Abstract
Limited data on the relative contributions of different routes of transmission for influenza virus are available. Person-to-person transmission is central to seasonal and pandemic spread; nevertheless, the modes of spread are a matter of ongoing debate. Resolution of this discussion is paramount to the development of effective control measures in health care and community settings. Using the guinea pig model, we demonstrated that transmission of influenza A/Panama/2007/1999 (H3N2) virus through the air is efficient, compared with spread through contaminated environmental surfaces (fomites). We also examined the aerosol transmission efficiencies of 2 human influenza virus A strains and found that A/Panama/2007/1999 influenza virus transmitted more efficiently than A/Texas/36/1991 (H1N1) virus in our model. The data provide new and much-needed insights into the modes of influenza virus spread and strain-specific differences in the efficiency of transmission
Influenza virus is responsible for annual epidemics associated with significant morbidity and mortality, particularly among young children and the elderly [1]. Influenza virus, compared with other respiratory viruses, contributes more to all-cause mortality, particularly in individuals >65 years old [2]. In addition, global pandemics secondary to novel influenza virus strains have claimed the lives of tens of millions of otherwise healthy individuals. The limitations of methods to abrogate the spread of influenza virus and the threat of a pandemic require that alternate means to control influenza be devised. The urgency of achieving this goal is evident, as underscored in a recent Institute of Medicine report [3]
Potential modes of transmission of influenza virus include direct contact with infected individuals, exposure to virus-contaminated objects (fomites), and inhalation of infectious aerosols. Fomites are inanimate objects (e.g., children’s toys) that can serve as vehicles for the spread of pathogens through indirect contact. Infectious aerosols consist of large respiratory droplets and droplet nuclei. Large respiratory droplets are >5–10 μm in diameter and are involved in short-range transmission. Droplet nuclei are <5 μm and are responsible for transmission over greater distances (long-range or airborne transmission) [3, 4]. Current recommendations from the Centers for Disease Control and Prevention for the prevention and control of influenza virus transmission in health care settings include adherence to standard precautions, maintenance of respiratory hygiene, droplet precautions, and enhanced airborne precautions in cases of suspected or confirmed avian influenza [5, 6]. These recommendations reflect the potential for influenza virus to be transmitted via fomites, large droplets, or droplet nuclei, but the relative contribution of each to overall transmission remains unknown
Although novel methods for the reduction of spread via fomites are being developed [7], the extent to which fomites contribute to the spread of influenza virus has not been rigorously addressed. Bean et al. [8] demonstrated the persistence of clinical isolates of influenza A and B in the environment. Transfer to hands from both porous and nonporous surfaces was also shown. In a study conducted by Boone and Gerba [9], 23%–59% of fomites from child care facilities and homes were positive for influenza virus RNA by polymerase chain reaction. Another study, by Thomas et al. [10], demonstrated the persistence of infectious influenza virus on Swiss banknotes for several days; it also demonstrated that addition of respiratory mucus permitted virus isolation after several weeks. Although fomites may play a role in transmission, human infections from contaminated surfaces have not been demonstrated in these investigations
Short- and long-range aerosol transmission of influenza virus has been observed in the ferret model [11]. Differences in transmission rates of various strains of influenza viruses have also been examined in ferrets [12–15]. Although the ferret model is well established, there are several limitations to its use, including animal size, cost, and temperament. Because of these disadvantages, few centers are capable of conducting large-scale transmission studies using this model. We recently described a novel mammalian model for the transmission of human influenza viruses—the guinea pig—which overcomes some of the limitations of the ferret model [16]. We demonstrated that guinea pigs are highly susceptible to infection with human influenza A/Panama/2007/1999 (H3N2) virus (hereafter, “Pan99”), which has an ID50 of 5 pfu, and that this virus grows to high titers in the upper respiratory tract and to moderate titers in the lungs [16]. We also showed 100% transmission of Pan99 virus by direct contact and aerosol in this system [16–18]
