory and binding: A review of the evidence. Binding in human memory: A neurocognitive approach. 2006:251–290. [Google Scholar]
- Burgos H, Mardones L, Campos M, Castillo A, Fernandez V, Hernandez A. Chronic treatment with clomipramine and desipramine induces deficit in long-term visuo-spatial memory of rats. Int J Neurosci. 2005;115:47–54. doi: 10.1080/00207450490512641. [DOI] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–71. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Lydic R, Baghdoyan HA, Hobson JA. Pontogeniculooccipital waves: spontaneous visual system activity during rapid eye movement sleep. Cell Molec Neurobiol. 1987;2:105–49. doi: 10.1007/BF00711551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–6. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Cheng R, Williams C, Meck W. Oscillatory bands, neuronal synchrony and hippocampal function: Implications of the effects of prenatal choline supplementation for sleep-dependent memory consolidation. Brain Research. 2008;1237:176–194. doi: 10.1016/j.brainres.2008.08.077. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000a;20:9187–94. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000b;885:303–21. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. Consciousness and neuroscience. Cereb Cortex. 1998;8:97–107. doi: 10.1093/cercor/8.2.97. [DOI] [PubMed] [Google Scholar]
- Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–4. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- Datta S. Avoidance Task Training Potentiates Phasic Pontine-Wave Density in the Rat: A Mechanism for Sleep-Dependent Plasticity. Journal of Neuroscience. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2004;24:1416–27. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:611–21. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30:409–23. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song Replay During Sleep and Computational Rules for Sensorimotor Vocal Learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–7. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- De Koninck J, Lorrain D, Christ G, Proulx G, Coulombe D. Intensive language learning and increases in rapid eye movement sleep: evidence of a performance factor. Int J Psychophysiol. 1989;8:43–7. doi: 10.1016/0167-8760(89)90018-4. [DOI] [PubMed] [Google Scholar]
- Derégnaucourt S, Mitra PP, Fehér O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433:710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Brown GG. Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res. 2001;10:85–92. doi: 10.1046/j.1365-2869.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Guerrien A, Leconte P. Sleep, brain activation and cognition. Physiol Behav. 1990;47:1271–8. doi: 10.1016/0031-9384(90)90382-e. [DOI] [PubMed] [Google Scholar]
- Emrick J, Riley B, Cropp E, Zheng C, Booth V, Poe G. Society for Neuroscience. Washington, D.C.: 2008. Cortical and hippocampal EEG show different simultaneous sleep states after learning. Society for Neuroscience Abstract. [Google Scholar]
- Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30:1617–30. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69:86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ferrara M, Iaria G, Tempesta D, Curcio G, Moroni F, Marzano C, De Gennaro L, Pacitti C. Sleep to find your way: the role of sleep in the consolidation of memory for navigation in humans. Hippocampus. 2008;18:844–51. doi: 10.1002/hipo.20444. [DOI] [PubMed] [Google Scholar]
- Fishbein W, Kastaniotis C, Chattman D. Paradoxical sleep: prolonged augmentation following learning. Brain Res. 1974;79:61–75. doi: 10.1016/0006-8993(74)90566-6. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–3. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Fox SE, Wolfson S, Ranck JB., Jr Hippocampal theta rhythm and the firing of neurons in walking and urethane anesthetized rats. Exp Brain Res. 1986;62:495–508. doi: 10.1007/BF00236028. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, Stryker MP. Sleep Enhances Plasticity in the Developing Visual Cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Givens B. Stimulus-evoked resetting of the dentate theta rhythm: relation to working memory. Neuroreport. 1996;8:159–63. doi: 10.1097/00001756-199612200-00032. [DOI] [PubMed] [Google Scholar]
