Summary
Enveloped viruses acquire their membrane from the host by budding at, or wrapping by, cellular membranes. Transmission electron microscopy (TEM) images, however, suggested that the prototype member of the poxviridae, vaccinia virus (VACV), may create its membrane ‘de novo’ with free open ends exposed in the cytosol. Within the frame of the German-wide priority programme we re-addressed the biogenesis and origin of the VACV membrane using electron tomography (ET), cryo-EM and lipid analysis of purified VACV using mass spectrometry (MS). This review discussed how our data led to a model of unconventional membrane biogenesis involving membrane rupture and the generation of a single open membrane from open membrane intermediates. Lipid analyses of purified virus by MS suggest an ER origin with a relatively low cholesterol content compared with whole cells, confirming published data. Unlike previous reports using thin-layer chromatography, no depletion of phosphatidylethanolamine was detected. We did detect, however, an enrichment for phosphatidic acid, diacylglycerol and phosphatidylinositol in the virion. Our data are discussed in the light of other pathogens that may require cellular membrane rupture during their intracellular life cycle.
Introduction
Viruses are small parasites of the cell that rely entirely on their host for replication. Many RNA or DNA viruses consists of just about 5–10 proteins and require the cell for vital functions such as protein synthesis, transport and membrane acquisition. With a genome encoding for about 200 proteins the prototype member of the Poxviridae, vaccinia virus (VACV), is no exception to this rule and cannot replicate without the cells it infects.
Enveloped viruses acquire their membrane from the host by budding at a cellular membrane or, less commonly, by wrapping of a cellular cisterna.
In 1968, however, Samuel Dales postulated that the biogenesis of the precursor membrane of VACV may be an exception to this rule. Based on conventional transmission electron microscopy (TEM), using embedding methods that were common at that time, he postulated that the VACV membrane consists of a single membrane made ‘de novo’, with open ends in the cytoplasm (Dales and Mosbach, 1968). Ever since this proposal the biogenesis and origin of the VACV membrane has been the subject of controversies. The general consensus in cell biology is that all membranes are made from pre-existing membranes and are closed upon themselves, mediated by specific membrane fission and fusion reactions. Budding or wrapping of viruses complies to this cell biological dogma and both resemble cellular processes; the formation of transport vesicles (budding) and autophagy (wrapping). So why should VACV be an exception?
On the biogenesis of the crescent membrane; what are the problems with EM?
Let us take one step back and consider what can be observed by TEM in VACV-infected cells. Unlike other DNA viruses, poxviruses are able to replicate their DNA in the cytoplasm since their large genome encodes for proteins that enable for DNA replication independent of the cellular nucleus. By EM the sites of replication appear from about 2–3 h post infection as large areas surrounded by the ER (see, e.g. Tolonen et al., 2001). From about 5–6 h post infection these DNA areas display small half-moon-shaped membranes, also called ‘crescents’ (Fig. 1A). These develop to form spheres with a diameter of approximately 350 nm, the so-called immature virus (IV; Fig. 1A). When the IV takes up DNA it undergoes a dramatic rearrangement to form a quasi brick-shaped particle, the mature virus (MV; Fig. 1B), a process that is accompanied by, and requires, the cleavage of several structural proteins of the virion (Katz and Moss, 1970; Ansarah-Sobrinho and Moss, 2004). MVs typically move out of the DNA site and leave the cell as reviewed in detail elsewhere (Smith and Law, 2004; Condit et al., 2006).
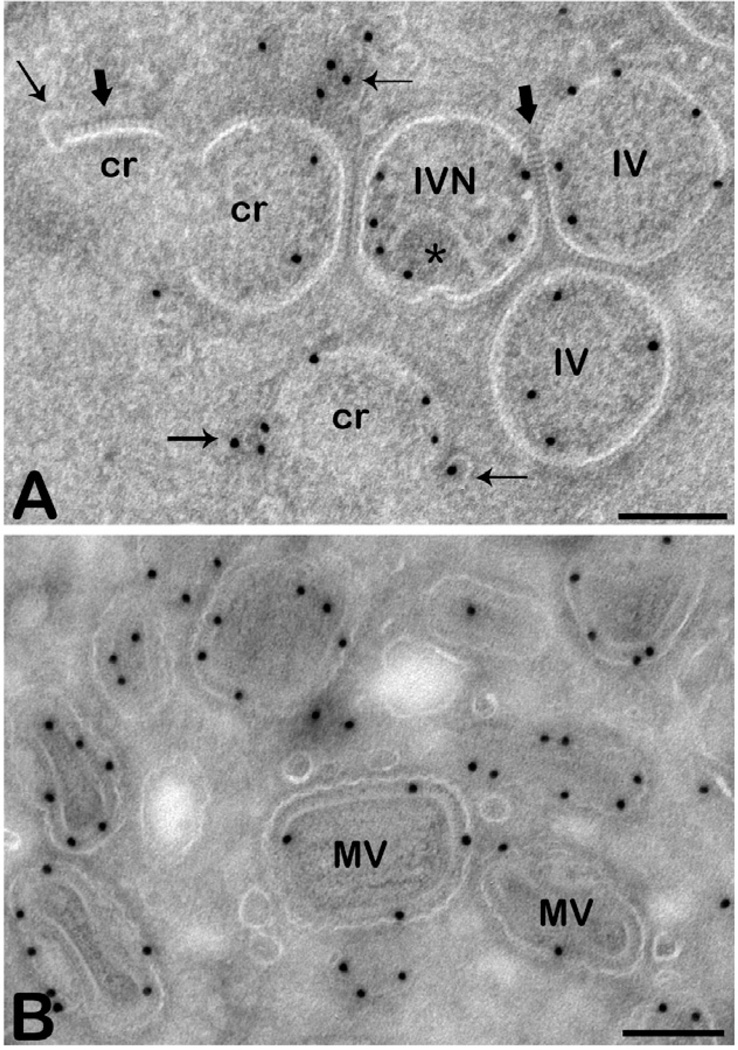
Fig. 1.
Different viral forms. The images show thawed cryo-sections of HeLa cells prepared at 12 h post infection with VACV and immunolabelled with anti-A14, a major membrane protein of VACV.
