Abstract
Hypothesis
A tissue response in the form of foreign body or a hypersensitivity reaction to cochlear implantation is common and may be one possible cause of a soft failure of cochlear implantation.
Background
Following a successful cochlear implantation, delayed failure may occur. The causes of a “soft” failure, that is one in which device malfunction cannot be proven, are unknown.
Methods
The histopathology of the temporal bones of a patient who in life had suffered a soft failure following cochlear implantation was described. In addition, the temporal bones of 8 other subjects who in life had undergone cochlear implantation were studied for evidence of a foreign body or hypersensitivity reaction.
Results
In the case report, a necrotizing granulomatous giant cell reaction surrounded the cochlear implant electrode track through the mastoid, middle ear, and into the cochlea in both ears. There was osteolysis of the cribrose area, otic capsule and bone between the facial nerve and cochlea, and destruction of the organ of Corti and spiral ganglion. In the additional 8 cases studied, a similar, although less pronounced, foreign body or hypersensitivity reaction was seen in 6 (75%) of the cases.
Conclusions
A foreign body or hypersensitivity reaction in the form of giant cells and lymphocytic cell infiltration is common following cochlear implantation and may be one possible cause of soft failure.
Introduction
Following a successful cochlear implantation, delayed failure may occur. These failures may be categorized as either a “hard failure” or as a “soft failure”. In a report of 33 revision cochlear implant operations in 30 patients Buchman et al (2004) found that in 24 percent, the speech processor had failed to lock with the internal device (hard failure) whereas in 76 percent, lock was maintained with a variety of auditory, nonauditory, or other performance related issues (soft failure). The European consensus statement on cochlear implant failures (2005) categorized device failures as (1) those cases in which the electrical characteristics of the cochlear implant were outside the manufacturer’s specifications, resulting in loss of clinical benefit (nearly equivalent to the definition of hard failures (Buchman et al 2004) and (2) those with performance decrements unexplained based on manufacturers specifications. The cochlear implant soft failures consensus development conference (Balkany et al 2005) defined a soft failure as a suspected but not proven device malfunction. The characteristic symptoms in such patients included shocking sensations, popping sounds, intermittency or an unexplained progressive decrement in performance. The experience of a cochlear implant program in Quebec (Cote et al 2007) found that the need for explantation was caused by hard failures in 53.3% and by soft failures in 11.1%. Other reasons for explantation in their series included extrusion, infection, intratemporal pathology or perilymph fistula.
Buchman et al (2005) reported that in soft failures, auditory symptoms were resolved in 20 out of 23 (87 percent) patients undergoing revision cochlear implantation of the same ear.
In this report, we present the case history of a 71-year old man who underwent a right cochlear implant 13 years prior to death. One year following implantation there was evidence of a soft failure. Implantation and subsequent explantation and reimplantation of the contralateral left ear were performed with no substantial improvement in speech recognition.
Histopathologic study of the temporal bones demonstrated a necrotizing giant cell granulomatous process in both cochleae with degeneration of the spiral ganglion cells and the organ of Corti. The histopathology was consistent with either a foreign body or hypersensitivity giant cell reaction.
Although animal testing of the biocompatibility of cochlear implants has demonstrated only mild tissue reactions, (RK Shepherd et al 1984), there have been several case reports in humans documenting either histopathologic evidence of either a foreign body or hypersensitivity giant cell reaction to the implant (Bertuleit et al 1999, Kronenberg et al 2001, Kunda et al 2006, Migirov et al 2007) or clinical allergy testing consistent with a delayed hypersensitivity response (Puri et al 2005; Kunda et al 2006). In addition, in other cases an apparently progressive osteolysis of the cochlear capsule has been described radiographically (Ho et al 2007) and histopathologically (Doherty and Linthicum 2004), although the pathogenesis in these cases was ascribed to pressure necrosis or a focal inflammatory reaction.
In addition, in this report a review of the temporal bones of other patients who in life had undergone cochlear implantation revealed a cellular immune response consisting of giant cells and lymphocytes in 6 out of 8 (75%) patients, substantiating that a cellular immune response is a common finding.
Materials and Methods
Case report
This 71-year old man developed a bilateral sensorineural hearing loss at approximately age 16 years of probable genetic etiology given that two of his siblings and one nephew had a similar pattern of hearing loss. By the age of 57, he was severely to profoundly hearing impaired bilaterally. A CT scan of the temporal bones was normal.
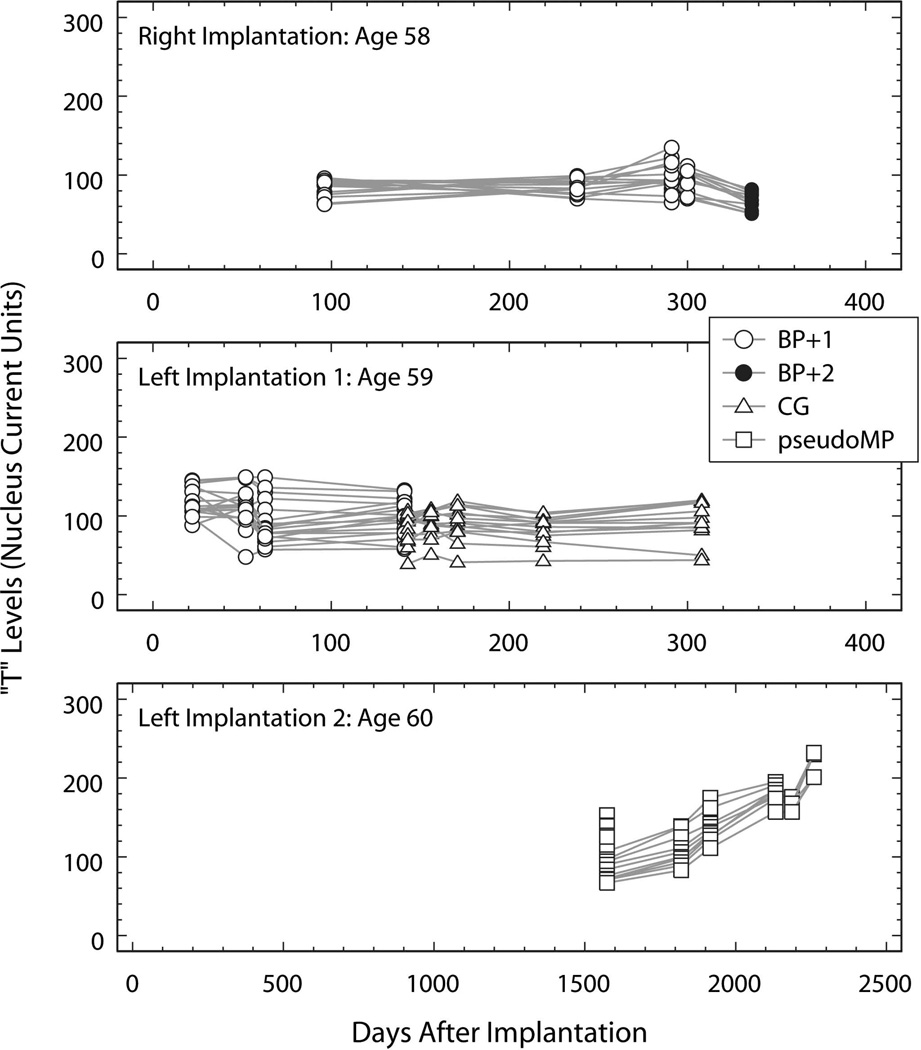
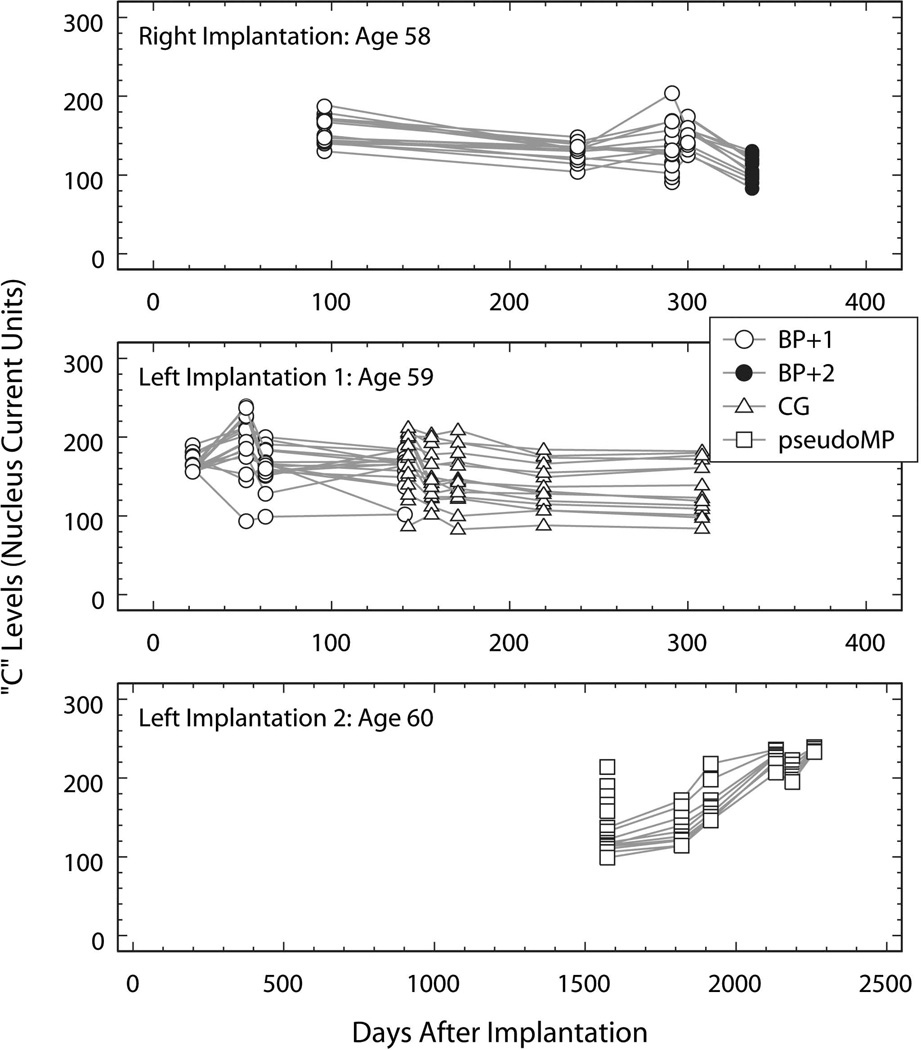
At age 58, he underwent a right cochlear implant using a 22-channel Nucleus® device with full insertion including 4 stiffening rings. T and C levels measured at five testing sessions were preserved in the subject’s medical records and are plotted as a function of time (days after implantation: DAI) in the top panels of Fig. 1 and Fig. 2. The first four testing sessions were conducted using the bipolar plus one (BP+1; open circles) stimulus configuration. Regression models computed for T and C as a function of DAI accounted for less than 10% of the variance in T (R2=0.04; p=0.10) and C (R2=0.09; p=0.02) with only the model for C reaching significance. At the DAI-300 testing session, sound-processor C levels for electrodes 16, 17 and 19 were reduced because of “eye twitching.” At the DAI-336 testing session, additional electrodes elicited eye twitching at stimulus amplitudes below the BP+1 C levels. As a result, the stimulus configuration was changed to bipolar plus two (BP+2) and electrodes 16, 17, 19 and 20 were turned off. As expected, the T and C levels measured using the BP+2 configuration at DAI 336 (filled circles in Fig. 1 and Fig. 2) were significantly lower than those measured using the BP+1 configuration at DAI 300 (for T levels: t=−5.10, df=23, p<0.001; and for C levels: t=−6.77, df=23, p<0.001).
