Abstract
Problem
Galectin-3 is a β-galactoside binding protein with immunomodulatory properties and exerts its extracellular functions via interactions with glycoconjugate ligands. Therefore, to elucidate the function of galectin-3, binding ligands in human seminal plasma were investigated.
Method of Study
Galectin-3 binding proteins were isolated from seminal plasma by affinity chromatography, and candidate ligands were identified by MS/MS. Biochemical methods were used to characterize the ability of galectin-3 to bind its ligands.
Results
Identified galectin-3 ligands included CD13, MUC6, PAP, PSA and ZAG. 1D and 2D electrophoretic analysis of seminal plasma demonstrated that CD13, PAP, PSA, and ZAG immunoreactivity co-migrated with galectin-3-reactive protein bands and spots at expected molecular weights and pIs. Inhibition assays indicated that CD13, PSA, PAP, and ZAG, interact with galectin-3 in a protein-carbohydrate manner.
Conclusion
The galectin-3 binding ligands identified in this study indicate multiple roles for galectin-3 in the reproductive and immunological functions of seminal plasma.
Keywords: immunomodulation, lectin, inflammation, proteomics, semen
INTRODUCTION
Galectin-3 is a carbohydrate binding protein with extracellular functions that include immunomodulation, inflammation, cell adhesion, host-pathogen interactions, and the progression of multiple cancers, such as prostate cancer.1–3 The ~30 kDa galectin-3 monomer contains a single carbohydrate recognition domain (CRD), and the monomer can multimerize in the presence of carbohydrate ligands via self-association of the galectin-3 non-lectin domain.1–4 Many galectin-3 functions are dependent on the cross-linking of glycoconjugate ligands. Therefore, the functional characterization of galectin-3 in a given cell type or body fluid includes the identification of its target glycoconjugate ligands. In the current study, the reproductive and immunological function of galectin-3 in human semen was investigated by the identification of galectin-3 binding ligands in seminal plasma.
Seminal plasma is the fluid component of semen and plays a major role in supplying nutrients to sperm and protecting sperm from the immune system in the female reproductive tract.5 Seminal plasma also induces an inflammatory response in the female reproductive tract that promotes tissue remodeling, implantation, and maternal immune tolerance.6 Our laboratory previously identified galectin-3 in semen as a component of seminal plasma and prostasomes. Prostasomes are exosome-like vesicles in seminal plasma that are secreted by the prostatic epithelium and are proposed to have immunomodulatory functions in the female reproductive tract.7 We characterized galectin-3 binding ligands in human prostasomes and demonstrated that galectin-3 is a proteolytic substrate for prostate specific antigen (PSA) in seminal plasma8 and on the surface of prostasomes.9 We now report the results of a proteomic study that investigated galectin-3 function in seminal plasma by characterizing proteins that bound to immobilized galectin-3. Biochemical analysis confirmed the identification of selected galectin-3 binding ligands in seminal plasma. Galectin-3 interactions with these ligands in seminal plasma and their potential roles in reproduction and immunity are discussed.
MATERIALS AND METHODS
Purification of galectin-3 binding ligands from human seminal plasma
Semen samples from healthy men were obtained following protocols approved by the University of Arkansas for Medical Sciences (UAMS) Institutional Review Board. Clarified seminal plasma was prepared from liquefied semen by centrifugation at 1,000 g for 20 min to remove cellular components, and the collected supernatant was centrifuged at 10,000 g at 4 °C for 30 min. The subsequent supernatant was ultracentrifuged at 100,000 g at 4 °C for two hours to remove membrane and particulate material. Recombinant human galectin-3 was covalently bound to cyanogen bromide-activated Sepharose (Sigma, St. Louis, MO, USA) following the manufacturer’s instructions, and galectin-3 affinity column chromatography was performed as previously described.9
Proteomic analysis of galectin-3 binding ligands and criteria for selection of candidate ligands for further characterization
Galectin-3 binding proteins isolated from human seminal plasma were separated by SDS-PAGE and stained with Coomassie blue. Coomassie-stained protein bands were excised with a razor blade, and trypsin digestion was performed with a ProGest in-gel enzymatic digestion robot (Genomic Solutions, Ann Arbor, MI, USA) using sequence grade modified trypsin (Promega, Madison, WI, USA). The proteins were analyzed by MS/MS on a high-performance linear ion trap mass spectrometer (LTQ XL; Thermo Scientific). Data were processed using the Scaffold 3 Proteome Software (http://www.proteomesoftware.com/). The resulting peak list was used to search the Mascot protein database (www.matrixscience.com) and assigned a Mascot score. Mascot scores are a summation of the scores for the individual peptides. Higher scores translate to greater sequence probability.
Spectral counts for identified proteins were calculated using the equation:
(number of assigned spectra) / (molecular weight of protein)
Percent of total spectral count for each protein was calculated using the equation:
(spectral count) / (total spectral count for all proteins) × 100
Proteins with 10% or greater of the total spectral count were considered as candidates for further characterization. A protein-protein interaction map for galectin-3 and identified binding ligands was assembled using the Cytoscape software program (www.cytoscape.org), which extracts interaction data from the Human Protein Reference Database, the Biological General Repository for Interaction Datasets, the open source molecular interaction database (IntAct) and the NCI/Nature Pathway Interaction Database10. Candidate galectin-3 binding ligands in seminal plasma were selected for further investigation based on satisfaction of two of the three following criteria: (i) prevalence in the galectin-3 affinity-purified sample, (ii) not previously reported as a galectin-3 binding ligand and (iii) functional relevance to the reproductive tract.
Immunoblot and lectin blot analysis
For 1D SDS-PAGE, protein samples were separated using reducing conditions on 12% polyacrylamide gels.11 Precision Plus Protein Standards (Bio-Rad, Hercules, CA, USA) were used to estimate apparent protein molecular mass. Immunoblot analysis was performed as previously described.9 Electroblots were incubated with the following primary antibody dilutions: anti-CD13 (1:5,000; Gene Tex Inc., Irvine, CA, USA.), anti-Mac-2-binding protein (M2BP) (1:1,000; R&D Systems, Minneapolis, MN, USA), anti-prostatic acid phosphatase (PAP) (1:50,000; Gene Tex Inc.), anti-PSA (1:5,000; Abcam, Cambridge, MA, USA), or anti-zinc α2-glycoprotein (ZAG) (1:50,000; Gene Tex Inc.).
Lectin blot analysis was performed as previously described,9 using biotinylated intact galectin-3 (10 µg/mL) or galectin-3 CRD (5.7 µg/mL). For competitive inhibition experiments, biotinylated galectin-3 was pre-incubated with 2 mg/mL asialofetuin, 200 mM lactose, or 200 mM sucrose in PBS-Tween 20 for two hours at 4 °C.