- Golding NL, Kath WL, Spruston N. Dichotomy of action-potential backpropagation in CA1 pyramidal neuron dendrites. J Neurophysiol. 2001;86:2998–3010. doi: 10.1152/jn.2001.86.6.2998. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Guerrien A, Dujardin K, Mandai O, Sockeel P, Leconte P. Enhancement of memory by auditory stimulation during postlearning REM sleep in humans. Physiol Behav. 1989;45:947–50. doi: 10.1016/0031-9384(89)90219-9. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, Mcginty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci. 2005;22:2111–6. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Suntsova N, Stewart DR, Gong H, Szymusiak R, Mcginty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol. 2003;549:563–71. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Novati A, Keijser JN, Van Der Zee EA, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res. 2010;19:280–8. doi: 10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, Heller HC. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–33. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Faraguna U, Vyazovskiy VV, Tononi G, Cirelli C. Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep. 2009;32:719–29. doi: 10.1093/sleep/32.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hars B, Hennevin E. Reminder abolishes impairment of learning induced by paradoxical sleep retardation. Physiol Behav. 1983;30:831–6. doi: 10.1016/0031-9384(83)90244-5. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Acetylcholine and memory. Trends Neurosci. 1993;16:218–22. doi: 10.1016/0166-2236(93)90159-j. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Hars B, Bloch V. Improvement of learning by mesencephalic reticular stimulation during postlearning paradoxical sleep. Behav Neural Biol. 1989;51:291–306. doi: 10.1016/s0163-1047(89)90948-5. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Hars B, Maho C, Bloch V. Processing of learned information in paradoxical sleep: relevance for memory. Behav Brain Res. 1995;69:125–35. doi: 10.1016/0166-4328(95)00013-j. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Leconte P, Bloch V. Paradoxical sleep increase triggered by learning, extinction and relearning of a response based on a positive reinforcement. Brain Res. 1974;70:43–54. doi: 10.1016/0006-8993(74)90210-8. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–63. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci. 2003;23:11725–31. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JG, Dallenbach KM. Obliviscence during Sleep and Waking. The American Journal of Psychology. 1924;35:605–612. [Google Scholar]
- Jones M, Wilson M. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biology. 2005;3:2187. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet M. Recherches sur les structures nerveuses et les mechanismes responsables des différentes phases du sommeil physiologique. Arch Ital Biol. 1962;100:125–206. [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–8. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJM, Sagi D. Dependence on rem Sleep of Overnight Improvement of a Perceptual Skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Izumi Y, Zorumski CF. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol. 1997;77:3013–20. doi: 10.1152/jn.1997.77.6.3013. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–7. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Krug M, Lossner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, Mcnaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression. J Neurosci. 1991;11:2931–7. doi: 10.1523/JNEUROSCI.11-09-02931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairy GC, Goldsteinas L, Guennoc A. Disturbances of sleep in patients with confusional and demential syndromes. Electroencephalogr Clin Neurophysiol. 1967;23:286. [PubMed] [Google Scholar]
- Lapierre JL, Kosenko PO, Lyamin OI, Kodama T, Mukhametov LM, Siegel JM. Cortical acetylcholine release is lateralized during asymmetrical slow-wave sleep in northern fur seals. J Neurosci. 2007;27:11999–2006. doi: 10.1523/JNEUROSCI.2968-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Mccormick DA. Abolition of Spindle Oscillations by Serotonin and Norepinephrine in the Ferret Lateral Geniculate and Perigeniculate Nuclei In Vitro. Neuron-Cambridge Ma- 1996;17:309–321. doi: 10.1016/s0896-6273(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Leonard BJ, Mcnaughton BL, Barnes CA. Suppression of hippocampal synaptic plasticity during slow-wave sleep. Brain Res. 1987;425:174–7. doi: 10.1016/0006-8993(87)90496-3. [DOI] [PubMed] [Google Scholar]