A. The immature forms; crescents (cr) that can appear as small arcs or as incomplete circles, immature virions (IVs) that appear as circles and immature virions with nucleoid (IVN, star indicates the DNA). Note that the crescents are often in close proximity to labelled membranes with which they sometimes seem to be continuous (thin arrows). The thick arrows point to the spike-like appearing scaffold on the convex side of the crescent.
B. Different views of mature virus (MV); although the MV is homogenous in size and shape the particle is asymmetric (brick-shaped) leading to different appearances by thin section EM. Scale bars-200 nm.
How is the crescent made? Using conventional embedding (see for instance Fig. 2A) Samuel Dales proposed that the crescent is composed of only one membrane since his images displayed only one structure representing a unit membrane. Since this membrane seemingly exposed both ends into the cytoplasm (see for instance Fig. 1A) and since it was not obviously connected to other membranes in his images, he proposed that it was made ‘de novo’ (Dales and Mosbach, 1968). While this model prevailed for quite some years in the poxvirus field, it was seriously challenged in the early nineties by Sodeik and colleagues (Sodeik et al., 1993). From a cell biological point of view this model did not make sense; within cells membranes are made from pre-existing membranes and all membranes are closed upon themselves. There is no mechanism to explain how phospholipids are transported through the hydrophilic cytoplasm to create a new membrane and how open membrane ends are stabilized in this environment (reviewed in Sodeik and Krijnse Locker, 2002).
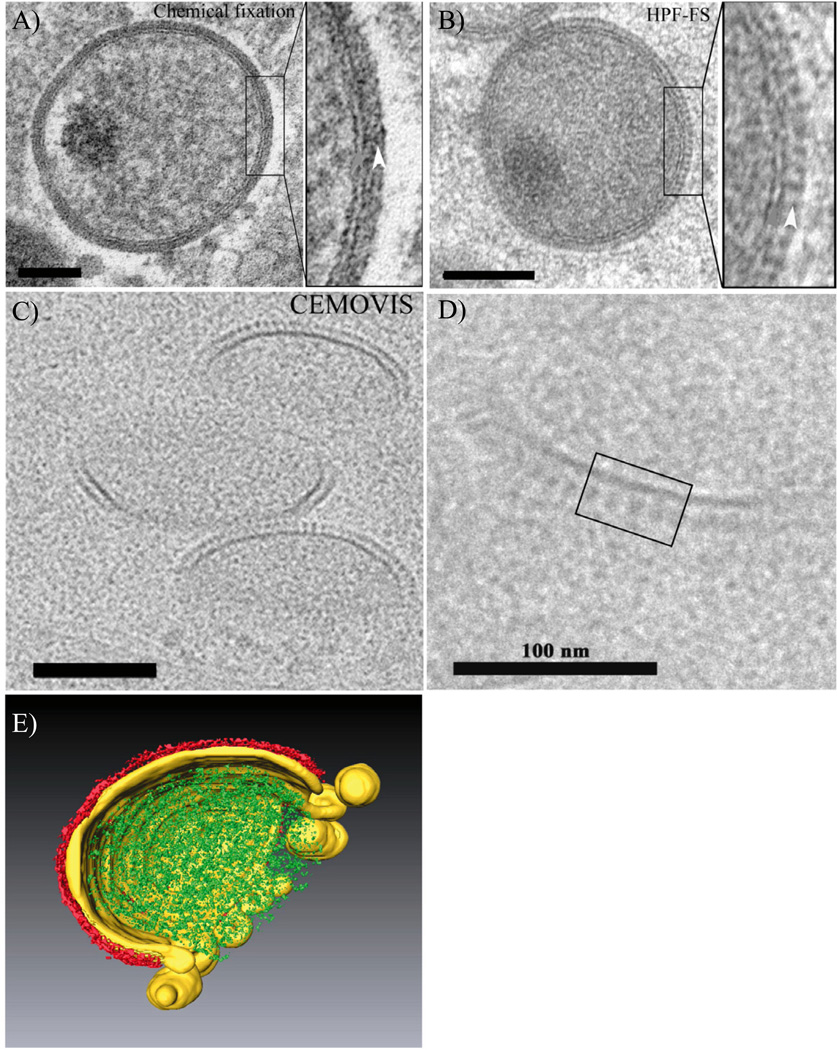
Fig. 2.
Appearance of the crescent by conventional embedding, CEMOVIS and by 3D rendering.
A–D. The images are from HeLa cells infected for 8 h with VACV. In (A) the cells were chemically fixed and embedded at room temperature in epoxy resin. In (B) to (D) the cells were preserved by high pressure freezing (HPF) and in (B) embedded in lowicryl after freeze substitution (FS) whereas in (C) and (D) the cells were imaged by CEMOVIS. The latter reveals two separate layers with different appearances that are not seen the same way after dehydration and resin embedding.
E. A typical crescent analysed by ET and the tomogram was subjected to volume segmentation. It shows how the growing crescent membrane (yellow), coated on the outside by the scaffold protein (red), is connected to a collection of open membrane structures that contribute to its formation. The connected membranes typically curve in the opposite direction compared with the forming crescent and are not coated with scaffold. The growing crescent forms around the viral core proteins (green).
The images are from Chlanda et al. (2009) reproduced with permission.
Based on these cell biological dogmas Sodeik et al. went on to challenge the hypothesis of Dales. From their data they concluded that the crescent is a membrane cisterna derived from the ER, membrane acquisition of VACV occurs by wrapping, thus surrounding the virus by two, rather than one, membranes (Sodeik et al., 1993). The most convincing piece of evidence in this study was the use of proteases, applied to cells of which the plasma membrane was permeabilized, showing that under these conditions the crescent membrane appeared as two separate layers, which the authors interpreted as two membranes (Sodeik et al., 1993). The proposal by Sodeik et al. in 1993 was subsequently challenged mainly by two publications proposing one membrane only (Hollinshead et al., 1999) (Heuser, 2005). Thus Hollinshead and colleagues (Hollinshead et al., 1999) re-analysed the crescent membrane and compared it to cellular membranes using essentially the same embedding method as Dales. They saw only one membrane and concluded that this membrane was coated by an 8-nm-thick protein coat.