For 2D electroblot analysis of human seminal plasma, protein samples were separated in the first dimension using a Biorad-Protean IEF Cell isoelectric focusing apparatus. Isoelectrically focused proteins were subsequently run in the second dimension by SDS-PAGE to resolve proteins by molecular weight. Immunoblot and lectin analyses were performed a minimum of three times.
RESULTS
Proteomic analysis of candidate galectin-3 binding ligands in human seminal plasma
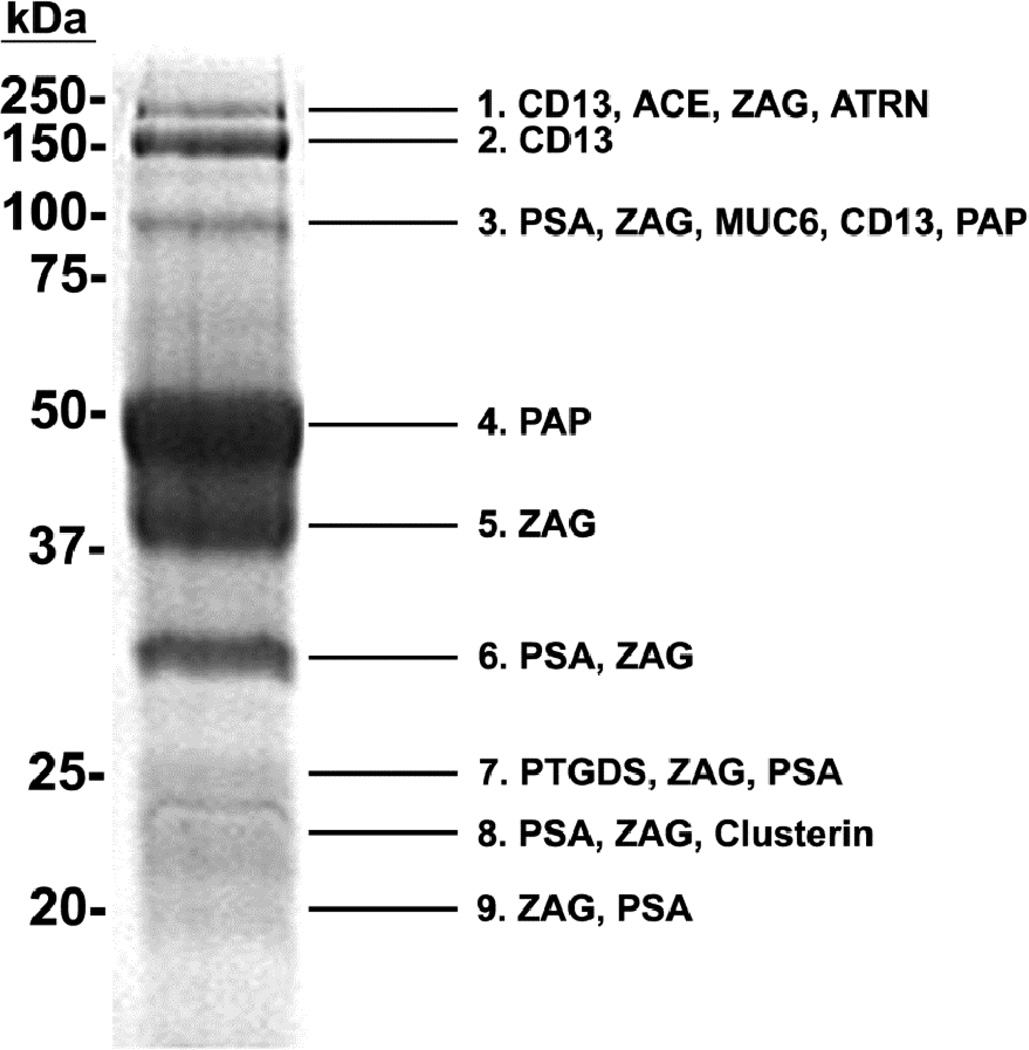
Galectin-3 binding proteins were subjected to SDS-PAGE and stained with Coomassie blue (Fig. 1). Nine Coomassie-stained protein bands ranging in molecular mass from ~20 to 150 kDa were excised for analysis by MS/MS. Spectral counts for identified proteins were calculated, and proteins with 10% or greater of the total spectral count and Mascot score higher than 50 were considered significant. Candidate galectin-3 binding proteins identified in human seminal plasma included angiotensin converting enzyme (ACE), attractin (ATRN), CD13, clusterin, mucin 6 (MUC6), PAP, prostaglandin H2 D-isomerase (PTGDS), PSA, and ZAG. Table 1 lists the sequence coverage (the percent of peptides identified by MS/MS that match the full-length protein sequence), Mascot score, percent spectral count, and previously described functions for each of the candidate galectin-3 binding ligands.
Figure 1.
Proteomic analysis of galectin-3 binding ligands purified from seminal plasma. Affinity-purified, galectin-3 binding proteins were separated by SDS-PAGE and stained with Coomassie blue. Protein bands were excised, and the isolated proteins were identified by MS/MS. Spectral counts for identified proteins were calculated, and proteins with 10% or greater of the total spectral count for each band are shown in order of increasing percent spectral count.
Table 1.
Protein matches, percent sequence coverage, Mascot scores, percent spectral count, and functions of galectin-3 binding proteins purified by affinity chromatography of human seminal plasma and analyzed by MS/MS.
