Abstract
Galectin-3 is a multi-functional carbohydrate binding protein that was previously characterized as a proteolytic substrate for prostate specific antigen (PSA) and was shown to be associated with prostasomes in human semen. Prostasomes are exosome-like vesicles that are secreted by the prostatic epithelium and have multiple proposed functions in normal reproduction and prostate cancer. In the current study, galectin-3 binding ligands in human prostasomes were identified and characterized with the goal to investigate galectin-3 function in prostasomes. Galectin-3 binding proteins were isolated by affinity column chromatography. Candidate ligands identified by MS/MS were PSA, prostatic acid phosphatase (PAP), zinc alpha-2-glycoprotein (ZAG), dipeptidyl peptidase-4 (CD26), aminopeptidase N (CD13), neprilysin, clusterin, antibacterial protein (FALL-39), and alpha-1-acid glycoprotein (ORM1). Biochemical methods were used to characterize the ability of galectin-3 to bind to selected ligands, and galectin-3 cleavage assays were utilized to investigate the protease(s) in prostasomes that cleaves galectin-3. CD26, CD13, PSA, PAP, and ZAG immunoreactivity was detected in extracts of purified prostasomes. One-dimensional electroblot analysis of prostasomes demonstrated that CD26, PAP, and CD13 immunoreactivity co-migrated with galectin-3-reactive protein bands. PSA and ZAG were found to be associated with the surface of prostasomes. Both intact and cleaved galectin-3 were detected in prostate and prostasome extracts. Cleavage and inhibition assays indicated that PSA in prostasomes proteolytically cleaves galectin-3. The identification of these glycoproteins as galectin-3 ligands lays the groundwork for future studies of galectin-3 and prostasome function in reproduction and prostate cancer.
INTRODUCTION
Prostasomes are exosome-like vesicles that are secreted by the prostatic epithelium and are incorporated into seminal plasma during ejaculation. The fusion of prostasomes with sperm increases sperm motility by delivery of intra-prostasomal calcium stores and functions to prevent the premature maturation of sperm (Arienti et al. 2004). Prostasomes have also been proposed to protect sperm from the female immune system by inhibiting leukocyte function and serving as a reservoir for complement inhibitors, such as CD59. The immunoinhibitory effects of prostasomes have also been implicated in facilitating sexually transmitted infections (Kelly and Critchley 1997). Moreover, prostate cancer cell lines have been shown to secrete prostasomes, and prostasomes have been proposed to play a role in angiogenesis, tumor invasion, and immunosuppression during prostate cancer progression (Burden et al. 2006).
We previously identified galectin-3 in semen and on the surface of human prostasomes (Jones et al. 2010) and demonstrated that galectin-3 is a proteolytic substrate for the serine protease prostate specific antigen (PSA) in human semen (Saraswati et al. 2011). Galectin-3 is an ~30 kDa carbohydrate-binding protein (lectin) that is composed of a C-terminal carbohydrate recognition domain (CRD) linked to an N-terminal non-lectin domain via a collagen-like linker sequence (Dumic et al. 2006). Although the role of galectin-3 in reproduction is not known, the extracellular functions of galectin-3 in other systems include cell-cell and cell-matrix adhesion, immunomodulation, inflammation, cell signaling, and pathogen-host interactions (Rabinovich et al. 2002; Dumic et al. 2006; Vasta 2009). Furthermore, galectin-3 is implicated in the regulation of immunomodulation, apoptosis, angiogenesis, and metastatic cell adhesion and invasion in multiple cancers, including prostate cancer (Dumic et al. 2006).
The extracellular functions of galectin-3 are largely dependent on the ability of the lectin to cross-link its glycoconjugate ligands. Therefore, the functional characterization of galectin-3 in a given cell type or exosome includes identification of its target glycoconjugate ligands. Previously, Block et al. [2010] characterized candidate galectin-3 binding ligands, including Mac-2 binding protein (M2BP), in prostasomes based on their co-purification with galectin-3 during lactose-affinity chromatography. The current study utilized a proteomic approach to investigate the function of galectin-3 in prostasomes by identifying galectin-3 binding ligands that bound directly to immobilized galectin-3. Biochemical analyses examined the association of selected galectin-3 binding ligands with prostasomes, and galectin-3 was investigated as a substrate for PSA in prostasomes. Potential roles for galectin-3 interactions with the identified ligands in reproduction and prostate cancer are discussed.
MATERIALS AND METHODS
Antibodies and Protein Extracts
Anti-prostatic acid phosphatase (PAP; Clone EPR4066) and anti-CD13 (Clone EPR4059) rabbit monoclonal antibodies and rabbit polyclonal anti-zinc alpha 2 glycoprotein (ZAG) antibodies were purchased from Gene Tex Inc. (Irvine, CA). Rabbit polyclonal anti-PSA antibodies were obtained from ABcam (Cambridge, MA), and an anti-PSA neutralization mouse monoclonal antibody (Clone 181827) was purchased from R&D Systems (Minneapolis, MN). Anti-CD26 mouse monoclonal antibodies (Clone 202-36) were purchased from Lab Vision (Fremont, CA). All of the above antibodies were generated against human antigens. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit and goat anti-mouse secondary antibody F(ab′)2 fragments and HRP-conjugated streptavidin were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Protein extracts of human testis, epididymis, seminal vesicle, and prostate were purchased from Biochain Institute, Inc. (Hayward, CA); these extracts were prepared from liquid nitrogen fresh frozen tissues using a proprietary mixture containing HEPES (pH 7.9), sodium deoxycholate, NP-40, and protease inhibitors.
Recombinant galectin-3
The full-length 250 amino acid polypeptide galectin-3 was expressed as a recombinant protein in bacteria and purified by lactose affinity column chromatography as previously described (Jones et al. 2010). For some experiments, recombinant galectin-3 was biotinylated with twenty-fold molar excess EZLink® Sulfo-NHS-LC-Biotin (Thermo Fisher Scientific, Rockford, IL) in phosphate buffered saline (PBS)/4 mM β-mercaptoethanol (βME) for 30 minutes at room temperature (Jones et al. 2010).
Preparation of human sperm extracts, clarified seminal plasma, and isolated prostasomes
Semen samples from healthy human males were obtained following protocols approved by the University of Arkansas for Medical Sciences (UAMS) Institutional Review Board (IRB). Sperm were harvested from semen by centrifugation and sperm extracts were prepared as previously described (Jones et al. 2010). To prepare clarified seminal plasma and enriched prostasomes, semen was centrifuged at 1000 × g for 20 minutes to remove the cellular component, and the supernatant was centrifuged at 10,000 × g at 4 °C for 30 minutes. The subsequent supernatant was ultracentrifuged at 100,000 × g at 4 °C for two hours to obtain clarified seminal plasma (supernatant) and a membrane-enriched fraction (pellet). The resulting pellet containing the seminal plasma membrane fraction was resuspended in PBS at the original volume of the seminal plasma, subjected to column chromatography on Sephacryl S300 at 4 °C, and prostasomes were collected in the void volume (Jones et al. 2010).