In the present study, we evaluate the efficiency of spread of a human H3N2 isolate via short- and long-range aerosols and via fomites, using the more-accessible guinea pig model. Furthermore, we demonstrate differences in transmission efficiency between 2 influenza virus strains of human origin
Methods
Guinea pigsFemale Hartley (outbred) guinea pigs (300–350 g) were purchased from Charles River Laboratories, and strain 13 (inbred) guinea pigs (male and female, 350–550 g) were purchased from the US Army Medical Research Institute of Infectious Diseases. All experiments were done in accordance with Mount Sinai School of Medicine Institutional Animal Care and Use Committee regulations and were conducted in a negative-pressure biohazard suite with high-efficiency particulate air–filtered exhaust. Care was taken to change gloves and decontaminate work surfaces between the handling of exposed animals. Nasal washes were performed with animals under anesthesia, and viral titers were determined by plaque assay on Madin-Darby canine kidney (MDCK) cells. For transmission experiments, preimmune and convalescent serum samples were collected, and serology was performed by hemagglutination inhibition
VirusesStocks of influenza viruses, including Pan99 and influenza A/Texas/36/1991 (H1N1) virus (hereafter, “Tx91”), were generated after a single passage in MDCK cells. Recombinant Pan99 (rPan99) virus was produced using reverse genetics [15], and stocks were generated after passage in MDCK cells
Transmission experimentsThe majority of transmission experiments were performed in Caron environmental chambers (model 6030) to maintain temperature at 20°C and relative humidity at 20%; air in the chamber is recirculated. Experiments involving aerosol inoculations and fomite transmission experiments were performed under ambient conditions
For short- and long-range aerosol transmission experiments, anesthetized Hartley guinea pigs were intranasally inoculated with 1×103 pfu of influenza virus in 300 μL (150 μL per nare) of PBS supplemented with penicillin-streptomycin (100 U/mL and 100 μg/mL, respectively) and 0.3% bovine serum albumin (large-droplet inoculation). Twenty-four hours later, 4 naive Hartley guinea pigs were placed in separate transmission cages with wire tops and 1 perforated side. Cages were placed either next to transmission cages containing inoculated animals, with perforated sides facing each other (short-range transmission experiments), or at a distance (80 or 107 cm) above the inoculated animals housed in regular cages with wire lids (long-range transmission experiments). In this manner, air flowed freely between cages, but direct contact between inoculated and exposed animals was prohibited
For fomite transmission experiments, guinea pigs were inoculated intranasally with 1×103 pfu of Pan99 virus. After 48 h, each inoculated animal was removed from its cage, and an uninoculated (exposed) animal was placed in its stead. Bedding, food dishes, and water bottles were not changed at this point. Nasal wash samples were collected from inoculated animals to ensure viral shedding at the time of exposure, and these animals were subsequently placed in separate cages. Exposed animals remained in their respective cages for 24 h, at which time nasal wash samples were collected and animals moved to clean cages (occupation of cages for a total of 72 h necessitated changing)
Aerosol inoculationAnimals were placed in an inhalation exposure system (model 099C A4212; Glas-Col) for 30 min, during which time Pan99 virus in PBS supplemented with penicillin-streptomycin (100 U/mL and 100 μg/mL, respectively) and 0.3% bovine serum albumin was nebulized into the chamber, forming a cloud composed of droplet nuclei that were ∼1–2 μm in size. This was followed by a 600-s cloud decay cycle and subsequent air decontamination cycle, after which animals were removed from the inhalation exposure system and housed in cages