| Protein and Band Number | Percent Sequence Coverage |
Mascot Score |
Percent Spectral Count |
Function |
|---|---|---|---|---|
| CD13 | Aminopeptidase N, CMV receptor23, 24 | |||
| Band 1 | 8 | 412 | 22.69 | |
| Band 2 | 28 | 1456 | 83.01 | |
| Band 3 | 3 | 216 | 13.05 | |
| Angiotensin Converting Enzyme | Exopeptidase36 | |||
| Band 1 | 13 | 672 | 16.64 | |
| Attractin | Soluble human plasma protein similar to dipeptidyl peptidase IV37 | |||
| Band 1 | 9 | 440 | 10.47 | |
| Mucin 6 | HIV transfer25 | |||
| Band 3 | 4 | 372 | 15.19 | |
| Prostatic Acid Phosphatase | Semen liquefaction, Enhanced HIV infectivity20, 38 | |||
| Band 3 | 6 | 67 | 10.63 | |
| Band 4 | 50 | 1161 | 50.11 | |
| Zinc α2 Glycoprotein | Stimulates lipolysis, sperm motility21 | |||
| Band 1 | 14 | 157 | 16.31 | |
| Band 3 | 14 | 131 | 28.14 | |
| Band 5 | 61 | 1512 | 87.50 | |
| Band 6 | 51 | 638 | 13.42 | |
| Band 7 | 43 | 470 | 26.14 | |
| Band 8 | 25 | 249 | 16.13 | |
| Band 9 | 33 | 324 | 44.81 | |
| Prostate Specific Antigen | Semen liquefaction, Prostate cancer8, 18 | |||
| Band 3 | 13 | 159 | 32.99 | |
| Band 6 | 44 | 623 | 48.62 | |
| Band 7 | 38 | 354 | 19.36 | |
| Band 8 | 37 | 323 | 50.44 | |
| Band 9 | 14 | 205 | 39.40 | |
| Prostaglandin H2 D-Isomerase | Conversion of PGH2 to PGD2 and lipid binding28 | |||
| Band 7 | 40 | 486 | 42.77 | |
| Clusterin | Apoptosis, cell adhesion, and male infertility39–41 | |||
| Band 8 | 13 | 255 | 14.07 | |
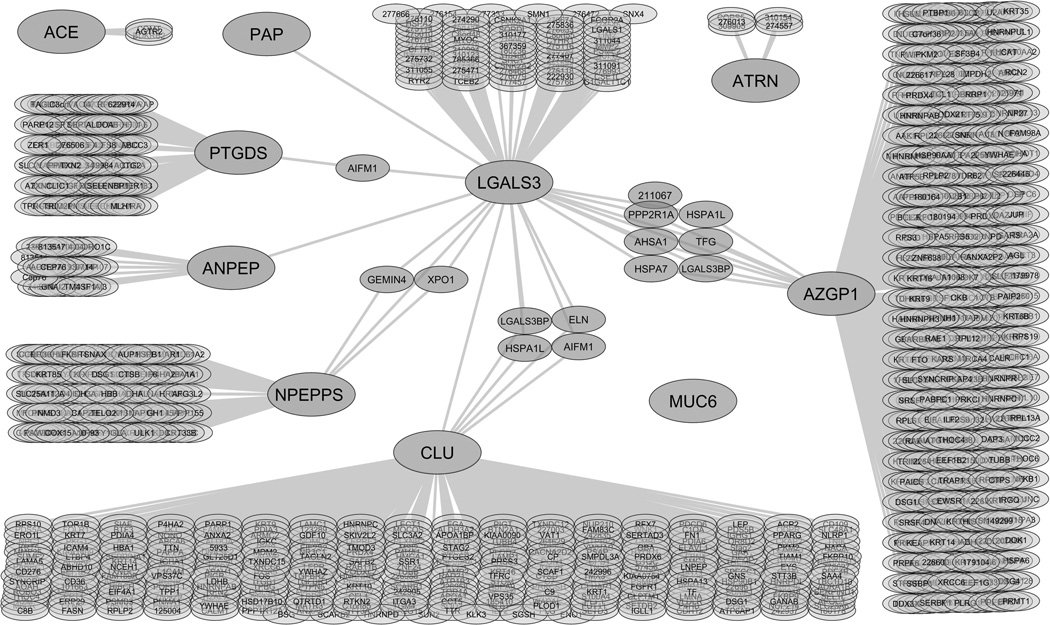
Protein-protein interaction network of the candidate galectin-3 binding ligands
A protein-protein interaction map was generated from reported interaction data.10 The map represents an overlay of the interaction networks for each of the galectin-3 binding ligands and the known proteins that interact with each ligand (Fig. 2). Large nodes indicate the galectin-3 affinity-purified proteins identified in this study. Small nodes indicate proteins from the database that interact with galectin-3 and/or the candidate galectin-3 binding ligands identified in this study. Connecting lines indicate protein interactions, and each protein is shown by its gene symbol. The interaction map indicates that galectin-3 and PTGDS both interact with apoptosis-inducing factor 1. Galectin-3 and PSA interact with the GEMIN4 gene product and exportin 1. Galectin-3 and clusterin both interact with M2BP, heat shock 70 kDa protein 1L, elastin, and apoptosis-inducing factor 1. Galectin-3 and ZAG both interact with protein phosphatase 2 regulatory subunit A, activator of 90 kDa heat shock protein ATPase homolog 1, heat shock 70 kDa protein 7, heat shock protein 70kDa 1L, protein TFG, and M2BP. Protein interactions with MUC6 were not identified by database search. Interaction lines were added between galectin-3 and its ligands CD13, clusterin, PAP, PSA, and ZAG to indicate the interactions previously identified in the literature,8, 9, 12, 13 but not identified by the database search. Based on this analysis, direct interactions with galectin-3 have not been identified previously for ACE, ATRN, MUC6, or PTGDS.
Figure 2.
Protein-protein interaction network of the identified candidate galectin-3 binding ligands in seminal plasma. A protein-protein interaction map was generated using data obtained from the HPRD, BioGRID, IntAct database, and NCI/Nature Pathway Interaction Database. Large nodes indicate the galectin-3 affinity purified proteins identified in this study. Small nodes represent proteins that interact with the candidate galectin-3 binding ligands based on the database search. Connecting lines indicate protein interactions. Each protein is shown by its gene symbol or Entrez identification number. Angiotensin-converting enzyme (ACE), attractin (ATRN), CD13 (ANPEP), clusterin (CLU), galectin-3 (LGALS3), mucin 6 (MUC6), prostatic acid phosphatase (PAP), prostate specific antigen (NPEPPS), prostaglandin-H2 D-isomerase (PTGDS), and zinc α2-glycoprotein (AZGP1).