The core of the collective data described above was based on TEM, which inherently carries two main limitations; the samples should be thin and dry. Considering the latter, biological samples generally contain about 80% water thus (most) embedding techniques require complete dehydration. An important consideration in EM should thus be whether this creates unwanted artefacts; for membranes, dehydration by ethanol or acetone could result in membrane collapse and lipid extraction, making two membranes appear as one. The second limitation of TEM is a relatively small electron penetration through the sample and cells that are on average 10 mm thick must be sectioned in to 60–70 nm thin sections for routine TEM. Thus TEM images are two-dimensional projections (of thin slices) of cells that contain three-dimensional structures. In the case of VACV-infected cells conventional TEM displays the crescent or IV as an arc or a circle (Fig. 1A) whereas in reality it is a partial or complete sphere respectively.
In his study John Heuser applied different EM techniques relying on deep etching and freeze fracture. His study concluded that the crescent is a single membrane coated on its convex side by a scaffold protein adopting a honeycomb-like structure (Heuser, 2005). However, whether the outer layer contains a membrane or is a modified membrane, which may not split upon freeze fracture was unclear.
Within the frame of the German scientific network ‘dynamics of membranes and its exploitation by viruses’ we addressed these limitations by applying two EM techniques that were relatively novel in 2007; cryo-EM of vitreous sections and electron tomography.
Cryo-electron microscopy of vitreous sections (CEMOVIS) and electron tomography to dissect the biogenesis of the VACV membrane
CEMOVIS is an EM technique mainly developed by the group of Jacques Dubochet, the father of cryo-EM. In the early eighties Dubochet revolutionized EM techniques by showing that samples vitrified in a thin layer of ice can be observed in a frozen state by EM (Adrian et al., 1984; reviewed in Dubochet, 2012). The advantage of this technique is obvious; samples are observed in a fully hydrated state without chemical fixation and dehydration used in conventional embedding. Already early on Dubochet and colleagues expanded the technique of cryo-EM to sections of cells, but were confronted with the formidable challenge of freezing cells without creating ice-crystals causing serious structural damage (McDowall et al., 1983). The preservation of cells by high pressure freezing (HPF) becoming a routine technique opened the way for cryo-EM of vitreous sections (reviewed in Al-Amoudi et al., 2004). Cells are rapidly frozen using a specialized machine (high pressure freezer, such as to create amorphous ice up to 200 mm in thickness; reviewed in Studer et al., 2008), cryo-sectioned and observed by cryo-EM in a fully hydrated state, enabling (so is the prospect) to observed the true nature of structures.
CEMOVIS showed the two crescent layers as two entities displaying two different features; one layer was continuous, the other discontinuous with a spike-like appearance (Chlanda et al., 2009). CEMOVIS also enabled us to measure the size of the layers without putative dehydration (shrinking) artefacts. This showed that the inner layer had an average diameter of 6 nm, consistent with the thickness of a membrane, whereas the discontinuous outer layer formed by the scaffold protein (and putative scaffold-associated proteins) was 11 nm thick. It thus seemed likely that the crescent consisted of only one membrane, shaped by the scaffold protein located on its convex side. To investigate whether the scaffold layer disguises an additional (second) membrane we used the drug rifampicin that specifically blocks the assembly of the VACV scaffold protein, the gene product of D13, on the crescent membrane (McNulty-Kowalczyk and Paoletti, 1993) and results in structures (rifampicin bodies) surrounded by membranes. By CEMOVIS the latter appeared as a single unit, with features of a membrane and not coated by the, thicker, spike-like layer (Chlanda et al., 2009). We thus proposed that the crescent contains one membrane only. By conventional EM techniques the two distinct layers were not well resolved, possibly because they collapse during dehydration.
If the crescent consists of one membrane how is this membrane made; is it made ‘de novo’ as proposed originally by Dales and colleagues? Similar to the original images of Dales ‘thin section-CEMOVIS’ displayed the crescent as an arc with open ends and without obvious continuities with other membranes (Fig. 2C and D). However, whether this curved line, which in fact is a part of a sphere, is continuous with other structures in three dimensions cannot be appreciated on thin sections. We used electron tomography (ET) to overcome this limitation of conventional TEM. ET relies on the use of slightly thicker samples (about 300 nm) and a specialized intermediate voltage (200–300 kV) TEM that is able to tilt the specimen and automatically acquire tilt series of two-dimensional projections, which are computationally aligned and used to reconstruct a tomogram (reviewed in Grünewald et al., 2003). This way one can obtain a three dimensional image of cellular structures, in particular of continuities. In the case of the crescent this led to two astonishing observations. In three dimensions its membrane appeared continuous with small membrane structures that obviously contributed to the formation of the crescent membrane (Fig. 2E). An even more striking observation was that many of these membrane structures were open. Apparently in VACV-infected cells some factor(s) was able to produce single open membrane structures that contributed to a single open membrane sphere, shaped by the assembly on its convex side of the viral scaffold protein (Fig. 2E). Thus, as summarized in Fig. 3 we proposed that the single membrane of VACV involves membrane biogenesis via open membrane intermediates, generated by membrane rupture.