Fig. 1.
T levels (Nucleus current units) plotted as a function of time (days after implantation of the device tested). Top panel: measurements made using the device implanted in the right ear at age 58. Middle panel: measurements made using the device implanted in the left ear at age 59. Bottom panel: measurements made using the device implanted and replacing the first left-ear implant at age 60. The legend identifies the stimulus configuration used for each measure and applies to all panels. Open circles: bipolar plus one (BP+1); filled circles: bipolar plus two (BP+2); open triangles: common ground (CG); open squares: pseudomonopolar using electrode 1 (most basal) as the common return electrode (pseudoMP).
Fig. 2.
C levels (Nucleus current units) plotted as a function of time (days after implantation of the device tested). Top panel: measurements made using the device implanted in the right ear at age 58. Middle panel: measurements made using the device implanted in the left ear at age 59. Bottom panel: measurements made using the device implanted and replacing the first left-ear implant at age 60. The legend identifies the stimulus configuration used for each measure and applies to all panels. Open circles: bipolar plus one (BP+1); filled circles: bipolar plus two (BP+2); open triangles: common ground (CG); open squares: pseudomonopolar using electrode 1 (most basal) as the common return electrode (pseudoMP).
Eight months following implantation (DAI 238) the subject scored 78% on the CID sentence test and by report he was able to use the telephone. However, one year following implantation (DAI 392), his CID sentence score had dropped to 18%. Integrity testing revealed normal functioning of the internal device.
At age 59 he underwent a left cochlear implant using a Nucleus® 22 channel device with full insertion. T and C levels measured at nine testing sessions using this implant are plotted as a function of time (days after left device implantation; DAI) in the middle panels of Fig. 1 and Fig. 2. Measures made using the BP+1 stimulus configuration are represented by open circles and by triangles when the common ground (CG) configuration was employed. The BP+1 measures show a modest downward trend for T (slope: −0.15 level units/DAI; p=0.02) that accounts for only 7% of the variance in T (R2=0.07; p=0.02) and no significant trend in C.
“Twitching” was first noted in the subject’s medical record at DAI 141 and DAI 143 in response to stimulation of electrodes 16, 17, 18, 19 or 20. At DAI-143, in order to avoid twitching, the stimulus configuration was changed from BP+1 to common ground (CG, open triangles in Fig. 1 and Fig. 2) and electrode 19 was turned off. Regression models computed for the T and C measures made in the CG stimulus configurations showed T stable with time (slope: 0.03; p=0.454) and a modest decreasing trend in C measures (slope: −0.16 level units/DAI; p=0.01) that accounted for only 8% of the variance in C (R2=0.08; p=0.01).
Because of poor performance and the question of kinking of the electrode array on the left, he underwent explantation of the left ear and reimplantation using a Cochlear Corporation Nucleus® 22 channel device with full insertion at age of 60. The six sets of T and C measures available from the subject’s medical record are plotted as a function of time (days after left device reimplantation; DAI) in the bottom panels of Fig. 1 and Fig. 2. All of these measures were made using the pseudomonopolar (pseudoMP) stimulation mode using the most basal electrode 1 as the return electrode. Both the T and C measures show a substantial increasing trend. Regression models confirmed the significance of these trends with a slope of 0.16 level units/DAI (p<0.0001) accounting for 73% of the variance in T (R2=0.73; p<0.0001) and a slope of 0.15 level units/DAI (p<0.0001) accounting for 69% of the variance in C (R2=0.69; p<0.0001).
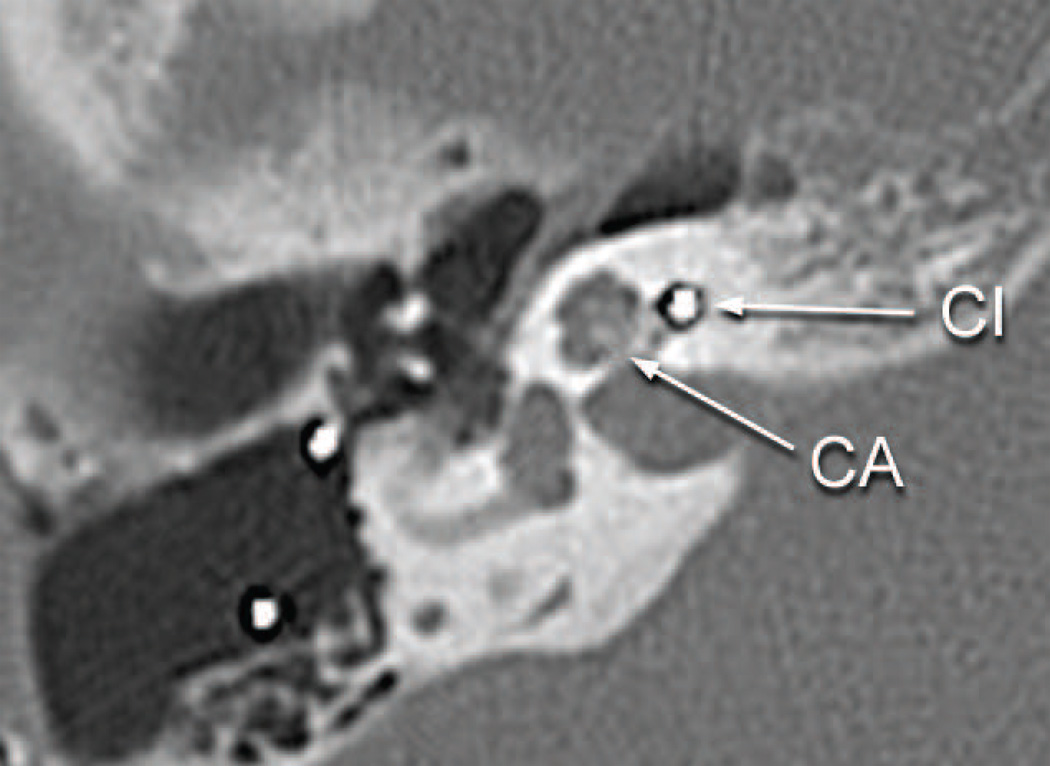
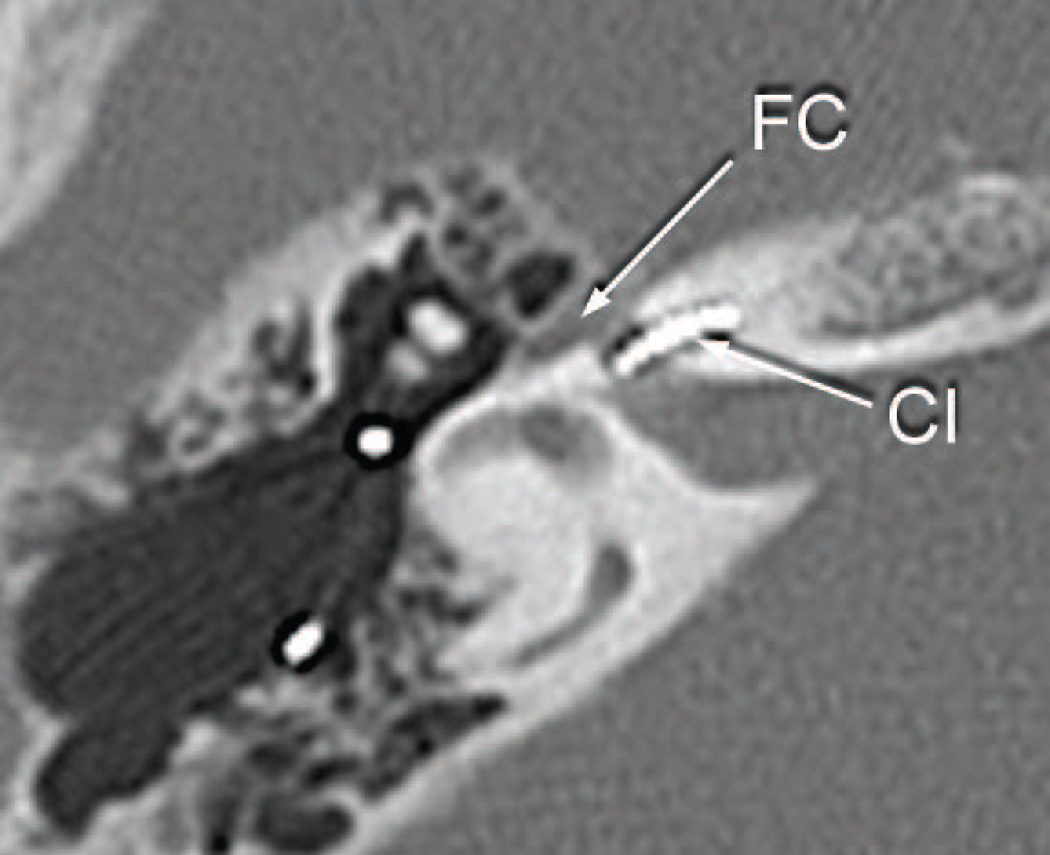
The subject’s performance with both implantations of the left ear was poor and he developed delayed onset of stimulation of the facial nerve starting at age 59. Integrity testing of the left implant at age 65 (1992 DAI) demonstrated proper functioning of the device. A CT scan of the temporal bones done at the age of 66 demonstrated that both implants were in proper location, and there was no significant abnormality of the cochlea, other than some reduced mineralization of the cochlear capsule on both sides (Fig. 3). The bone separating the geniculate ganglion from the cochlea was said to be thin but present bilaterally (Fig. 3). On the right side the implant extended approximately 270 degrees into the cochlea and on the left to approximately 250 degrees. The right sided implant was reactivated in 2003, again with poor results. The patient’s past medical history included insulin dependent diabetes mellitus. He had no known allergies. He died at the age of 71 of intracranial hemorrhage.