- Llinas R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci U S A. 1993;90:2078–81. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally Structured Replay of Awake Hippocampal Ensemble Activity during Rapid Eye Movement Sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Lucero MA. Lengthening of REM sleep duration consecutive to learning in the rat. Brain Res. 1970;20:319–22. doi: 10.1016/0006-8993(70)90299-4. [DOI] [PubMed] [Google Scholar]
- Mahowald MW, Schenck CH. Dissociated states of wakefulness and sleep. Neurology. 1992;42:44–51. discussion 52. [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–44. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai O, Guerrien A, Sockeel P, Dujardin K, Leconte P. REM sleep modifications following a Morse code learning session in humans. Physiol Behav. 1989;46:639–42. doi: 10.1016/0031-9384(89)90344-2. [DOI] [PubMed] [Google Scholar]
- Marks CA, Wayner MJ. Effects of sleep disruption on rat dentate granule cell LTP in vivo. Brain Res Bull. 2005;66:114–9. doi: 10.1016/j.brainresbull.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, FA M, Giagheddu M, Imperato A, Gessa GL. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–32. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- Mavanji V, Datta S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: a mechanism for sleep-dependent plasticity. Eur J Neurosci. 2003;17:359–70. doi: 10.1046/j.1460-9568.2003.02460.x. [DOI] [PubMed] [Google Scholar]
- Mcclelland JL, Mcnaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–57. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Mcdermott CM, Lahoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–95. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcginty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–75. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Mcgrath MJ, Cohen DB. REM sleep facilitation of adaptive waking behavior: a review of the literature. Psychological Bulletin. 1978;85:24–57. [PubMed] [Google Scholar]
- Mednick SC, Nakayama K, Cantero JL, Atienza M, Levin AA, Pathak N, Stickgold R. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2002;5:677–81. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- Melton AW, Irwin JM. The influence of degree of interpolated learning on retroactive inhibition and the overt transfer of specific responses. American Journal of Psychology. 1940;53:175–203. [PubMed] [Google Scholar]
- Meneses A, Hong E. Effects of 5-HT4 receptor agonists and antagonists in learning. Pharmacol Biochem Behav. 1997;56:347–51. doi: 10.1016/s0091-3057(96)00224-9. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Perez GM, Alvarado MC, Barnes CA, Mcnaughton BL. Reversible inactivation of the medial septum differentially affects two forms of learning in rats. Brain Res. 1990;528:12–20. doi: 10.1016/0006-8993(90)90188-h. [DOI] [PubMed] [Google Scholar]
- Molle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol. 2006;96:62–70. doi: 10.1152/jn.00014.2006. [DOI] [PubMed] [Google Scholar]
- Nakao K, Ikegaya Y, Yamada MK, Nishiyama N, Matsuki N. Hippocampal long-term depression as an index of spatial working memory. Eur J Neurosci. 2002;16:970–4. doi: 10.1046/j.1460-9568.2002.02159.x. [DOI] [PubMed] [Google Scholar]
- Nicholson AN, Belyavin AJ, Pascoe PA. Modulation of rapid eye movement sleep in humans by drugs that modify monoaminergic and purinergic transmission. Neuropsychopharmacology. 1989;2:131–43. doi: 10.1016/0893-133x(89)90016-x. [DOI] [PubMed] [Google Scholar]
- Nielsen TA. A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci. 2000;23:851–66. doi: 10.1017/s0140525x0000399x. discussion 904-1121. [DOI] [PubMed] [Google Scholar]
- Orr G, Rao G, Houston FP, Mcnaughton BL, Barnes CA. Hippocampal synaptic plasticity is modulated by theta rhythm in the fascia dentata of adult and aged freely behaving rats. Hippocampus. 2001;11:647–54. doi: 10.1002/hipo.1079. [DOI] [PubMed] [Google Scholar]