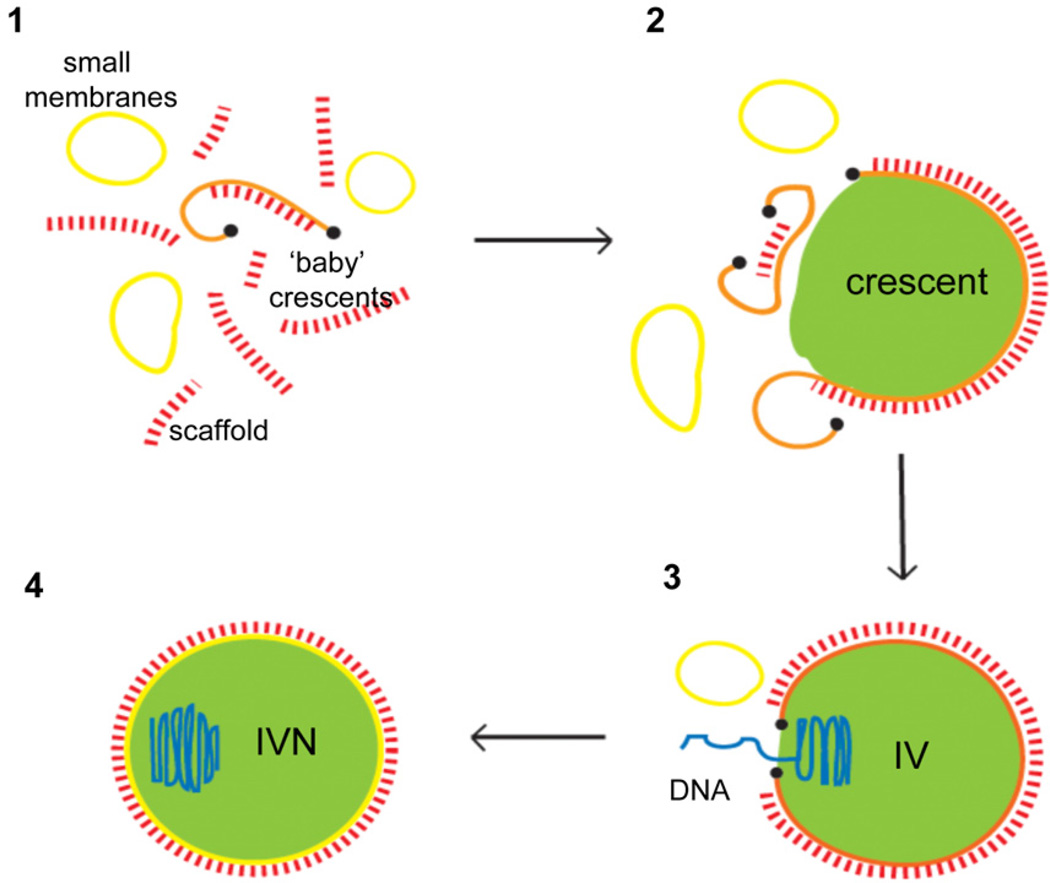
Fig. 3.
Model on the biogenesis of the crescent. 1. Viral membrane-formation (orange) is initiated in an area of pre-assembled honey-comb like scaffold protein, composed of the gene product of D13 of VACV, that forms rod-like structures (red) and that is surrounded by smooth closed membrane structures (yellow). These structures rupture to form open membrane sheets. 2. The open sheets are transported to the growing crescent membrane shaped by the assembly of the scaffold on its convex side. The crescent grows as more of the open membranes, transported from their site of rupture, fuse with the crescent membrane. The newly formed crescent associates with the virosome (green), which is composed of soluble viral proteins. We speculate that a protein-lipid complex (black dot) stabilizes the ends of the open membrane sheet and prevents re-sealing (fusion). Disassembly of this complex could trigger fusion of ruptured membranes with the growing crescent membrane or the sealing of the IV after DNA-uptake. 3. The sphere remains open and forms a pore of heterogeneous size through which the viral DNA (blue) is inserted into the particle. 4. After DNA take-up, the membrane sphere is sealed and forms a closed compartment, likely after disassembly of the putative lipid/protein complex at the membrane ends (black dots). IV: immature virion, IVN: immature virion containing the nucleoid (from Chlanda et al., 2009 reproduced with permission).
On the origin of the VACV membranes; lipid analysis by mass spectrometry
Membranes that accumulate in close proximity to the crescents have been observed before. The original images from Samuel Dales shows membrane structures in close proximity to the crescent that became more abundant when cells were treated with an inhibitor of RNA synthesis (Dales and Mosbach, 1968). However, on routine thin sections it is impossible to determine whether these are continuous with the crescent. Where do these open membranes come from? There are basically three possibilities to address this question: (i) analyse whether the membrane structures are connected to cellular membranes by ET, (ii) analyse if antibodies to compartment-specific cellular proteins localize to the crescents and their associated membranes, or (iii) analyse their lipid composition and compare it to organelles isolated from cells after fractionation. By ET, we were unable to detect connections of the small open membranes or the crescents to cellular organelles (Chlanda et al., 2009). Thus, most likely these membrane structures were budded off and transported from a cellular organelle to the site of crescent formation. Using immunolabelling collective data suggest that the crescent may be derived from the early secretory pathway, smooth ER or ER-Golgi intermediate compartment (see for instance Sodeik et al., 1993; Salmons et al., 1997; Risco et al., 2002). It should be mentioned, however, that the crescent itself excludes host proteins (a phenomenon common to many viruses) and the observations were based on the presence in close proximity of membranes labelled for ER/IC markers (Sodeik et al., 1993; Risco et al., 2002).
Since the crescents cannot be purified from cells, lipid analyses rely on purified and concentrated MV. In VACV-infected cells more than one infectious form is made that differ by the amount and origin of their membranes (reviewed in Smith and Law, 2004). For lipid analyses we considered the MV only since it is made from crescents/IVs and is likely to have a similar lipid composition. In previous studies lipids were extracted from purified virus (MVs) by organic solvents {typically a Folch mixture [methanol : chloroform (1:2)] and separated by thin-layer chromatography [TLC]}. In one of the earliest publications it was observed that the MV is relatively depleted in phosphatidylethanolamine (PE) compared with cells and that a relatively large fraction of the viral lipids ran at the solvent front (Stern and Dales, 1974). This relative depletion of the virus in PE and the appearance of a lipid running at the solvent front was recapitulated in most other lipid analyses using TLC (Hiller et al., 1981; Sodeik et al., 1993; Cluett and Machamer, 1996). It suggested that a (substantial) fraction of PE, about 10–25% of the total lipids of the virus, was modified to a lipid that ran faster on TLC plates. Indeed, Sodeik et al., proposed that N-acyl-PE could be such a candidate (Sodeik et al., 1993), but they did not further analyse this possibility. Compared with total cellular lipids, Sodeik et al. also found that the relative amounts of phosphatidylserine (PS), sphingomyelin (SM) and PE were reduced, compensated by an increase in phosphatidylinositol (PI) and the unidentified lipid. Two other studies proposed the latter to be acyl-bis(monoacylglycerol)phosphate (acyl-BMP), also called semi-lysobisphosphatidic acid (SLBPA) or hemi-acyl-bis(monoacylglycerol)phosphate (further abbreviated as acyl-BMP/SLBPA; Hiller et al., 1981; Cluett and Machamer, 1996), a lipid proposed to be present in endosomes and the Golgi complex. In addition, low amounts of N-acyl-PE were found in the viral membrane (Cluett and Machamer, 1996). Other studies concluded that the MV membrane must be enriched in PS (36% of total phospholipids) (Ichihashi and Oie, 1983) and that this lipid plays an essential role in the infectivity (Oie, 1985) and the entry of the virus (Mercer and Helenius, 2008).