Fig. 3.
Postoperative CT scan of patient presented as a case report.
a. Right ear eight years following cochlear implantation and five years before death. The electrode array (CI) was seen in the basal turn. No abnormality of the cochlea was identified. The bone of the cribrose area (CA) was intact.
b. Right ear eight years following cochlear implantation. The facial canal in its horizontal segment (FC) was of normal caliber near the electrode of the cochlear implant (CI) in the basal turn.
c. Left ear six years following cochlear implantation, explantation and reimplantation and five years before death. An area of demineralization of the cochlear capsule medial to the basal turn was seen (arrow). The basal diameter of the basal turn of the cochlea was expanded anteriorly.
d. Left ear six years following cochlear implantation. The electrode (CI) was within the basal turn of the cochlea near the carotid canal (CC).
Histological Preparation
Both temporal bones were removed 58.5 hours after death and fixed in 10% buffered formalin. After a CT scan of the temporal bone specimens was done, the specimens were decalcified in ethylenediamine tetra acetic acid, dehydrated in graded alcohols, and embedded in celloidin. The specimens were serially sectioned in the axial plane with an average thickness of 20 micrometers, and every 10th section was stained with hematoxylin and eosin and mounted on a glass slide for histologic examination and two-dimensional graphic reconstruction.
PCR and histochemical stains for mycobacteria were performed. A search for the presence of particles of a foreign body was done using polarized light.
Eight additional temporal bone specimens from the collection of the Massachusetts Eye and Ear Infirmary from patients who in life had undergone cochlear implantation and which were prepared in a similar fashion, embedment in celloidin, serial sectioning and staining with hematoxylin and eosin (Table) were reviewed. Every 10th section of the cochlea throughout the electrode track from the entrance near the round window to the termination of the electrode track was evaluated for the presence of foreign body giant cells and lymphocytes in the vicinity of the cochlear implant.
Table.
Clinical data and histologic findings in 8 cases from the temporal bone collection of the Massachusetts Eye and Ear Infirmary in patients who in life had undergone cochlear implantation.
L=lymphocytes; GC=giant cells; CI=cochlear implant; FB=foreign body, Y=yes, N=no
| Case | Age/Sex | CI Electrode Ear/Manufacturer/Type |
# Years Implanted |
F.B. Giant Cells Y/N |
Monocytes Y/N |
Location of Cellular Infiltrate |
|---|---|---|---|---|---|---|
| 1 | 67 M | L Nucleus 22 | 8 years | N | N | None |
| 2 | 83 M | R Nucleus 22 | 7 years | Y | Y | Cochleostomy only |
| 3 | 81 M | L Clarion | 3 years | N | N | None |
| 4 | 70 M | R Nucleus 22 | 12 years | Y | Y | Cochleostomy and full extent of CI |
| 5 | 84 M | R Nucleus 22 | 12 years | Y | Y | Cochleostomy only |
| 6 | 91 M | L Nucleus 22 | 10 years | Y | Y | Cochleostomy and full extent of CI |
| 7 | 84 F | R Nucleus 22 | 2 years | Y | Y | Cochleostomy only |
| 8 | 72 F | L Nucleus 24 | 2 years | Y | Y | Cochleostomy and full extent of CI |
FB=foreign body; Y=yes; N-no; CI=cochlear implant
Results
Case report
Right ear
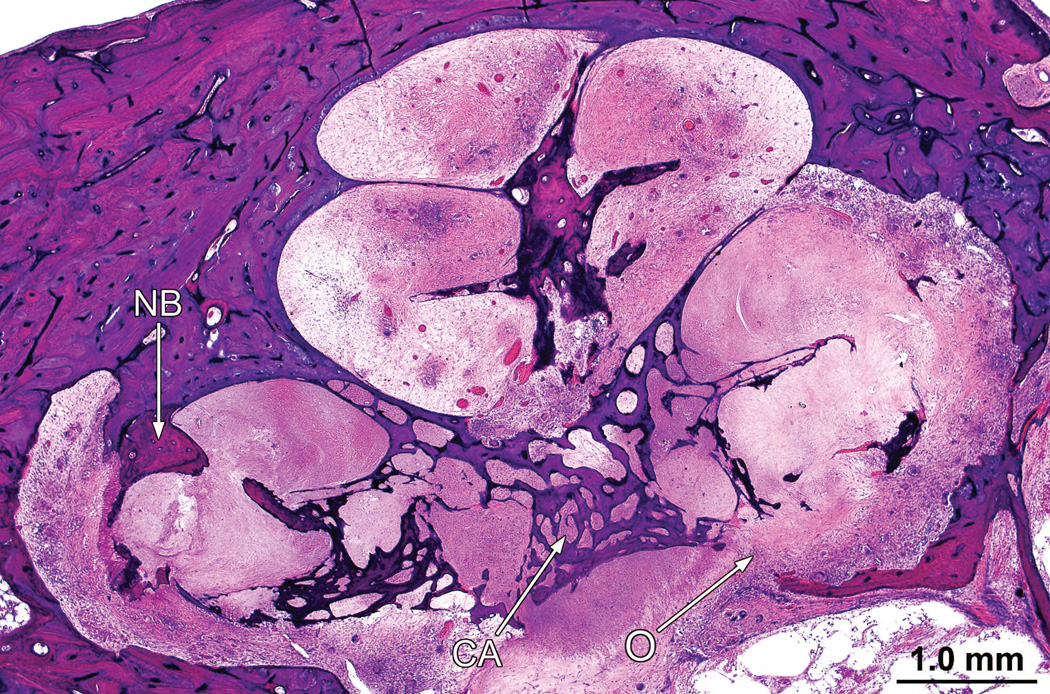
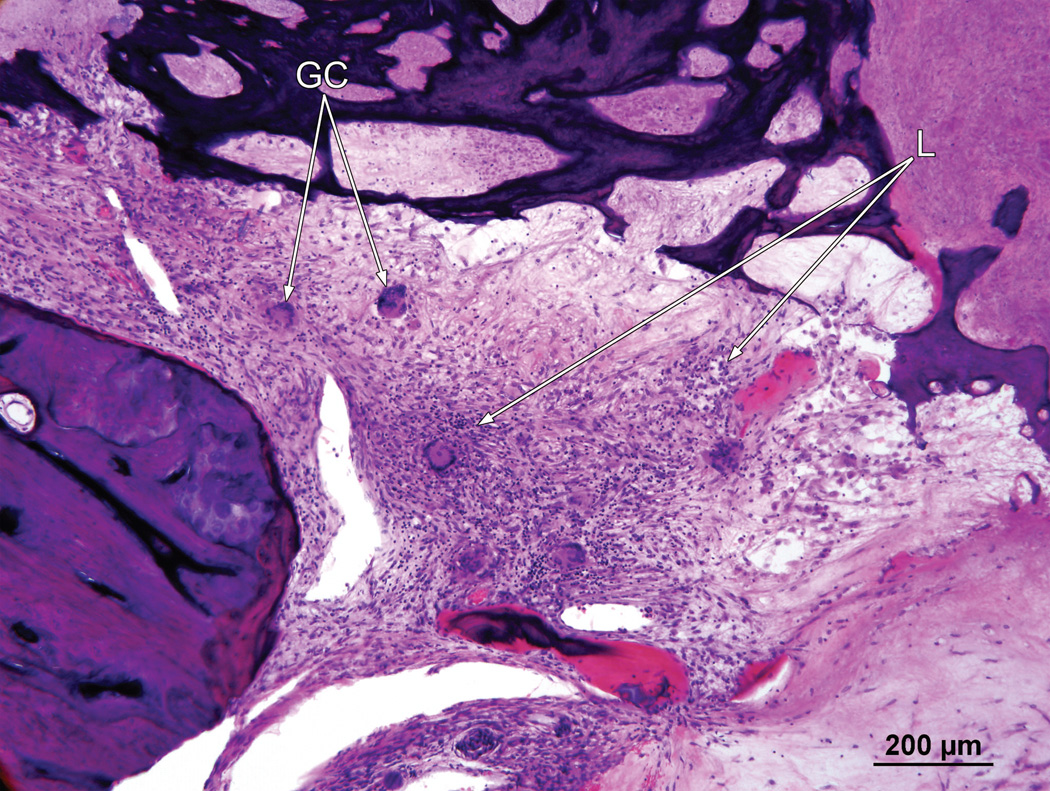
The cochlear implant track entered the scala tympani at approximately 5.5 mm from the round window and terminated 18.5 mm from the round window. The entire cochlea was filled with a necrotizing granulomatous process (Fig. 4). There was no evidence of a residual organ of Corti. There were few remaining spiral ganglion cells (Fig. 5). There was osteolysis of the otic capsule particularly in the basal turn (Fig. 5) and the granulomatous process demonstrated both giant cells and lymphocytes (Fig. 6).