Air samplingIntranasally inoculated guinea pigs were placed in a cage modified for air sampling. This sampling chamber consisted of an air inlet, a sampling port, and a sealed lid. The sampling chamber was placed in the environmental chamber to control the temperature and relative humidity of the air entering the sampling chamber (20°C and 20%, respectively). Animals were placed inside for 10 min, followed by a 10-min sampling period. A glass liquid impinger (Ace Glass model 7542–10) containing 5 mL of PBS supplemented with penicillin-streptomycin (100 U/mL and 100 μg/mL, respectively), amphotericin B (1.25 μg/mL), and 0.3% bovine serum albumin was attached to the sampling port. A Gast pump (Westech Instruments) pulled air from the chamber at a rate of ∼8 L/min. Samples were concentrated using centrifugal filter devices (Millipore Amicon Ultra-4 Ultracel 100K). The titer of infectious virus was determined by plaque assay on MDCK cells. Two air samples were obtained daily, and the final aerosol viral titer was based on the average. Nasal wash samples were collected on alternate days, and upper respiratory tract viral titers were determined by plaque assay on MDCK cells
Results
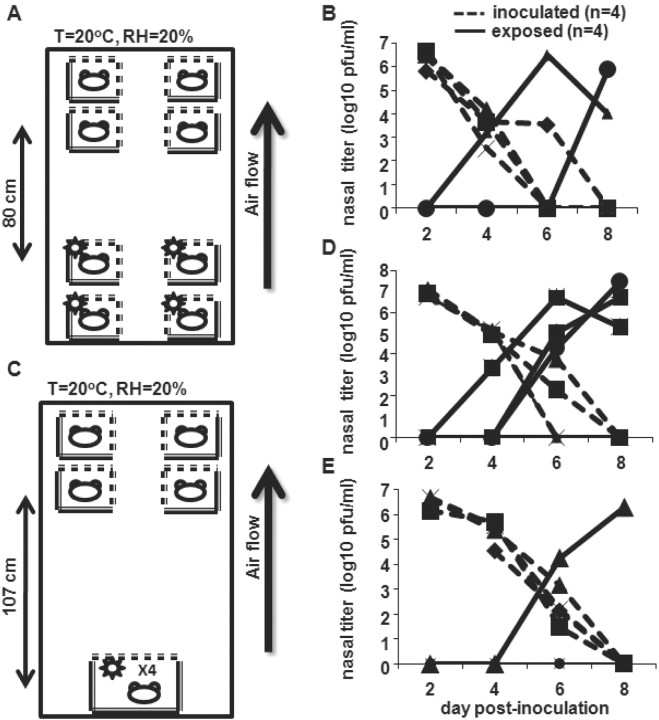
Long-range transmission of human influenza Pan99 virus among Hartley guinea pigsTo explore the possibility of airborne transmission of influenza virus in our model, we determined whether transmission occurred when naive animals were placed above infected animals. In the first experiment, 4 Pan99-inoculated guinea pigs were placed on the lower 2 shelves in the Caron environmental chamber. Twenty-four hours later, 4 naive guinea pigs were placed ⩾80 cm above inoculated animals (figure 1A), and nasal washes were performed as described in Methods on alternate days. Two transmission events occurred during the course of the experiment (figure 1B). The experiment was repeated in a similar manner, except inoculated animals were grouped in 1 large cage placed on the bottom shelf of the chamber. This permitted a distance of 107 cm between inoculated and exposed animals (figure 1C). A total of 3 transmission events occurred during the course of this experiment (figure 1D). When this experiment was repeated, 1 transmission event occurred (figure 1E). Transmission in an upward direction and over distance (long-range transmission) suggests that smaller droplets, and potentially droplet nuclei, play a role in the spread of influenza virus in the guinea pig model
Figure 1.
Long-range transmission of influenza A/Panama/2007/1999 (Pan99) virus, suggesting that airborne transmission occurs. A Experiment at 80 cm. Four Hartley guinea pigs were intranasally inoculated with 1×103 pfu of Pan99 and placed on the bottom 2 shelves of an environmental chamber that maintained constant temperature and relative humidity. Twenty-four hours later, 4 uninoculated animals were placed on the upper 2 shelves of the chamber. Temperature (T) and relative humidity (RH) in the chamber are indicated. B Nasal wash titers. Nasal washes were performed on alternate days, and viral titers were determined by plaque assay on MDCK cells. C Experiment at 107 cm. Inoculated animals were placed in the same large cage, and a distance of >1 m separated 4 inoculated and 4 exposed animals. Nasal wash titers are shown in panel D, and the experiment was reproduced, with results shown in panel E
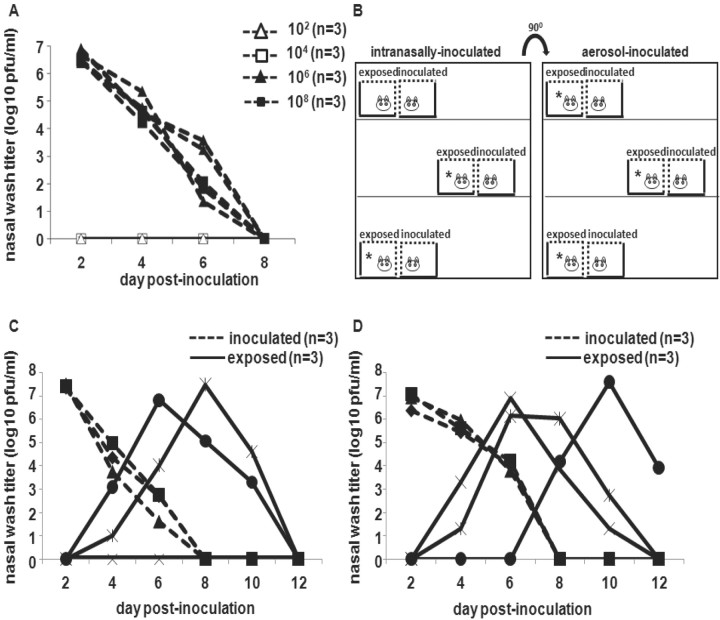
Efficient short-range transmission of Pan99 virus from aerosol- and droplet-inoculated strain 13 guinea pigsWe have previously shown 100% transmission of Pan99 virus by short-range aerosol spread from intranasally inoculated Hartley guinea pigs [16]. Strain 13 guinea pigs were obtained because enhanced replication of respiratory syncytial virus has been reported in this strain of guinea pig, compared with other strains of guinea pigs [19]. To establish whether aerosol-inoculated guinea pigs were also capable of transmitting to exposed animals, we examined short-range aerosol spread among both intranasally inoculated and aerosol-inoculated strain 13 guinea pigs. Because of the limited availability of strain 13 guinea pigs, we initially used Hartley strain animals to establish that this species could be infected through the aerosol route. Three Hartley guinea pigs were exposed to aerosols containing 1×102, 1×104, 1×106, or 1×108 pfu of Pan99 virus, as described in Methods. All animals exposed to 1×106 or 1×108 pfu of Pan99 virus became infected (figure 2A)
Figure 2.