Within the frame of this network we took advantage of our expertise in lipid analyses by mass spectrometry. An important advantage of mass spectrometric analysis of lipids is that it not only covers lipid classes, but also all individual lipid species within each lipid class. For virus purification we took advantage of a protocol established previously and which was aimed at analysing the protein content of virions by mass spectrometry (Jensen et al., 1996). As before, this resulted in highly purified and concentrated virus with a protein concentration of around 1.5 mg ml−1 (not shown; for details see Jensen et al., 1996). To analyse to what extent this virus preparation was contaminated with cellular membranes, uninfected cells were subjected to the same purification protocol and mock fractions analysed by quantitative mass spectrometry of lipids as described (Brügger et al., 2006; Haag et al., 2012). This suggested that the virus fractions contained as little as 3% contaminating membranes (data not shown) which was confirmed by negative staining EM. The relative lipid composition of purified MV was then compared with uninfected HeLa cells, used to grow the virus. Parallel experiments showed that the relative lipid composition of HeLa cells was not altered after infection, even though we did observe an overall increase of total lipids at later times post infection (data not shown).
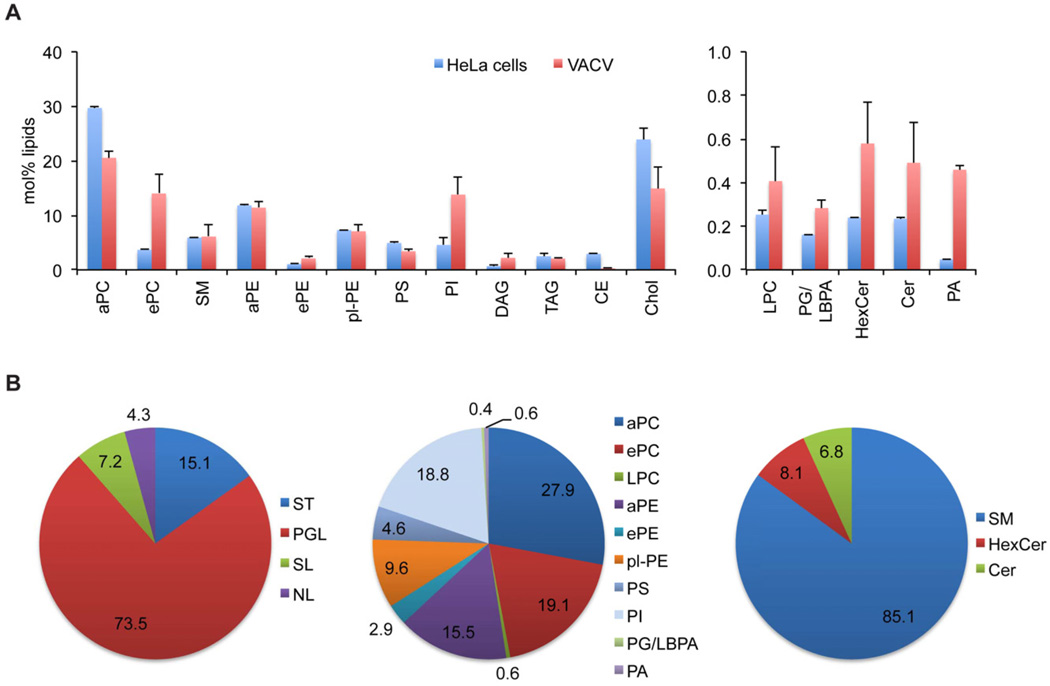
Shotgun lipidomics analysis of viral membranes showed that phosphoglycerolipids made up 74% to total lipids measured (Fig. 4A and B). Within the class of phosphoglycerolipids, phosphatidylcholine (PC) and ethanolamine-containing lipids were found in comparable amounts, adding up to approximately 55 mol%. With 19 mol% of total phosphoglycerolipids, PI is a major lipid of viral membranes, while only low amounts of PS and only traces (below 1 mol%) of lyso-phosphatidylcholine (lyso-PC), phosphatidic acid (PA) and phosphatidylglycerol/lysobisphosphatidic acid (PG/LBPA) were detected (Fig. 4A and B). Sphingolipids presented about 7 mol% of total lipids, with SM as major lipid (Fig. 4A and B). In comparison with total cellular lipids, we found a strong enrichment (≥ 3-fold) of PA, PI, diacylglycerol (DAG) and a relative depletion of cholesterol in the viral membranes. We also observed an enrichment for the ether species of PC and PE, but not for the respective diacyl species (Fig. 4A and B).
Fig. 4.
Lipid composition of VACV. Viral lipids were extracted in the presence of endogenous lipid standards. Lipid analysis was performed by nanoelectrospray ionization tandem mass spectrometry, employing lipid class-specific scan procedures. Due to the low abundance of PG and/or LBPA an unequivocal identification as either PG or LBPA via the mass spectrometric fragmentation pattern was not possible.
A. Lipid profile of VACV displayed as mol% of measured lipids.
B. Lipid distributions (in mol%) displayed in class categories.
aPC, diacyl-phosphatidylcholine; ePC, ether-acyl-phosphatidylcholine; SM, sphingomyelin; aPE, diacyl-phosphatidylethanolamine; ePE, ether-acyl-phosphatidylethanolamine; pl-PE, plasmalogen-phosphatidylethanolamine; PS, phosphatidylserine; PI, phosphatidylinositol; DAG, diacylglycerol; TAG, triacylglycerol; CE, cholesterolester; Chol, cholesterol; LPC, lyso- phosphatidylcholine; PG, phosphatidylglycerol; LBPA, lysobisphosphatidic acid; HexCer, hexosylceramide; Cer, ceramide; PA, phosphatidic acid; ST, sterol; PGL, phosphoglycerolipids; SL, sphingolipids; NL, neutral storage lipids.
