Abstract
Evidence suggests that anger and pain are related, yet it is not clear by what mechanisms anger may influence pain. We have proposed that effects of anger states and traits on pain sensitivity are partly opioid-mediated. In this study, we tested the extent to which analgesic effects of acute anger arousal on subsequent pain sensitivity were opioid-mediated by subjecting healthy participants to anger-induction and pain either under opioid blockade (oral naltrexone) or placebo. Participants were 160 healthy individuals. A double-blind, placebo-controlled, between-subjects opioid blockade design was used, with participants assigned randomly to one of two Drug conditions (placebo or naltrexone), and to one of two Task Orders (anger-induction followed by pain or vice versa). Results of ANOVAs showed significant Drug Condition × Task Order interactions for sensory pain ratings (MPQ-Sensory) and angry and nervous affect during pain-induction, such that participants who underwent anger-induction prior to pain while under opioid blockade (naltrexone) reported more pain, and anger and nervousness than those who underwent the tasks in the same order, but did so on placebo. Results suggest that for people with intact opioid systems, acute anger arousal may trigger endogenous opioid release that reduces subsequent responsiveness to pain. Conversely, impaired endogenous opioid function, such as that found among some chronic pain patients, may leave certain people without optimal buffering from the otherwise hyperalgesic affects of anger arousal, and so may lead to greater pain and suffering following upsetting or angry events.
Keywords: Anger, Acute Pain, Endogenous Opioids, Harassment, Opioid Blockade
Introduction
Anger states and traits appear to affect pain sensitivity. Associations between anger and pain have been reported in studies of acute pain induced in the laboratory [9,13,14,25], and evidence suggests that anger variables can exacerbate the severity of chronic pain [8,13,26,43]. Recently, models have been proposed to explain how anger variables may influence pain (e.g.,[12]).
One model focuses on whether endogenous opioid functioning may underlie the connection between anger variables and pain sensitivity [9]. Findings regarding the role of negative affect in endogenous opioid-mediated analgesia provide indirect support. Frew and Drummond [23] reported that in healthy women, discouragement induced by a mental arithmetic task performed under threat of electric shock was associated with endogenous opioid release that tempered the otherwise hyperalgesic effects of this negative affect. Related work in depressed patients similarly indicated that increased depression induced by stressful mental arithmetic was associated with opioid release that reduced subsequent pain responsiveness [24]. Other results suggest that acute anger arousal may have similar analgesic effects. Subjects undergoing a computer tracking task with experimenter harassment reported greater increases in anger than subjects not experiencing harassment, and also displayed significant increases in subsequent pain tolerance compared to non-harassed subjects [25]. These effects are consistent with those anticipated if anger arousal triggered opioid release producing subsequent analgesia, although the design of this study did not test specifically for opioid mechanisms.
More directly, findings indicate that pharmacological blockade of endogenous opioids produces increased anger, suggesting an association specifically between anger and opioids, although the absence of an experimental anger manipulation in this work limits the conclusions that can be drawn [28,32]. Findings of brain imaging studies are also relevant, suggesting that endogenous opioid activity in the orbitofrontal cortex, rostral anterior cingulate cortex, amygdala, insula, and periaqueductal gray regions contribute to modulation of both pain and negative affect, including anger [5,6,34,40,44,46,47]. Taken together, findings support the hypothesis that acute arousal of negative affect states including anger may have pain-related effects via opioid mechanisms.
The present study sought to replicate and extend findings of Janssen et al. [25] regarding anger-induced analgesia by directly testing for endogenous opioid mediation. Here, we induced anger and pain in the context of an opioid blockade paradigm. If endogenous opioids, released during anger arousal, contribute to analgesic effects during subsequent painful stimuli, then participants under opioid blockade who undergo anger-induction prior to pain will report greater pain intensity than participants who undergo anger-induction prior to pain while under placebo. Given findings of Frew and Drummond [23] that negative affect-induced opioid analgesia occurred in females but not males, the role of gender as a possible moderator was also examined.
Method
Participants
Participants were 165 healthy normal volunteers recruited through posted flyers, media advertisements, and e-mail announcements. Participants were paid $75 for their participation. Exclusion criteria were: a) history of cardiovascular disorder; b) history of renal or hepatic disease; c) current use of medications that affect cardiovascular function (e.g., beta blockers); d) history of chronic pain (e.g., low back pain, frequent headaches); e) current alcohol or substance abuse problems; f) a history of psychotic or bipolar disorders, or posttraumatic stress disorder; g) a current diagnosis of major depression; and h) pregnancy (as determined by pregnancy test conducted by investigators on all female potential participants). Five participants discontinued the procedure during the forearm exercise prior to inflation of the cuff (see below), three of whom were in the naltrexone condition, and two other participants had incomplete affect ratings for the maze task. Thus, the final sample was 158 participants (n = 79 women). The mean age of the sample was 27.2 years (SD = 5.6). The sample was comprised of 5.6% Hispanic or Latino(n = 9), 10.6% Asian (n = 17), 8.1% African American (n = 13), and 75.3% White (n = 119)participants.
Design Overview
A double-blind, placebo-controlled, between-subjects opioid blockade design was used. A between-subjects design rather than a within-subjects design was used due to the necessity of debriefing participants immediately following the experimental session regarding the deception involved in the harassment-based anger induction procedure (see below). This debriefing would render the anger manipulation invalid on repetition in a within-subject design. Participants were assigned randomly to one of two Drug conditions (placebo or naltrexone), and both participants and the experimenters were blind to participants' Drug condition. Participants were also assigned randomly to one of two Task Orders of a computer maze task (anger-induction through harassment) and a forearm ischemia task (pain-induction). They performed either the computer maze first and then underwent the forearm ischemia (Maze/Forearm Ischemia), or they underwent the forearm ischemia first and then performed the computer maze task (Forearm Ischemia/Maze). Self-report measures of pain and negative affect were collected at relevant points.