Short-range spread of influenza A/Panama/2007/1999 (Pan99) virus through the air from droplet- and aerosol-infected strain 13 guinea pigs. A Shedding from the upper respiratory tract of groups of 3 Hartley guinea pigs inoculated by aerosol with 1×102, 1×104, 1×106, or 1×108 pfu of Pan99, as described in Methods. B Cage arrangement for intranasally and aerosol-inoculated guinea pigs and exposed counterparts, indicating which naive animals became infected (asterisks). C Short-range aerosol transmission from 3 strain 13 guinea pigs intranasally inoculated with 1×103 pfu of Pan99 to 2 of 3 exposed strain 13 guinea pigs. D Short-range aerosol transmission from 3 strain 13 guinea pigs inoculated by aerosol with 1×106 pfu of Pan99 virus to 3 exposed strain 13 guinea pigs
We then sought to determine whether aerosol-inoculated strain 13 guinea pigs were capable of transmitting influenza A virus. An experiment using 14 guinea pigs was set up. Three guinea pigs were inoculated intranasally with 1×103 pfu of Pan99 virus. An additional 3 animals were inoculated via aerosolization with 1×106 pfu of Pan99 virus. The inoculated guinea pigs were then placed in individual transmission cages. Two mock-infected animals were housed in separate enclosed cages (data not shown). After 24 h, 6 uninoculated animals were placed in transmission cages that were juxtaposed to the cages of inoculated animals, such that the wire mesh surfaces opposed each other, as described in Methods and illustrated in figure 2B. All animals were housed in the same room
Body temperature and weight were recorded daily, and animals were observed for lethargy, ruffled fur, trembling, and respiratory distress. No signs of illness were observed in any of the animals, and the 2 mock-infected animals did not shed virus in nasal wash samples (data not shown). Short-range noncontact exposure of uninoculated strain 13 guinea pigs to inoculated animals led to the infection of 5 of 6 exposed animals, including 2 of 3 guinea pigs placed in proximity to intranasally inoculated animals (figure 2C) and all 3 guinea pigs placed next to aerosol-inoculated animals (figure 2D). Thus, similar to our findings in Hartley strain guinea pigs inoculated with Pan99 virus by the droplet (intranasal) route, short-range aerosol transmission of Pan99 virus to strain 13 guinea pigs from aerosol- and droplet-inoculated animals was efficient
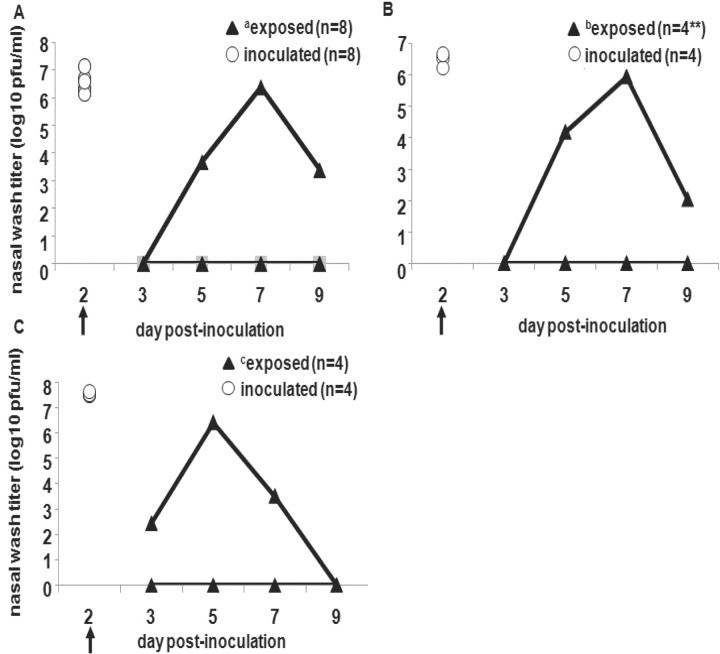
Inefficient transmission of human influenza Pan99 virus via fomites among guinea pigsTo examine the role of fomite transmission, we performed a series of experiments in which susceptible guinea pigs were exposed to surfaces contaminated with influenza virus. In the first experiment, 8 Hartley guinea pigs were inoculated intranasally and placed in individual cages. Forty-eight hours after inoculation, infected animals were replaced by naive guinea pigs, as described in Methods. Only 1 of 8 exposed animals became infected (figure 3A) and seroconverted (data not shown), shedding virus from day 5 to day 9 after inoculation, with a peak titer on day 7. The experiment was repeated in the same manner with 4 inoculated Hartley guinea pigs. Only 1 of 4 exposed guinea pigs became infected (figure 3B) and seroconverted (data not shown). To confirm our findings in Hartley guinea pigs, we also conducted a similar fomite transmission experiment with strain 13 guinea pigs. Only 1 of the 4 exposed strain 13 guinea pigs became infected (figure 3C) and seroconverted (data not shown), with peak titers from nasal wash samples at day 5 after inoculation. Again, fomite transmission was not as efficient in this system as transmission through the air