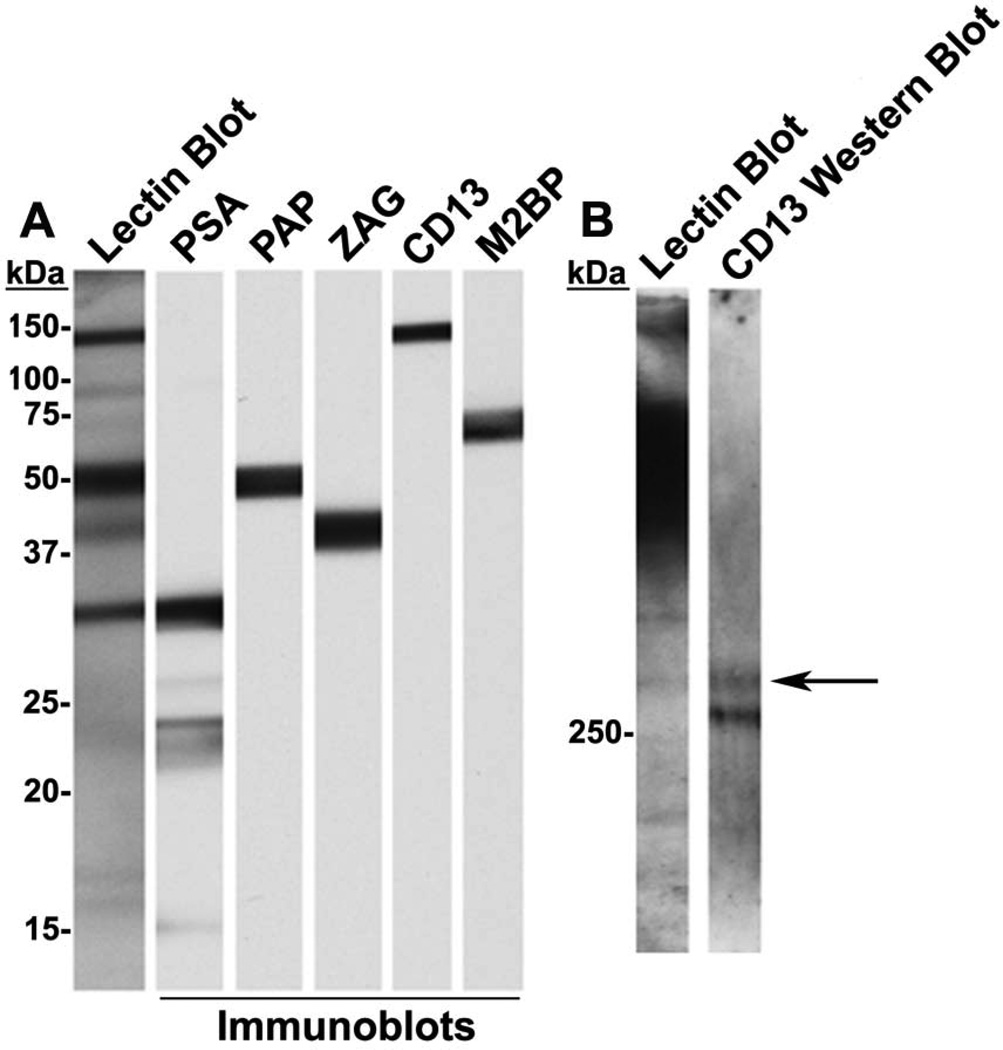
Co-migration of ligand immunoreactivity with galectin-3-reactive bands on 1D lectin blots of seminal plasma
Human seminal plasma was subjected to lectin blot analysis with biotinylated galectin-3 and immunoblot analysis for PSA, PAP, ZAG, CD13, and M2BP (Fig. 3A & 3B) using electroblot strips prepared from the same electrophoretic gel. Seven major galectin-3-reactive bands were identified, and CD13, M2BP, PAP, ZAG, and PSA immunoreactive bands co-migrated with lectin-detected bands at ~150, ~75, ~50, ~40, and ~30 kDa, respectively. None of the galectin-3 immunoreactive bands co-migrated with the galectin-3-reactive ~100 kDa band.
Figure 3.
Co-migration of galectin-3-reactive and immunoreactive bands on 1D electroblots of seminalplasma. (A) Seminal plasma was subjected to 1D immunoblot and galectin-3 lectin blot analysis. The identified PSA (~30 kDa), PAP (~50 kDa), ZAG (~40 kDA), CD13 (~150 kDa), and M2BP (~75 kDa) immunoreactive bands co-migrated with galectin-3-reactive bands.(B) Seminal plasma was subjected to SDS-PAGE on 4% acrylamide followed by galectin-3 lectin blot and CD13 immunoblot analysis. An identified CD13 immunoreactive band co-migrated with a galectin-3-reactive band on the lectin blot (arrow). A large, strongly stained, smeary band above 250 kDa was detected on the lectin blot.
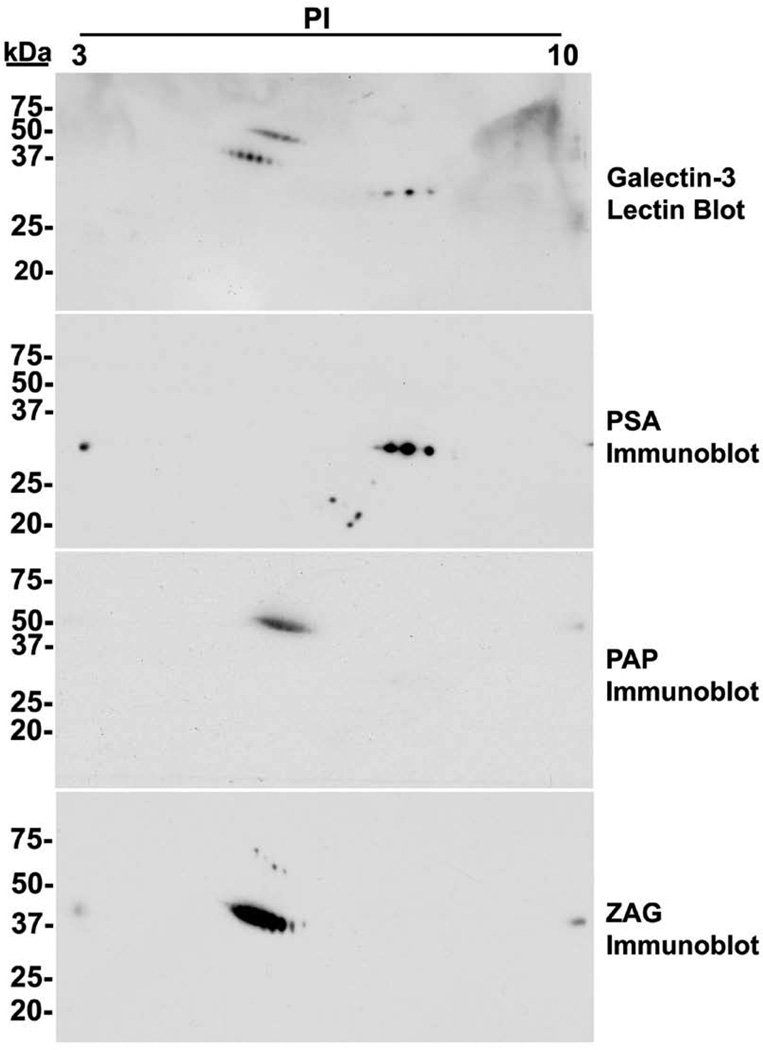
Co-migration of ligand immunoreactivity with galectin-3-reactive spots on 2D lectin blots of seminal plasma
Two-dimensional immunoblot analysis for PSA, PAP, and ZAG and 2D lectin blot analysis with biotinylated galectin-3 (Fig. 4) were performed to further examine the interaction between galectin-3 and the identified ligands. Three galectin-3-reactive spot patterns were identified with molecular weights corresponding to ~50, ~40, and ~30 kDa. Immunoreactive spots for PSA, PAP, and ZAG co-migrated with lectin-detected spots at the expected molecular weights and pIs:14 ~30 kDa/pI ~7, ~50 kDa/pI ~5, and ~40 kDa/pI ~5, respectively.