Purification of galectin-3 binding ligands
Recombinant galectin-3 was covalently bound to cyanogen bromide-activated (CNBr) Sepharose (Sigma) following the manufacturer’s instructions and used to prepare a galectin-3 affinity chromatography column. The column was washed extensively with 0.1 M NaHCO3 (pH 8.5), 0.5 M NaCl, washed again with 0.1 M sodium acetate (pH 6.0), 0.5 M NaCl, and equilibrated with column buffer: 20 mM methyl-β-cyclodextrin (MβCD), 0.1 % SDS, 0.1% octyl β glucoside, and 4 mM βME in Tris-buffered saline (TBS;10 mM Tris, pH 7.4, 150 mM NaCl). Prostasome protein extracts were prepared with lysis buffer (column buffer with 1% octyl β glucoside), clarified by ultracentrifugation, and applied to the affinity column. The column was washed extensively with column buffer, and bound material was eluted with 250 mM lactose. Eluted proteins were pooled, dialyzed to remove lactose, and reapplied to the re-equilibrated affinity column to optimize affinity purification.
Proteomic analysis of galectin-3 binding ligands isolated from human prostasomes and selection of candidate ligands for further characterization
Galectin-3 affinity-purified proteins isolated from human prostasomes were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue. Coomassie-stained protein bands were excised using a razor blade, and trypsin digestion was performed with a ProGest in-gel enzymatic digestion robot (Genomic Solutions, Ann Arbor, MI) using sequence grade modified trypsin (Promega, Madison, WI). The proteins were analyzed by tandem mass spectrometry (MS/MS) on a high performance linear ion trap mass spectrometer (LTQ XL; Thermo Scientific). Data were processed using the Proteome Software, Scaffold 3 (http://www.proteomesoftware.com/). The resulting peak list was used to search the Mascot protein database (www.matrixscience.com) and assigned a Mascot score. Mascot scores are a summation of the scores for the individual peptides. Higher scores translate to greater probability for a sequence being correct.
Spectral counts for identified proteins were calculated using the equation:
Percent of total spectral count for each protein was calculated using the equation:
Proteins with 10% or greater of the total spectral count were considered as candidates for further characterization. A protein-protein interaction map for galectin-3 and identified binding ligands was assembled using the Cytoscape software program (www.cytoscape.org), which extracts interaction data from the Human Protein Reference Database (HPRD), the Biological General Repository for Interaction Datasets (BioGRID), the open source molecular interaction database (IntAct), and the NCI/Nature Pathway Interaction Database (Smoot et al. 2011).
Candidate galectin-3 binding ligands in human prostasomes were selected for further investigation in the current study based on satisfaction of two of the three following criteria: 1) prevalence in the galectin-3 affinity purified sample, 2) not previously reported as a galectin-3 binding ligand, 3) functional relevance to the reproductive tract.
Surface biotinylation and avidin affinity chromatography of prostasome proteins
Proteins on the prostasome surface were labeled with membrane-impermeable sulfo-NHS-biotin (Block et al. 2010). Surface-biotinylated prostasomes were incubated in lysis buffer and ultracentrifuged at 100,000 × g at 4 °C. Biotinylated proteins were isolated from the clarified supernatant by affinity column chromatography on avidin-Sepharose (Thermo Scientific). Bound proteins were eluted with 2 mM biotin and 4 mM βME in PBS (Block et al. 2010).
Electrophoresis and electroblot analysis
Protein samples were separated by one-dimensional SDS-PAGE under reducing conditions on 12% polyacrylamide gels (Laemmli 1970). Precision Plus Protein Standards (Bio-Rad, Hercules, CA) were used to estimate apparent protein molecular mass. For immunoblot analysis, electrophoresed proteins were transferred to nitrocellulose (Bio-Rad), and the membrane was blocked in 5% milk solution: nonfat powdered milk in PBS with 0.1% Tween 20 (PBS-Tween 20). Electroblots were incubated with the following primary antibody dilutions: anti-CD26 (1:5,000), anti-CD13 (1:200,000), anti-PAP (1:2,500), anti-ZAG (1:2,500), or anti-PSA (1:5,000) in PBS-Tween 20 overnight at 4 °C. Blots were washed three times with PBS-Tween 20 and incubated with goat anti-rabbit (1:2,500) or anti-mouse (1:2,500) secondary antibodies in PBS-Tween 20. Blots were washed twice with PBS-Tween 20 and once with PBS before development with enhanced chemiluminescence (ECL; GE Healthcare, Piscataway, NJ) on X-ray film.
For lectin blot analysis, proteins were separated by one-dimensional SDS-PAGE under reducing conditions on 12% polyacrylamide gels (Laemmli 1970), transferred to nitrocellulose, and blocked in 1.35% cold water fish gelatin (Sigma-Aldrich, St. Louis, MO) in PBS-Tween 20. Blots were washed three times with PBS-Tween 20 and incubated in 10 μg/mL biotinylated, recombinant galectin-3 in PBS-Tween 20 overnight at 4 °C. Blots were washed three times with PBS-Tween 20 and incubated in streptavidin-HRP (1:100,000) in PBS-Tween 20. Blots were washed twice with PBS-Tween 20 and once with PBS before development with ECL on X-ray film.
Galectin-3 cleavage and inhibition assays
Galectin-3 cleavage assays were performed as previously described (Saraswati et al. 2011). For each 25 μl reaction, 25 ng biotinylated, recombinant galectin-3 was incubated at 37 °C with 50 ng purified PSA (Calbiochem) or 10.75 μg purified prostasomes (based on protein content) and subjected to electroblot analysis with streptavidin-HRP. For inhibitor studies, separate prostasome aliquots were pre-incubated for 30 minutes with protease inhibitors: 5 mM phenylmethylsulfonyl fluoride (PMSF), 50 mM ZnCl2, 1 mM tosyllysine chloromethyl ketone (TLCK), or 20 mM 1,10-phenanthroline. For PSA neutralization studies, prostasome samples were pre-incubated with 80 ng/μl anti-PSA neutralization antibody for 45 minutes before addition of biotinylated galectin-3. Following proteolysis and electroblot analysis with streptavidin-HRP, the ImageJ program (Schneider et al. 2012) was used to quantitate individual protein bands from three separate experiments. Calculation of percent cleavage of galectin-3 was performed as previously described (Saraswati et al. 2011). P-values were calculated using an unpaired t-test in the Graph Pad software package.