Figure 3.
Inefficiency of transmission of influenza A/Panama/2007/1999 (Pan99) virus via fomites in Hartley and strain 13 guinea pigs. Arrows indicate the time point for each experiment when intranasally inoculated animals were removed from the cages and replaced with naive guinea pigs. White circles represent nasal wash viral titers for inoculated animals on day 2 after inoculation, and black triangles represent nasal wash viral titers for exposed animals on days 3, 5, 7, and 9 after inoculation. In panel A, 8 Hartley guinea pigs were intranasally inoculated with Pan99 and then replaced with 8 uninfected Hartley guinea pigs (a7 of 8 exposed animals did not become infected). In panel B, the experiment was repeated using 4 inoculated and 4 exposed Hartley guinea pigs (b3 of 4 exposed animals did not become infected). In panel C, the same experimental approach was used with 4 Pan99-inoculated and 4 exposed strain 13 guinea pigs (c3 of 4 exposed animals did not become infected)
We then attempted to infect guinea pigs by depositing Pan99 virus on numerous nonporous surfaces of 6 cages. A total of 5×105 pfu of Pan99 in 30-μL volumes was deposited on 5 surfaces in each cage: the nozzle of the water bottle, the area under the food dish where animals burrow when hiding, and 3 sides of the cage. One Hartley guinea pig was placed in each cage immediately after cage application, and the animals remained in these cages for 48 h. Two animals were inoculated intranasally with 1×103 pfu of the same virus preparation of Pan99, as positive controls for virus replication in guinea pigs. None of the 6 animals housed in the contaminated cages became infected or seroconverted, whereas the 2 control animals shed high titers (on the order of 1×106 pfu/mL) and seroconverted (data not shown). Lack of active infection after the application of virus to the surrounding environment provides additional evidence that fomites play a limited role in transmitting influenza virus in the guinea pig model
Transmission efficiencies of Pan99 and Tx91We next determined whether there were differences in transmission rates between the human influenza virus strains. Short-range aerosol transmission experiments were conducted as described in Methods. In separate experiments, 4 Hartley guinea pigs were inoculated with rPan99 or Tx91, and 4 naive animals were exposed to them. Compared with rPan99, Tx91 transmitted less efficiently through the air (figure 4A and 4B). In addition, a direct contact transmission experiment was set up, wherein 4 Hartley guinea pigs received 100-fold higher inocula of Tx91 (1×105 pfu). Naive guinea pigs were placed in the same cage as inoculated animals, permitting direct exposure. Again, only 1 of 4 exposed animals became infected (data not shown)
Figure 4.