Materials
Opioid antagonist
Naltrexone hydrochloride (Mallinckrodt Pharmaceuticals, Inc.) was given in the standard oral therapeutic dose (50mg) which achieves peak blood concentrations within 60 minutes (Product Information, Mallinckrodt Pharmaceuticals, Inc.). Naltrexone is a nonselective opioid receptor antagonist that temporarily blocks endogenous opioid activity at all three major classes of opioid receptors. The mean elimination half-life for naltrexone and its major active metabolite (6-beta-naltrexol) are 4 and 13 hours respectively, and the standard dose has been shown to block the effects of intravenously administered heroin for up to 24 hours (Product Information, Mallinckrodt Pharmaceuticals, Inc.). To maintain blinding, both naltrexone and the placebo were placed in identical capsules prepared by the Vanderbilt University Investigational Pharmacy. All participants rested quietly for 60 minutes following administration of the drug to allow peak blockade activity to be achieved before undergoing the anger-induction and pain tasks. Given the half-life of naltrexone, it is unlikely that significant escape from opioid blockade occurred during the anger- and pain-induction procedures of the experimental session.
Hand dynamometer
Pain-induction was accomplished through an ischemic pain task (see below). This task used a hand dynamometer in order to exercise dominant forearm muscles prior to inflation of a standard manual sphygmomanometer cuff to create temporary forearm ischemia.
Questionnaires
The McGill Pain Questionnaire-Short Form (MPQ; [31]) is a well-validated scale that was used to assess sensory and affective qualitative aspects of the ischemic task. Analyses presented below used the MPQ sensory score (MPQ-Sensory) and MPQ affective score (MPQ- Affect). The MPQ also includes a 100mm visual analog scale of overall pain intensity (VAS-Intensity). Numeric rating scales (NRS; [26]) tapped the degree to which participants felt nervous (NRS-Nervous) and angry (NRS-Anger; 0 = “Not at all;” 100 = “Extremely“) during each epoch of the protocol.
Anger-Induction Task
Anger was induced with a procedure in which participants were required to take instruction from an antagonistic confederate during performance of an ostensible computerized maze task. The task was portrayed as a collaborative task for two people (the participant and a trained study confederate presented as another study participant). Instructions were presented describing the object of the task as being for the participant to use a computer mouse to move a computer icon as quickly as possible and with as few errors as possible from the entry to the exit of a “complex computer-generated maze.” The participant operating the mouse was unable to see the maze on the screen and had to perform the task based solely on guidance provided by the other study “participant” (the confederate) who was able to view the computer screen.
The confederate assumed an unfriendly attitude from the outset. He or she followed a semi-standardized script that included instructions to move the cursor in certain directions, exclamations about errors, derogatory comments about the participant's ability, and comments indicating that the confederate blamed the participant for all mistakes. Trained male and female university students served as confederates. To avoid confounds involving participant-confederate gender matches, approximately equal numbers of same sex, male participant-female confederate, and female participant-male confederate matches were used. This task and the harassment manipulation were adopted from Engebretson et al. [21]. The maze task was 5-min in duration.
Pain-Induction
Pain was induced with an ischemic pain procedure based on that described by Maurset et al. [29]. Participants were first asked to raise their dominant forearm over their head for 30 seconds followed by two minutes of dominant forearm muscle exercise using a hand dynamometer at 50% of his or her maximal grip strength (as determined prior to beginning the laboratory procedures). Immediately following this, a blood pressure cuff was inflated on the participant's dominant bicep to 200 mmHg. The cuff remained inflated until subjects indicated that their maximal pain tolerance had been reached (up to a maximum of 5 minutes). Because 78 of the participants (49.4%) reached this 5 minute limit, a skewed distribution owing to an apparent ceiling effect prevented valid analysis of pain tolerance data (see below).
Procedure
Participants were screened for exclusion criteria and asked not to consume caffeine for 3 hours prior to their appointments, nor use analgesics or medications potentially affecting blood pressure (e.g., pseudoephedrine) for 12 hours prior to their appointments. When they arrived at the laboratory, the equipment, procedures, and function (and risks) of naltrexone were explained, and maximum grip strength was determined. Informed consent was obtained. A “cover story” for the true purpose of the study was given. In brief, participants were told that the maze task would be used to assess the effects of “stress during cooperation” on pain responses, and so the maze task would be completed in conjunction with another research participant. Participants were told that they would be assigned by flip of a coin to serve either as the “guide” or the “runner.” The guide would direct the other person's efforts to negotiate the maze, whereas the runner would operate the computer mouse. Participants always served as the runner. They were told that the maze task would be timed, and that their performance would be evaluated based upon how quickly the maze was completed and how many errors were made. Participants were told that they would switch roles, and perform the task again after a short rest period.
Participants were seated in a comfortable chair in an upright position throughout all experimental procedures. A 10-min resting adaptation period commenced, after which participants completed the baseline NRS ratings of current affective state. Participants received the appropriate randomized drug (placebo or naltrexone), and then rested quietly for 60-min to allow peak blockade activity to be achieved.
Next, for participants in the Maze/Forearm Ischemia task order condition, the confederate entered the room and sat 2 meters from the participant on the opposite side of the computer table. They were told not to speak Instructions for both tasks were given, but the instructions for the maze task were emphasized and directed to both the confederate and participant. The maze task then began. After the task, the confederate left the room, and the participant again provided NRS ratings of current affective state. Instructions for the ischemic task were then briefly reiterated and it began approximately 90 sec after the computer maze was completed. Immediately after the ischemic task, participants provided pain ratings using the MPQ and completed the NRS ratings of current affective state.
For participants in the Forearm Ischemia/Maze task order, after the 60-min drug absorption period, instructions for both tasks were given, but the instructions for the ischemic task were emphasized. The ischemic pain task was then conducted, and immediately following this, participants completed the MPQ and the NRS ratings of current affective state. The confederate then entered the room, and instructions for the maze task were given and directed to both the confederate and participant. The maze task then began. After the task, the confederate left the room, and the participants provided NRS ratings of their current affective state.