RESULTS
Proteomic analysis of candidate galectin-3 binding ligands in human prostasomes
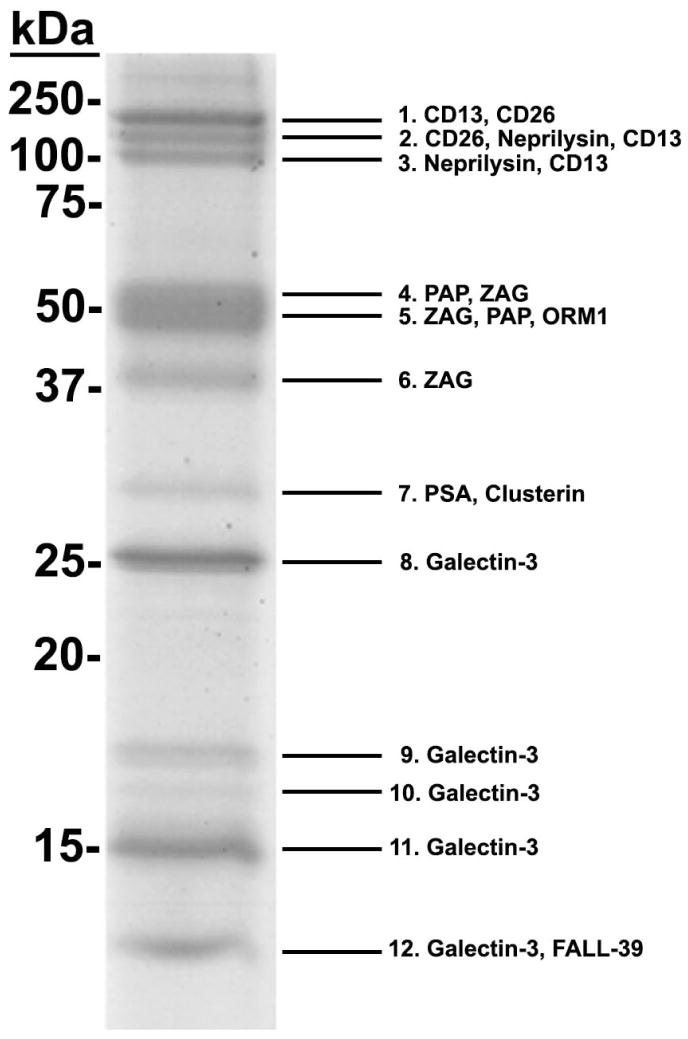
Galectin-3 binding proteins were isolated from purified human prostasomes on immobilized galectin-3. Subsequently, proteins were pooled, separated by SDS-PAGE, and stained with Coomassie blue (Fig. 1). Twelve Coomassie-stained protein bands ranging in molecular mass from ~14–150 kDa were excised and analyzed by MS/MS. Spectral counts for identified proteins were calculated, and proteins with 10% or greater of the total spectral count and mascot score higher than 50 were considered significant. Candidate galectin-3 binding proteins identified in human prostasomes included PSA, PAP, ZAG, CD26, CD13, neprilysin, clusterin, FALL-39, ORM1, and galectin-3. Table 1 lists the sequence coverage (the percent of peptides identified by MS/MS that match the full length protein sequence), mascot score, percent spectral count, and previously described functions for each of the candidate galectin-3 binding ligands.
Figure 1.
Proteomic analysis of candidate galectin-3 binding ligands in human prostasomes.
Affinity-purified, galectin-3 binding proteins were separated by SDS-PAGE and stained with Coomassie blue. Protein bands were excised, and the isolated proteins were identified by MS/MS. Spectral counts for identified proteins were calculated, and proteins with 10% or greater of the total spectral count for each band are shown in order of increasing percent spectral count.
Table 1.
Protein Matches, Percent Sequence Coverage, Mascot Score, Percent Spectral Count, and Functions of Tandem Mass Spectrometry Proteins Purified by Affinity Chromatography of Human Prostasomes
| Protein and Band Number | Percent Sequence Coverage | Mascot Score | Percent Spectral Count | Function |
|---|---|---|---|---|
| CD13 | Aminopeptidase N, CMV receptor (Arienti et al. 1997a; Kasman 2005) | |||
| Band 1 | 36 | 2485 | 41.23 | |
| Band 2 | 14 | 956 | 16.69 | |
| Band 3 | 9 | 791 | 10.10 | |
|
| ||||
| CD26 | Dipeptidyl peptidase IV, T cell activation (Burden et al. 2006; Yu et al. 2010) | |||
| Band 1 | 19 | 961 | 13.61 | |
| Band2 | 22 | 1008 | 29.44 | |
|
| ||||
| Neprilysin | Membrane metalloendopeptidase, Amyloid β regulation (Hersh and Rodgers 2008) | |||
| Band 2 | 25 | 1086 | 9.83 | |
| Band 3 | 36 | 1720 | 43.20 | |
|
| ||||
| Prostatic Acid Phosphatase | Semen Liquefaction, Enhanced HIV infectivity (Hassan et al. 2010; Kim et al. 2010) | |||
| Band 4 | 24 | 824 | 31.35 | |
| Band 5 | 24 | 584 | 26.61 | |
|
| ||||
| Zinc α2-glycoprotien | Stimulation of lipolysis, Sperm motility (Hassen et al. 2008) | |||
| Band 4 | 40 | 674 | 12.77 | |
| Band 5 | 35 | 688 | 28.17 | |
| Band 6 | 61 | 1428 | 60.64 | |
|
| ||||
| Alpha-1-acid glycoprotein | Immunosuppression (Lee et al. 2010) | |||
| Band 5 | 15 | 241 | 11.41 | |
|
| ||||
| PSA | Semen liquefaction, Prostate cancer (Williams et al. 2007; Jones et al. 2010) | |||
| Band 7 | 31 | 470 | 36.45 | |
|
| ||||
| Clusterin | Apoptosis (Li et al. 2010) | |||
| Band 7 | 23 | 671 | 13.11 | |
|
| ||||
| Galectin-3 | Cell adhesion, Immunomodulation, Cancer progression (Dumic et al. 2006) | |||
| Band 8 | 41 | 784 | 69.76 | |
| Band 9 | 41 | 702 | 71.72 | |
| Band 10 | 41 | 655 | 57.10 | |
| Band 11 | 41 | 790 | 73.30 | |
| Band 12 | 35 | 512 | 45.66 | |
|
| ||||
| Antibacterial Protein | Antibacterial peptide (Zu et al. 2003) | |||
| Band 12 | 33 | 308 | 41.10 | |
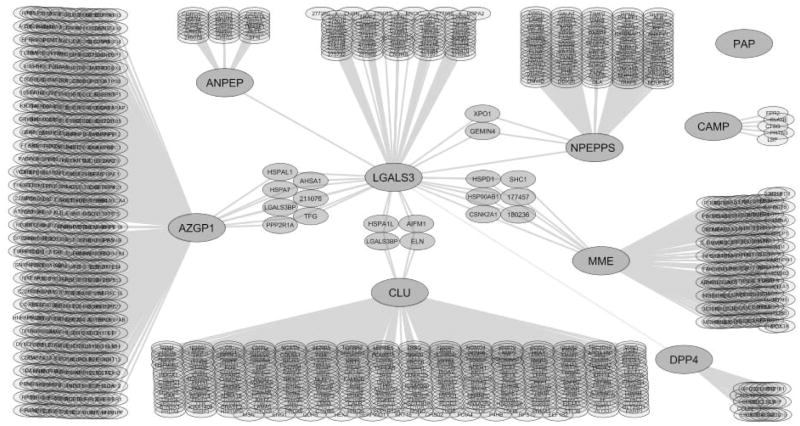
Protein-protein interaction network of the candidate galectin-3 binding proteins
Cytoscape software was used to generate a protein-protein interaction map from reported interaction data (Smoot et al. 2011). The generated interaction map represents an overlay of the interaction networks for each of the galectin-3 binding ligands and the known proteins that interact with each ligand. Large nodes indicate the galectin-3 affinity-purified proteins identified in this study. Small nodes indicate interacting proteins with the candidate galectin-3 binding ligands retrieved from the databases. Connecting lines indicate protein interactions. Each protein is shown by its gene symbol or Entrez identification number (Fig. 2). The interaction map indicated that galectin-3 and neprilysin both interact with casein kinase 2-alpha 1 and heat shock protein (HSP) 90-beta. Galectin-3 and clusterin interact with apoptosis inducing factor, elastin, HSP60, and M2BP. Interaction lines were added between CD13 and galectin-3, CD26 and galectin-3, and PSA and galectin-3 to indicate the interactions previously demonstrated by Yang et al. [2007], Block et al. [2010], and Saraswati et al. [2011], respectively, that were not identified in the interaction databases. Based on this analysis, direct interactions with galectin-3 have not been identified for clusterin, Fall-39, neprilysin, ORM-1, PAP, or ZAG.