Transmission efficiency and recovery from aerosols of influenza A/Panama/2007/1999 (H3N2) virus (Pan99) and influenza A/Texas/36/1991 (H1N1) virus (Tx99). Short-range transmission experiments were conducted with 4 inoculated and 4 exposed guinea pigs, as described in Methods. A Recombinant Pan (rPan99) virus. B Tx/91 virus. C Nasal wash titers for 8 of 16 guinea pigs intranasally inoculated with Pan99 or Tx/91 influenza viruses. D Titers in air of Pan99 or Tx91 from aerosols generated by inoculated guinea pigs and sampled using a glass liquid impinger, as described in Methods. Data shown in panels C and D are compiled from 4 independent experiments; data in panel D are means ± SDs
To determine whether viral titers in an infectious bioaerosol may contribute to the difference in transmission efficiencies, the following experiments were performed. First, 8 Hartley guinea pigs were inoculated with 1×103 pfu of Pan99 or Tx91. Air sampling was performed daily for 6 days, as described in Methods. On alternate days (days 2, 4, and 6 after inoculation), nasal wash samples were collected from 4 of the 8 inoculated guinea pigs to determine viral titers in the upper respiratory tract. Experiments were done in duplicate with a total of 16 Pan99-inoculated and 16 Tx/91-inoculated guinea pigs; nasal wash samples were collected from 8 animals in each group. Peak nasal wash titers were higher and occurred earlier in Pan99-inoculated animals compared with the peak viral shedding of Tx91-inoculated animals (figure 4C). Similarly, Pan99 influenza virus was recovered from the air in higher titers and was observed earlier in the time course compared with Tx91 (figure 4D). No virus was recovered from the air on days 1 and 6 after inoculation
Discussion
The field of influenza virus transmission has largely lain fallow for decades since Andrews and Glover’s original experiments in the 1940s [11]. The paucity of data addressing the role of droplet nuclei in airborne transmission of influenza virus is responsible for substantial debate [20, 21]. Several lines of evidence raise the possibility of airborne transmission but fail to establish a definite role. Early volunteer-based studies revealed that humans may be infected by the aerosol route [22] (reviewed in [4]). Epidemiological evidence includes descriptions of outbreaks of influenza illness in health care settings, in military barracks, and aboard aircraft after the introduction of a single infected individual, and the explosive nature of these outbreaks is suggestive of airborne transmission [4, 23–25]
It has also been demonstrated that humans produce aerosols when talking or quietly breathing and that individuals naturally infected with influenza virus produce small particles containing viral RNA [26–28]. In a meta-analysis of experimental influenza virus infections in humans, Carrat et al. [29] concluded that peak viral shedding precedes peak symptom scores by ∼1 day. It is possible that humans are generating infectious bioaerosols in the absence of symptoms, thus contributing to the spread of influenza virus during the incubation period. Controlled human transmission studies are not possible from a research ethics standpoint, so mammalian models are required. The development of a novel model using the guinea pig, a smaller mammal than the ferret, allows for numerous larger-scale experiments addressing the spread of influenza viruses
We have previously established that transmission of human Pan99 is highly efficient by direct contact and at short range through the air in the guinea pig model, despite the absence of expulsion events, such as coughing or sneezing [16–18]. The results described in this article suggest that long-range airborne transmission of influenza virus may occur, on the basis of the upward spread of virus over a distance of 80 or 107 cm (figure 1). In each of these experiments, at least 1 primary transmission event occurred, although we cannot exclude the possibility that subsequent infections may have resulted from secondary, short-range transmissions. We also demonstrated that animals infected by intranasal (droplet) inoculation or by aerosolization transmitted human influenza virus efficiently through the air at short range (figure 2)
In contrast, transmission via fomites was inefficient, despite a known low ID50 for Pan99 in guinea pigs [16]. This suggests that transmission events observed during direct contact and short-range transmission experiments are not due to contaminated particulate matter. In fact, the 2 of 12 Hartley guinea pigs (and 1 of 4 strain 13 guinea pigs) that became infected in the cage-swap experiments could have been infected by aerosol transmission, because the uninfected animals were placed into the cages from which the infected animals had just been removed