Fig. 4.
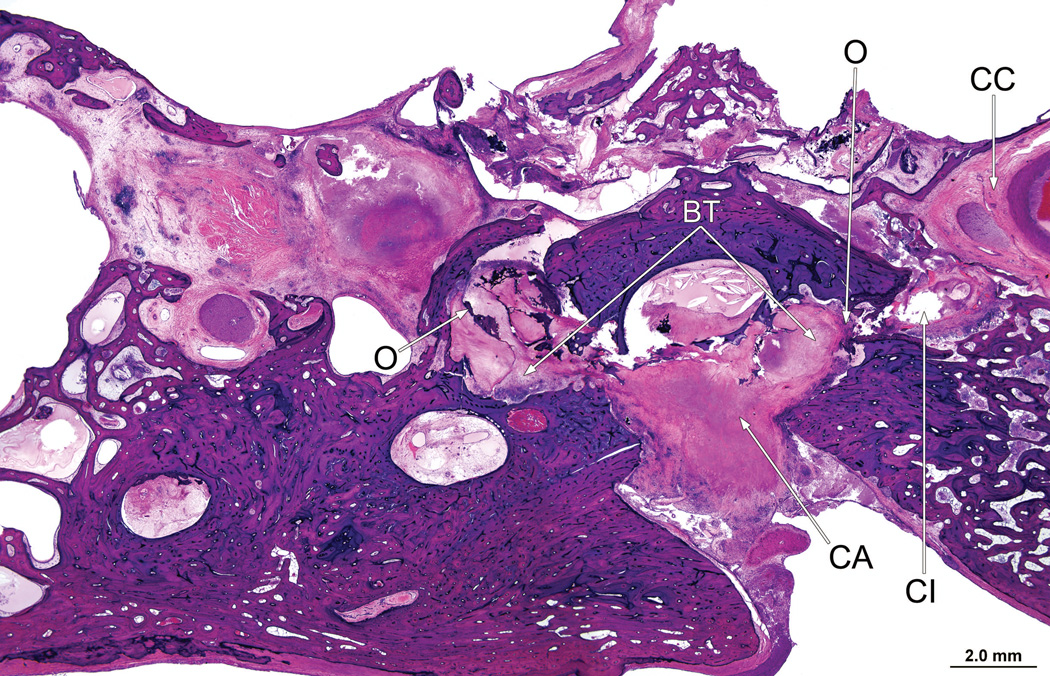
In the right ear (case report), the entire cochlea was filled with a necrotizing granulomatous process. There had been osteolysis (O) of the bone in the cribrose area (CA). There was scattered new bone formation (NB)
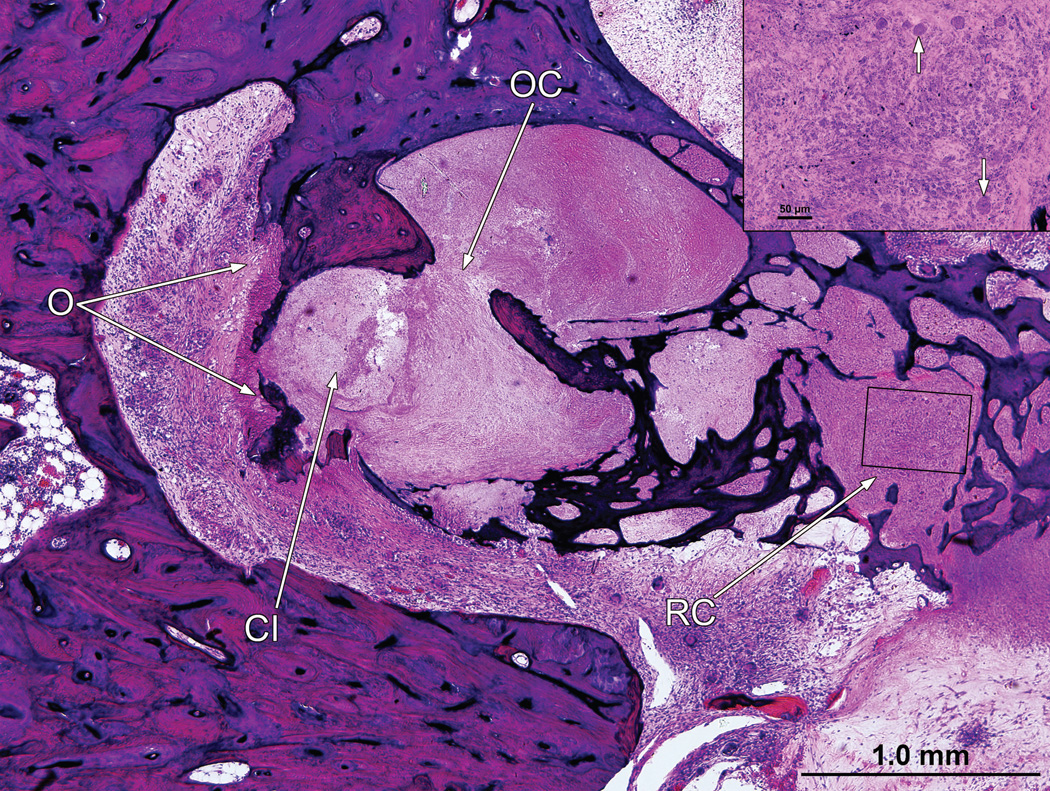
Fig. 5.
Right ear (case report). There had been osteolysis (O) of the otic capsule. The organ of Corti (OC) has been destroyed, and there were only a few remaining spiral ganglion cells in Rosenthal’s canal (RC) (insert). The track of the cochlear implant (CI) was seen.
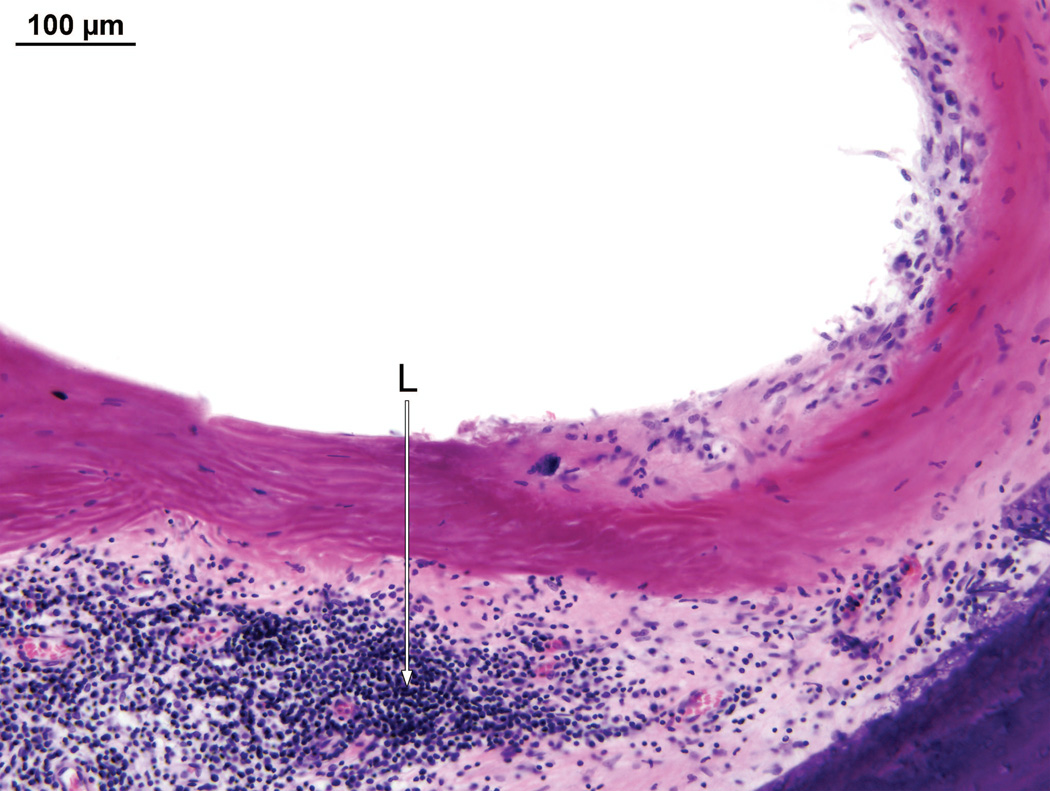
Fig. 6.