Figure 2.
Protein-protein interaction network of the identified candidate galectin-3 binding ligands in prostasomes.
A protein-protein interaction map was generated from interaction data obtained from the HPRD, BioGRID, IntAct, and NCI/Nature Pathway Interaction Database. Large nodes indicate the galectin-3 affinity purified proteins identified in this study. Small nodes indicate proteins known to interact with the candidate galectin-3 binding ligands. Connecting lines indicate protein interactions. Each protein is shown by its gene symbol or Entrez identification number. Apoptosis inducing factor (AIFM1), casein kinase 2-alpha (CSNK2A1), CD13 (ANPEP), CD26 (DPP4), elastin (ELN), FALL-39 (CAMP), Galectin-3 (LGALS3), HSP60 (HSPA1L), HSP90-beta (HSP90AB1), M2BP (LGALS3BP), neprilysin (MME), PSA (NPEPPS), PAP (PAP), and ZAG (AZGP1).
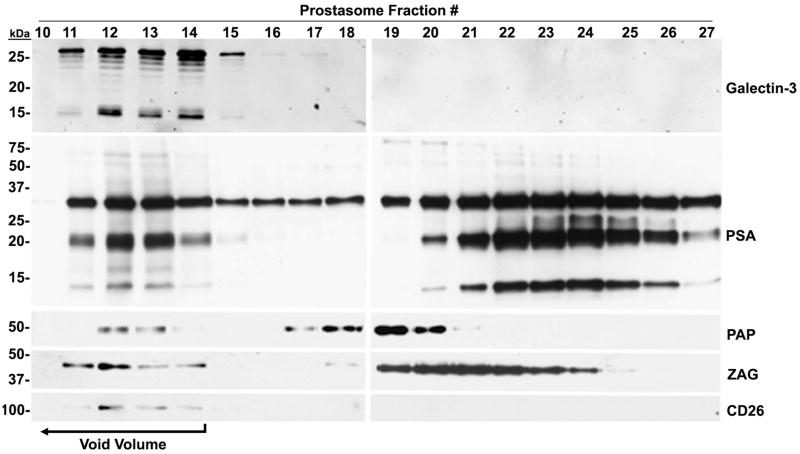
Galectin-3 and ligand immunoreactivity in purified human prostasomes
PSA, PAP, ZAG, CD26, and CD13 were selected for further characterization based on the criteria established for the study. The membrane fraction from seminal plasma was subjected to size exclusion column chromatography, and prostasomes were collected in the void volume. Electroblots of collected fractions were immunostained for galectin-3, PSA, PAP, and ZAG immunoreactivity (Fig. 3). CD26 (Arienti et al. 1997b) and CD13 (Burden et al. 2006) are known glycoproteins on the prostasome surface; therefore, CD26 was included as a positive control. CD26 was only present in the void volume (fractions 10–14) at its expected molecular mass of ~140 kDa. Immunoreactivity for galectin-3 was detected in fractions 11–17, with stronger staining in fractions 12–14, at ~30 and 16 kDa. PSA immunoreactivity was identified in fractions 10–27 at ~30 kDa for intact PSA with multiple lower molecular mass forms ranging from ~10–20 kDa. PAP immunoreactivity was detected in fractions 12–14 and 17–21 at an expected molecular mass of ~50 kDa. ZAG immunoreactivity was found in fractions 11–15 and 18–25 at its expected molecular mass of ~40 kDa. Identification of immunoreactivity for PSA, PAP, ZAG, and CD26 in the void volume indicates that these galectin-3 binding ligands are associated with human prostasomes. The detection of galectin-3, PSA, PAP, and ZAG in fractions 16–27 suggests their association with the amorphous material, particulate material in seminal plasma that is not associated with prostasomes.
Figure 3.
Association of galectin-3 binding ligands with purified prostasomes.
The membrane fraction from seminal plasma was subjected to size exclusion column chromatography on Sephacryl 300, and prostasomes were collected in the void volume (fractions 10–14; bracketed arrow). Electroblots of collected fractions were immunostained for galectin-3, PSA, PAP, ZAG, and CD26 immunoreactivity.
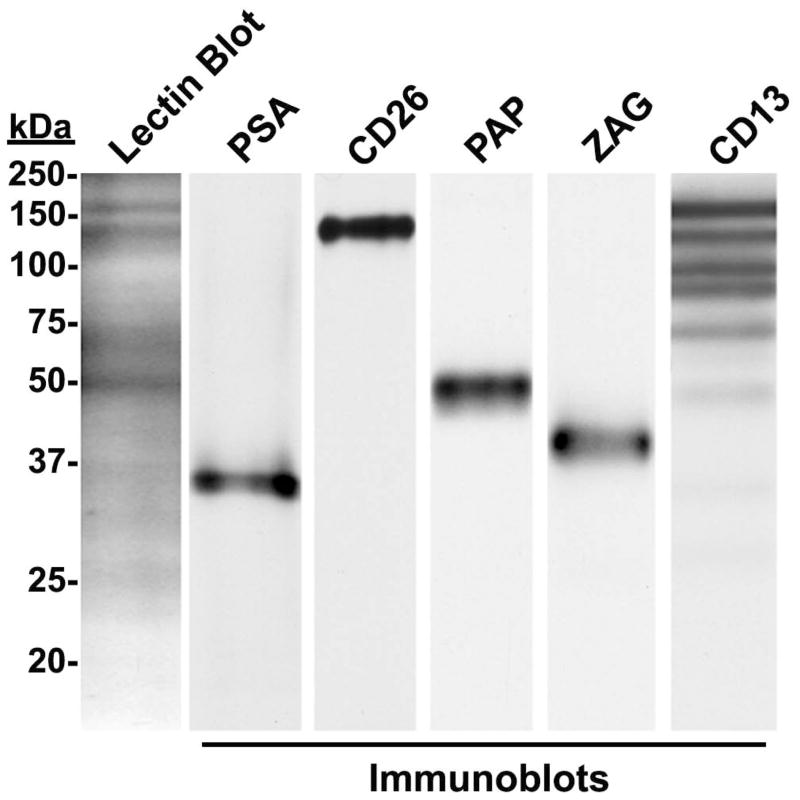
Co-migration of ligand immunoreactivity with galectin-3-reactive bands on lectin blots of human prostasomes
Human prostasomes were subjected to lectin blot analysis with biotinylated galectin-3 and immunoblot analysis for PSA, PAP, ZAG, CD26, and CD13 immunoreactivity (Fig. 4) using electroblot strips prepared from the same electrophoretic gel. Four major galectin-3-reactive bands with molecular masses of ~150, ~140, ~70, and ~50 kDa were identified. Immunoreactivity for PSA, PAP, ZAG, CD26, and CD13 was identified at their expected apparent molecular masses. The identified CD26 and PAP immunoreactive band co-migrated with lectin-detected bands at ~140 kDa and ~50 kDa, respectively. CD13 had several immunoreactive bands, and the major immunoreactive band of CD13 co-migrated with a lectin-detected band at ~150 kDa. The ZAG and PSA immunoreactive bands did not co-migrate with galectin-3-reactive bands on the lectin blots. Furthermore, the somewhat smeary ~70 kDa galectin-3 reactive band did not co-migrate with immunoreactivities for any of the identified ligands, nor was an ~70 kDa protein isolated by affinity purification on immobilized galectin-3.