We also focused on the PE and acyl-BMP/SLBPA content of the MV, because they were previously shown by TLC to be significantly altered. The amount of all PE species [diacyl-PE, diether-PE and plasmalogen-PE (pl-PE)] added up to about 28 mol% of total PE, indicating no significant reduction of this lipid in the viral membrane (Fig. 4A and B). Compared with uninfected cells none of the three different PE species was significantly reduced or increased in the MV (with the exception of a small increase in ePE). This discrepancy (the failure to detected a depletion of PE) likely relates to the different methods of lipid detection, TLC versus mass spectrometry. We were unable to directly analyse acyl-BMP/SLBPA, which was reported to contribute with approximately 25% to the total phospholipids, as appropriate lipid standards were not available. Our preliminary data suggested that rather than direct infusion a liquid chromatography-mass spectrometry approach is more suitable, as both lipids were not detected by shotgun lipidomics in viral membranes. It should be noted, however, that acyl-BMP/SLBPA is a minor lipid in cells and thus the proposed 25% of total lipids in the MV seen by others would imply a formidable enrichment of this specific lipid in the viral membrane.
Taken together, the overall relative content of phospholipids, in particular of cholesterol (approximately 15% of the total lipids) was indicative of membranes derived from the early secretory pathway, as previously suggested (Sodeik et al., 1993).
Can the phospholipid composition of the MV reveal insight into the mechanism of VACV membrane biogenesis in particular membrane rupture and the stabilization of open membrane ends? Water molecules minimize the contact with phospholipid acyl chains, which drives the phospholipids to assemble into a lipid bilayer and to form a closed compartment. Membrane curvature may depend on the spontaneous curvature of the individual phospholipids and their spatial distribution in the membrane (reviewed in Frolov et al., 2011). The rim of the crescent membrane is likely to be stabilized by phospholipids with positive spontaneous curvature such as lyso-phospholipids or phosphatidylinositol phosphates. In contrast phospholipids with a small polar head group as for instance PE or DAG tend to negatively bend a membrane. Thanks to its shape, PE is an important fusogenic lipid when present in the outer leaflet or apposing membranes but is probably not able to induce membrane rupture or stabilize an open membrane. Accordingly, our study shows that the overall PE content is not altered in the viral membrane. We observed a significant increase in PI, PA, DAG and ePE in the viral membrane. PI is a precursor of negatively charged phosphatidylinositol phosphates, which are important signalling lipids involved in membrane trafficking (Di Paolo and De Camilli, 2006) and have positive spontaneous curvature (Khelashvili et al., 2009). The contents of different phosphatidylinositol phosphates of the MV membrane, however, await further lipid analyses. Lyso-phospholipids have only one acyl chain and thus also have positive spontaneous curvature. Lyso-PC, which was slightly enriched in the MV, has properties of a detergent and can partition between a membrane and a solvent where it forms micelles. Depending on the concentration, lyso-PC is able to reversibly block membrane fusion or exocytosis (Chernomordik et al., 1993; Vogel et al., 1993), induce a pore and destabilize a membrane (Wilson-Ashworth et al., 2004). Because of these features we speculate that this lipid could be enriched in the open membrane sheets and the crescent ends but not in the MV (since it has a closed membrane) perhaps explaining the slight increase in the MV membrane in comparison with cellular membranes. Its putative enrichment, however, awaits the development of a protocol to isolate and purify crescents or the open membrane intermediates from infected cells.
We also observed an increase in PA and concomitant increase in DAG, to which PA can be rapidly converted (Morris et al., 1997). Both PA and DAG have negative spontaneous curvature and PA has been linked to fusion and fission in the presence of Ca2+ or at low pH (Morris et al., 1997), thus it is not clear how this property would contribute to the biogenesis of the viral membrane.
In summary, our lipid analyses did not confirm a depletion of PE reported previously while a putative enrichment of BMP/SLBPA seen by others, remains to analysed. The role of specific lipids in the biogenesis of the viral membranes, in particular those found to be enriched in the MV, also awaits further experiments.
Membrane rupture: not so uncommon
The biogenesis of the VACV membrane points to an unprecedented mechanism involving open membrane intermediates. This mechanism is likely to be highly controlled; our data show that membranes are ruptured and we speculated that they fuse with the growing crescent membrane creating open ends. We showed that the crescent membrane remains open until the viral DNA is inserted upon which the membrane closes. One approach we initiated is to make use of available inducible viruses to analyse the role of individual viral proteins in membrane rupture and crescent formation. This showed that neither the D13 scaffold protein (Chlanda et al., 2009) nor the major membrane protein A17 (Chlanda et al., 2011) is involved. Thus what regulates all of these processes is currently the subject of our research.
While membrane rupture seems at odds with cellular membrane dynamics regulated by fission and fusion processes, it may also play a role for other pathogens. It is not clear how non-enveloped viruses gain access to the cytosol; the formation of a membrane pore large enough to accommodate the viral particle or membrane lysis have been proposed (reviewed in Tsai, 2007; Moyer and Nemerow, 2011). For several non-enveloped viruses the viral protein required for cytosolic penetration has been identified and needs activation by cellular cues during entry in order to acquire its membrane-modifying properties. This principle is perhaps best exemplified for adenoviruses, with a size of about 100 nm, the largest non-enveloped virus. Binding to the cell surface mediates the loss of the fibre protein (Nakano et al., 2000; Burck-hardt et al., 2011), exposing protein VI. After internalization, the partly disassembled virion is able to escape from endosomes in to the cytosol, a process that requires protein VI and low pH that may induce additional changes to the viral capsid (Greber et al., 1993; Wiethoff et al., 2005). Escape may be mediated by endosomal lysis, based on the cytosolic delivery of endosomal content upon adeno-infection and release of lumenal content of liposomes incubated with protein VI (Wiethoff et al., 2005; Maier et al., 2010; Moyer et al., 2011).
Similarly, intracellular bacteria that replicate in the cellular cytoplasm are able to escape from phagosomes that mediate their entry into cells. Because of the size of these pathogens (about 1–2 µm) their escape is most likely mediated by rupture rather than pore formation. While cellular factors involved in this process remain elusive, several bacterial factors required for phagosomal escape have been identified (see Matsuda et al., 2012 for a recent example; reviewed in Ray et al., 2009). For most non-enveloped viruses and all intracellular bacteria lysis/rupture occurs on membranes of endocytic origin. An exception is provided by the small polyoma virus SV40 that after internalization reaches the ER lumen from which it may escape using its VP2 protein, ER-localized chaperons and factors involved ER-associated degradation (reviewed in Tsai, 2007; Geiger et al., 2011; Moyer and Nemerow, 2011). Whether VACV may use a similar mechanism to rupture membranes of ER origin is unknown and putative viral and cellular proteins/lipids involved in this process remain to be identified.