These findings are contrary to what is known for other respiratory viruses and what has been commonly believed about influenza virus. Rhinovirus is likely transmitted by autoinoculation of ocular or nasal mucous membranes after contact with fomites [30]. Nonenveloped viruses, such as rotavirus, may persist in the environment for several months [31]. Influenza is an enveloped virus, potentially rendering it more susceptible to inactivation by environmental stressors. The presence of a lipid envelope is not solely responsible for poor fomite transmission, however, because spread via fomites has been demonstrated for respiratory syncytial virus [32], and environmental persistence has been shown for the severe acute respiratory syndrome coronavirus [33], both enveloped viruses
We also address differences in transmission efficiencies between different strains of influenza viruses. Pan99 appeared to transmit more efficiently than Tx91, even when a higher dose of Tx91 was administered to inoculated animals and direct contact between inoculated and exposed animals was allowed. This observation may be related to higher peak shedding titers of Pan99 virus, leading to higher titers in the air occupied by Pan99-inoculated animals than in the air occupied by Tx91-inoculated guinea pigs. The difference observed between viral strains may result from viral, host, or environmental factors or from a combination of these. Further research is necessary to identify the precise determinants of transmission efficiency influenza viruses in this system and to determine whether differences in efficiencies are mainly dependent on subtype
Although the guinea pig model offers advantages over other mammalian models for the study of influenza virus transmission, there are clear differences in the spread of influenza between this model and humans. Specifically, influenza virus–infected guinea pigs do not generate expulsion events, such as coughing or sneezing. Thus, the infectious bioaerosols produced by them may differ in composition and distribution from those produced by symptomatic humans. Similarly, with respect to transmission via fomites, substantial behavioral differences between humans and guinea pigs preclude the extrapolation to humans of the findings on fomite transmission among guinea pigs
In summary, we demonstrate efficient aerosol transmission and inefficient fomite transmission of human influenza viruses in guinea pigs. The guinea pig model is thus well suited for the study of influenza virus transmission through the air. Our results indicate that a susceptible mammalian host can produce bioaerosols laden with influenza virus, that influenza viruses can survive in infectious aerosols, and that productive infection of a susceptible host results when influenza virus is transmitted by the aerosol route. We also show that, in the guinea pig model, epidemic strains differ in the efficiency of aerosol transmission, suggesting that viral transmission phenotypes may affect the severity of annual outbreaks
Acknowledgments
We are indebted to Dr. T. Moran (Mount Sinai School of Medicine [MSSM]) for the use of the Glas-Col inhalation exposure system and to Cpt. C. Koeller (US Army Medical Research Institute of Infectious Diseases) for the strain 13 guinea pigs. We are also grateful to Dr. Nicole Bouvier (MSSM) for her contributions to experimental design and execution
Footnotes
Potential conflicts of interest: none reported
Presented in part: Options for the Control of Influenza VI, Toronto, Ontario, 17–23 June 2007 (abstract P1203); X International Symposium on Respiratory Viral Infections, Singapore, 28 February–3 March 2008
Financial support: Center for Research on Influenza Pathogenesis (National Institute of Allergy and Infectious Diseases contract HHSN266200700010C); W. M. Keck Foundation; National Institutes of Health (grant P01 AI158113; Ruth L. Kirschstein Physician Scientist Research Training in Pathogenesis of Viral Diseases Award 5T32A1007623-07 to S.M.); Northeast Biodefense Center (grant U54 AI057158); Center for Investigating Viral Immunity and Antagonism (grant U19 AI62623); Sunnybrook Health Sciences Centre, Toronto (S.M.); Parker B. Francis Fellowship in Pulmonary Research (A.C.L.)
References
- 1.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Committee on Personal Protective Equipment for Healthcare Workers during an Influenza Pandemic. Institute of Medicine . Preparing for an influenza pandemic: personal protective equipment for healthcare workers. In: Goldfrank LR, Liverman CT, editors. Washington, DC: National Academies Press; 2008. [Google Scholar]
- 4.Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in health care settings. Clin Infect Dis. 2003;37:1094–101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Infection control guidance for the prevention and control of influenza in acute-care facilities. Available at: http://www.cdc.gov/flu/professionals/infectioncontrol/healthcarefacilities.htm. Accessed 21 January 2009.
- 6.Centers for Disease Control and Prevention Interim recommendations for infection control in health-care facilities caring for patients with known or suspected avian influenza. Available at: http://www.cdc.gov/flu/avian/professional/infect-control.htm. Accessed 21 January 2009.