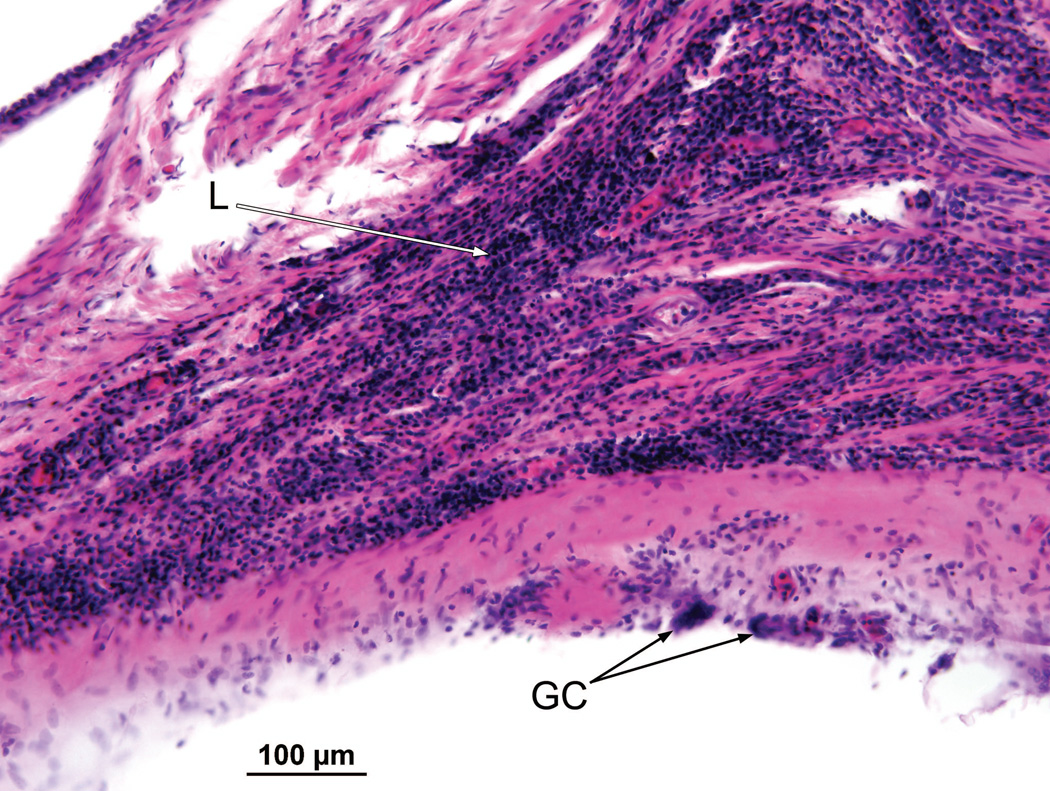
Right ear (case report). The granulomatous process contained both foreign body giant cells (GC) and lymphocytes (L).
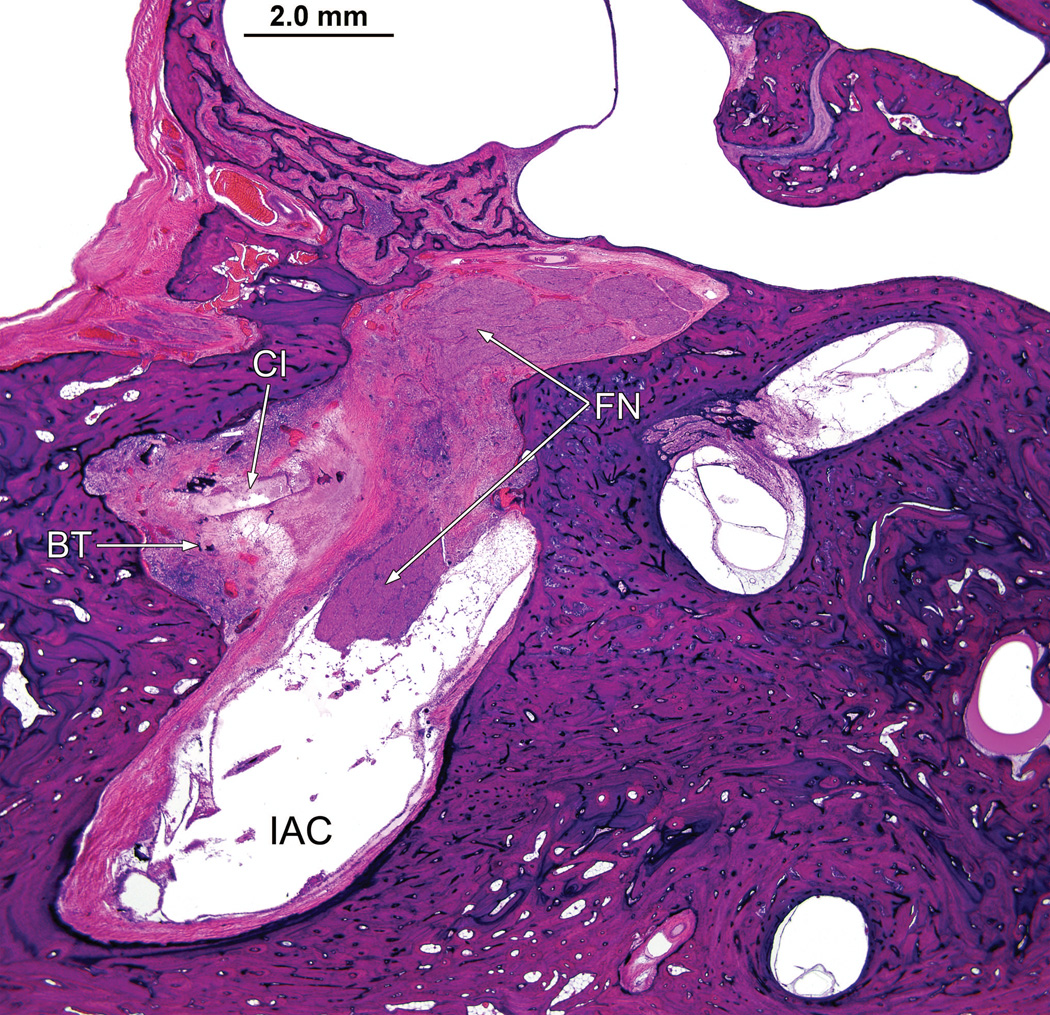
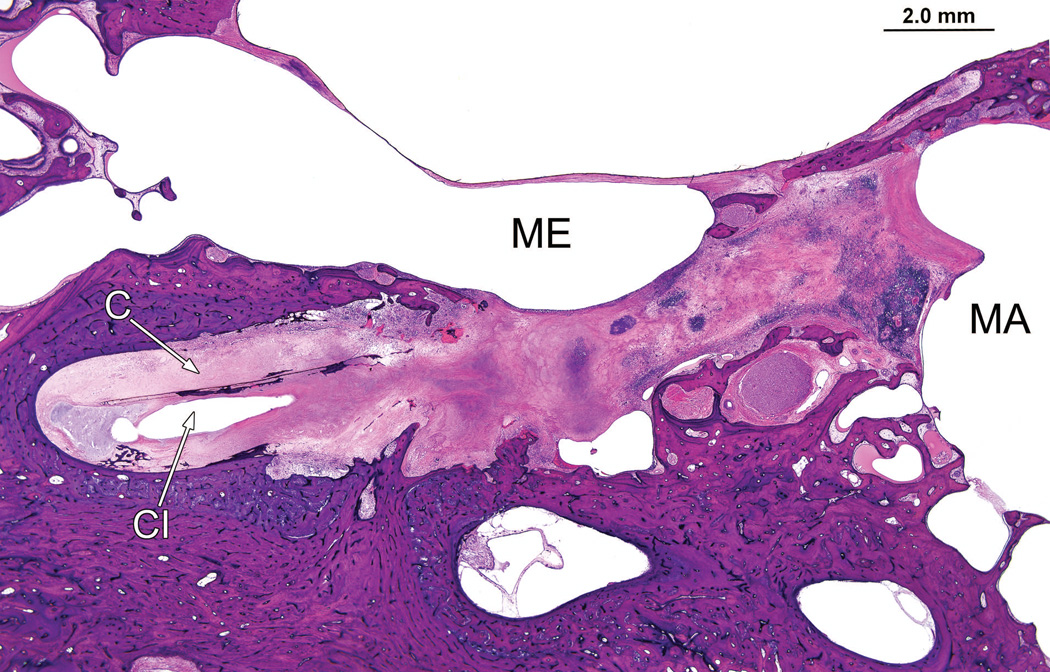
There was osteolysis of the otic capsule between the basal turn of the cochlea and the internal auditory canal in the vicinity of the labyrinthine and horizontal segments of the facial nerve (Fig. 7). The necrotizing giant cell granulomatous process followed the electrode track through otherwise uninvolved mastoid and middle ear cavities into the inner ear, (Fig. 8) and was particularly prominent in the basal turn and extended to the macula sacculi but did not involve the semicircular canals or utricle.
Fig. 7.
Right ear (case report). The bone between the basal turn (BT) of the cochlea and the internal auditory canal (IAC) had been destroyed by the granulomatous process. The cochlear implant electrode track (CI) was in proximity to the facial nerve (FN).
Fig. 8.
Right ear (case report). The granulomatous process surrounds the cochlear implant track (CI) in the mastoid (MA) and middle ear (ME) and cochlea (C). However the remainder of the middle ear and mastoid air cell system were uninvolved.
