Abstract
Brain concussions are a serious public concern and are associated with neuropsychiatric disorders, such as depression. Patients with concussion who suffer from depression often experience distress. Nevertheless, few pre-clinical studies have examined concussion-induced depression, and there is little information regarding its pharmacological management. Edaravone, a free radical scavenger, can exert neuroprotective effects in several animal models of neurological disorders. However, the effectiveness of edaravone in animal models of concussion-induced depression remains unclear. In this study, we examined whether edaravone could prevent concussion-induced depression. Mice were subjected to a weight-drop injury and intravenously administered edaravone (3.0 mg/kg) or vehicle immediately after impact. Serial magnetic resonance imaging showed no abnormalities of the cerebrum on diffusion T1- and T2-weighted images. We found that edaravone suppressed concussion-induced depressive-like behavior in the forced swim test, which was accompanied by inhibition of increased hippocampal and cortical oxidative stress (OS) and suppression of 5-lipoxygenase (5-LOX) translocation to the nuclear envelope in hippocampal astrocytes. Hippocampal OS in concussed mice was also prevented by the nicotinamide adenine dinucleotide phosphate oxidase inhibitor, apocynin, and administration of BWB70C, a 5-LOX inhibitor, immediately and 24 h after injury prevented depressive-like behaviors in concussed mice. Further, antidepressant effects of edaravone were observed in mice receiving 1.0 or 3.0 mg/kg of edaravone immediately after impact, but not at a lower dose of 0.1 mg/kg. This antidepressant effect persisted up to 1 h after impact, whereas edaravone treatment at 3 h after impact had no effect on concussion-induced depressive-like behavior. These results suggest that edaravone protects against concussion-induced depression, and this protection is mediated by suppression of OS and 5-LOX translocation.
Key words: : concussion, depression, edaravone, 5-lipoxygenase
Introduction
Traumatic brain injury (TBI) is a widespread public health concern. Individuals with TBI suffer from a broad range of short- and long-term disabilities that are attributed to physiological and neuropsychiatric impairments1 that include depression, anxiety, and cognitive dysfunction. Among these conditions, depression is the most common and distressing condition occurring after TBI because it adversely affects rehabilitation efforts and decreases the individual's quality of life.1,2 Although depression most often occurs in the first year after TBI, individuals with TBI remain at risk for developing depression for decades following their injury.3 This is a particular problem with concussive brain injuries (CBIs) in which patients have no clinically apparent neurological brain lesions. These patients are often not able to get adequate treatment and care and may suffer from long-lasting TBI-associated conditions.4
Free radicals, such as superoxide and nitric oxide (NO), are produced within minutes of brain injury.5 Normally, superoxide is scavenged by superoxide dismutase (SOD) however, when NO levels increase, the reaction between NO and superoxide forms peroxynitrite.6,7 In fact, nitric oxide synthase (NOS) activity increases at the lesion site in rats subjected to fluid percussion-induced TBI.8 Moreover, NO increases significantly in rats after controlled cortical impact9 or parasagittal cortical fluid percussion-induced TBI.10 Peroxynitrite is a powerful oxidant that can form nitrate phenolic moieties, including tyrosine residues, in protein.7 Therefore, nitrotyrosine formation is considered a biomarker of peroxynitrite production. Several studies have demonstrated increased peroxynitrite-mediated tyrosine nitration after TBI.11–13 Interestingly, the hippocampus is most susceptible to oxidative stress (OS)-induced damage,14 and hippocampal changes have been implicated in patients suffering from depression.15 In a mouse model of depression, chronic mild stress could induce nitrotyrosine formation in the hippocampus, whereas NOS inhibitor prevented depressive-like behavior.16 Nevertheless, there is no direct evidence that postconcussive free radical production contributes to depression.
Edaravone is a scavenger that interacts biochemically with many free radicals17 and attenuates ischemic brain injury in both humans and animal models.18 Recent studies have explored whether edaravone shows neuroprotective effects in other neurological disorders, such as epilepsy.19 Previously, we demonstrated that edaravone suppresses axonal injury and OS in traumatized mouse brains and protects mice from TBI-induced memory deficits.20 In patients with TBI, 5-lipoxygenase (5-LOX) immunoreactivity increased in glial fibrillary acidic protein (GFAP)-positive glial cells.21 Moreover, pharmacological inhibition or genetic disruption of 5-LOX has antidepressant-like effects in mice.22 Li and colleagues also demonstrated that edaravone inhibits 5-LOX activation mediated by OS occurring with oxygen-glucose deprivation-induced ischemic-like injury.23 We hypothesized that edaravone could prevent concussion-induced depression by suppressing OS and, subsequently, 5-LOX activation.
In this study, we developed an animal model of concussion to induce depressive-like behavior in the absence of remarkable histopathological brain injury. A secondary aim of this study was to investigate whether edaravone could protect mice from concussion-induced depressive behavior. Further, we examined whether the antidepressant-like effects of edaravone could prevent oxidative damage and 5-LOX activation.
Methods
Animals
Male C57BL/6N mice (12–16 weeks old; CLEA Japan, Tokyo, Japan) were housed and allowed to habituate for more than 1 week before the start of experimentation. Food and water were available ad libitum throughout the experiments. Animals were maintained in a temperature- (23±1°C) and humidity-controlled room (55±2%) on a 12-h light-dark cycle. All experimental protocols conformed to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996) and were approved by the committee for care and use of laboratory animals at Kochi University (Kochi, Japan).
Experimental concussion and drug treatment
Experimental concussion was induced using the concussive head trauma device. Detailed descriptions of this device can be found in previously published articles.24,25 Briefly, the device consisted of a vertical metal guide tube (diameter, 15 mm; length, 90 cm) through which a metal weight (40 g) was dropped from the full length of the tube. The right temporal region of the mouse's head, between the corner of the eye and the ear, was positioned under the tube while the head was manually supported and immobilized by a sponge. Sham control animals were anesthetized, but did not undergo concussion.
Mice were divided randomly into four groups for each treatment: 1) concussion plus vehicle-treated group; 2) concussion plus edaravone, selective 5-LOX inhibitor (BWB70C), or nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor (apocynin)-treated group; 3) sham plus vehicle-treated group; and 4) sham plus edaravone, BWB70C, or apocynin-treated group.