After completion of both tasks, participants were thoroughly debriefed (especially with regard to the computer maze and harassment), asked whether they had believed the “other subject” was part of the study, and information regarding possible side effects of naltrexone were reviewed. They were given a “side effects” checklist, which listed 10 physical symptoms occasionally experienced by people taking naltrexone (e.g., nausea, sweating). They indicated the degree to which they were experiencing each symptom on a 1 (not at all) to 10 (the most possible) scale. Participants were kept under observation until 3 hours post-drug administration to monitor for additional side effects, and were discharged after this time unless side effects required further monitoring.
Data Reduction and Analysis
First, unpleasant physical symptoms experienced by participants receiving naltrexone, even at a low level, may have impacted their mood, and thus may have represented a confound. Responses on the 10-item “side effects” checklist were summed to comprise a total Symptoms Score. A Drug Condition (placebo, naltrexone) × Task Order (Maze/Forearm Ischemia, Forearm Ischemia/Maze) × Gender (male, female) between-subjects ANOVA was performed to determine whether participants varied in symptoms according to both drug and task order condition, and to determine whether the Symptoms Score should be used as a covariate. Second, as a manipulation check to determine whether the harassment manipulation significantly aroused primarily anger, within-subject ANOVAs were performed on NRS-Anger and NRS-Nervous values from baseline to the maze task. Although our primary focus was on drug and task order effects on pain indexes, drug and task order effects may also have been exerted on anger and nervousness ratings during the harassment of the maze task. If so, then high levels of blockade-induced anger and/or nervousness during the maze task relative to those in the placebo condition may partly account for any observed differences in pain responses. To evaluate this possibility, two Drug Condition (placebo, naltrexone) × Task Order (Maze/Forearm Ischemia, Forearm Ischemia/Maze) × Gender (male, female) between-subjects ANOVAs were performed with NRS-Anger and NRS-Nervous ratings as the dependent measures.
Primary analyses consisted of Drug Condition (placebo, naltrexone) × Task Order (Maze/Forearm Ischemia, Forearm Ischemia/Maze) between-subjects ANOVAs for the MPQ-Sensory, MPQ-Affect and VAS-Intensity pain measures, and the NRS-Anger and NRS-Nervous responses to pain-induction. Main and interactive effects of gender were also included in these analyses to examine possible gender moderation of anger-related opioid analgesic effects.
Results
Drug × Task Order Effects on Naltrexone Side Effects (Symptoms Score)
A Drug Condition (placebo, naltrexone) × Task Order (Maze/Forearm Ischemia, Forearm Ischemia/Maze) × Gender (male, female) ANOVA was performed on Symptoms Score. All main and interaction effects were nonsignificant, except for the main effect of Drug Condition. Here, participants in the naltrexone condition reported significantly more side effects (M = 4.05; SD = 7.5) than those receiving placebo (M = 2.03; SD = 2.6) [F(1,158) = 5.66; p < .03], irrespective of task order. Even though the effect size was rather small (η2 = .03), on the basis of this significant difference, Symptoms Score was used as a covariate in primary analyses.
Effects of the Computer Maze Task on Anger and Nervousness
A within-subject ANOVA showed a significant baseline (M = 3.06; SD = 4.6) to maze task (M = 11.14; SD = 16.0) increase [F(1,157) = 47.34; p < .01] in reports of anger. This increase reflected an effect size of η2 = .23. For feelings of nervousness, the increase from baseline (M = 6.26; SD = 8.3) to maze task (M = 11.02; SD = 14.4) was also significant [F(1,157) = 21.06; p < .01], but represented an effect size of only η2 = .12. Results suggest that the maze task primarily elicited increased anger as intended.
To evaluate the possibility that drug effects on anger and/or nervousness during the maze task could affect pain during the pain task, two Drug Condition (placebo, naltrexone) × Task Order (Maze/Forearm Ischemia, Forearm Ischemia/Maze) × Gender (male, female) between-subjects ANOVAs were performed for NRS-Anger and NRS-Nervous ratings during the maze task. For both of these indexes, all interaction and main effects were nonsignificant [F's < 2.26].Results suggest that anger and nervousness levels during the maze task were not significantly affected by drug condition, and so the possibility that pain ratings during the forearm ischemiat ask were differentially affected by blockade-related effects on anger and/or nervousness during the maze task appeared small.
Pain Tolerance
With nearly 50% of the sample reaching the 5-min limit for forearm ischemia pain exposure, using “pain tolerance” as a continuous variable in analyses was not appropriate. Still, drug and task order effects could be assessed by examining frequencies of participants in each condition who reached the 5-min limit. For participants receiving placebo, the frequencies of participants reaching this limit for the Maze/Forearm Ischemia and Forearm Ischemia/Maze conditions were 16 and 19, respectively. For participants receiving naltrexone, the frequencies for the Maze/Forearm Ischemia and Forearm Ischemia/Maze conditions were 20 and 23, respectively. A Drug Condition × Task Order χ2 analysis was performed on these relative frequencies, with results showing a nonsignificant effect [χ2 (1, n = 78) = .05; p > .10]. Results suggest that drug and task order conditions did not significantly affect the number of participants who reached the 5-min tolerance limit.
Drug × Task Order Effects on MPQ Pain Indexes
Two Drug Condition (placebo, naltrexone) × Task Order (Maze/Forearm Ischemia, Forearm Ischemia/Maze) × Gender (male, female) between-subjects ANOVAs were performed with acute pain ratings as the dependent measure.