Figure 4.
Comparison of galectin-3 reactive lectin blot bands with immunoreactive bands in human prostasomes.
Human prostasome extracts were subjected to galectin-3 lectin blot analysis and anti-PSA, PAP, ZAG, CD26, and CD13 immunoblot analysis. CD26, PAP, and CD13 immunoreactive bands co-migrated with galectin-3 reactive bands. However, galectin-3 reactive bands did not co-migrate with PSA and ZAG immunoreactive bands.
Electroblot analysis of surface-biotinylated prostasome proteins
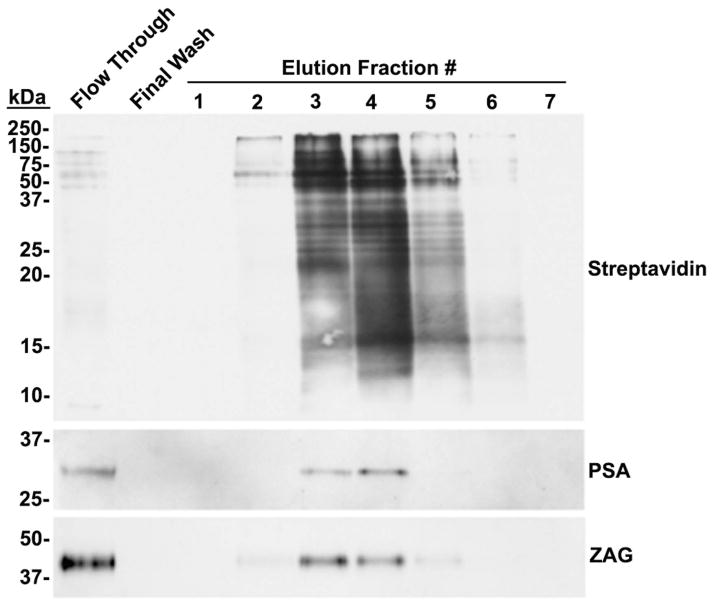
The surface of isolated prostasomes was biotinylated with membrane-impermeable sulfo-NHS-LC-biotin, and biotinylated surface proteins were affinity-purified on immobilized avidin. The column flow-through, last wash, and eluted proteins were subjected to electroblot analysis with streptavidin-HRP (Fig. 5). Streptavidin reactive bands were detected in the flow-through and eluted fractions 2–6. The presence of faint biotinylated bands in the column flow-through lane suggested an incomplete capture of the biotinylated proteins on the avidin column. Immunoreactivity for PSA was identified at its reported molecular mass of ~30 kDa in the flow-through and eluted fractions 3 and 4. Immunoreactivity for ZAG was identified at its reported molecular mass of ~ 40 kDa in the flow-through and eluted fractions 2–5. Anti-PAP and anti-CD26 immunoreactivity were not detected in the flow through, eluted fractions, or final wash of the biotinylated prostasomes (Not shown). Identification of anti-PSA and anti-ZAG immunoreactivity in the streptavidin-purified fractions indicates that PSA and ZAG are associated with the surface of human prostasomes.
Figure 5.
Galectin-3 binding ligands on the surface of human prostasomes.
Prostasomes were surface-labeled with biotin, and biotinylated proteins were affinity purified on immobilized avidin. The column flow-through, last wash, and eluted proteins were subjected to electroblot analysis with streptavidin-HRP and anti-PSA and anti-ZAG antibodies.
Characterization of galectin-3 proteolytic cleavage activity in prostasomes
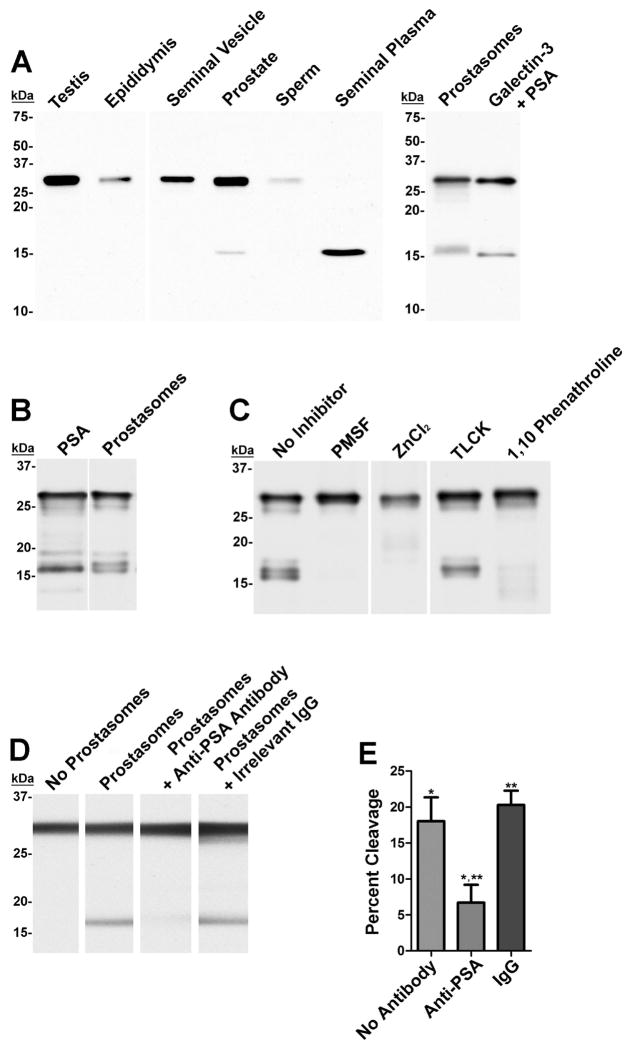
To evaluate galectin-3 cleavage in the male reproductive tract, clarified seminal plasma and protein extracts of human testis, epididymis, seminal vesicle, prostate, sperm, and prostasomes were evaluated by anti-galectin-3 immunoblot analysis (Fig. 6A). Galectin-3 immunoreactivity was identified as an ~30 kDa band in the testis, epididymis, seminal vesicle, and sperm extracts. In clarified seminal plasma, galectin-3 immunoreactivity was identified as an ~16 kDa band. In the prostate and prostasome extracts, galectin-3 bands were detected at both ~30 kDa and ~16 kDa. Immunoreactive bands of similar molecular mass were detected for recombinant galectin-3 that was proteolytically cleaved with PSA in vitro.
Figure 6.
Proteolytic cleavage of galectin-3 in prostasomes.
A: Human testis, epididymis, vas deferens, seminal vesicle, prostate, sperm, prostasome extracts, clarified seminal plasma, and PSA-cleaved recombinant galectin-3 were evaluated for galectin-3 immunoreactivity by immunoblot analysis. An ~16 kDa immunoreactive band indicative of cleaved galectin-3 was detected in the prostate and prostasomes extracts, seminal plasma, and the PSA-treated galectin-3 sample. B: Biotinylated galectin-3 was cleaved with purified PSA or prostasomes. Galectin-3 cleavage products were visualized by electroblot analysis with streptavidin-HRP. C: The galectin-3 cleavage assay was performed with prostasomes that were pre-incubated with 5 mM PMSF, 50 mM ZnCl2, 1 mM TLCK, or 20 mM 1,10-phenanthroline. D: The galectin-3 cleavage assay was performed with untreated prostasomes and prostasomes that were pre-incubated with PSA neutralization antibody or irrelevant antibody. Untreated galectin-3 was included as a negative control for electroblot analysis. E: Percent cleavage of galectin-3 was calculated for the neutralized PSA reaction and controls in D. Each bar represents the mean of three independent experiments; * p-value <0.05, ** p-value <0.005.