Edaravone, which was donated by Mitsubishi Tanabe Pharm (Osaka, Japan), was dissolved in 1 M of NaOH and titrated with 1 M of HCl to a pH of 7.4. This solution was then diluted with saline to an edaravone concentration of 0.3 mg/mL. Immediately after the concussive brain injury, mice received a single 3.0-mg/kg intravenous (i.v.) injection of either edaravone or vehicle.
BWB70C (Sigma-Aldrich, Louis, MO) was dissolved in dimethyl sulfoxide (DMSO). Immediately and 24 h after concussive brain injury, mice received 2 consecutive i.v. injections of either BWB70C (0.1 and 3 mg/kg) or vehicle. Mice received a single intraperitoneal (i.p.) injection of apocynin (50 mg/kg; Sigma-Aldrich) dissolved in DMSO or vehicle 30 min before concussion.
For the dose-response study, mice were divided randomly into five groups and were subjected to concussion following the procedures described earlier. Mice were then administered edaravone at 0.1, 1.0, or 3.0 mg/kg. For the therapeutic time-window study, mice were divided randomly into six groups, subjected to concussion, underwent the same procedure of concussion as described, and administered edaravone at 1, 3, or 6 h after concussion.
Neurological assessment
The neurological status of each mouse was assessed with an extensive set of tests 1 and 24 h after the concussive brain injury.24 The tests evaluated the hind-leg flexion reflex (when raised by the tail), righting reflex (ability to fall on all four legs after a short drop), secreting signs (around the mouth and eye), balance beam task, beam walking coordination task, and exploration and locomotor activity tests previously described by Royle and colleagues and Shapira and colleagues.26,27 Importantly, none of the injured mice showed any abnormalities in these neurological tests. Mice were allowed to recover from the concussion procedure for 2 days before performing the forced swim test (FST) to avoid influence of neurological assessment.
Magnetic resonance imaging
Animals were examined with a 9.4-T scanner (Varian NMR Systems 400WB; Agilent Technologies, Santa Clara, CA) with an actively shielded gradient coil (ID=25 cm). A custom-built birdcage resonator (ID=55 mm) was used as a radiofrequency coil. Magnetic resonance imaging (MRI; diffusion-weighted imaging [DWI], T1, and T2) was conducted during the 4 h after the CBI and was repeated at 24 and 72 h. A DWI was acquired for each animal using a spin echo/echo planar imaging sequence with the following parameters: field of view (FOV)=2×2 cm; matrix=128×128; slice thickness (THK)=1 mm; number of slices (NS)=9; repetition time (TR)=2500 ms; echo time (TE)=38 ms; number of averages (NA)=1; b-values=1.94, 64.38, 251.71, 563.92, and 1001.01 s/mm2; Δ=5 ms; and Δ=25 ms. T1-weighted images were acquired using a multiple spin echo sequence (FOV=2×2 cm; matrix=128×128; THK=1 mm; NS=9; NA=1; TR=290 ms; and TE=14 ms); T2-weighted MRIs were acquired using a multiple spin echo sequence (FOV=2×2 cm; matrix=128×128; THK=1 mm; NS=9; NA=1; TR=3000 ms; and TE=60 ms).
Forced swim test
Using previously described procedures, we conducted the FST 3 days after the CBI to assess depressive-like behavior.23,25 Briefly, each mouse was placed in a glass cylinder (height, 24 cm; diameter, 13 cm) containing 14 cm of water maintained at 22–23°C. The mouse remained in the cylinder for 6 min, and the duration of immobility was recorded during the last 4 min of the 6-min testing period. A mouse was considered immobile when it stopped swimming and floated in the water motionless, except for movements necessary to keep its head above water. Two observers blinded to the treatment condition scored the immobility times. The observers' scores agreed in over 94% of the behavioral tests.
Tissue preparation and immunohistochemistry
Mice were killed 24 h postconcussion or 3 h after the last BWB70C injection. Brains were removed after transcardial perfusion (TCP) with 0.9% saline and 4% paraformaldehyde (PFA). Brains were postfixed overnight in 4% PFA and then cryoprotected by immersion in 20% sucrose for 24 h. Coronal sections were cut with a 30-μm thickness for hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC).
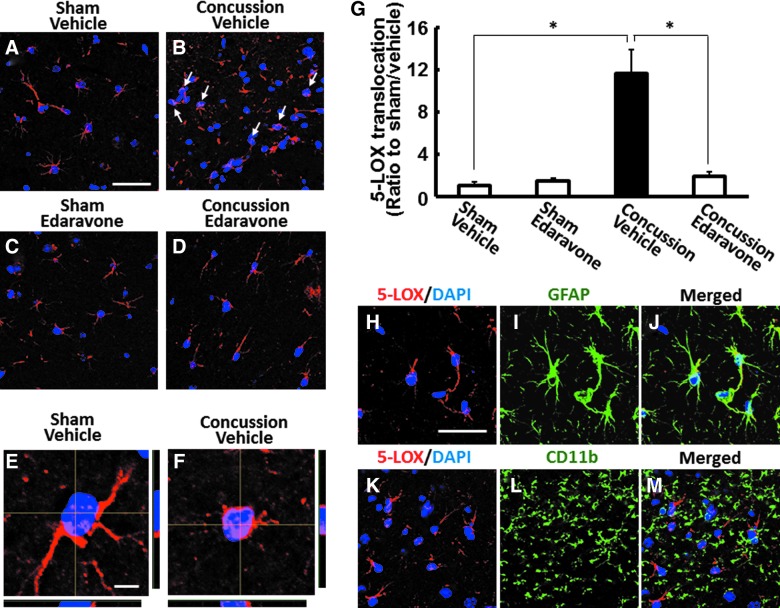
IHC was performed on corresponding serial sections. In brief, sections were blocked for 30 min in 3% bovine serum albumin and 0.1% Triton X-100 in 10 mM of phosphate-buffered saline. For single labeling of 5-LOX, sections were incubated with goat anti-5-LOX antibody (Ab; dilution, 1:200; Santa Cruz Biotechnology, Dallas, TX) overnight at 4°C followed by the addition of Alexa Fluor 594–conjugated donkey anti-goat immunoglobulin G (IgG; dilution, 1:500; Invitrogen, Carlsbad, CA) at room temperature for 2 h. For 5-LOX/CD11b or 5-LOX/GFAP double-labeling immunofluorescence (IF), sections were incubated with goat anti-5-LOX Ab and rat anti-CD11b Ab (1:100; Bio-Rad Laboratories, Hercules, CA), or rabbit anti-GFAP Ab (1:500; Santa Cruz Biotechnology) overnight at 4°C, which was followed by the addition of Alexa Fluor 594–conjugated donkey anti-goat IgG (dilution, 1:500; Invitrogen) and Alexa Fluor 488–conjugated donkey anti-rat IgG, or Alexa Fluor 488–conjugated donkey anti-rabbit IgG (dilution, 1:500; Invitrogen) at room temperature for 2 h. Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies, Carlsbad, CA), and fluorescent signals from double-labeled sections were analyzed using a confocal microscope (FV-100D; Olympus, Tokyo, Japan) at 400× magnification. Nuclei double labeled with DAPI and 5-LOX were counted in representative micrographs of each hippocampal region to quantify the fluorescent signal.