Left ear
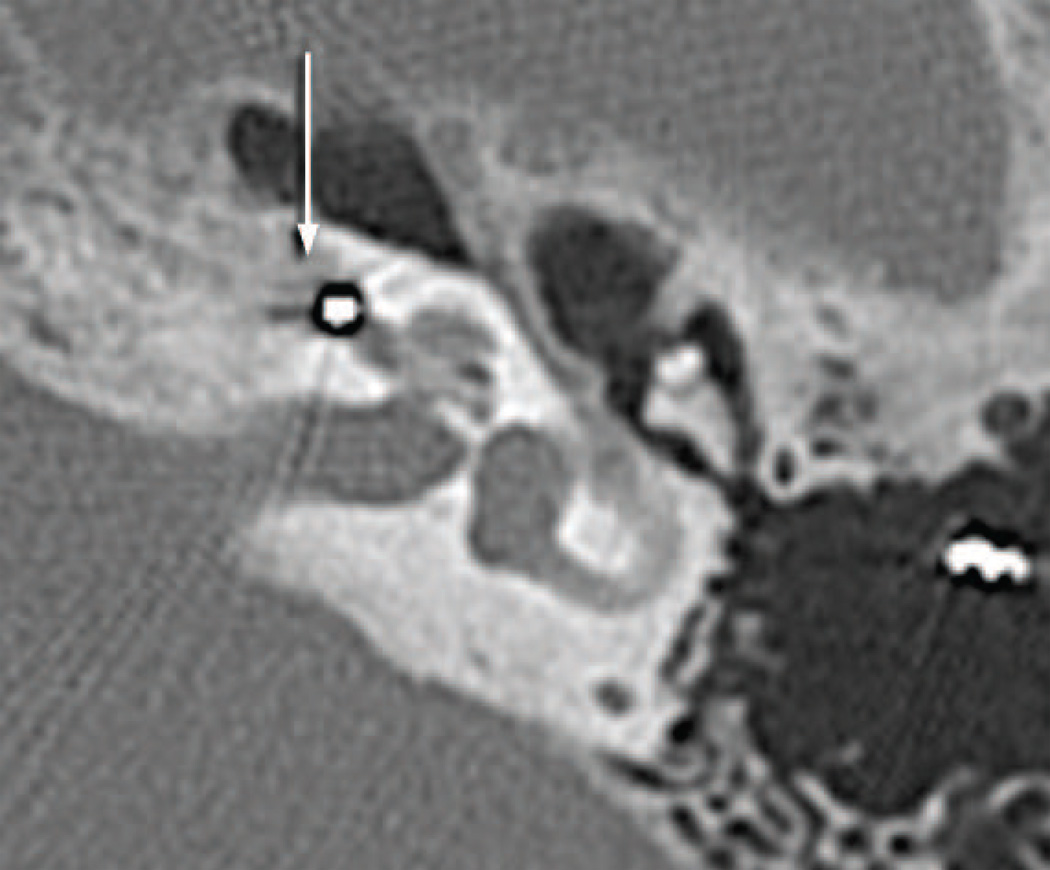
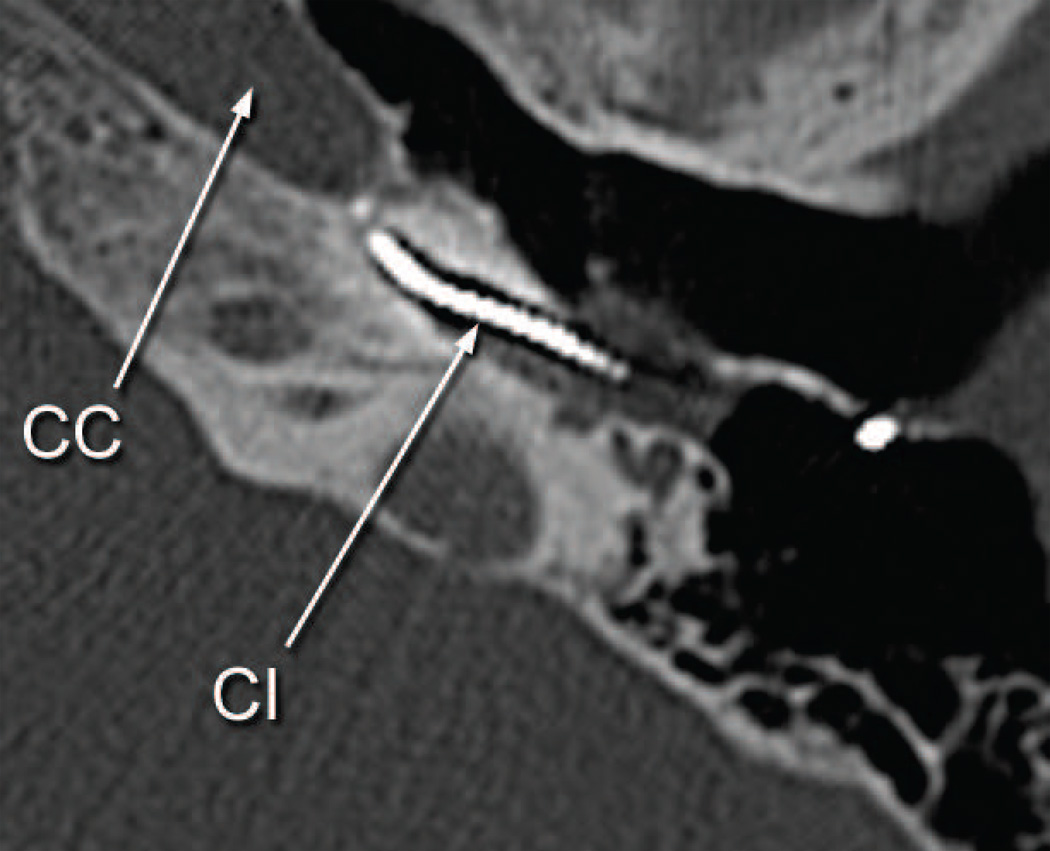
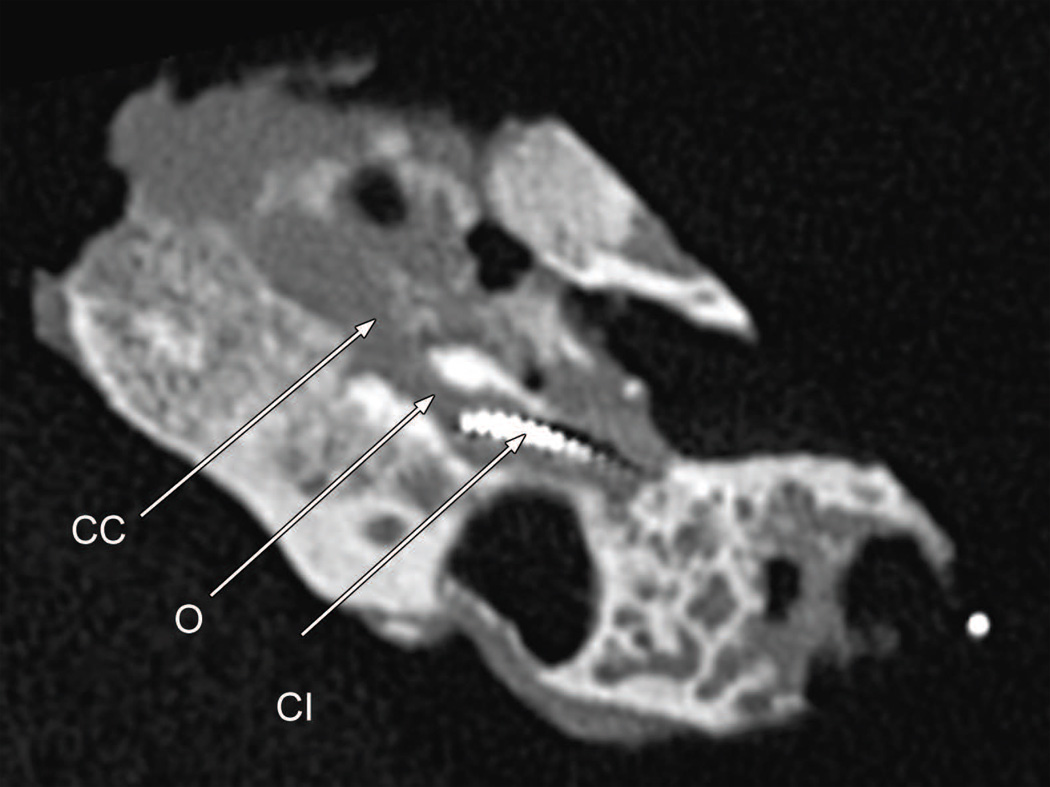
Postmortem CT scans (Fig. 9) as compared with those done at age 66 (Fig. 3) suggested progressive osteolysis during this 5 year period. The cochlear implant track entered the scala tympani at approximately 3.5 mm from the round window. The track then extended only a short distance within the basal turn and then exited the cochlea anteriorly in proximity to the carotid artery and extended to approximately the level of millimeter 12 of the cochlea in this extra-cochlear position (Figs. 8 and 9). In the cribrose area of the cochlea there was total dissolution of the bony partitions separating the scala tympani from the internal auditory canal (Fig. 10). In addition there was osteolysis of the otic capsule not only in the area of the carotid canal (Fig. 11) but also of the posterior aspect of the basal turn and between the basal turn and the labyrinthine and horizontal segments of the facial nerve. As on the right side, the necrotizing giant cell granuloma followed the electrode tract from the mastoid into the cochlea. It also extended to the vestibule and petrous apex anterior to the cochlea. There was no recognizable organ of Corti or spiral ganglion cells in any turn. The granulomatous process displaced the facial nerve anteriorly in the internal auditory canal. There was new bone formation in the superior semicircular canal, crus commune, and ampullated end of the posterior semicircular canal but no evidence of involvement by the granulomatous process.
Fig. 9.
Postmortem CT scan of the left temporal bone (case report). There had been progression osteolysis (O) between the basal turn containing the cochlear implant (CI) and the carotid canal (CC). Compare Fig. 3D.
Fig. 10.