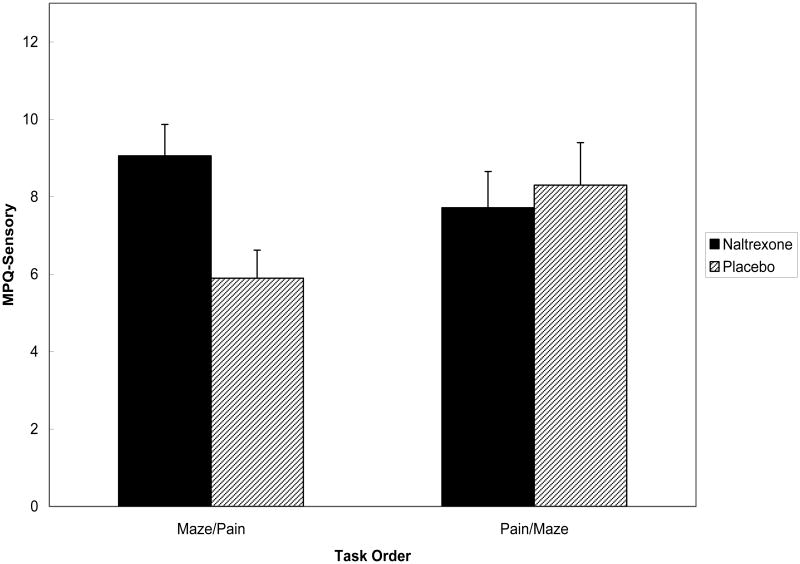
The 3-way interaction was nonsignificant for MPQ-Sensory [F < 1], as was the Drug × Gender interaction [F(1,150) = 1.99; p > .10]. However, the Drug Condition × Task Order effect was significant [F(1,150) = 4.86; p < .03], and the source of this 2-way interaction is portrayed in Figure 1. Simple effect analyses revealed that for participants in the Forearm Ischemia/Maze order, the Drug Condition effect was nonsignificant [F < 1], whereas among participants in the Maze/Forearm Ischemia order, the effect for Drug conition was significant [F(1,76) = 7.21; p < .01]. Because variability in pain tolerance times could affect the magnitude of pain ratings, these analyses were rerun with pain tolerance as a covariate. Symptoms Score was also evaluated as a covariate. Results were virtually unchanged with these factors controlled. Findings indicated that participants who were angered prior to pain induction while under the effects of opioid blockade reported higher MPQ-Sensory ratings than those who performed the tasks in the same order but under placebo.
Figure 1.
Mean (± S.E.) MPQ-Sensory pain ratings by drug condition and task order.
The 3-way interaction was nonsignificant for MPQ-Affect [F < 1], as was the Drug Condition × Task Order effect [F(1,150) = 1.98; p > .10]. Because of the significant interaction effect for MPQ-Sensory, the simple effects analyses described above were conducted here for exploratory purposes. Similar to effects for the MPQ-Sensory variable, analyses revealed that for participants in the Forearm Ischemia/Maze order, the Drug Condition effect for MPQ-Affect was nonsignificant [F < 1], whereas among participants in the Maze/Forearm Ischemia order, the effect for Drug condition was significant [F(1,76) = 5.12; p < .03], with mean differences highlighting elevated affective pain among those receiving naltrexone (M = 1.71; SD = 2.5)compared to those receiving placebo (M = .88; SD = 1.6). Although exploratory, results parallel findings for the sensory dimension of pain.
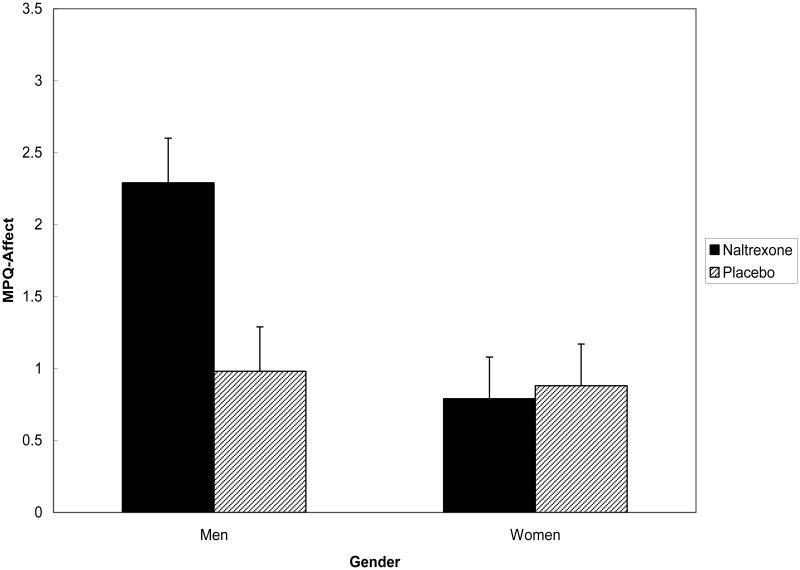
Further, the Drug × Gender interaction was significant for MPQ-Affect ratings [F(1,151) = 5.50; p < .02]. The source of this 2-way interaction is portrayed in Figure 2. Simple effect tests revealed that for participants in the Naltrexone condition, the Gender effect was significant [F(1,80) = 8.47; p < .01], whereas among participants in the Placebo condition, the effect for Gender was nonsignificant [F < 1]. Although men and women did not differ significantly on the frequency with which they reached the 5-min tolerance limit [χ2 (1, n = 78) = .06; p > .10], analyses were rerun with pain tolerance time as a covariate. Symptoms Score was also used as a covariate. Results were again virtually unchanged. Results indicated that men reported higher MPQ-Affect ratings than women only when under opioid blockade.
Figure 2. Mean (± S.E.) MPQ-Affect pain ratings by drug condition and gender.
For analyses of VAS Intensity, all interaction and main effects were nonsignificant [F's < 1.51].