Galectin-3 cleavage assays were utilized to investigate the protease(s) in human prostasomes that cleaves galectin-3. Electroblot analysis identified biotinylated galectin-3 bands of ~30 kDa and ~16 kDa in samples incubated with prostasomes or PSA (Fig. 6B). The ~16 kDa band in the treated samples were detected as a doublet with a faint band at ~19 kDa. Galectin-3 cleavage in the presence of prostasomes was inhibited with PMSF, ZnCl2, and 1,10-phenanthroline, but was resistant to TLCK (Fig 6C). Furthermore, pre-incubation of prostasomes with an anti-PSA neutralization antibody decreased the galectin-3 cleavage activity by ~63% (<0.05; Fig. 6D and E). Irrelevant mouse IgG had no inhibitory effect on galectin-3 cleavage in the presence of prostasomes.
DISCUSSION
The multiple functions of galectin-3 are exerted through ligand binding, and the consequential effects are dependent on the specific galectin-3 ligands involved (Ochieng et al. 2004). Galectin-3 ligands in human prostasomes were purified, identified, and characterized to gain insight into the function of galectin-3 in these exosomes, which function in normal reproduction and prostate cancer progression. The candidate galectin-3 binding ligands in prostasomes identified using a proteomic approach were PSA, PAP, ZAG, CD26, CD13, neprilysin, clusterin, FALL-39, and ORM1. With the exception of FALL-39 and ORM1, these proteins were previously detected in human prostasomes by proteomic characterization (Utleg et al. 2003; Poliakov et al. 2009). Not surprisingly, galectin-3 was identified in the pool of affinity purified galectin-3 binding proteins. Galectin-3 likely bound the immobilized galectin-3 column because of its ability to self-associate via its N-terminal domain (Dumic et al. 2006).
Protein interactions for galectin-3 with CD13 (Yang et al. 2007), PSA (Saraswati et al. 2011), and CD26 (Block et al. 2010) have previously been described. Mapping of protein-protein interactions map for the candidate galectin-3 binding ligands in context with known galectin-3 binding interactions indicated that neprilysin and clusterin interact with proteins that interact with galectin-3. However, no direct interactions have been previously identified for galectin-3 with PAP, ZAG, neprilysin, clusterin, FALL-39, or ORM1. Therefore, these results are the first to indicate these proteins as galectin-3 binding ligands.
PSA, PAP, ZAG, CD26, and CD13 were selected for further investigation as galectin-3 ligands in prostasomes based on satisfaction of two out of three criteria: 1) prevalence in the galectin-3 affinity purified sample, 2) not previously identified as a galectin-3 binding ligand, 3) functional relevance to reproduction. CD26 (Arienti et al. 1997b) and CD13 (Arienti et al. 1997a) are well characterized as prostasome proteins. Using CD26 as a positive control for prostasomes, immunoblot analysis identified PSA, PAP, ZAG, and CD26 in prostasome fractions isolated from seminal plasma, indicating their association with prostasomes. PSA, PAP and ZAG were also associated with amorphous material that was collected by ultracentrifugation but did not elute with prostasomes during size exclusion chromatography. Co-migration of CD26, PAP, and CD13 immunoreactive bands with galectin-3-reactive bands on electroblots of purified prostasomes suggested that galectin-3 interacts with glycans on these glycoproteins in prostasomes. The ZAG and PSA immunoreactive bands did not co-migrate with galectin-3-reactive bands indicating that the interaction of galectin-3 with these ligands does not involve the glycan on these glycoproteins. Galectin-3 is a proteolytic substrate for PSA (Saraswati et al. 2011); therefore, the binding interactions between galectin-3 and PSA may involve the PSA active site. Furthermore, the collagen-binding ability of ZAG (Hassan et al. 2008) suggests that ZAG may bind to the collagen-like linker sequence in galectin-3. Significantly, the CD13 immunoblots had multiple bands, with the most intense band at ~150 kDa. This is a novel finding because previous laboratories only showed a portion of the Western blot corresponding to the intense ~150 kDa band (Alponti et al. 2011; Gao et al. 2011). Both intact CD13 and fragments of the protein may be present in prostasomes.
Prostasomes are too small (40–500 nm) for adequate visualization by light microscopy and immunofluoresence (Burden et al. 2006). Therefore, biotinylation of the prostasome surface coupled with avidin-affinity purification was used to examine localization of galectin-3 binding ligands to the surface of human prostasomes. Sulfo-NHS-LC-biotin is not membrane permeable; therefore, only molecules on the prostasome surface are biotinylated (Block et al. 2010). PSA and ZAG were identified in the fraction of surface-biotinylated proteins purified from prostasomes. Definitive conformation of binding ligand localization to the prostasome surface will require electron microscopy. Nevertheless, these results indicate that PSA and ZAG are present on the surface of human prostasomes. The lack of anti-PAP and anti-CD26 immunoreactivity in both the flow through and the streptavidin-purified fractions suggests that biotinylation of PAP and CD26 may have inhibited antibody binding. The ability of biotinylation to block anti-CD26 antibody binding has previously been described (Kahne and Ansorge 1994). Nevertheless, CD26 was previously reported on the prostasome surface (Schrimpf et al. 1999). Localization of PAP in prostasomes requires further study.
Block et al. [2010] previously reported M2BP, CD26, prolactin-inducible protein (PIP), olfactomedin-4 (OLF4), and semenogelins I and II (SgI and SgII) as candidate galectin-3 binding ligands in human prostasomes. The difference in candidate galectin-3 binding ligands identified in this study and by Block et al. [2010] may have occurred due to the different experimental approaches employed. The previous study characterized candidate ligands that co-purified with galectin-3 during lactose affinity chromatography. Block et al. [2010] used mascot scores to evaluate potential binding ligands, while a more stringent semi-quantitative method was used for this study. When the Block et al. [2010] data was reviewed using the semi-quantitative method utilized in the current study, M2BP was the only ligand with a percent spectral count higher than 10 (results not shown). Moreover, lectin blot analysis detected an ~70 kDa protein in prostasomes that was not identified by the proteomic approach used in this study. Significantly, a M2BP polypeptide fragment is typically detected as a somewhat smeary ~67–75 kDa protein band under denaturing conditions (Muller et al. 1999). Therefore, the ~70 kDa galectin-3 reactive band in prostasomes may represent M2BP. Serum M2BP levels can vary significantly between individuals (Peehl et al. 2011), suggesting that M2BP levels in semen may also vary. The overwhelming amounts of PSA, PAP, and ZAG in semen and differences in galectin-3 binding affinities may have prevented M2BP binding to immobilized galectin-3 and the detection of M2BP as a potential ligand in this study. Therefore, the results of the current study do not contradict the identification of M2BP as a galectin-3 ligand in prostasomes.