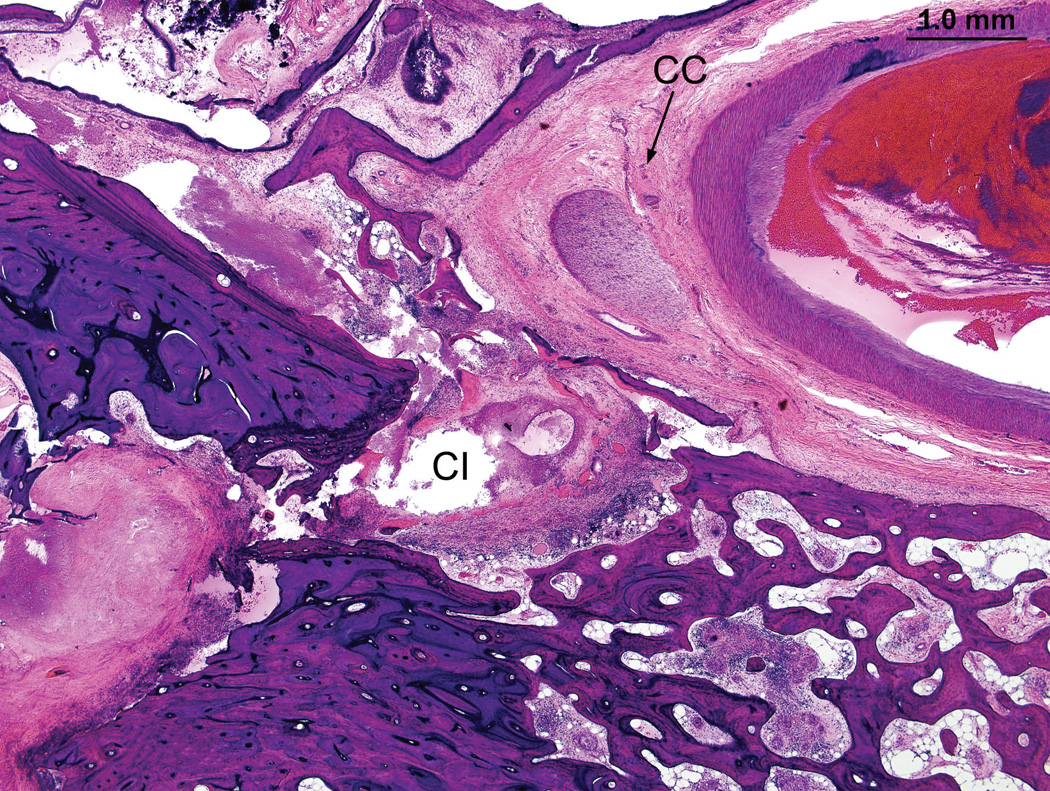
Left ear (case report). There was a necrotizing granulomatous process that filled the cochlea particularly in the basal turn (BT). The electrode track (CI) left the basal turn of the cochlea anteriorly to enter the carotid canal (CC). The bone of the cribrose area (CA) had been destroyed, and there was also osteolysis (O) of the otic capsule, particularly in the basal turn.
Fig. 11.
Left ear (case report). The cochlear implant track (CI) enters the carotid canal (CC).
In both ears there was no indication of particles of a foreign body as studied by polarized light. PCR and histochemical stains for mycobacteria were negative. There was no evidence of vasculitis.
Other specimens
Semiserial sections from 8 patients who in life had undergone cochlear implantation (Table) were examined for the presence of foreign body giant cells and lymphocytes. In 6 of 8 specimens (75%) both cell types were found, either at the cochleostomy only (n=3) or throughout the full length of the track of the cochlear implant (n=3) (Fig. 12). In these specimens the cellular immune response was found as early as two years following implantation, whereas the two patients without this cellular response had been implanted at 3 and 8 years before death. There was no evidence of soft failure in these cases, although the severity of the hypersensitivity reaction was far less than in the subject of the case report.
Fig. 12.
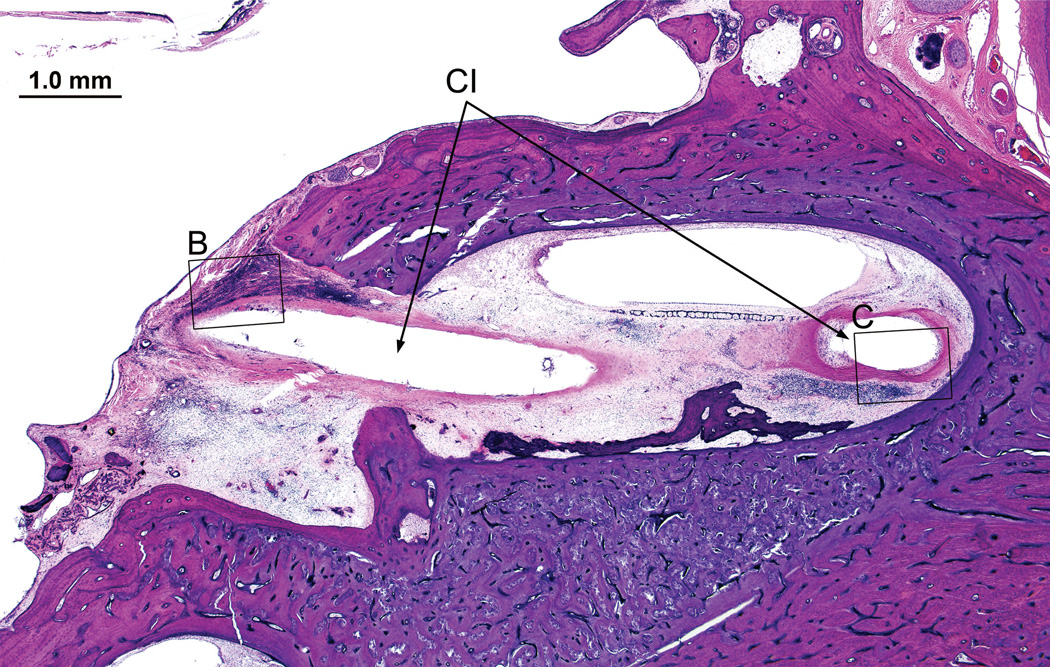
Left temporal bone from a 91-year old man who had undergone cochlear implantation of the left ear at age 80 years. (Case 6, Table). The cochlear implant electrode array had been removed but its track (CI) was visible within the specimen. There was an intense lymphocytic cell (L) and multinucleated giant cell (GC) infiltration near the implant track here in the basal turn near the cochleostomy. Seen in Fig. 12B & 12C (boxed areas).
Discussion
Delayed failure of cochlear implantation may be ascribed to a device failure, either 1) failure to lock or 2) failure to otherwise meet manufacturer’s specifications, described as a “hard failure” or may be due to a performance decrement unexplained by integrity testing (“soft failure”) (Buchman et al 2004, Balkany et al 2005, European Concensus statement 2005, Cote et al 2007). There are very little data on the success rate of reversing the decrement in auditory function by reimplantation in soft failures, although Buchman et al (2004) suggest that auditory symptoms were resolved in 87% of such patients. Thus there is a subset of soft failures in which explantation and reimplantation do not result in significant improvement of auditory symptoms or speech recognition.
The causes of such failures, particularly those who fail despite reimplantation and which therefore cannot be reasonably ascribed to device failure are unknown. We present a case report herein of a well documented soft failure following cochlear implantation of the right ear in a 71 year old man and simultaneous development of twitching of the facial musculature during stimulation by a progressively increasing number of electrodes. The predominant histopathology was a necrotizing granulomatous process following the electrode track through the mastoid into the cochlea and filling the entire cochlear duct. This resulted in total loss of the organ of Corti and most spiral ganglion cells, osteolysis of the otic capsule including bone between the basal turn and labyrinthine segment of the facial nerve and extension to the vestibule. There were similar findings, albeit somewhat more severe, on the contralateral left side which had undergone two unsuccessful cochlear implantations. In addition on the left, there was evidence of a progressive osteolysis of the otic capsule with presumed migration of the electrode array from the basal turn into the carotid canal.
Seventy-five percent of other specimens from patients who in life had undergone cochlear implantation demonstrated a similar but less pronounced biological response, similar to that described by Nadol and Eddington (2004).
The differential diagnosis of this granulomatous process includes Wegener’s granulomatosis, mycobacterial infection, foreign body reaction, or a hypersensitivity response. Wegener’s granulomatosis is less likely given the fact that no vasculitis was found in the specimens. PCR and histochemical stains were negative for mycobacteria, and an infectious process in general is unlikely given the bilaterality of the involvement and limitation of the process to the electrode track as it passed through the mastoid and into the cochlea. Furthermore, although there was osteolysis of the cribrose areas between the basal turn and internal auditory canal in both ears, there was no history consistent with meningitis as would have been expected in an infectious process. A foreign body response is possible, but the presence of necrosis and the absence of foreign material seen in polarized light make this less likely. The most likely explanation, therefore, is a delayed hypersensitivity reaction.
Similar histopathology has been described in other cochlear implant cases (Bertuleit et al 1999, Kronenberg et al 2001, Kunda et al 2006, Migirov 2007). In addition, Ho et al described delayed erosion of the cochlear capsule of the basal turn of the cochlea in two patients (2007) and a similar finding was described by Doherty and Linthicum (2004) in a temporal bone from a patient who had undergone cochlear implantation three years prior to death. These three cases were attributed to a focal inflammatory process.
Similar histopathology, including giant cell granuloma attributed to a foreign body or hypersensitivity response has been described following implantation of silicone-containing prostheses including skin expanders (Maturri et al 1991), implants for arthroplasty, (Kistler et al 2005), cardiac pacemakers (Maushagen et al 1994), cerebrospinal fluid shunts (Snow et al 1989, Jimenez et al 1994, Hashimoto et al 2004); breast implants (Busch et al 1994, Meyer et al 1998) and an endolymphatic subarachnoid shunt (Pulec 1998).