In these examples above the fate of the lysed membranes remain mostly elusive. In the case of Shigella flexneri membrane remnants produced by phagosomal lysis were shown to play a role in damping cellular response to infection by inducing an autophagic response followed by their destruction (Dupont et al., 2009). To date no other examples are known in which open membrane remnants are used to build a new membrane.
With analogies existing to other pathogens it would be interesting to study whether these membrane-rupturing processes share an evolutionary conserved mechanism or whether these are totally unrelated processes. We are currently in the process of addressing some of these questions. Finally, for none of the described examples above membrane rupture has been observed directly and was implied by indirect evidence (release of lumenal content, modification of artificial membranes). Thus, our study on VACV is the first to visualize this process directly, made possible by combining several EM techniques, in particular cellular tomography. Needless to say that the work greatly benefitted from the priority programme 1175 by providing opportunities for exchange of both ideas and methods.
Acknowledgements
We like to thank Iris Leibrecht for excellent technical assistance. The electron microscopy core facility at the EMBL for providing the opportunity to expand to new EM techniques.
References
- Adrian M, Dubochet J, Lepault J, McDowall AW. Cryo-electron microscopy of viruses. Nature. 1984;308:32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Al-Amoudi A, Chang JJ, Leforestier A, McDowall A, Salamin LM, Norlén LP, et al. Cryo-electron microscopy of vitreous sections. EMBO J. 2004;23:3583–3588. doi: 10.1038/sj.emboj.7600366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansarah-Sobrinho C, Moss B. Role of I7 protein in proteolitic processing of vaccinia virus membrane and core components. J Virol. 2004;78:6335–6343. doi: 10.1128/JVI.78.12.6335-6343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brügger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krausslich H-G. The HIV lipidome: a raft with an unusual composition. Proc Natl Acad Sci USA. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt CJ, Suomalainen M, Schoenenberger P, Boucke K, Hemmi S, Greber UF. Drifting motions of the adenovirus receptor CAR and immobile integrins initiate virus uncoating and membrane lytic protein exposure. Cell Host Microbe. 2011;10:105–117. doi: 10.1016/j.chom.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Vogel SS, Sokoloff A, Onaran HO, Leikina EA, Zimmerberg J. Lysolipids reversibly inhibit Ca(2+)-, GTP- and pH-dependent fusion of biological membranes. FEBS Lett. 1993;318:71–76. doi: 10.1016/0014-5793(93)81330-3. [DOI] [PubMed] [Google Scholar]
- Chlanda P, Carbajal MA, Cyrklaff M, Griffiths G, Krijnse Locker J. Membrane rupture generates single open membrane sheets during vaccinia virus assembly. Cell Host Microbe. 2009;6:81–90. doi: 10.1016/j.chom.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Chlanda P, Carbajal MA, Kolovou A, Hamasaki M, Cyrk-laff M, Griffiths G, Krijnse-Locker J. Vaccinia virus lacking A17 induces complex membrane structures composed of open membrane sheets. Arch Virol. 2011;156:1647–1653. doi: 10.1007/s00705-011-1012-1. [DOI] [PubMed] [Google Scholar]
- Cluett EB, Machamer CE. The envelope of vaccinia virus reveals an unusual phospholipid in Golgi complex membranes. J Cell Sci. 1996;109:2121–2131. doi: 10.1242/jcs.109.8.2121. [DOI] [PubMed] [Google Scholar]
- Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- Dales S, Mosbach EH. Vaccinia as a model for membrane biogenesis. Virology. 1968;35:564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Dubochet J. Cryo-EM-the first thirty years. J Microsc. 2012;245:221–224. doi: 10.1111/j.1365-2818.2011.03569.x. [DOI] [PubMed] [Google Scholar]
- Dupont N, Lacas-Gervais S, Bertout J, Paz I, Freche B, Van Nhieu GT, et al. Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe. 2009;6:137–149. doi: 10.1016/j.chom.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Frolov VA, Shnyrova AV, Zimmerberg J. Lipid polymorphisms and membrane shape. Cold Spring Harb Perspect Biol. 2011;3:a004747. doi: 10.1101/cshperspect.a004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger R, Andritschke D, Friebe S, Herzog F, Luisoni S, Heger T, Helenius A. BAP31 and BiP are essential for dislocation of SV40 from the endoplasmic reticulum to the cytosol. Nat Cell Biol. 2011;13:1305–1314. doi: 10.1038/ncb2339. [DOI] [PubMed] [Google Scholar]
- Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Grünewald K, Medalia O, Gross A, Steven AC, Baumeister W. Prospects of electron cryotomography to visualize macromolecular complexes inside cellular compartments: implications of crowding. Biophys Chem. 2003;100:577–591. doi: 10.1016/s0301-4622(02)00307-1. [DOI] [PubMed] [Google Scholar]
- Haag M, Schmidt A, Sachsenheimer T, Brügger B. Quantification of signaling lipids by nanoelectrospray ionization tandem mass spectrometry (Nano-ESI MS/MS) Metabolites. 2012;2:57–76. doi: 10.3390/metabo2010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Deep-etch EM reveals that the early pox-virus envelope is a single membrane bilayer stabilized by a geodetic ‘honeycomb’ surface coat. J Cell Biol. 2005;169:269–283. doi: 10.1083/jcb.200412169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller G, Eibl H, Weber K. Acyl bis(monoacylglycero)phosphate, assumed to be a marker for lysosomes, is a major phospholipid of vaccinia virions. Virology. 1981;113:761–764. doi: 10.1016/0042-6822(81)90204-x. [DOI] [PubMed] [Google Scholar]
- Hollinshead M, Vanderplasschen A, Smith GL, Vaux DJ. Vaccinia virus intracellular mature virions contain only one lipid membrane. J Virol. 1999;73:1503–1517. doi: 10.1128/jvi.73.2.1503-1517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y, Oie M. The activation of vaccinia virus infectivity by the transfer of phosphatidylserine from the plasma membrane. Virology. 1983;130:306–317. doi: 10.1016/0042-6822(83)90085-5. [DOI] [PubMed] [Google Scholar]