Drug × Task Order Effects on Anger and Nervousness in Response to Acute Pain
For NRS-Anger values during forearm ischemic pain, a within-subject ANOVA showed a significant baseline (M = 3.06; SD = 4.6) to task (M = 6.03; SD = 10.6) increase [F(1,157) = 17.98; p < .01], with an effect size of η2 = .10. For NRS-Nervous values, the increase from baseline (M = 6.26; SD = 8.3) to ischemic pain task (M = 8.61; SD = 10.3) was also significant [F(1,157) = 13.40; p < .01], with an effect size of η2 = .08. Thus, the ischemic pain task elicited similar increases in anger and nervousness.
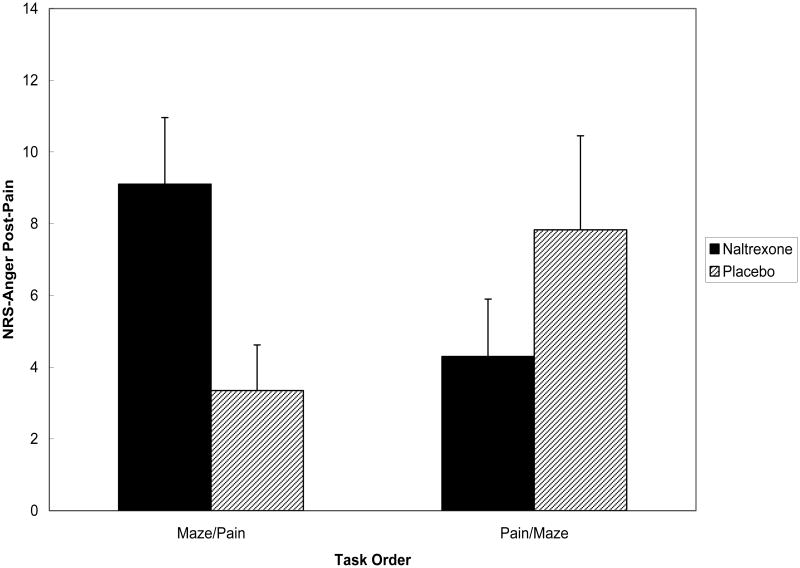
The Drug × Condition × Gender interaction was nonsignificant for NRS-Anger ratings during pain-induction [F = 1.71; p > .10], but the Drug Condition × Task Order effect was significant [F(1,150) = 7.43; p < .01]. The source of this significant interaction is portrayed in Figure 3. Simple effect tests revealed that for participants in the Forearm Ischemia/Maze order, the Drug Condition effect was nonsignificant [F = 2.21; p > .10], whereas the effect for Drug condition among participants in the Maze/Forearm Ischemia was significant [F(1,77) = 5.57; p < .03]. Because variability in pain tolerance and Symptom Score could also affect anger ratings during ischemic pain, these analyses were rerun with these factors as covariates. No appreciable change in results was found. Results suggested that participants who were angered prior to undergoing pain while under opioid blockade reported greater anger during pain than those who performed the tasks in the same order after receiving placebo.
Figure 3.
Mean (± S.E.) NRS-Anger ratings following pain stimulation by drug condition and task order.
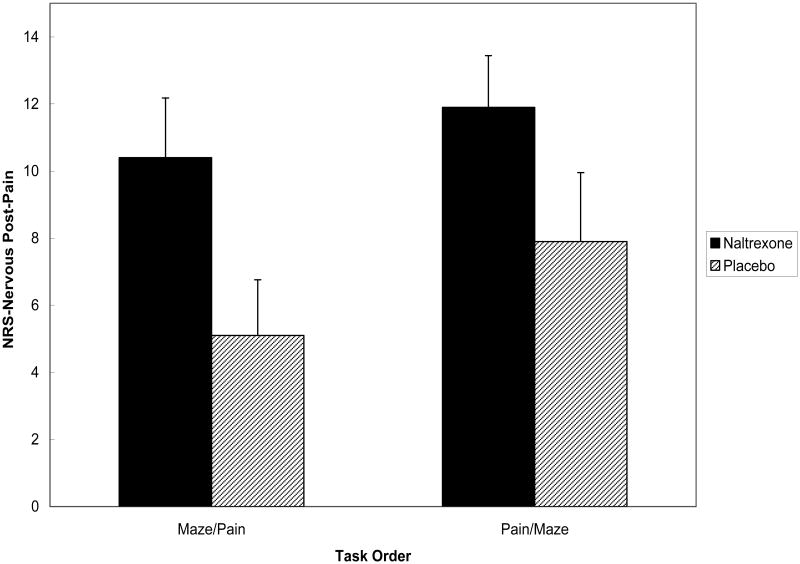
Similar to anger, the Drug × Condition × Gender interaction was nonsignificant for NRS-Nervous values during pain-induction [F < 1], whereas the Drug Condition × Task Order effect was significant [F(1,150) = 8.06; p < .01]. The source of this 2-way interaction is presented in Figure 4. Again following the pattern for anger, simple effect tests showed that for those in the Forearm Ischemia/Maze order, the Drug Condition effect was nonsignificant [F = 2.35; p > .10], but that this effect among1 participants in the Maze/Forearm Ischemia order was significant [F(1,76) = 6.98; p < .01]. As with NRS-Anger, pain tolerance time and Symptom Score were used as covariates in rerun analyses for NRS-Nervous, with no changes to results. These findings indicate that participants who were angered prior to undergoing pain while under opioid blockade reported greater feelings of nervousness during pain than those who were angered prior to pain but received placebo.
Figure 4.
Mean (± S.E.) NRS-Nervous ratings following pain stimulation by drug condition and task order.