The candidate galectin-3 binding ligands identified in human prostasomes by the current study and their potential relevance to reproduction, prostate cancer, and sexually-transmitted diseases are discussed below:
PSA (~30 kDa) is a chymotrypsin-like serine protease secreted by the prostatic epithelium and normally functions in liquefaction of semen following ejaculation (Williams et al. 2007). Galectin-3 is a proteolytic substrate for PSA (Saraswati et al. 2011); therefore, the binding interactions between galectin-3 and PSA may involve the PSA active site. PSA has also been implicated in the promotion of localized prostate tumors and bone metastases by its roles in immunomodulation, invasion, and apoptosis (Williams et al. 2007). Likewise, multiple roles have been proposed for galectin-3 in prostate cancer (Burden et al. 2006; Dumic et al. 2006). Therefore, the regulation of galectin-3 by PSA may be involved in galectin-3 function in prostate cancer progression, and galectin-3 may play a role with PSA during semen liquefaction.
PAP (~50 kDa) is a glycosylated enzyme secreted by the prostatic epithelium and functions in semen liquefaction (Ortlund et al. 2003). PAP is also proposed to play a role in enhanced human immunodeficiency virus (HIV) infectivity by forming amyloid aggregates called semen-derived enhancer of viral infection (SEVI) that promote virion attachment to target cells (Kim et al. 2010). Therefore, in prostasomes, galectin-3 and PAP may be involved in the transfer of HIV to the female reproductive tract. Furthermore, galectin-3 may interact with PAP during semen liquefaction.
ZAG (~40 kDa) is a glycoprotein that is secreted by prostatic epithelial cells into seminal plasma. ZAG has been shown to play a role in sperm motility via the cAMP/PKA signaling pathway and may be required for fertilization (Qu et al. 2007; Hassan et al. 2008). Furthermore, ZAG has been identified in prostate cancer tumors. Significantly, ZAG interacts with multiple binding ligands including collagen (Hassan et al. 2008) and thus, may interact with galectin-3 via its collagen-like linker. The interaction of ZAG with galectin-3 in prostasomes may be involved in the facilitation of sperm motility, fertilization, and/or prostate cancer progression.
The CD26 glycoprotein (110–140 kDa) is an ectopeptidase expressed by most human epithelial cells either as an intrinsic membrane protein and/or as a soluble enzyme (Yu et al. 2010). CD26 is present on the surface of human prostasomes (Arienti et al. 1997b) and plays diverse roles in immunity and cancer. Therefore, CD26 and its interactions with galectin-3 may be involved in the immunomodulatory activities and cancer-associated functions of prostasomes.
The CD13 glycoprotein (aminopeptidase N; ~150 kDa) is a membrane-bound metalloproteinase (Arienti et al. 1997a). Galectin-3 specifically binds to CD13 both in vitro and in vivo in a carbohydrate recognition-dependent manner, and CD13 is a crucial mediator of galectin-3-induced angiogenesis in endothelial cells (Yang et al. 2007). Therefore, CD13 and galectin-3 interactions may contribute to the angiogenic properties of prostasomes which are implicated in prostate cancer progression. Furthermore, CD13 is implicated in infection and pathogenesis of cytomegalovirus (CMV), a virus that can be sexually-transmitted (Kasman 2005). The identification of CD13 in prostasomes suggests that galectin-3 and prostasomes may be involved in sexually-transmitted infections (STIs).
Neprilysin, clusterin, FALL-39, and ORM1 were also identified as galectin-3 binding ligands by our proteomic approach. Although these candidate ligands did not meet the criteria established for further characterization in this study, their interactions with galectin-3 may be significant for prostasome function. Neprilysin is proposed to play a role in the androgen-independent progression of prostate cancer (Burden et al. 2006); thus, galectin-3 may interact with neprilysin in this disease. Clusterin is associated with prostasomes and was identified as a major antigen for sperm agglutination auto-antibodies in infertile men (Carlsson et al. 2004). The bacteriostatic function of FALL-39 (Zhu et al. 2003) suggests that the FALL-39 interaction with galectin-3 contributes to the bacteriostatic properties of prostasomes. Furthermore, galectin-3 and ORM1 are implicated as inflammatory mediators (Kratz et al. 2003; Dumic et al. 2006).
Galectin-3 is proteolytically cleaved by PSA in human seminal plasma (Saraswati et al. 2011), and Jones et al. [2010] detected cleaved galectin-3 by proteomic analysis of lactose-binding proteins isolated from prostasomes. In the current study, similarity in immunoreactive banding patterns with PSA-cleaved recombinant galectin-3 suggested that galectin-3 proteolytic cleavage occurs in the prostate, seminal plasma, and prostasomes. A nearly identical banding pattern was detected for galectin-3 cleaved with PSA or cleaved in the presence of prostasomes. PSA and the protease that cleaves galectin-3 in prostasomes exhibit identical susceptibility to the protease inhibitors tested (Cohen et al. 1992; Robert et al. 1997). Furthermore, addition of a PSA neutralizing antibody significantly inhibited galectin-3 cleavage in prostasomes. Collectively, these results indicate that galectin-3 is a proteolytic substrate for PSA in human prostasomes and demonstrate that the PSA associated with prostasomes is enzymatically active. In addition, these results indicate a potential role of galectin-3 and PSA in normal reproductive function and prostate cancer.
In summary, the affinity purification of galectin-3 binding proteins from human prostasomes identified PSA, PAP, ZAG, CD13, CD26, neprilysin, clusterin, FALL-39, and ORM1 as potential galectin-3 binding ligands. Except for CD13, PSA, and CD26, interactions between these proteins and galectin-3 have not been previously reported. The described biochemical analyses confirmed PSA, PAP, ZAG, CD13, and CD26 as candidate galectin-3 binding ligands and implicated PSA in the proteolytic cleavage of galectin-3 in human prostasomes. We anticipate that these results will contribute to the future investigation of prostasomes and galectin-3 binding ligand interactions in prostate cancer progression, STI transmission, infertility, and normal reproductive function.
Acknowledgments
The project described was supported by Award Number R01HD050540 to ABD from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD. The authors wish to thank Drs. Mari Davidson and Robert Eoff for their valuable advice, David Schoen for his critical review of the manuscript, and Drs. Alan Tackett and Samuel Mackintosh of the UAMS Proteomics Core Facility. The Proteomics Core Facility is supported by the Translational Research Institute (TRI), grant UL1TR000039 through the NIH National Center for Advancing Translational Sciences.
Footnotes
DISCLOSURES
None
AUTHORS CONTRIBUTIONS
Matthew Kovak, Sarika Saraswati, Sabrina Goodard, and Alan Diekman performed the research and analyzed the data. Matthew Kovak and Alan Diekman wrote the paper.