- Jensen ON, Houthaeve T, Shevchenko A, Cudmore S, Mann M, Griffiths G, Krijnse Locker J. Identification of the major membrane and core proteins of vaccinia virus by two-dimensional electrophoresis. J Virol. 1996;70:7485–7497. doi: 10.1128/jvi.70.11.7485-7497.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E, Moss B. Vaccinia virus structural polypeptide from a higher molecular weight precursor: formation and integration into virus particles. J Virol. 1970;6:717–726. doi: 10.1128/jvi.6.6.717-726.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelashvili G, Harries D, Weinstein H. Modeling membrane deformations and lipid demixing upon protein-membrane interaction: the BAR dimer adsorption. Biophys J. 2009;97:1626–1635. doi: 10.1016/j.bpj.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowall AW, Chang JJ, Freeman R, Lepault J, Walter CA, Dubochet J. Electron microscopy of frozen hydrated sections of vitreous ice and vitrified biological samples. J Microsc. 1983;131:1–9. doi: 10.1111/j.1365-2818.1983.tb04225.x. [DOI] [PubMed] [Google Scholar]
- McNulty-Kowalczyk A, Paoletti E. Mutations in ORF D13L and other genetic loci alter the rifampicin phenotype of vaccinia virus. Virology. 1993;194:638–646. doi: 10.1006/viro.1993.1303. [DOI] [PubMed] [Google Scholar]
- Maier O, Galan DL, Wodrich H, Wiethoff CM. An N-terminal domain of adenovirus protein VI fragments membranes by inducing positive membrane curvature. Virology. 2010;402:11–19. doi: 10.1016/j.virol.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Okada N, Kodama T, Honda T, Iida T. A cytotoxic type III secretion effector of Vibrio para-haemolyticus targets vacuolar H(+)-ATPase subunit c and ruptures host cell lysosomes. PLoS Pathog. 2012;8:e1002803. doi: 10.1371/journal.ppat.1002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Morris AJ, Frohman MA, Engebrecht J. Measurement of phospholipase D activity. Anal Biochem. 1997;252:1–9. doi: 10.1006/abio.1997.2299. [DOI] [PubMed] [Google Scholar]
- Moyer CL, Nemerow GR. Viral weapons of membrane destruction: variable modes of membrane penetration by non-enveloped viruses. Curr Opin Virol. 2011;1:44–49. doi: 10.1016/j.coviro.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer CL, Wiethoff CM, Maier O, Smith JG, Nemerow GR. Functional genetic and biophysical analyses of membrane disruption by human adenovirus. J Virol. 2011;85:2631–2641. doi: 10.1128/JVI.02321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MY, Boucke K, Suomalainen M, Stidwill RP, Greber UF. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J Virol. 2000;74:7085–7095. doi: 10.1128/jvi.74.15.7085-7095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie M. Reversible inactivation and reactivation of vaccinia virus by manipulation of viral lipid composition. Virology. 1985;142:299–306. doi: 10.1016/0042-6822(85)90338-1. [DOI] [PubMed] [Google Scholar]
- Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol. 2009;7:333–340. doi: 10.1038/nrmicro2112. [DOI] [PubMed] [Google Scholar]
- Risco C, Rodríguez JR, López-Iglesias C, Carrascosa JL, Esteban M, Rodríguez D. Endoplasmic reticulum-Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly. J Virol. 2002;76:1839–1855. doi: 10.1128/JVI.76.4.1839-1855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons T, Kuhn A, Wylie F, Schleich S, Rodriguez JR, Rodriguez D, et al. Vaccinia virus membrane proteins p8 and p16 are co-translationally inserted into the rough ER and retained in the intermediate compartment. J Virol. 1997;71:7404–7420. doi: 10.1128/jvi.71.10.7404-7420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Law M. The exit of vaccinia virus from infected cells. Virus Res. 2004;106:189–197. doi: 10.1016/j.virusres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Sodeik B, Krijnse Locker J. Assembly of vaccinia virus revisited: de novo membrane synthesis or acquisition from the host? Trends Microbiol. 2002;10:15–24. doi: 10.1016/s0966-842x(01)02256-9. [DOI] [PubMed] [Google Scholar]
- Sodeik B, Doms RW, Ericsson M, Hiller G, Machamer CE, van ‘t Hof W, et al. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993;121:521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern W, Dales S. Biogenesis of vaccinia: concerning the origin of the envelope phospholipids. Virology. 1974;62:293–306. doi: 10.1016/0042-6822(74)90393-6. [DOI] [PubMed] [Google Scholar]
- Studer D, Humbel BM, Chiquet M. Electron microscopy of high pressure frozen samples: bridging the gap between cellular ultrastructure and atomic resolution. Histochem Cell Biol. 2008;130:877–889. doi: 10.1007/s00418-008-0500-1. [DOI] [PubMed] [Google Scholar]
- Tolonen N, Doglio L, Schleich S, Krijnse Locker J. Vaccinia virus DNA-replication occurs in ER-enclosed cytoplasmic mini-nuclei. Mol Biol Cell. 2001;12:2031–2046. doi: 10.1091/mbc.12.7.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai B. Penetration of nonenveloped viruses into the cytoplasm. Annu Rev Cell Dev Biol. 2007;23:23–43. doi: 10.1146/annurev.cellbio.23.090506.123454. [DOI] [PubMed] [Google Scholar]
- Vogel SS, Leikina EA, Chernomordik LV. Lysophosphatidylcholine reversibly arrests exocytosis and viral fusion at a stage between triggering and membrane merger. J Biol Chem. 1993;268:25764–25768. [PubMed] [Google Scholar]
- Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J Virol. 2005;79:1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Ashworth HA, Judd AM, Law RM, Freestone BD, Taylor S, Mizukawa MK, et al. Formation of transient non-protein calcium pores by lysophospholipids in S49 lymphoma cells. J Membr Biol. 2004;200:25–33. doi: 10.1007/s00232-004-0691-x. [DOI] [PubMed] [Google Scholar]