Western blotting
Western blotting was used to detect nitrotyrosine or 4-hydroxynonenal (4-HNE) levels in mouse brains subjected to either concussion or sham operation. Briefly, the cortex and hippocampus were rapidly dissected on an ice-chilled stage 1 day postconcussion and were then lysed using M-PER mammalian protein extraction reagent (Thermo Fisher Scientific, Waltham, MA), supplemented with protease inhibitor cocktail (Thermo Fisher Scientific). Samples were centrifuged for 10 min (15,000 rpm at 4°C), and the supernatants were collected. Protein concentrations were determined with a bicinchoninic acid assay (Thermo Fisher Scientific).29 An aliquot from each protein sample was separated on a sodium dodecyl sulfate polyacrylamide gel electrophoresis precast gel and then transferred onto nitrocellulose membranes. Membranes were incubated with primary anti-nitrotyrosine Ab (1:2000; Millipore, Billerica, MA), anti-4-HNE Ab (1:5000; R&D Systems, Minneapolis, MN), or anti-β-actin Ab (1:1000; Santa Cruz Biotechnology) at 4°C overnight. The following day, membranes were incubated with horseradish-peroxidase–linked anti-mouse IgG (1:10,000; GE Healthcare, Little Chalfont, UK) for 1 h at room temperature and developed by enhanced chemiluminescence (GE Healthcare). Quantitation was performed with a densitometric analysis using ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical analysis
Data are expressed as the mean±standard error of the mean, and the statistical analyses were performed using Statview (SAS Institute Inc., Cary, NC). Quantification of IHC and depressive-like behaviors were analyzed with a one- or two-way analysis of variance (ANOVA), followed by a post-hoc Fischer's protected least significant difference multiple comparison test.
Results
Model of concussive traumatic brain injury
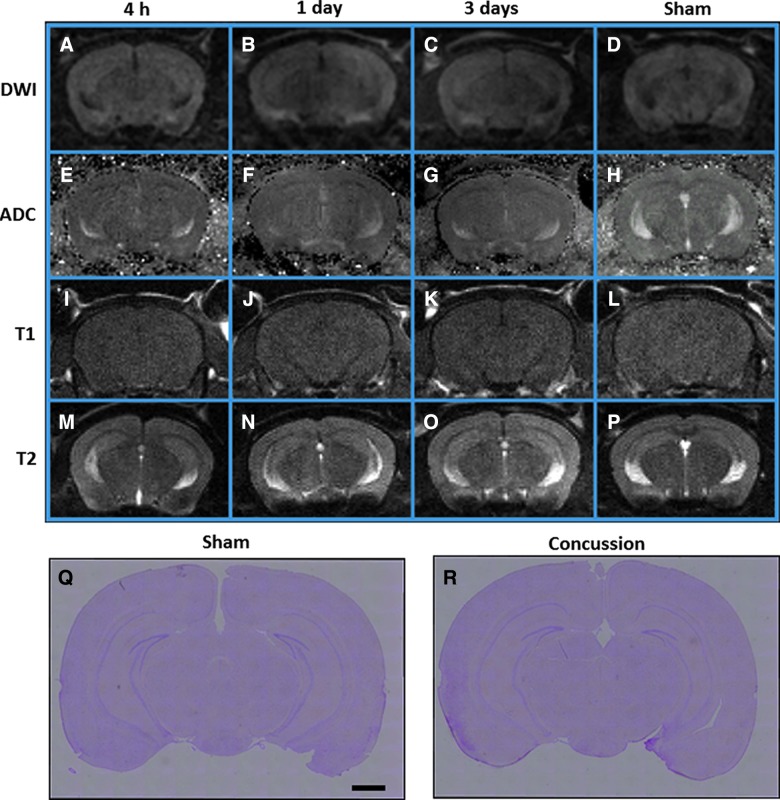
The MRI images obtained 4 h, 1 day, and 3 days after concussion appeared normal. No significant high-signal lesions indicative of cytotoxic or brain edema were evident on DWI or T1- and T2-weighted images, respectively (Fig. 1A–P). In both vehicle-treated concussed and sham-operated mice, no remarkable tissue damage was observed in the cortex and hippocampus with H&E staining 3 days after concussion (Fig. 1Q,R). These results indicate that the hippocampus and cortex did not suffer sufficient injury using the current concussion paradigm.
FIG. 1.