Figure 4.
Co-migration of galectin-3-reactive and immunoreactive spots on 2D electroblots of seminal plasma. Clarified seminal plasma was subjected to 2D immunoblot and galectin-3 lectin blot analysis. Three distinct immunoreactvie spot patterns ~30 kDa/~pI 7, ~50 kDa/~pI 5, and ~40 kDa/~pI 5 were identified by galectin-3 lectin blot analysis. These three distinct galectin-3-reactive spot patterns comigrated with the immunoblot spot patterns for PSA, PAP, and ZAG at their expected apparent molecular weight and pI.
Competitive inhibition of galectin-3 ligand interactions with galectin-3 carbohydrate-binding inhibitors
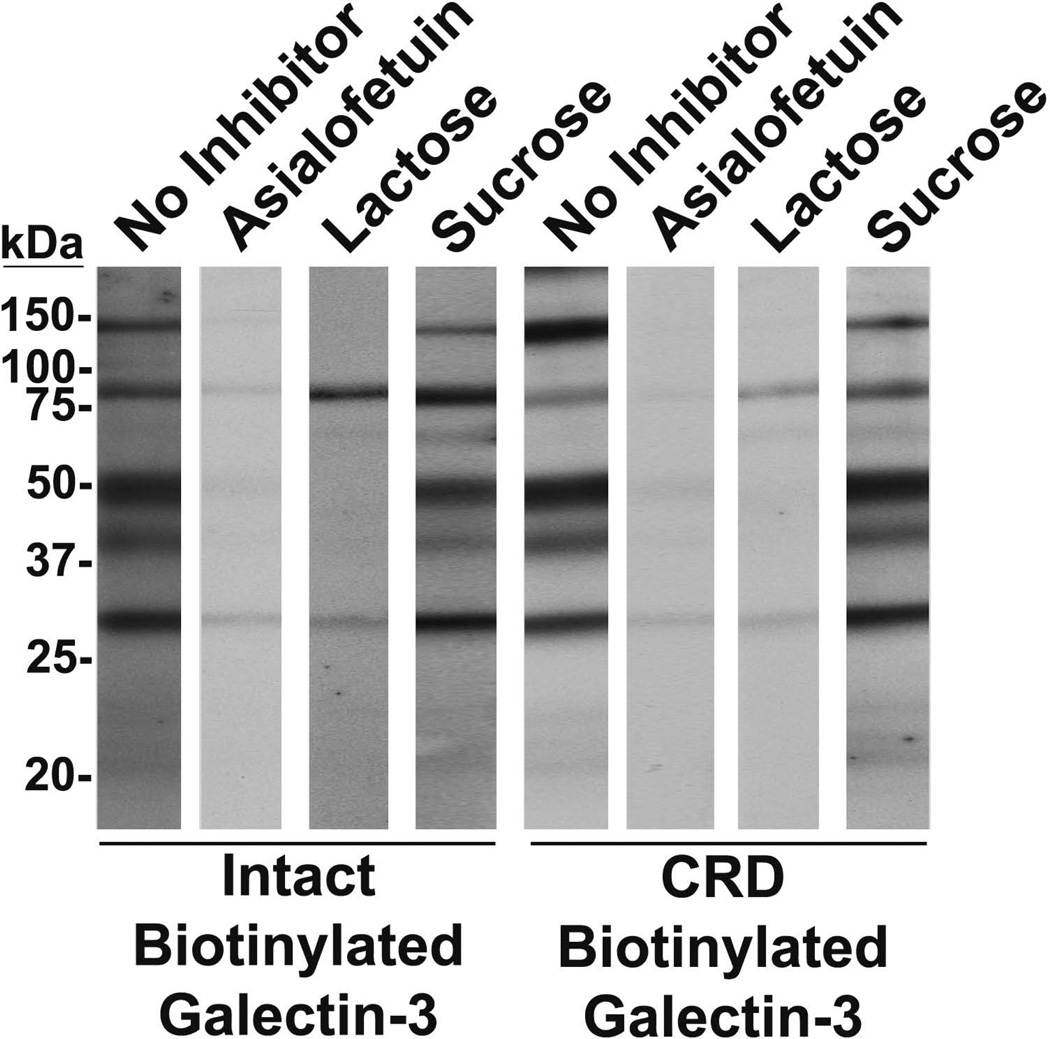
Lectin blot analysis was performed to assess the inhibitory effects of asialofetuin and lactose on galectin-3 binding with its ligands (Fig. 5). In the presence of asialofetuin, reactivity of intact galectin-3 and the galectin-3 CRD with bands at ~150, ~100, ~50, ~40, and ~30 kDa was greatly diminished, and the band at ~75 kDa was abolished. In the presence of lactose, reactivity of intact galectin-3 with bands at ~75, ~50, ~40, and ~30 kDa was greatly diminished, the ~150 kDa band was abolished, and with the ~100 kDa band was not affected. However, lactose greatly diminished the reactivity of the galectin-3 CRD with for all bands. Pre-incubation with sucrose as a negative control had no effect on intact galectin-3 or the galectin-3 CRD binding.
Figure 5.
Galectin-3 interacts with the identified binding ligands in a protein-carbohydrate manner. On lectin blots of seminal plasma, intact galectin-3 and the galectin-3 CRD detected bands at ~150, ~100,~75, ~50, ~40, and ~30 kDa. Following pre-incubation of galectin-3 with asialofetuin, bands at ~150,~100, ~50, ~40, and ~30 kDa were diminished, and the band at ~75 kDa was abolished. When galectin-3 was pre-incubated with lactose, bands at ~75, ~50, ~40, and ~30 kDa were diminished, the ~150 kDa band was abolished, and the ~100 kDa band was unaffected. Pre-incubation with sucrose had no effect.
DISCUSSION
The extracellular functions of galectin-3 include immunomodulation, inflammation, cell adhesion, host-pathogen interactions, and prostate cancer progression.1 In the current study, galectin-3 function in the reproductive tract was investigated by characterizing galectin-3 binding ligands in seminal plasma. The identified candidate galectin-3 binding ligands included ACE, ATRN, CD13, clusterin, MUC6, PAP, PSA, PTGDS, and ZAG. Each of these proteins were previously identified in human seminal plasma using a proteomic approach.15 Furthermore, we previously identified PSA, PAP, ZAG, CD13, and clusterin as galectin-3 binding ligands in prostasomes from human semen. However, no direct interactions were identified between galectin-3 and ACE, ATRN, or MUC6, or PTGDS. Therefore, these findings are the first to indicate these proteins as galectin-3 binding ligands.