Common Effects of Drug and Task Order
Because the Drug × Task Order interactions were significant for MPQ-Sensory, NRS-Anger and NRS-Nervous ratings, and simple effects tests suggested the same pattern of effects across these measures, analyses were performed to explore whether opioid blockade affected a common substrate underlying both pain and negative affect responses to acute pain. We focused only on the effects of Drug among those in the Maze/Forearm Ischemia task order (those who were angered prior to undergoing pain), wherein significant effects were found in the analyses above. First, note that the effect sizes for the differences between naltrexone and placebo cells for participants in this task order were η2 = .087 for MPQ-Sensory, η2 = .066 for NRS-Anger, and η2 = .073 for NRS-Nervous when considered separately. Second, we conducted a series of ANCOVAs (analogous to a Roy-Bargmann step-down procedure; [42]) with Drug as the between-subjects factor and with MPQ-Sensory, NRS-Anger and NRS-Nervous values serving as dependent variables in turn, and then serving as covariates in turn.
An ANCOVA with NRS-Anger and NRS-Nervous values entered as covariates showed that the effect of Drug condition on MPQ-Sensory was reduced to nonsignificance [F(1,75) = 2.25; p > .10] with the effect size η2 reduced from .087 to .029. The latter effect size index indicates the unique effect of Drug condition on MPQ-Sensory with the effects of negative affect controlled. The ANCOVA for NRS-Anger with MPQ-Sensory and NRS-Nervous values controlled was reduced to nonsignificance [F < 1] with the effect size η2 reduced from .066 to .010. The ANCOVA for NRS-Nervous with MPQ-Sensory and NRS-Anger values controlled was also reduced to nonsignificance [F(1,75) = 2.29; p > .10] with the effect size η2 reduced from .073 to .030.
Taken together, these results imply that the effects of anger provocation on responses to subsequent pain induction while participants were under opioid blockade were exerted on a common factor underlying both pain perception and arousal of negative emotions.
Discussion
Prior work in healthy individuals using opioid blockade methodology suggested that experimental induction of negative affect in general (“stress”; [4]) and discouragement specifically [23] triggered endogenous opioid analgesia that reduced subsequent acute pain responsiveness. These latter findings were not replicated in healthy individuals in a follow-up study, although similar effects were observed in individuals with major depressive disorder [24]. Other work indicated that experimental harassment procedures designed to elicit anger in particular also resulted in reduced responsiveness to the pain task that followed, although this study did not test directly for opioid mechanisms [25]. Results of the current study replicate and extend these latter findings. We found that participants under opioid blockade reported greater sensory and affective pain when angered prior to pain-induction than participants under placebo who were angered prior to pain, suggesting that acute anger arousal among those with intact opioid systems may have triggered endogenous opioid release that reduced subsequent responsiveness to pain. The pattern of findings in both the current study and previous work [4,23] suggest that endogenous opioids buffer the otherwise hyperalgesic effects of anger and other forms of negative affect on responsiveness to subsequent pain stimuli.
Prior work indicates that acute anger arousal is typically associated with increased blood pressure [15,36,37]. Acutely elevated blood pressure, in turn, can trigger reduced pain sensitivity via activation of baroreceptor-related analgesic mechanisms [33]. There is evidence that blood-pressure related analgesia, at least in animals, has an opioid-mediated component [37,38]. At least two human studies are consistent with this opioid hypothesis [30,35], with other work further suggesting a role for alpha-2 adrenergic mechanisms [18]. Taken together, results of this work suggest that the opioid analgesia apparently elicited by the acute anger arousal in this study could have been, in part, derived from anger-induced activation of blood pressure-related descending opioid inhibitory systems. Additional research is needed to shed light on this hypothesis.
In addition to anger-triggered endogenous opioid effects on intensity of perceived sensory qualities of pain, in particular, and on the affective dimension to at least a marginal extent, our findings suggest further that anger-induced opioid release modulated negative emotional responses to being hurt. That is, feelings of anger and nervousness in response to painful stimulation appeared also to be affected by opioid blockade. These findings are consistent with recent work suggesting opioid modulation of affective responses to acute pain [8] and mental stressors [24]. Interestingly, exploratory analyses in the current study suggested a high degree of statistical overlap between anger-induced opioid modulation of sensory and affective qualities of pain and pain-related emotional responses. This pattern of findings may be viewed as support for the idea that pain and emotional responses to that pain are both modulated by endogenous opioid activity in overlapping brain circuitry; a hypothesis consistent with existing brain imaging research [7,19,20,34].
A secondary aim of this study was to further evaluate previously reported gender differences in negative affect-induced opioid release. Frew and Drummond [23] reported that in a healthy sample, experimentally-induced discouragement triggered significant opioid-mediated analgesia in females but not males. However, in contrast to the findings of Frew and Drummond [23], previous brain imaging work suggested that pain-induced endogenous opioid analgesia was significantly greater in males than in females [48]. Results of the current study were more consistent with the latter findings, with results suggesting that males evinced greater opioid analgesia in response to acute anger arousal than females. Opposing findings between those of the current study and those of Frew and Drummond [23] may be due to differences in the negative emotion induction technique (harassment versus mental arithmetic with threat of electric shock), duration of negative emotion induction (5 minutes versus 30 minutes), or even differences between studies in menstrual phase of female participants (e.g., [39]). Alternatively, differing patterns of gender moderation effects on negative emotion-induced opioid release between the current study and prior work may simply indicate that such effects are not reliable, although this latter interpretation may be less likely given other work also indicating gender-related opioid analgesic response differences [3,17]. If there are gender differences in negative emotion-induced opioid analgesia, then future work will need to address situational determinants that may influence how these differences are manifested.
The potential clinical relevance of these laboratory findings may best be highlighted by examining results from diary studies of associations between negative affect and pain. Analyses reported in several such studies indicate that increased negative affect can result in subsequent increases in chronic pain intensity [1,2,22]. The current findings and related prior work [4,23,24] suggest that these hyperalgesic effects of negative affect on chronic pain intensity may be magnified to the extent that endogenous opioid analgesic function is impaired. In light of evidence that chronic pain may be associated with reduced endogenous opioid function [11], particularly in those with greater pain-related disability [10], it is possible that degree of chronic pain-related opioid dysfunction may be a factor that modulates the hyperalgesic effects of negative affect on chronic pain. Patients with chronic pain and impaired endogenous opioid function may be particularly susceptible to upsetting or angering events, perhaps setting the stage for vicious cycles in which distress begets greater chronic pain which in turn makes future stressful events more difficult to bear.