Assessment of brain damage in mice after traumatic brain injury. (A–P) Representative magnetic resonance images (DWI [A–D], ADC [E–H], T1 [I–L], and T2 [M and N] scan) were taken 4 h, 1 day, and 3 days after injury. The images do not indicate any gross pathology in brain tissue of mice subjected to concussion (A–C, E–G, I–K, and M–O) or sham operation (D, H, L, and P) at any time point. Control images obtained 1 day after sham operation. (Q and R) Hematoxylin and eosin staining performed on brains of mice subjected to concussion (R) and sham-operated mice (Q) did not show significant histopathological changes 1 day after concussion. Scale bar=1 mm. DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient. Color image is available online at www.liebertpub.com/neu
Depressive-like behavior prevented by treatment with edaravone after concussion
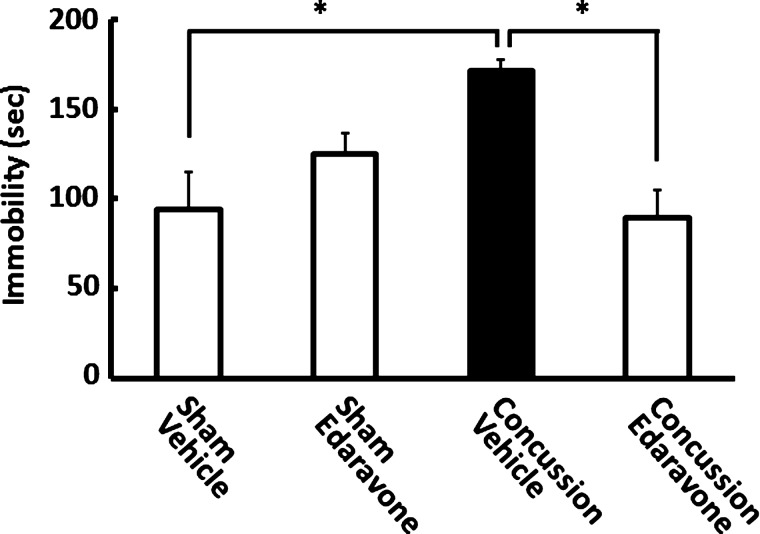
We conducted the FST 3 days after concussion to determine whether concussion increased the immobility time of concussed mice (Fig. 2). When compared to controls, the injured mice showed a significant increase in immobility time (p<0.01), indicating that depressive-like behavior was present after concussion. Importantly, the current concussion paradigm did not produce any observable motor deficits (see Methods).
FIG. 2.
Concussion-induced depressive-like behavior was prevented by edaravone treatment. Mice were injected with edaravone (3.0 mg/kg) or vehicle immediately after concussion. The forced swim test was performed 3 days postconcussion, and edaravone treatment prevented higher immobility time in vehicle-treated concussed mice. Data are expressed as the mean±standard error of the mean (sham operation plus vehicle-treated group, n=4; sham operation plus edaravone, n=4; concussion plus vehicle-treated group, n=5; concussion plus edaravone-treated group, n=6). *p<0.01, compared to the saline-treated concussed mice group (black bar).
We then examined whether edaravone could prevent depressive-like behavior by i.v. injecting mice with a single 3.0-mg/kg dose of edaravone immediately after concussion. The FST was then performed 3 days after concussion. As shown in Figure 2, the immobility time of concussed mice that received edaravone was significantly less than that for concussed mice that were vehicle treated (p<0.01). Further, the immobility time of edaravone-treated concussed mice was comparable to those of sham-operated mice treated with or without edaravone. These findings suggest that edaravone treatment prevented the concussion-induced depressive-like behavior. Two-way ANOVA revealed a main effect of concussion (F(1, 15)=4.922; p<0.05) and concussion×treatment with edaravone (F(1, 15)=10.683; p<0.01), but not with edaravone treatment alone (F(1, 15=0.849; p=0.371).
Effects of edaravone treatment on concussion-induced nitrotyrosine and 4-hydroxynonenal formation
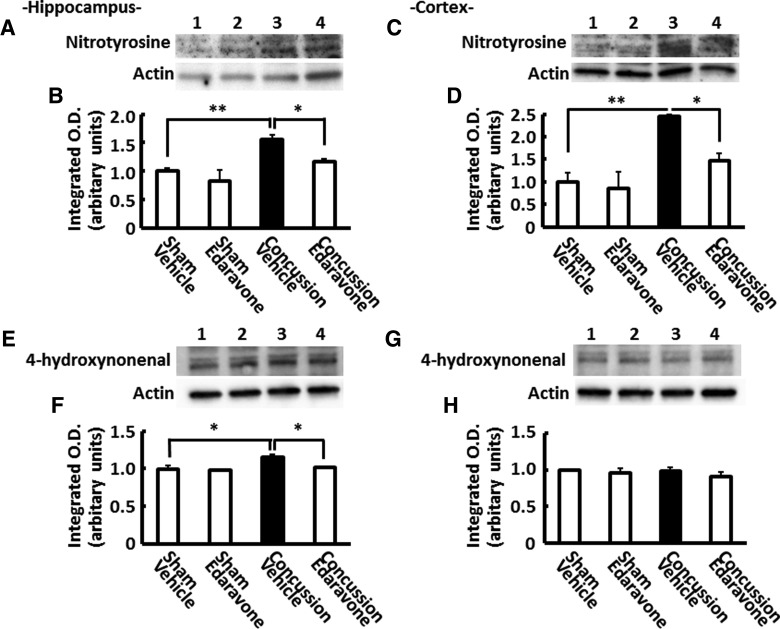
As shown in Figure 3A–D, we observed an increase in hippocampal and cortical levels of nitrotyrosine-modified protein bands between 75 and 100 kDa 1 day after concussion. These increased nitrotyrosine levels were significantly higher than those of sham-operated mice. Moreover, the ratio of the integrated optical density of nitrotyrosine to that of β-actin showed that nitrotyrosine levels were 1.56- and 2.44-fold higher in the hippocampus and cortex of concussed mice than those of sham-operated mice treated with vehicle, respectively (p<0.01; Fig. 3A–D). Considering that the radical scavenger, edaravone, suppressed peroxynitrite-induced oxidative neurotoxicity,30 we investigated whether edaravone affected the concussion-induced increases of nitrotyrosine in the hippocampus and cortex. As expected, edaravone significantly suppressed the increased nitrotyrosine levels (p<0.05; Fig. 3).
FIG. 3.
Nitrotyrosine and 4-hydroxynonenal (4-HNE) production in concussed mouse brains. Representative western blottings of hippocampal (A and B; E and F) and cortical (C and D; G and H) extracts with anti-nitrotyrosine antibody (A–D) and anti-4-HNE antibody (E–H). Lane 1: sham plus vehicle-treated group; lane 2: sham plus edaravone-treated group; lane 3: concussion plus vehicle-treated group; lane 4: concussion plus edaravone-treated group. Quantification of nitrotyrosine staining in the hippocampus (B and F) and cortex (D and H). Data are expressed as the mean±standard error of the mean (sham plus vehicle-treated group, n=4; sham plus edaravone, n=4; concussion plus vehicle-treated group, n=4; concussion plus edaravone-treated group, n=4). *p<0.05; **p<0.01, compared to the vehicle-treated concussed mice group (black bar).