References
- Alponti RF, Nogueira MI, Mendes MT, de Abreu C, Silveira PF. APM/CD13 and FOS in the hypothalamus of monosodium glutamate obese and food deprived rats. Regul Pept. 2011;166(1–3):98–104. doi: 10.1016/j.regpep.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Arienti G, Carlini E, Saccardi C, Palmerini CA. Role of human prostasomes in the activation of spermatozoa. J Cell Mol Med. 2004;8(1):77–84. doi: 10.1111/j.1582-4934.2004.tb00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arienti G, Carlini E, Verdacchi R, Cosmi EV, Palmerini CA. Prostasome to sperm transfer of CD13/aminopeptidase N (EC 3.4.11.2) Biochim Biophys Acta. 1997a;1336(3):533–538. doi: 10.1016/s0304-4165(97)00071-8. [DOI] [PubMed] [Google Scholar]
- Arienti G, Polci A, Carlini E, Palmerini CA. Transfer of CD26/dipeptidyl peptidase IV (E.C. 3.5.4.4) from prostasomes to sperm. FEBS Lett. 1997b;410(2–3):343–346. doi: 10.1016/s0014-5793(97)00655-8. [DOI] [PubMed] [Google Scholar]
- Block AS, Saraswati S, Lichti CF, Mahadevan M, Diekman AB. Co-purification of Mac-2 binding protein with galectin-3 and association with prostasomes in human semen. Prostate. 2010;71(7):711–721. doi: 10.1002/pros.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden HP, Holmes CH, Persad R, Whittington K. Prostasomes--their effects on human male reproduction and fertility. Hum Reprod Update. 2006;12(3):283–292. doi: 10.1093/humupd/dmi052. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Ronquist G, Nilsson BO, Larsson A. Dominant prostasome immunogens for sperm-agglutinating autoantibodies of infertile men. J Androl. 2004;25(5):699–705. doi: 10.1002/j.1939-4640.2004.tb02844.x. [DOI] [PubMed] [Google Scholar]
- Cohen P, Graves HC, Peehl DM, Kamarei M, Giudice LC, Rosenfeld RG. Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J Clin Endocrinol Metab. 1992;75(4):1046–1053. doi: 10.1210/jcem.75.4.1383255. [DOI] [PubMed] [Google Scholar]
- Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760(4):616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Gao JJ, Gao ZH, Zhao CR, Yuan Y, Cui SX, Zhang XF, Cheng YN, Xu WF, Tang W, Qu XJ. LYP, a novel bestatin derivative, inhibits cell growth and suppresses APN/CD13 activity in human ovarian carcinoma cells more potently than bestatin. Invest New Drugs. 2011;29(4):574–582. doi: 10.1007/s10637-010-9391-9. [DOI] [PubMed] [Google Scholar]
- Hassan MI, Waheed A, Yadav S, Singh TP, Ahmad F. Zinc alpha 2-glycoprotein: a multidisciplinary protein. Mol Cancer Res. 2008;6(6):892–906. doi: 10.1158/1541-7786.MCR-07-2195. [DOI] [PubMed] [Google Scholar]
- Jones JL, Saraswati S, Block AS, Lichti CF, Mahadevan M, Diekman AB. Galectin-3 is associated with prostasomes in human semen. Glycoconj J. 2010;27:227–236. doi: 10.1007/s10719-009-9262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahne T, Ansorge S. Non-radioactive labelling and immunoprecipitation analysis of leukocyte surface proteins using different methods of protein biotinylation. J Immunol Methods. 1994;168(2):209–218. doi: 10.1016/0022-1759(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Kasman LM. CD13/aminopeptidase N and murine cytomegalovirus infection. Virology. 2005;334(1):1–9. doi: 10.1016/j.virol.2005.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RW, Critchley HO. Immunomodulation by human seminal plasma: a benefit for spermatozoon and pathogen? Hum Reprod. 1997;12(10):2200–2207. doi: 10.1093/oxfordjournals.humrep.a019559. [DOI] [PubMed] [Google Scholar]
- Kim KA, Yolamanova M, Zirafi O, Roan NR, Staendker L, Forssmann WG, Burgener A, Dejucq-Rainsford N, Hahn BH, Shaw GM, Greene WC, Kirchhoff F, Munch J. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology. 2010;7:55. doi: 10.1186/1742-4690-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz E, Poland DC, van Dijk W, Katnik-Prastowska I. Alterations of branching and differential expression of sialic acid on alpha-1-acid glycoprotein in human seminal plasma. Clin Chim Acta. 2003;331(1–2):87–95. doi: 10.1016/s0009-8981(03)00084-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Muller SA, Sasaki T, Bork P, Wolpensinger B, Schulthess T, Timpl R, Engel A, Engel J. Domain organization of Mac-2 binding protein and its oligomerization to linear and ring-like structures. J Mol Biol. 1999;291(4):801–813. doi: 10.1006/jmbi.1999.2996. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19(7–9):527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- Ortlund E, LaCount MW, Lebioda L. Crystal structures of human prostatic acid phosphatase in complex with a phosphate ion and alpha-benzylaminobenzylphosphonic acid update the mechanistic picture and offer new insights into inhibitor design. Biochemistry. 2003;42(2):383–389. doi: 10.1021/bi0265067. [DOI] [PubMed] [Google Scholar]
- Peehl DM, Chen Z, Nolley R. Serum Mac-2BP does not distinguish men with high grade, large volume prostate cancer from men with benign prostatic hyperplasia. Prostate. 2011;71(1):26–31. doi: 10.1002/pros.21218. [DOI] [PubMed] [Google Scholar]
- Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. 2009;69(2):159–167. doi: 10.1002/pros.20860. [DOI] [PubMed] [Google Scholar]
- Qu F, Ying X, Guo W, Guo Q, Chen G, Liu Y, Ding Z. The role of Zn-alpha2 glycoprotein in sperm motility is mediated by changes in cyclic AMP. Reproduction. 2007;134(4):569–576. doi: 10.1530/REP-07-0145. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002;23(6):313–320. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- Robert M, Gibbs BF, Jacobson E, Gagnon C. Characterization of prostate-specific antigen proteolytic activity on its major physiological substrate, the sperm motility inhibitor precursor/semenogelin I. Biochemistry. 1997;36(13):3811–3819. doi: 10.1021/bi9626158. [DOI] [PubMed] [Google Scholar]
- Saraswati S, Block AS, Davidson MK, Rank RG, Mahadevan M, Diekman AB. Galectin-3 is a substrate for prostate specific antigen (PSA) in human seminal plasma. Prostate. 2011;71(2):197–208. doi: 10.1002/pros.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrimpf SP, Hellman U, Carlsson L, Larsson A, Ronquist G, Nilsson BO. Identification of dipeptidyl peptidase IV as the antigen of a monoclonal anti-prostasome antibody. Prostate. 1999;38(1):35–39. doi: 10.1002/(sici)1097-0045(19990101)38:1<35::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27(3):431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utleg AG, Yi EC, Xie T, Shannon P, White JT, Goodlett DR, Hood L, Lin B. Proteomic analysis of human prostasomes. Prostate. 2003;56(2):150–161. doi: 10.1002/pros.10255. [DOI] [PubMed] [Google Scholar]
- Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7(6):424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Singh P, Isaacs JT, Denmeade SR. Does PSA play a role as a promoting agent during the initiation and/or progression of prostate cancer? Prostate. 2007;67(3):312–329. doi: 10.1002/pros.20531. [DOI] [PubMed] [Google Scholar]
- Yang E, Shim JS, Woo HJ, Kim KW, Kwon HJ. Aminopeptidase N/CD13 induces angiogenesis through interaction with a pro-angiogenic protein, galectin-3. Biochem Biophys Res Commun. 2007;363(2):336–341. doi: 10.1016/j.bbrc.2007.08.179. [DOI] [PubMed] [Google Scholar]
- Yu DM, Yao TW, Chowdhury S, Nadvi NA, Osborne B, Church WB, McCaughan GW, Gorrell MD. The dipeptidyl peptidase IV family in cancer and cell biology. Febs J. 2010;277(5):1126–1144. doi: 10.1111/j.1742-4658.2009.07526.x. [DOI] [PubMed] [Google Scholar]
- Zhu L, Feng Y, Huang N, Wang B, Chen J. Effects of Fall-39 mRNA expression and antibacterial activity of human pulmonary gland epithelial cells induced by BCG. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34(4):618–621. 649. [PubMed] [Google Scholar]