One limitation of the current study is that we used a between-subjects rather than a within-subjects design, thereby reducing statistical power. This less powerful design was used because of the ethical necessity of revealing the deception used in the harassment manipulation immediately after the study session, thereby precluding a within-subjects design. Despite the relatively large sample size, this power issue may have contributed to some of the interaction effects on pain responses not reaching conventional levels of statistical significance. In short, within-person changes in pain intensity and pain qualities between placebo and opioid blockade would have provided more sensitive tests of our hypotheses. Second, because we used a tolerance paradigm during pain-induction, partcipants received varying lengths of exposure to the forearm ischemia pain stimulus. The interpretation of the MPQ-Sensory and MPQ-Affect ratings is therefore not entirely straightforward because the magnitude of pain ratings may have been affected by length of exposure. A fixed latency paradigm may have been more appropriate, in this regard. We attempted to redress this difficulty by statistically controlling for tolerance times in all analyses. Nonetheless, caution is needed when drawing conclusions from our results. Finally, the question of the emotional specificity of the harassment-induced analgesia must be considered. In the current study, while the harassment procedure was designed to elicit primarily anger and irritation – which results of analyses suggested was indeed the case -- we cannot rule out the possibility that what appears to be anger-related opioid analgesia is in fact analgesia induced by more general negative affect. The extent to which analgesia induced by anger specifically or negative affect more generally might overlap with the more general concept of stress-induced analgesia remains to be addressed. Future research will need to use experimental designs that allow teasing apart whether opioid-mediated effects are stronger when anger is aroused compared to when other negative emotions (e.g., anxiety) are aroused.
In summary, arousal of acute anger may trigger endogenous analgesic processes that affect subsequent acute pain responses, and these analgesic processes may be at least partly opioid-mediated. In the absence of effective endogenous opioid function (under opioid blockade), acute anger appears to exert hyperalgesic effects on subsequent pain. This anger-induced opioid analgesia appeared to be stronger in males than in females. Anger-related endogenous opioid release affected not only the sensory qualities of the acute pain experienced, but also modulated the negative emotional responses to experiencing acute pain. These opioid-mediated effects on sensory pain qualities and emotional responses to pain overlap substantially, consistent with previously reported overlap in brain circuits modulating both pain and negative affect.
Acknowledgments
This work was supported by grants R01-MH071260, R01-NS050578, and Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, National Institutes of Health. The authors have no conflicts of interest to report. The authors wish to acknowledge the assistance of Dr. Kari Baber, Elizabeth Miller, Kevin Stone, Sarah Lashley, Heather Cairl, Andrew Rosen, Jessica Gerfen, Nancy Beckman, and Erika Gray for their assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Affleck G, Urrows S, Tennen H, Higgins P. Daily coping with pain from rheumatoid arthritis: Patterns and correlates. Pain. 1992;51:221–229. doi: 10.1016/0304-3959(92)90263-B. [DOI] [PubMed] [Google Scholar]
- 2.Arena JG, Sherman RA, Bruno GM, Smith JD. The relationship between situational stress and phantom limb pain: Cross-lagged correlational data from six month pain logs. J Psychosom Res. 1990;34:71–77. doi: 10.1016/0022-3999(90)90009-s. [DOI] [PubMed] [Google Scholar]
- 3.Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156–60. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Bandura A, Cioffi D, Taylor CB, Brouillard ME. Perceived self-efficacy in coping with cognitive stressors and opioid activation. J Pers Soc Psychol. 1988;55:479–488. doi: 10.1037//0022-3514.55.3.479. [DOI] [PubMed] [Google Scholar]
- 5.Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, Valet M, Berthele A, Tolle TR. The runner's high: opioidergic mechanisms in the human brain. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- 7.Bruehl S, Burns JW, Chung O, Chont M. Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruehl S, Burns JW, Chung OY, Quartana P. Anger management style and emotional reactivity to noxious stimuli among chronic pain patients and healthy controls: the role of endogenous opioids. Health Psychol. 2008;27:204–214. doi: 10.1037/0278-6133.27.2.204. [DOI] [PubMed] [Google Scholar]
- 9.Bruehl S, Burns JW, Chung OY, Ward P, Johnson B. Anger and pain sensitivity in chronic low back pain patients and pain-free controls: The role of endogenous opioids. Pain. 2002;99:223–233. doi: 10.1016/s0304-3959(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 10.Bruehl S, Chung OY, Ward P, Johnson B. Endogenous opioids and chronic pain intensity: interactions with level of disability. Clin J Pain. 2004;20:283–292. doi: 10.1097/00002508-200409000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Bruehl S, McCubbin JA, Harden RN. Theoretical review: altered pain regulatory systems in chronic pain. Neurosci Biobehav Rev. 1999;23:877–890. doi: 10.1016/s0149-7634(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 12.Burns JW. Arousal of negative emotions and symptom-specific reactivity in chronic low back pain patients. Emotion. 2006;6:309–319. doi: 10.1037/1528-3542.6.2.309. [DOI] [PubMed] [Google Scholar]
- 13.Burns JW, Bruehl S, Caceres C. Anger management style, blood pressure reactivity and acute pain sensitivity: Evidence for a Trait × Situation model. Ann Behav Med. 2004;27:195–204. doi: 10.1207/s15324796abm2703_7. [DOI] [PubMed] [Google Scholar]