The peroxynitrite-derived free radicals can initiate lipid peroxidation (LPO). Therefore, we also evaluated 4-HNE, a marker for LPO, in the hippocampus and cortex 1 day after impact. As is indicated in Figure 3E–H, 1 day after concussion, we observed a significant increase only in hippocampal levels of 4-HNE-modified protein bands between 50 and 100 kDa. The increased 4-HNE levels were small, but were significantly higher in concussed mice than sham-operated mice. The ratio of the integrated optical density of 4-HNE to that of β-actin showed that 4-HNE levels in the hippocampus were 1.15-fold higher in concussed mice than sham-operated mice treated with vehicle (p<0.05). These increased 4-HNE levels were significantly suppressed when mice received edaravone i.v. immediately after concussion (Fig. 3E–F).
Nicotinamide adenine dinucleotide phosphate oxidase participates in concussion-induced 4-hydroxynonenal formation
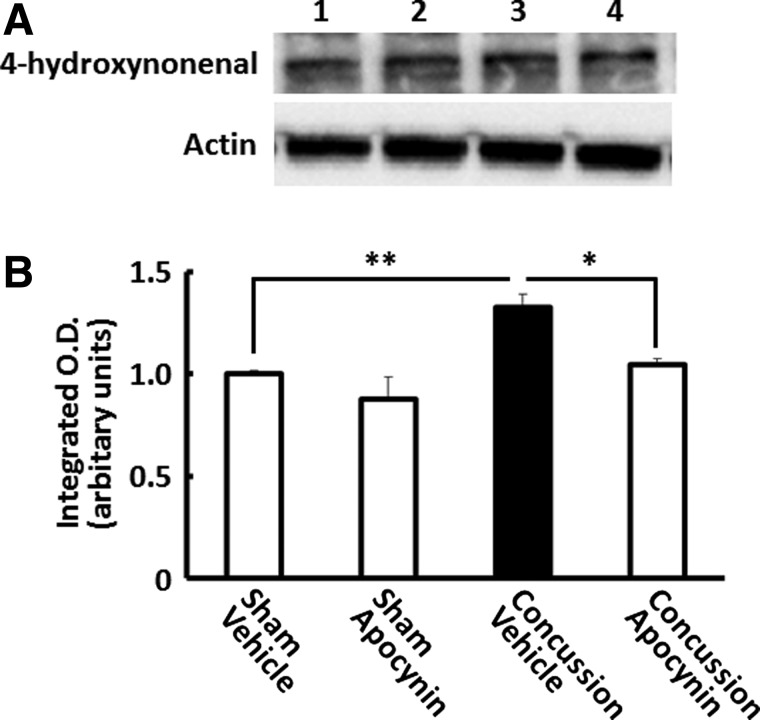
Next, we examined whether NADPH oxidase, which can produce superoxide, mediates peroxynitrite generation after concussion. Mice received a single 50-mg/kg i.p. injection of apocynin, an NADPH oxidase inhibitor, 30 min before concussion. A western blotting analysis with anti-4-HNE Ab showed that 4-HNE levels increased significantly in the hippocampus 3 h after concussions, which were almost completely suppressed by apocynin (p<0.05; Fig. 4).
FIG. 4.
Effect of nicotinamide adenine dinucleotide phosphate oxidase inhibitor on concussion-induced 4-hydroxynonenal (4-HNE) formation in the hippocampus. Representative Western blottings of hippocampal (A) extracts prepared from mice 3 h after concussion, with anti-4-HNE antibody. Lane 1: sham plus vehicle-treated group; lane 2: sham plus apocynin-treated group; lane 3: concussion plus vehicle-treated group; lane 4: concussion plus apocynin-treated group. Quantification of 4-HNE staining in the hippocampus (B). Data are expressed as the mean±standard error of the mean (sham plus vehicle-treated group, n=3; sham plus apocynin, n=3; concussion plus vehicle-treated group, n=3; concussion plus apocynin-treated group, n=3). *p<0.05; **p<0.01, compared to the vehicle-treated concussed mice group (black bar).
Edaravone prevented concussion-induced hippocampal 5-lipoxygenase translocation
OS induces 5-LOX translocation from the cytosol to the nuclear envelope, where it interacts with cofactors and becomes enzymatically active.23 In the current study, we determined whether our TBI mouse model exhibited 5-LOX translocation to the nuclear envelope. One day postinjury, 5-LOX protein expression, as detected by immunostaining with anti-5-LOX Ab, was observed only in the hippocampus (Fig. 5), but not the cortex (data not shown), of mice subjected to either concussion or sham operation. In addition, concussion caused 5-LOX translocation from the cytosol to the nuclear envelope of hippocampal cells 1 day postconcussion, whereas 5-LOX localization remained predominantly in the cytosol of sham-operated mice (p<0.01; Fig. 5A–G). Because 5-LOX inhibition can have antidepressive effects in rodent models,22,31 we evaluated whether edaravone affected concussion-induced 5-LOX translocation to the nuclear envelope. As shown in Figure 5A–G, 5-LOX translocation was significantly suppressed in mice that received a single 3.0-mg/kg i.v. injection of edaravone immediately after concussion (p<0.01). To further determine the cellular localization of 5-LOX protein, we performed double-IF staining to detect the colocalization of 5-LOX with the astrocytic marker, GFAP, or the microglial marker, CD11b. In the hippocampus of concussed mice, we found that 5-LOX was localized in GFAP-positive astrocytes, but not CD11b-positive microglia (Fig. 5H–M). Thus, these results suggest that hippocampal astrocytes are the primary cells responsible for 5-LOX activation after concussion.
FIG. 5.