CD13, MUC6, PAP, PSA, and ZAG were selected for further investigation as galectin-3 binding ligands in human seminal plasma based on satisfaction of the described criteria. M2BP was included in the characterization because it is a known galectin-3 binding ligand that has also been identified in seminal plasma.12 On electroblots of seminal plasma, CD13, M2BP, PAP, PSA, and ZAG immunoreactive bands co-migrated with galectin-3-reactive bands at their expected apparent molecular weight. Moreover, the spot pattern of a 2D galectin-3 lectin blot of seminal plasma matches the spot pattern of PSA, PAP, and ZAG on 2D immunoblots of in seminal plasma. Collectively, these results confirm PSA, PAP, ZAG, CD13, and M2BP as galectin-3 binding ligands and suggest that galectin-3 interacts with glycans on these glycoproteins in seminal plasma.
MUC6 was identified by MS/MS in a ~100 kDa protein band isolated from seminal plasma at 15.19% of the total spectral count. Given that the apparent molecular weight of MUC6 is greater than 250 kDa, the MUC6 sequence identified in the ~100 kDa band likely represents a fragment of the intact glycoprotein. Therefore, electroblot analysis with a low percentage gel was used to further investigate MUC6 as a galectin-3 binding ligand. Significantly, a galectin-3-reactive band above 250 kDa was identified by lectin blot analysis, as would be expected if galectin-3 was binding to a mucin. Immunoblot analysis of seminal plasma with two different anti-MUC6 antibodies was unsuccessful despite the presence of MUC6 in seminal plasma, likely due to the heavy glycosylation on MUC6 preventing antibody binding. Nevertheless, the detection of galectin-3 reactivity of appropriate molecular mass for MUC6 and the identification of a significant sequence match for MUC6 in the affinity-purified sample implicate MUC6 as a binding ligand for galectin-3 in seminal plasma.
Competition experiments with asialofetuin and lactose were utilized to characterize the molecular interactions between galectin-3 and its identified ligands. In the presence of these inhibitors, reactivity of both intact galectin-3 and the galectin-3 CRD with protein bands corresponding to CD13, M2BP, PAP, ZAG, and PSA was diminished. These results suggest that galectin-3 ligand binding occurs, at least in part, via interactions between the CRD’s carbohydrate-binding site and glycan moieties on the glycoprotein ligands. However, galectin-3 reactivity with the PSA band was not totally abolished by the carbohydrate inhibitors. Galectin-3 is a proteolytic substrate for PSA,8 and binding interactions between galectin-3 and PSA in seminal plasma may also involve the PSA active site. Thus, galectin-3 may interact with PSA via both protein-carbohydrate and protein-protein interactions. Furthermore, reactivity of intact galectin-3 and the galectin-3 CRD with an ~100 kDa band was diminished in the presence of asialofetuin, but not lactose. Galectin-3 binds with higher affinity to asialofetuin than to the simple disaccharide lactose.16, 17 Therefore, inhibition of galectin-3 binding to all but the ~100 kDa band indicates that galectin-3 binds with greater affinity to a component(s) of the ~100 kDa band than to proteins present in the other bands. Interestingly, the only protein identified in the ~100 kDa band that was not identified in the other bands was MUC6, suggesting that galectin-3 may bind with MUC6 with relatively high affinity. Further studies are required to investigate MUC6/galectin-3 interactions.
The identified galectin-3 binding ligands and their relevance to male reproduction are discussed below.
PSA (~30 kDa) is a chymotrypsin-like serine protease secreted by the prostatic epithelium and normally functions in liquefaction of the semen coagulum.18 We previously identified galectin-3 as a proteolytic substrate for PSA and demonstrated that a significant portion of galectin-3 in semen is present in its PSA-cleaved form.8 Proteolytic cleavage of galectin-3 abrogates the ability of the galectin-3 to crosslink its target ligands, thereby down-regulating galectin-3 function. PSA has also been implicated in the promotion of localized prostate tumors and bone metastases by its roles in immunomodulation, invasion, angiogenesis, and apoptosis.18 Likewise, multiple roles have been proposed for galectin-3 in prostate cancer.1 Therefore, the regulation of galectin-3 by PSA may be involved in galectin-3 function in prostate cancer progression.
PAP (~50 kDa) is a glycosylated non-specific phosphomonoesterase19 that hydrolyzes a wide spectrum of substrates. PAP is secreted by the prostatic epithelium into semen and plays a role in semen liquefaction.20 PAP is also proposed to enhance human immunodeficiency virus (HIV) infectivity by forming amyloid aggregates, known as semen-derived enhancer of viral infection (SEVI), that promote virion attachment to target cells.20
ZAG (~40 kDa) is a glycoprotein that is secreted by prostatic epithelial cells into seminal plasma. ZAG has been shown to play a role in sperm motility via the cAMP/PKA signaling pathway and may be required for fertilization.21, 22 Furthermore, ZAG has been identified in prostate cancer tumors. Interestingly, ZAG is a collagen-binding protein21 and therefore, may interact with galectin-3 via its collagen-like linker in addition to the protein-carbohydrate interactions identified in this study.
Significantly, PSA, PAP, ZAG, and prolactin inducible protein (PIP) have been shown to be components of a high molecular weight zinc-binding multi-protein complex in human seminal plasma.14 This study identified PAP, PSA, and ZAG as galectin-3 ligands in seminal plasma, and Block et al.12 previously proposed PIP as a candidate galectin-3 ligand in prostasomes. Yadav et al.14 noted that glycosylation of the proteins is the only distinguishing characteristic in common among the proteins in the identified complex. Therefore, galectin-3 may interact with this aggregate and/or be involved in the formation of the complex in seminal plasma.
The CD13 glycoprotein (aminopeptidase N; ~150 kDa) is a membrane-bound metalloproteinase.23 Galectin-3 specifically binds to CD13 in a carbohydrate recognition-dependent manner, and CD13 is a crucial mediator of galectin-3-induced angiogenesis in endothelial cells.13 Furthermore, CD13 is implicated in infection and pathogenesis of cytomegalovirus, a virus that can be sexually transmitted.24
MUC6 is a high molecular weight protein (>250 kDa) that is heavily glycosylated and is a ligand for the lectin dendritic cell-specific intracellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) in seminal plasma.25 DC-SIGN is a pattern recognition receptor involved in innate immunity against a variety of pathogens. Clusterin is another DC-SIGN ligand19 that was identified as a candidate galectin-3 ligand in this study. MUC6 and clusterin in seminal plasma have been proposed to interfere with the sexual transmission of HIV-1 and other DC-SIGN co-opting viruses.19, 25 Significantly, PAP and M2BP are also DC-SIGN ligands in seminal plasma.26 This commonality in binding ligands suggests that galectin-3 and DC-SIGN have competing or complementary immune-related functions in seminal plasma.