- Otani S, Marshall CJ, Tate WP, Goddard GV, Abraham WC. Maintenance of long-term potentiation in rat dentate gyrus requires protein synthesis but not messenger RNA synthesis immediately post-tetanization. Neuroscience. 1989;28:519–26. doi: 10.1016/0306-4522(89)90001-8. [DOI] [PubMed] [Google Scholar]
- Park SP, Lopez-Rodriguez F, Wilson CL, Maidment N, Matsumoto Y, Engel J., Jr In vivo microdialysis measures of extracellular serotonin in the rat hippocampus during sleep-wakefulness. Brain Res. 1999;833:291–6. doi: 10.1016/s0006-8993(99)01511-5. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Greenstein YJ, Grudman M, Winson J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 1988;439:383–7. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9:2907–18. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe GR, Nitz DA, McNaughton BL, Barnes CA. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855:176–80. doi: 10.1016/s0006-8993(99)02310-0. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Cirelli C, Arrighi P, Tononi G. c-Fos expression during wakefulness and sleep. Neurophysiol Clin. 1995;25:329–41. doi: 10.1016/0987-7053(96)84906-9. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Cirelli C, Ronca-Testoni S, Tononi G. NGFI-A expression in the rat brain after sleep deprivation. Brain Res Mol Brain Res. 1997;46:143–53. doi: 10.1016/s0169-328x(96)00295-1. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Cirelli C, Tononi G. Immediate-early genes in spontaneous wakefulness and sleep: expression of c-fos and NGFI-A mRNA and protein. J Sleep Res. 1994;3:80–96. doi: 10.1111/j.1365-2869.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Portell-Cortes I, Marti-Nicolovius M, Segura-Torres P, Morgado-Bernal I. Correlations between paradoxical sleep and shuttle-box conditioning in rats. Behav Neurosci. 1989;103:984–90. doi: 10.1037//0735-7044.103.5.984. [DOI] [PubMed] [Google Scholar]
- Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiol Behav. 1990;48:749–53. doi: 10.1016/0031-9384(90)90220-x. [DOI] [PubMed] [Google Scholar]
- Rashidy-Pour A, Motamedi F, Semnanian S, Zarrindast MR, Fatollahi Y, Behzadi G. Effects of reversible inactivation of the medial septal area on long-term potentiation and recurrent inhibition of hippocampal population spikes in rats. Brain Research. 1996;734:43–48. [PubMed] [Google Scholar]
- Rausch G, Scheich H. Dendritic spine loss and enlargement during maturation of the speech control system in the mynah bird (Gracula religiosa) Neuroscience Letters. 1982;29:129–133. doi: 10.1016/0304-3940(82)90341-x. [DOI] [PubMed] [Google Scholar]
- Reymann KG, Frey JU. The late maintenance of hippocampal LTP: requirements, phases, ‘synaptic tagging’, ‘late-associativity’ and implications. Neuropharmacology. 2007;52:24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Goyal V, Mello CV, Pavlides C. Brain gene expression during REM sleep depends on prior waking experience. Learn Mem. 1999;6:500–8. doi: 10.1101/lm.6.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J Neurosci. 2002;22:10914–23. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Nicolelis MA. Reverberation, storage, and postsynaptic propagation of memories during sleep. Learn Mem. 2004;11:686–96. doi: 10.1101/lm.75604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, Tononi G. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30:1643–57. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D, Berger M, Voderholzer U. Sleep and depression--results from psychobiological studies: an overview. Biol Psychol. 2001;57:67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- Romcy-Pereira R, Pavlides C. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci. 2004;20:3453–62. doi: 10.1111/j.1460-9568.2004.03808.x. [DOI] [PubMed] [Google Scholar]
- Seligman M. On the generality of the laws of learning. Psychol Rev. 1970;77:406–418. [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–51. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–8. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–63. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. Sleep states and learning: a review of the animal literature. Neurosci Biobehav Rev. 1985;9:157–68. doi: 10.1016/0149-7634(85)90042-9. [DOI] [PubMed] [Google Scholar]