Concussion-induced 5-lipoxygenase (5-LOX) translocation in hippocampal astrocytes was prevented by edaravone. Representative images demonstrating immunoreactivity for 5-LOX (red) and DAPI nuclear staining (blue) in the hippocampus of mice 24 h after sham operation (A and C) or concussion (B and D). The location of 5-LOX can be visualized in the cytosol in sham-operated mice treated with vehicle (A) or edaravone (C). Mice treated with vehicle immediately after concussion showed an increase in hippocampal 5-LOX translocation to the nuclear envelope (B, arrow), which was suppressed by edaravone treatment (D). Scale bar=25 μm. Z-stack confocal laser scanning micrographs of 5-LOX (red)-positive cells in the hippocampus of mice subjected to sham operation (E) or concussion (F). Nuclei were stained with DAPI (blue). Scale bar=5 μm. (G) Quantification of 5-LOX translocation to the nuclear envelope. Data are expressed as mean±standard error of the mean (sham injury plus vehicle-treated group, n=4; sham injury plus edaravone, n=4; concussive TBI plus vehicle-treated group, n=5; concussive traumatic brain injury plus edaravone-treated group, n=4). *p<0.01, compared to the vehicle-treated concussed mice group (black bar). Colocalization of 5-LOX (red) with GFAP (green) or CD11b (green) in mice 24 h postinjury (H–M). Immunoreactivity of 5-LOX translocated to the nuclear (blue) envelope was detected in GFAP-positive astrocytes (H–J), but not in CD11b-positive microglia (K–M). Scale bar=25 μm. DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein. Color image is available online at www.liebertpub.com/neu
5-lipoxygenase inhibition prevented concussion-induced depressive-like behavior
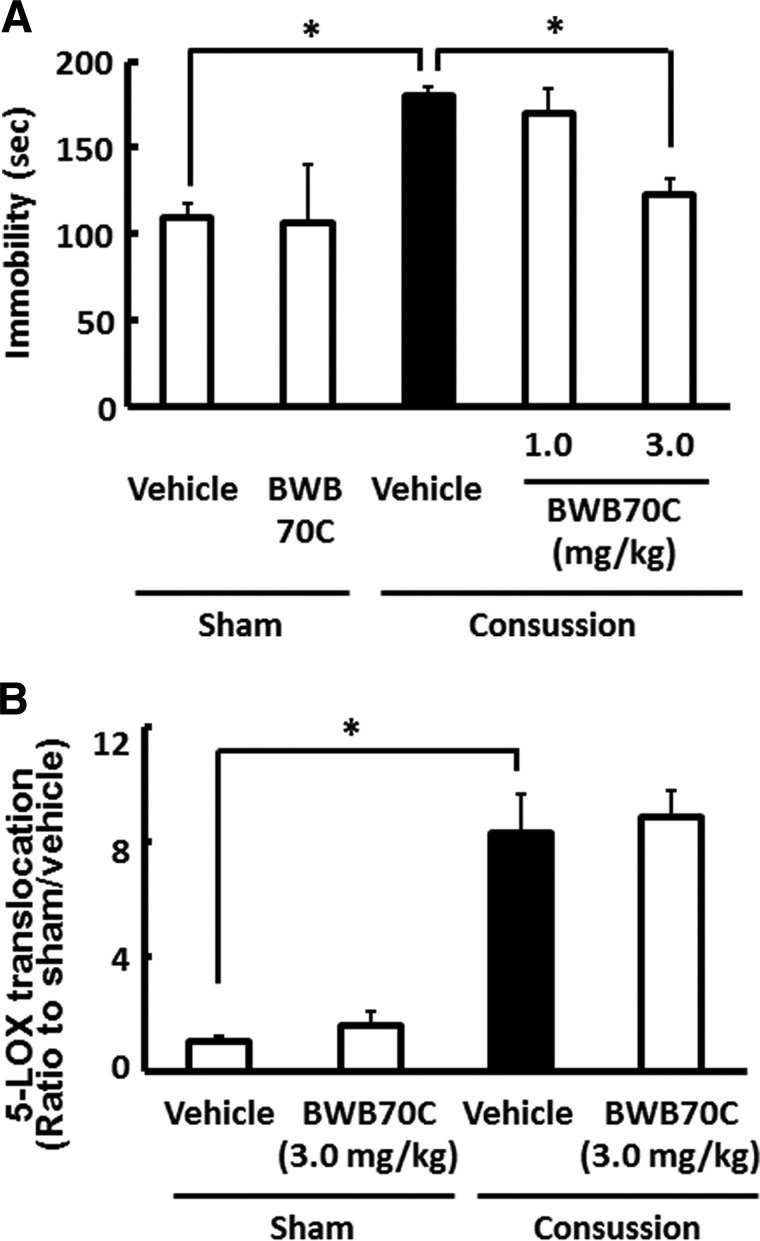
We evaluated whether 5-LOX activation in the hippocampal astrocytes of concussed mice contributed to the appearance of concussion-induced depressive-like behavior. Mice were i.v. injected with 1.0 and 3.0 mg/kg of BWB70C, a specific 5-LOX inhibitor, immediately and 24 h after concussion, and then performed the FST 3 days postconcussion. As shown in Figure 6A, concussed mice treated with BWB70C showed a dose-dependent reduction in immobility time, when compared to vehicle-treated concussed mice (p<0.01). Two-way ANOVA revealed a main effect of treatment with BWB70C (F(1, 18)=4.873; p<0.05), but not concussion×BWB70C treatment (F(1, 18)=3.734; p=0.069). Because BWB70C treatment showed protective effects against concussion-induced depressive-like behavior, we performed additional experiments to examine whether BWB70C interfered with 5-LOX translocation in hippocampal astrocytes of concussed mouse brains. Concussion-induced translocation of 5-LOX, however, was not affected in mice treated with 3.0 mg/kg of BWB70C immediately and 24 h after concussion (p=0.06; Fig. 6B). 5-LOX consists of a C-terminal catalytic domain that contains iron,32 and an N-terminal C-like β-barrel domain with regulatory functions that binds two Ca2+ ions,33 and targets 5-LOX to the nuclear membrane.34 BWB70C is an iron-chelating redox-type inhibitor of 5-LOX.35 It was recently reported that zileuton, another 5-LOX inhibitor with the same mechanism of action as BWB70C, does not suppress 5-LOX translocation to the nuclear envelope after oxygen-glucose deprivation.23,36 This is considered to provide reasonable explanation on the reason why BWB70C could not inhibit the 5-LOX translocation in our experiments. Therefore, these results suggest that concussion-induced 5-LOX activation in hippocampal astrocytes is associated with the appearance of depressive-like behavior.