ACE, PTGDS, and ATRN were also identified as candidate galectin-3 binding ligands. ACE is an exopeptidase and its release from human sperm during capacitation is associated with motility and fertilizing capability.27 The glycoprotein PTGDS converts prostaglandin H2 to prostaglandin D228, but is proposed to function in semen as a steroid and lipid carrier that provides thyroid hormones and retinoids to maturing sperm.29 ATRN is similar to CD26 (dipeptidyl peptidase IV), which we identified as a galectin-3 binding ligand in prostasomes,9 and is implicated in host defense in semen.30 Although these candidate ligands did not meet the criteria for further characterization in this study, their interactions with galectin-3 in seminal plasma may be functionally significant.
M2BP (~67–75 kDa, ~90–100 kDa) is a secreted glycoprotein normally present in epithelia, the extracellular matrix, and body fluids including semen.31 M2BP is proposed to function in host defense and cancer progression. We previously characterized M2BP on the surface of prostasomes in human semen,12 but we did not detect M2BP in the pool of galectin-3 binding proteins purified in this study. However, serum M2BP levels can vary significantly, suggesting that M2BP levels in semen may also vary.32 The overwhelming amounts of PSA, PAP, and ZAG in seminal plasma may have prevented M2BP binding to the galectin-3 affinity column. Furthermore, the heavy glycosylation of M2BP may have interfered with its identification by Coomassie stain. Nevertheless, electroblot analysis implicated M2BP as a galectin-3 ligand in seminal plasma.
Collectively, the galectin-3 binding ligands identified in this study indicate multiple roles for galectin-3 in the reproductive and immunological functions of seminal plasma. For example, galectin-3 interactions with PSA and PAP indicate a role for galectin-3 in semen liquefaction, and interactions with ZAG, ACE, and PTGDS implicate roles in sperm function. CD13/galectin-3 interactions may contribute to the angiogenic potential of seminal plasma in the female reproductive tract.33–35 The ligands identified in this study and galectin-3’s function as a pro-inflammatory mediator implicate multiple roles for galectin-3 in the immunomodulatory and inflammatory responses required for reproductive function and immune defense in the female reproductive tract. The identified interactions with CD13, PAP, and MUC6 also indicate that galectin-3 may be involved in pathogenic infection in the female genital tract. Moreover, PSA cleavage of galectin-3 and galectin-3 binding to MUC6 and clusterin, similar to binding of the lectin DC-SIGN, may serve to down-regulate galectin-3 function in seminal plasma and the female reproductive tract. Therefore, it is interesting to speculate that the initial concentration of functional galectin-3 in seminal plasma must be diminished to prevent detrimental effects in the female reproductive tract. Further study will be necessary to elucidate the functional roles of the identified ligand interactions in the mechanisms that regulate galectin-3 function in seminal plasma. We anticipate that our current findings will contribute to these future investigations into the reproductive and immunological functions of galectin-3 in semen and the female reproductive tract.
ACKNOWLEDGMENTS
The project described was supported by award number R01HD050540 to ABD from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD. The authors wish to thank Karah Bogoslavsky for critical review of the manuscript, Drs. Mari Davidson and Robert Eoff for their valuable advice, and Drs. Alan Tackett and Samuel Mackintosh of the UAMS Proteomics Core Facility. The Proteomics Core is supported by the Arkansas IDeA Network for Biomedical Research Excellence (P20GM103429), the University of Arkansas Center for Protein Structure and Function (P30GM103450), the UAMS Center for Microbial Pathogenesis and Host Inflammatory Responses (P20GM103625), and the Translational Research Institute, grant UL1TR000039 through the NIH National Center for Advancing Translational Sciences.
REFERENCES
- 1.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002;23:313–320. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- 3.Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7:424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 5.Edstrom AM, Malm J, Frohm B, Martellini JA, Giwercman A, Morgelin M, Cole AM, Sorensen OE. The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J Immunol. 2008;181:3413–3421. doi: 10.4049/jimmunol.181.5.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- 7.Aalberts M, Stout TA, Stoorvogel W. Prostasomes: extracellular vesicles from the prostate. Reproduction. 2014;147:R1–R14. doi: 10.1530/REP-13-0358. [DOI] [PubMed] [Google Scholar]
- 8.Saraswati S, Block AS, Davidson MK, Rank RG, Mahadevan M, Diekman AB. Galectin-3 is a substrate for prostate specific antigen (PSA) in human seminal plasma. Prostate. 2011;71:197–208. doi: 10.1002/pros.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovak MR, Saraswati S, Goddard S, Diekman AB. Proteomic identification of galectin-3 binding ligands and characterization of galectin-3 proteolytic cleavage in human prostasomes. Andrology. 2013 doi: 10.1111/j.2047-2927.2013.00099.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Block AS, Saraswati S, Lichti CF, Mahadevan M, Diekman AB. Co-purification of Mac-2 binding protein with galectin-3 and association with prostasomes in human semen. Prostate. 2011;71:711–721. doi: 10.1002/pros.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang E, Shim JS, Woo HJ, Kim KW, Kwon HJ. Aminopeptidase N/CD13 induces angiogenesis through interaction with a pro-angiogenic protein, galectin-3. Biochem Biophys Res Commun. 2007;363:336–341. doi: 10.1016/j.bbrc.2007.08.179. [DOI] [PubMed] [Google Scholar]
- 14.Yadav VK, Kumar V, Chhikara N, Kumar S, Manral P, Kashav T, Saini S, Srinivasan A, Singh S, Singh TP, Yadav S. Purification and characterization of a native zinc-binding high molecular weight multiprotein complex from human seminal plasma. J Sep Sci. 2011;34:1076–1083. doi: 10.1002/jssc.201000842. [DOI] [PubMed] [Google Scholar]