Allergy testing to various formulations of silicone used in cochlear implants has resulted in positive results in some patients (Puri et al 2005, Kunda et al 2006, Migirov et al 2007). In most cases the clinical presentation of the presumed hypersensitivity response was in the vicinity of the receiver/stimulator electronic package rather than the electrode array, although it was clinically unknown in these cases whether the process did or did not extend to the track of the cochlear implant within the cochlea. The presence of a granulomatous labyrinthitis following cochlear implantation was described by Bertuleit et al (1999). Furthermore the histopathology as described by Doherty and Linthicum (2004) in retrospect may well be attributable to a similar process as described here. Animal testing of the biocompatibility of silicone cochlear implants was described by Shepherd et al in 1984, and although foreign body giant cell reaction was not described, a mild tissue reaction in the form of fibrosis and a lymphocytic cell infiltration was described.
Other possible causes of the tissue response have been hypothesized including alteration of intrinsic proteins by ethylene oxide sterilization (Pittman et al 1994, Klykkenn and Curtis 2007), immunogenicity induced by tin moeties used in catalyzing silicone adhesives (Klykken and Curtis 2007) or a foreign body reaction (Klykken and Curtis 2007).
The formulation of silicone varies by specific components of the implant device such as the receiver/stimulator and electrode array (Kunda et al 2006). The manufacturers including Advanced Bionics, Cochlear Corporation, and Medel Corporation provide sample kits for allergic testing (Kunda et al 2006). In addition, silicone adhesives have also been implicated as possible allergens (Kunda et al 2006).
In retrospect, the available measures of T and C levels do not reveal early trends suggesting the granulomatous process identified in this case study. The increasing trend in T and C levels shown in the bottom panels of Fig. 1 and Fig. 2 wasn’t documented until almost 7 years from his first implantation and about 4 years (age 64) after reimplantation of the left ear. While it is possible that the “twitching” reported 300 days after the first implant (right ear; age 59) and/or the dramatic decrease in CID sentence reception (78% to 18%) documented 92 days later were the first indicators of some early aspect of the pathology we report, direct evidence does not exist to make such links. The earliest direct clinical evidence of this granulomatous process was demineralization and osteolysis of the bone of the otic capsule on both sides, eight years after the first and six years after the last cochlear implantation (Fig. 3). This osteolysis progressed until the time of death (Fig. 9). Similar CT abnormalities were reported by Bertuleit et al (1999) and by Ho et al (2007). In the latter report, radiographic evidence of migration of the implant array outside the anatomical limits of the cochlea was documented similar to the left ear in the case report presented herein.
In summary, the prevalence of foreign body giant cells and lymphocytic cell infiltration along the track of the cochlear implant in a series of patients presented herein, and the necrotizing granulomatous process within the cochlea of the case report with a history of “soft failure” suggests that at least in some patients a hypersensitivity or foreign body giant cell reaction may be responsible for failures not attributable to electrical failure, particularly for patients in whom no reversal of decrement in auditory performance is seen following reimplantation.
Acknowledgments
Supported by: NIH/NIDCD #R01 DC000152
Bibliography
- 1.Buchman CA, Higgins CA, Cullen R, Pillsbury HC. Revision cochlear implant surgery in adult patients with suspected device malfunction. Otol Neurotol. 2004;25(4):504–510. doi: 10.1097/00129492-200407000-00018. [DOI] [PubMed] [Google Scholar]
- 2.European concensus statement on cochlear implant failures and explantations. Otol Neurotol. 2005;26(6):1097–1099. [No authors listed] [PubMed] [Google Scholar]
- 3.Balkany TJ, Hodges AV, Buchman CA, Luxford WM, Pillsbury CH, Roland PS, et al. Cochlear implant soft failures consensus development conference statement. Otol Neurotol. 2005;26(4):815–818. doi: 10.1097/01.mao.0000178150.44505.52. [DOI] [PubMed] [Google Scholar]
- 4.Côté M, Ferron P, Bergeron F, Bussières R. Cochlear implantation: causes of failure, outcomes, and audiologic performance. Laryngoscope. 2007;117(7):1225–1235. doi: 10.1097/MLG.0b013e31805c9a06. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd RK, Webb RL, Clark GM, Pyman BC, Hirshorn MS, Murray MT, Houghton ME. Implanted material tolerance studies for a multiple-channel cochlear prosthesis. Acta Otolaryngol (Stockh) 1984;(Suppl 411):71–81. [PubMed] [Google Scholar]
- 6.Bertuleit H, Groden C, Schäfer HJ, Leuwer R. Removal of a cochlear implant with chronic granulation labyrinthitis and foreign body reaction. Laryngorhinootologie. 1999;78(6):304–306. doi: 10.1055/s-2007-996876. [DOI] [PubMed] [Google Scholar]
- 7.Kronenberg J, Wolf M, Migirov L, Shapira Y, Aviel-Ronen S, Hildesheimer M. Foreign body reaction to cochlear implant. Otorhinolaryngol Nova. 2001;11:207–209. [Google Scholar]
- 8.Kunda LD, Stidham KR, Inserra MM, Roland PS, Franklin D, Roberson JB., Jr Silicone allergy: a new cause for cochlear implant extrusion and its management. Otol Neurotol. 2006;27(8):1078–1082. doi: 10.1097/01.mao.0000235378.64654.4d. [DOI] [PubMed] [Google Scholar]
- 9.Migirov L, Taitelaum-Swead R, Hildesheimer M, Kronenberg J. Revision surgeries in cochlear implant patients: a review of 45 cases. Eur Arch Otorhinolaryngol. 2007;264:3–7. doi: 10.1007/s00405-006-0144-5. [DOI] [PubMed] [Google Scholar]
- 10.Puri S, Dornhoffer JL, North PE. Contact dermatitis to silicone after cochlear implantation. Laryngoscope. 2005;115(10):1760–1762. doi: 10.1097/01.mlg.0000172202.58968.41. [DOI] [PubMed] [Google Scholar]
- 11.Ho EC, Proops D, Andrews P, Graham J. Unexpected exit of a cochlear implant electrode through the wall of the basal turn of the cochlea-a report on two patients. Coch Implants Int. 2007;8(3):162–171. doi: 10.1179/cim.2007.8.3.162. [DOI] [PubMed] [Google Scholar]
- 12.Doherty JK, Linthicum FH., Jr Temporal Bone Histopathology Case of the Month. Cochlear endosteal erosion with focal osteomyelitis induced by cochlear implantation. Otol & Neurotol. 2004;25:1029–1030. doi: 10.1097/00129492-200411000-00029. [DOI] [PubMed] [Google Scholar]
- 13.Maturri L, Azzolini A, Campiglio GL, Tardito E. Are synthetic prostheses really inert? Preliminary results of a study on the biocompatibility of Dacron vascular prostheses and Silicone skin expanders. Int Surg. 1991;76(2):115–118. [PubMed] [Google Scholar]
- 14.Kistler U, Weiss AP, Simmen BR, Herren DB. Long-term results of silicone wrist arthroplasty in patients with rheumatoid arthritis. J Hand Surg (Am) 2005;30(6):1282–1287. doi: 10.1016/j.jhsa.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Maushagen E, Reichle B, Simon H. Circumscribed erythema after cardiac pacemaker implantation. Z Kardiol. 1994;83(5):340–342. [PubMed] [Google Scholar]
- 16.Snow RB, Kossovsky N. Hypersensitivity reaction associated with sterile ventriculoperitoneal shunt malfunction. Surg Neurol. 1989;31(3):209–214. doi: 10.1016/0090-3019(89)90119-5. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez DF, Keating R, Goodrich JT. Silicone allergy in ventriculoperitoneal shunts. Childs Nerv Syst. 1994;10(1):59–63. doi: 10.1007/BF00313586. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto M, Yokota A, Urasaki E, Tsujigami S, Shimono M. A case of abdominal CSF pseudocyst associated with silicone allergy. Childs Nerv Syst. 2004;20(10):751–754. doi: 10.1007/s00381-003-0904-0. [DOI] [PubMed] [Google Scholar]
- 19.Busch H. Silicon toxicology. Semin Arthritis Rheum. 1994;24(Suppl 1):11–17. doi: 10.1016/0049-0172(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 20.Meyer DR, Bui HX, Carlson JA, Ratliff CD, Guerarra MC, DelRosario AD, et al. Silicon granulomas and dermatomyositis-like changes associated with chronic eyelid edema after silicone breast implant. Ophthal Plast Reconstr Surg. 1998;14(3):182–188. doi: 10.1097/00002341-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Pulec JL. Endolymphatic subarachnoid shunt failure caused by Silastic allergy. Ear Nose Throat J. 1998;77(8):614–616. 619–620, 622. [PubMed] [Google Scholar]
- 22.Nadol JB, Jr, Eddington DK. Histologic evaluation of the tissue seal and biologic response around cochlear implant electrodes in the human. Otol Neurotol. 2004;25(3):257–262. doi: 10.1097/00129492-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Klykken PC, Curtis JM. Letter to the Editor. Re:“Silicone allergy: a new cause for cochlear implant extrusion and its management”. Otol Neurotol. 2007;28:1159–1161. doi: 10.1097/MAO.0b013e31815a8453. [DOI] [PubMed] [Google Scholar]
- 24.Pittman T, Williams D, Rathore M, Knutsen AP, Mueller KR. The role of ethylene oxide allergy in sterile shunt malfunctions. Br J Neurosurg. 1994;8(1):41–45. doi: 10.3109/02688699409002391. [DOI] [PubMed] [Google Scholar]