FIG. 6.
Effect of 5-lipoxygenase (5-LOX) inhibitor on depressive-like behavior and 5-LOX translocation after concussion. Mice were injected with either the 5-LOX inhibitor, BWB70C (1.0 or 3.0 mg/kg), or vehicle immediately and 1 day after concussion. (A) The forced swim test was performed 3 days postconcussion, and treatment with BWB70C prevented the higher immobility time in vehicle-treated concussed mice. Data are expressed as the mean±standard error of the mean (SEM; sham plus vehicle-treated group, n=4; sham plus BWB70C, n=3; concussion plus vehicle-treated group, n=6; concussion plus BWB70C-treated group, n=5). *p<0.01, compared to the vehicle-treated concussed mice group (black bar). (B) Quantification of 5-LOX translocation to the nuclear envelope 3 h after the last injection of BWB70C (3.0 mg/kg) or vehicle. Mice treated with BWB70C and vehicle showed no significant difference in the increased 5-LOX translocation in the hippocampal astrocytes 24 h after concussion. Data are expressed as the mean±SEM (sham plus vehicle-treated group, n=4; sham plus BWB70C group, n=5; concussion plus vehicle-treated group, n=5; concussion plus BWB70C-treated group, n=5). *p<0.01, compared to the vehicle-treated concussed mice group (black bar).
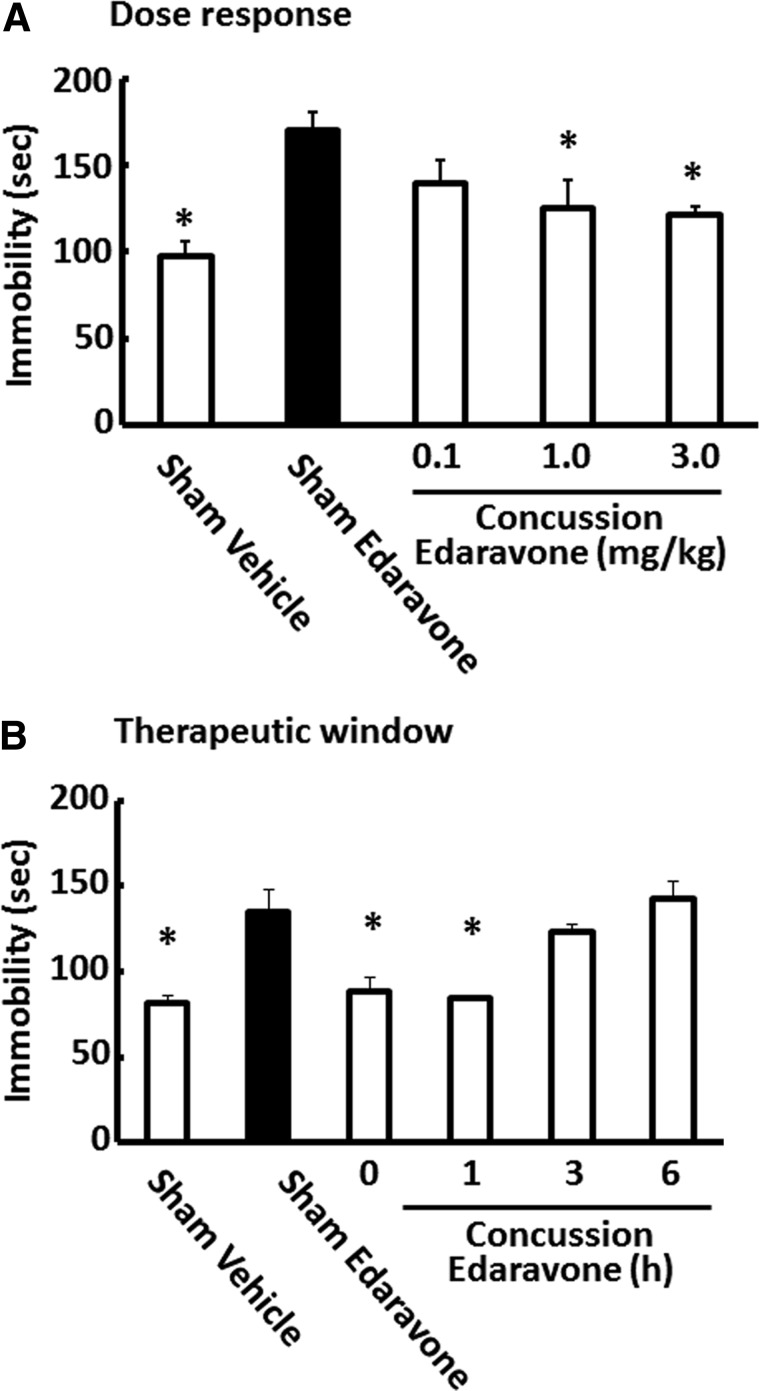
Dose response and therapeutic window for edaravone administration
To determine whether edaravone showed a dose-response relationship, we i.v. administered edaravone immediately after concussion at doses ranging from 0.1 to 3.0 mg/kg. As shown in Figure 7A, increased immobility durations in the FST were inversely related to the edaravone dose and was suppressed significantly in mice that received higher doses (1.0 or 3.0 mg/kg; F(5, 16)=9.11; p<0.001). However, there were no significant differences in the immobility duration in the FST between vehicle-treated concussed mice and 0.1-mg/kg edaravone-treated concussed mice.
FIG. 7.
Dose response and therapeutic window profiles for edaravone administration in depressive-like behavior after concussion. Dose-response examination showing the ability of immediate edaravone administration to suppress depressive-like behavior in mice 3 days after concussion (A). Data are expressed as the mean±standard error of the mean (SEM; sham plus vehicle-treated group, n=3; sham plus edaravone, n=4; concussion plus vehicle-treated group, n=4; concussion plus edaravone-treated group, n=4). *p<0.01, compared to the vehicle-treated concussed mice group (black bar). High dose of edaravone (1.0 and 3.0 mg/kg), but not saline or low dose of edaravone (0.1 mg/kg), suppressed the increased durations of immobility in the forced swim test. Therapeutic window profiles for administration and suppression of depressive-like behavior in mice 3 days after concussion (B). We found that immediate or 1-h postconcussion edaravone administration, but not vehicle or later edaravone administration (3 or 6 h), suppressed the increased immobility duration in the forced swim test. Data are expressed as the mean±SEM (sham plus vehicle-treated group, n=4; sham plus edaravone, n=4; concussion plus vehicle-treated group, n=4; concussion plus edaravone-treated group, n=4). *p<0.01, compared to the vehicle-treated concussed mice group (black bar).