- 7.Haldar J, An D, Alvarez de Cienfuegos L, Chen J, Klibanov AM. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc Natl Acad Sci USA. 2006;103:17667–71. doi: 10.1073/pnas.0608803103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN. Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982;146:147–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Boone SA, Gerba CP. The occurrence of influenza A virus on household and day care center fomites. J Infect. 2005;51:103–9. doi: 10.1016/j.jinf.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Thomas Y, Vogel G, Wunderli W, et al. Survival of influenza virus on banknotes. Appl Environ Microbiol. 2008;74:3002–7. doi: 10.1128/AEM.00076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews CH, Glover RE. Spread of infection from the respiratory tract of the ferret. I. Transmission of influenza A virus. Br J Exp Pathol. 1941;22:91–7. [Google Scholar]
- 12.Maines TR, Chen LM, Matsuoka Y, et al. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci USA. 2006;103:12121–6. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuman PD, Keely S, Schiff GM. Assessment of signs of influenza illness in the ferret model. J Virol Methods. 1989;24:27–34. doi: 10.1016/0166-0934(89)90004-9. [DOI] [PubMed] [Google Scholar]
- 14.Yen HL, Lipatov AS, Ilyushina NA, et al. Inefficient transmission of H5N1 influenza viruses in a ferret contact model. J Virol. 2007;81:6890–8. doi: 10.1128/JVI.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumpey TM, Maines TR, Van Hoeven N, et al. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science. 2007;315:655–9. doi: 10.1126/science.1136212. [DOI] [PubMed] [Google Scholar]
- 16.Lowen AC, Mubareka S, Tumpey TM, Garcia-Sastre A, Palese P. The guinea pig as a transmission model for human influenza viruses. Proc Natl Acad Sci USA. 2006;103:9988–92. doi: 10.1073/pnas.0604157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowen AC, Steel J, Mubareka S, Palese P. High temperature (30°C) blocks aerosol but not contact transmission of influenza virus. J Virol. 2008;82:5650–2. doi: 10.1128/JVI.00325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–6. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bramley AM, Khan MA, Manson HE, Hegele RG. Development of respiratory syncytial virus “bronchiolitis” in guinea pigs does not reflect an allergic predisposition in the host. Chest. 2003;124:671–81. doi: 10.1378/chest.124.2.671. [DOI] [PubMed] [Google Scholar]
- 20.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7:257–65. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 21.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–62. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alford RH, Kasel JA, Gerone PJ, Knight V. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med. 1966;122:800–4. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 23.Klontz KC, Hynes NA, Gunn RA, Wilder MH, Harmon MW. An outbreak of influenza A/Taiwan/1/86 (H1N1) infections at a naval base and its association with airplane travel. Am J Epidemiol. 1989;129:341–8. doi: 10.1093/oxfordjournals.aje.a115137. [DOI] [PubMed] [Google Scholar]
- 24.Blumenfeld HL, Kilbourne ED, Louria DB, Rogers DE. Studies on influenza in the pandemic of 1957–1958. I. An epidemiologic, clinical and serologic investigation of an intrahospital epidemic, with a note on vaccination efficacy. J Clin Invest. 1959;38:199–212. doi: 10.1172/JCI103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moser MR, Bender TR, Margolis HS, Noble GR, Kendal AP. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110:1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 26.Fabian P, McDevitt JJ, DeHaan WH, et al. Influenza virus in human exhaled breath: an observational study. PLoS ONE. 2008;3:e2691–e2691. doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10:105–16. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 28.Edwards DA, Man JC, Brand P, et al. Inhaling to mitigate exhaled bioaerosols. Proc Natl Acad Sci USA. 2004;101:17383–8. doi: 10.1073/pnas.0408159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167:775–85. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 30.Musher DM. How contagious are common respiratory tract infections. N Engl J Med. 2003;348:1256–66. doi: 10.1056/NEJMra021771. [DOI] [PubMed] [Google Scholar]
- 31.Abad FX, Pinto RM, Bosch A. Survival of enteric viruses on environmental fomites. Appl Environ Microbiol. 1994;60:3704–10. doi: 10.1128/aem.60.10.3704-3710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall CB. The nosocomial spread of respiratory syncycial viral infections. Ann Rev Med. 1983;34:311–9. doi: 10.1146/annurev.me.34.020183.001523. [DOI] [PubMed] [Google Scholar]
- 33.Lai MYY, Cheng PKC, Lim WWL. Survival of severe acute respiratory syndrome coronavirus. Clin Infect Dis. 2005;41:e67–71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]