- 14.Burns JW, Johnson BJ, Mahoney N, Devine J, Pawl R. Anger management style, hostility and spouse responses: gender differences in predictors of adjustment among chronic pain patients. Pain. 1996;64:445–453. doi: 10.1016/0304-3959(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 15.Burns JW, Katkin ES. Psychological, situational, and gender predictors of cardiovascular reactivity to stress: a multivariate approach. J Behav Med. 1993;16:445–465. doi: 10.1007/BF00844816. [DOI] [PubMed] [Google Scholar]
- 16.Burns JW, Kubilus A, Bruehl S. Emotion-induction moderates effects of anger management style on acute pain sensitivity. Pain. 2003;106:109–18. doi: 10.1016/s0304-3959(03)00298-7. [DOI] [PubMed] [Google Scholar]
- 17.Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg. 2003;97:1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- 18.Chung OY, Bruehl S, Diedrich L, Diedrich A, Chont M, Robertson D. Baroreflex sensitivity associated hypoalgesia in healthy states is altered by chronic pain. Pain. 2008;138:87–97. doi: 10.1016/j.pain.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation – a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 20.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 21.Engebretson TO, Matthews KA, Scheier MF. Relations between anger expression and cardiovascular reactivity: Reconciling inconsistent findings through a matching hypothesis. J Pers Soc Psych. 1989;57:513–521. doi: 10.1037//0022-3514.57.3.513. [DOI] [PubMed] [Google Scholar]
- 22.Feldman SI, Downey G. Schaffer-Neitz R Pain, negative mood, and perceived social support in chronic pain patients: a daily diary study of people with reflex sympathetic dystrophy syndrome. J Consult Clin Psychol. 1999;67:776–785. doi: 10.1037//0022-006x.67.5.776. [DOI] [PubMed] [Google Scholar]
- 23.Frew AK, Drummond PD. Negative affect, pain and sex: the role of endogenous opioids. Pain. 2007;132(Suppl 1):S77–85. doi: 10.1016/j.pain.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Frew AK, Drummond PD. Stress-evoked opioid release inhibits pain in major depressive disorder. Pain. 2008 doi: 10.1016/j.pain.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Janssen SJ, Spinhoven P, Brosschot JF. Experimentally induced anger, cardiovascular reactivity, and pain sensitivity. J Psychosom Res. 2001;51:479–485. doi: 10.1016/s0022-3999(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 26.Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. New York: Guilford Press; 1992. pp. 135–151. [Google Scholar]
- 27.Kerns RD, Rosenberg R, Jacob MC. Anger expression and chronic pain. J Behav Med. 1994;17:57–67. doi: 10.1007/BF01856882. [DOI] [PubMed] [Google Scholar]
- 28.Martin del Campo AF, Dowson JH, Herbert J, Paykel ES. Effects of naloxone on diurnal rhythms in moods and endocrine function: a dose-response study in man. Psychopharm. 1994;114:583–590. doi: 10.1007/BF02244988. [DOI] [PubMed] [Google Scholar]
- 29.Maurset A, Skoglung LA, Hustveit O, Klepstad P, Oye I. A new version of the ischemic tourniquet pain test. Meth Find Exp Clin Pharmacol. 1992;13:643–647. [PubMed] [Google Scholar]
- 30.McCubbin JA, Helfer SG, Switzer FS, 3rd, Galloway C, Griffith WV. Opioid analgesia in persons at risk for hypertension. Psychosom Med. 2006;68:116–120. doi: 10.1097/01.psy.0000195742.24850.79. [DOI] [PubMed] [Google Scholar]
- 31.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 32.Pickar D, Cohen MR, Naber D, Cohen RM. Clinical studies of the endogenous opioid system. Biol Psychiatry. 1982;17:1243–1276. [PubMed] [Google Scholar]
- 33.Rau H, Elbert T. Psychophysiology of arterial baroreceptors and the etiology of hypertension. Biol Psychiatr. 2001;57:179–201. doi: 10.1016/s0301-0511(01)00094-1. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1264–1280. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Sheps DS, Bragdon EE, Gray TF, Ballenger M, Usedom JE, Maixner W. Relationship between systemic hypertension and pain perception. Am J Cardio. 1992;70:3F–5F. doi: 10.1016/0002-9149(92)90181-w. [DOI] [PubMed] [Google Scholar]
- 36.Siegman AW, Snow SC. The outward expression of anger, the inward experience of anger and CVR: The role of vocal expression. J Behav Med. 1997;20:29–45. doi: 10.1023/a:1025535129121. [DOI] [PubMed] [Google Scholar]
- 37.Sitsen JM, DeJong W. Hypoalgesia in genetically hypertensive rats (SHR) is absent in rats with experimental hypertension. Hypertension. 1983;5:185–190. doi: 10.1161/01.hyp.5.2.185. [DOI] [PubMed] [Google Scholar]
- 38.Sitsen J, DeJong W. Observations on pain perception and hypertension in spontaneously hypertensive rats. Clin Exp Hypertension. 1984;A6:1345–1356. doi: 10.3109/10641968409039601. [DOI] [PubMed] [Google Scholar]
- 39.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26:5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprenger T, Valet M, Boecker H, Henriksen G, Spilker ME, Willoch F, Wagner KJ, Wester HJ, Tölle TR. Opioidergic activation in the medial pain system after heat pain. Pain. 2006;122:63–67. doi: 10.1016/j.pain.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Suarez EC, Kuhn CM, Schanberg SM, Williams RB, Zimmerman EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: The role of interpersonal challenge. Psychosom Res. 1998;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Tabachnick BG, Fidell LS. Using Multivariate Statistics. New York, NY: Harper Collins College Publishers; 1996. [Google Scholar]
- 43.Wade JB, Price DD, Hamer RM, Schwartz SM, Hart RP. An emotional component analysis of chronic pain. Pain. 1990;40:303–310. doi: 10.1016/0304-3959(90)91127-5. [DOI] [PubMed] [Google Scholar]
- 44.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156:842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 46.Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neuro transmission. Arch Gen Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- 47.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensionsof pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 48.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]