- Smith C. Sleep states and memory processes. Behav Brain Res. 1995;69:137–45. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- Smith C, Butler S. Paradoxical sleep at selective times following training is necessary for learning. Physiol Behav. 1982;29:469–73. doi: 10.1016/0031-9384(82)90268-2. [DOI] [PubMed] [Google Scholar]
- Smith C, Lapp L. Increases in number of REMS and REM density in humans following an intensive learning period. Sleep. 1991;14:325–30. doi: 10.1093/sleep/14.4.325. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav. 1996;59:93–7. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Smith C, Wong PT. Paradoxical sleep increases predict successful learning in a complex operant task. Behav Neurosci. 1991;105:282–8. doi: 10.1037//0735-7044.105.2.282. [DOI] [PubMed] [Google Scholar]
- Smith C, Young J, Young W. Prolonged increases in paradoxical sleep during and after avoidance- task acquisition. Sleep. 1980;3:67–81. [PubMed] [Google Scholar]
- Smith CT, Conway JM, Rose GM. Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiol Learn Mem. 1998;69:211–7. doi: 10.1006/nlme.1997.3809. [DOI] [PubMed] [Google Scholar]
- Steriade M. Acetylcholine systems and rhythmic activities during the waking-sleep cycle. Progress in Brain Research. 2004;145:179–196. doi: 10.1016/S0079-6123(03)45013-9. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O’Dell TJ. Activity Dependent Beta-Adrenergic Modulation of Low Frequency Stimulation Induced LTP in the Hippocampal CA1 Region. Neuron. 1996;17:475–482. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- Tonchev AB, Yamashima T. Differential neurogenic potential of progenitor cells in dentate gyrus and CA1 sector of the postischemic adult monkey hippocampus. Exp Neurol. 2006;198:101–13. doi: 10.1016/j.expneurol.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Tsukamoto-Yasui M, Sasaki T, Matsumoto W, Hasegawa A, Toyoda T, Usami A, Kubota Y, Ochiai T, Hori T, Matsuki N, Ikegaya Y. Active hippocampal networks undergo spontaneous synaptic modification. PLoS One. 2007;2:e1250. doi: 10.1371/journal.pone.0001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH, Stewart DJ. Joint cholinergic-serotonergic control of neocortical and hippocampal electrical activity in relation to behavior: effects of scopolamine, ditran, trifluoperazine and amphetamine. Physiol Behav. 1986;38:57–65. doi: 10.1016/0031-9384(86)90132-0. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brain stem generation of the hippocampal EEG. Prog Neurobiol. 1982;19:159–86. doi: 10.1016/0301-0082(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V, Borbely AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res. 2000;9:367–71. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30:1631–42. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallhausserfranke E, Nixdorfbergweiler B, Devoogd T. Song isolation is associated with maintaining high spine frequencies on zebra finch IMAN neurons. Neurobiology of Learning and Memory. 1995;64:25–35. doi: 10.1006/nlme.1995.1041. [DOI] [PubMed] [Google Scholar]
- Walter T. REM Illumination: Memory Consolidation. Grove City, Ohio: Lotus Magnus, LLC; 2007. [Google Scholar]
- Wamsley EJ, Tucker MA, Payne JD, Stickgold R. A brief nap is beneficial for human route-learning: The role of navigation experience and EEG spectral power. Learn Mem. 17:332–6. doi: 10.1101/lm.1828310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Mcnaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Winson J. Loss of Hippocampal Theta Rhythm Results in Spatial Memory Deficit in the Rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Winson J. Brain and Psyche: The Biology of the Unconscious. Garden City, NY: Anchor Press/Doubleday; 1985. [Google Scholar]
- Winson J. The meaning of dreams. Sci Am. 1990;263:86–8. 90–2, 94–6. doi: 10.1038/scientificamerican1190-86. [DOI] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394:891–4. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- Yang HW, Lin YW, Yen CD, Min MY. Change in bi-directional plasticity at CA1 synapses in hippocampal slices taken from 6-hydroxydopamine-treated rats: the role of endogenous norepinephrine. Eur J Neurosci. 2002;16:1117–28. doi: 10.1046/j.1460-9568.2002.02165.x. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]