- 15.Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, Yagi F, Kasai K. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 17.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1–2, and-3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams SA, Singh P, Isaacs JT, Denmeade SR. Does PSA play a role as a promoting agent during the initiation and/or progression of prostate cancer? Prostate. 2007;67:312–329. doi: 10.1002/pros.20531. [DOI] [PubMed] [Google Scholar]
- 19.Kong HY, Byun J. Emerging Roles of Human Prostatic Acid Phosphatase. Biomol Ther (Seoul) 2013;21:10–20. doi: 10.4062/biomolther.2012.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KA, Yolamanova M, Zirafi O, Roan NR, Staendker L, Forssmann WG, Burgener A, Dejucq-Rainsford N, Hahn BH, Shaw GM, Greene WC, Kirchhoff F, Munch J. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology. 2010;7:55. doi: 10.1186/1742-4690-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan MI, Waheed A, Yadav S, Singh TP, Ahmad F. Zinc alpha 2-glycoprotein: a multidisciplinary protein. Mol Cancer Res. 2008;6:892–906. doi: 10.1158/1541-7786.MCR-07-2195. [DOI] [PubMed] [Google Scholar]
- 22.Qu F, Ying X, Guo W, Guo Q, Chen G, Liu Y, Ding Z. The role of Zn-alpha2 glycoprotein in sperm motility is mediated by changes in cyclic AMP. Reproduction. 2007;134:569–576. doi: 10.1530/REP-07-0145. [DOI] [PubMed] [Google Scholar]
- 23.Arienti G, Carlini E, Verdacchi R, Cosmi EV, Palmerini CA. Prostasome to sperm transfer of CD13/aminopeptidase N (EC 3.4.11.2) Biochim Biophys Acta. 1997;1336:533–538. doi: 10.1016/s0304-4165(97)00071-8. [DOI] [PubMed] [Google Scholar]
- 24.Kasman LM. CD13/aminopeptidase N and murine cytomegalovirus infection. Virology. 2005;334:1–9. doi: 10.1016/j.virol.2005.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stax MJ, van Montfort T, Sprenger RR, Melchers M, Sanders RW, van Leeuwen E, Repping S, Pollakis G, Speijer D, Paxton WA. Mucin 6 in seminal plasma binds DC-SIGN and potently blocks dendritic cell mediated transfer of HIV-1 to CD4(+) T-lymphocytes. Virology. 2009;391:203–211. doi: 10.1016/j.virol.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Clark GF, Grassi P, Pang PC, Panico M, Lafrenz D, Drobnis EZ, Baldwin MR, Morris HR, Haslam SM, Schedin-Weiss S, Sun W, Dell A. Tumor biomarker glycoproteins in the seminal plasma of healthy human males are endogenous ligands for DC-SIGN. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.008730. M111 008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibahara H, Kamata M, Hu J, Nakagawa H, Obara H, Kondoh N, Shima H, Sato I. Activity of testis angiotensin converting enzyme (ACE) in ejaculated human spermatozoa. Int J Androl. 2001;24:295–299. doi: 10.1046/j.1365-2605.2001.00301.x. [DOI] [PubMed] [Google Scholar]
- 28.Heshmat SM, Mullen JB, Jarvi KA, Soosaipillai A, Diamandis EP, Hamilton RJ, Lo KC. Seminal plasma lipocalin-type prostaglandin D synthase: a potential new marker for the diagnosis of obstructive azoospermia. J Urol. 2008;179:1077–1080. doi: 10.1016/j.juro.2007.10.070. [DOI] [PubMed] [Google Scholar]
- 29.Leone MG, Haq HA, Saso L. Lipocalin type prostaglandin D-synthase: which role in male fertility? Contraception. 2002;65:293–295. doi: 10.1016/s0010-7824(02)00280-9. [DOI] [PubMed] [Google Scholar]
- 30.Radhakrishnan Y, Hamil KG, Tan JA, Grossman G, Petrusz P, Hall SH, French FS. Novel partners of SPAG11B isoform D in the human male reproductive tract. Biol Reprod. 2009;81:647–656. doi: 10.1095/biolreprod.109.077545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grassadonia A, Tinari N, Iurisci I, Piccolo E, Cumashi A, Innominato P, D'Egidio M, Natoli C, Piantelli M, Iacobelli S. 90K (Mac-2 BP) and galectins in tumor progression and metastasis. Glycoconj J. 2004;19:551–556. doi: 10.1023/B:GLYC.0000014085.00706.d4. [DOI] [PubMed] [Google Scholar]
- 32.Peehl DM, Chen Z, Nolley R. Serum Mac-2BP does not distinguish men with high grade, large volume prostate cancer from men with benign prostatic hyperplasia. Prostate. 2011;71:26–31. doi: 10.1002/pros.21218. [DOI] [PubMed] [Google Scholar]
- 33.Kaczmarek MM, Krawczynski K, Filant J. Seminal plasma affects prostaglandin synthesis and angiogenesis in the porcine uterus. Biol Reprod. 2013;88:72. doi: 10.1095/biolreprod.112.103564. [DOI] [PubMed] [Google Scholar]
- 34.Krawczynski K, Kaczmarek MM. Does seminal plasma affect angiogenesis in the porcine oviduct? Reprod Biol. 2012;12:347–354. doi: 10.1016/j.repbio.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Sales KJ, Sutherland JR, Jabbour HN, Katz AA. Seminal plasma induces angiogenic chemokine expression in cervical cancer cells and regulates vascular function. Biochim Biophys Acta. 2012;1823:1789–1795. doi: 10.1016/j.bbamcr.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Imai Y, Kuba K, Ohto-Nakanishi T, Penninger JM. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ J. 2010;74:405–410. doi: 10.1253/circj.cj-10-0045. [DOI] [PubMed] [Google Scholar]
- 37.Tang W, Gunn TM, McLaughlin DF, Barsh GS, Schlossman SF, Duke-Cohan JS. Secreted and membrane attractin result from alternative splicing of the human ATRN gene. Proc Natl Acad Sci U S A. 2000;97:6025–6030. doi: 10.1073/pnas.110139897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassan MI, Aijaz A, Ahmad F. Structural and functional analysis of human prostatic acid phosphatase. Expert Rev Anticancer Ther. 2010;10:1055–1068. doi: 10.1586/era.10.46. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths GS, Galileo DS, Aravindan RG, Martin-DeLeon PA. Clusterin facilitates exchange of glycosyl phosphatidylinositol-linked SPAM1 between reproductive luminal fluids and mouse and human sperm membranes. Biol Reprod. 2009;81:562–570. doi: 10.1095/biolreprod.108.075739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Qu J, Shelat H, Gao S, Wassler M, Geng YJ. Clusterin induces CXCR4 expression and migration of cardiac progenitor cells. Exp Cell Res. 2010;316:3435–3442. doi: 10.1016/j.yexcr.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Novak S, Smith TA, Paradis F, Burwash L, Dyck MK, Foxcroft GR, Dixon WT. Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology. 2010;74:956–967. doi: 10.1016/j.theriogenology.2010.04.025. [DOI] [PubMed] [Google Scholar]