We next examined whether there was a therapeutic window at which edaravone could attenuate concussion-induced depressive-like behavior. Briefly, mice were treated with edaravone (3.0 mg/kg) either immediately or 1, 3, or 6 h postconcussion, and their immobility in the FST were measured 3 days after impact. Figure 7B indicates that mice treated with edaravone immediately or 1 h after concussion showed significant reductions in their immobility times, when compared to vehicle-treated concussed mice (F(5, 18)=12.082; p<0.001). There was no significant difference in the immobility duration between concussed mice treated with vehicle or edaravone at 3 or 6 h after concussion.
Discussion
Depression is a significant consequence of TBI; however, few pre-clinical studies have investigated the pharmacological management of TBI-induced depression.2 The present study demonstrated the following: 1) Concussion induced by a weight drop resulted in depressive-like behavior in mice without histopathological brain alterations; 2) edaravone protected mice from concussion-induced depressive-like behavior; 3) edaravone suppressed OS by NADPH oxidase and 5-LOX translocation in the hippocampal astrocytes of concussed mice; and 4) pharmacological inhibition of 5-LOX prevented depressive-like behavior in mice subjected to concussion. These findings suggest that edaravone has neuroprotective effects against depressive-like behavior after concussion.
An excess of free radical production resulting from TBI can cause oxidation and nitration of cellular components, including proteins and DNA, which play a role in the pathogenesis of post-traumatic secondary injury, such as neuronal death and axonal injury.19,20 Free radicals recently have been recognized to a play role in depression pathogenesis in patients with major depression and animal models of depression.37 However, little is known about the contributions of OS to postconcussive depression. In this study, we observed depressive-like behavior and oxidative damage in the hippocampus of concussed mice that could be suppressed by administering edaravone immediately after concussion. Some antidepressants can induce hippocampal neuronal plasticity.38 Further, it has been demonstrated that stress and a glucocorticoid-induced reactive oxygen species (ROS) burst can cause structural changes in the limbic system.14 Thus, we suggest the protective effect of edaravone against concussion-induced depression might be attributed to a free radical scavenger.
The brain can produce ROS through several sources, including NADPH oxidase, xanthine oxidase, and P450 enzymes, all of which contribute to physiological and pathological functions.39 In the case of TBI, mitochondria are considered the major source of ROS production,40 but NADPH oxidase is also likely to play the role.41 In this study, we observed that NADPH oxidase was a source of ROS production in response to the concussion. NADPH oxidase activity can peak early at 1 h after TBI, followed by a secondary peak that appears between 24 and 96 h postinjury.42 Additionally, Seo and colleagues reported that hippocampal NADPH oxidase mediated depressive-like behavior in mice through chronic stress.44 Thus, hippocampal ROS production through NADPH oxidase might be the first step toward concussion-induced depression.
To date, we and other laboratories have evaluated the effects of edaravone on TBI-induced damage, including axonal injury20 and neuronal death;17 however, there are no reports that demonstrate edaravone prevents depression after TBI. Hippocampal 5-LOX is considered to play an important role in various pathophysiological processes in the central nervous system (CNS).45,46 Interestingly, pharmacological inhibition or genetic disruption of 5-LOX produces antidepressant-like effects in mice.22,31 On the other hand, OS can induce 5-LOX activation.23 As indirect evidence, OS can increase production of 5-oxo-6,8,11,14-eicosatetraenoic acid, which is synthesized from the 5-LOX product, 5-hydroxyeicosatetraenoic acid, in inflammatory47 and endothelial cells.48 Further, Li and colleagues reported that, in oxygen-glucose deprivation/recovery-induced ischemic-like injury, 5-LOX activation is mediated by OS through the p38 mitogen-activated protein kinase pathway in PC12 cells.23 In this study, astrocytic 5-LOX translocation to the nuclear envelope was found in the hippocampus 1 day postconcussion and was significantly suppressed by edaravone. This suggests that inhibiting 5-LOX translocation with a scavenging ROS during the early phases of concussion might help to prevent concussion-induced depression. This idea is supported by the present findings in that treatment with the 5-LOX inhibitor, BWB70C, suppressed concussion-induced depressive-like behavior. In the CNS, 5-LOX is expressed in various regions, but most prominently in the hippocampus and cerebellum.49 Further, 5-LOX expression levels can be lower in the younger (2 months), rather than older, rat brain (24 months).50 In this study, 5-LOX immunoreactivity was detected in the hippocampus, but not the cortex, regardless of concussion. This might be owing to the fact that expression levels of 5-LOX in the cortex were extremely low and we could not detect it. However, because clinical and experimental evidence suggests the the cortex is pathophysiologically related to depression,51 we could not rule out the possibility that 5-LOX expressed in the cortex contributes to concussion-induced depressive-like behavior. Further, it will be necessary, in future studies, to clarify the mechanism through which astrocytic 5-LOX translocation mediates induction of depressive-like behaviors in more detail.
TBI is associated with an increased risk of developing neuropsychiatric disorders52 and produces conditions or symptoms including depression, anxiety, and personality changes.53 Clinical pictures often reveal a strong interplay among symptoms,54 which complicates investigation of the pathophysiology and treatment of post-TBI depression. In this study, we used a minimal TBI method established by Zohar and collegues24 that was modified to produce a mouse model of CBI. Zohar and colleagues reported that a minimal TBI in mice resulted in an early emergence of depressive-like behaviors.55 Consistent with that report, we also found that mice subjected to concussion exhibited depressive-like behavior 3 days postconcussion, without evidence of anxiety (data not shown) or morphological brain damage. However, it is possible that concussion-induced OS causes neuronal cell death. To examine this possibility, we assessed cell death using Fluoro-Jade B (FJB) staining; however, we could not detect FJB-positive cells 24 h after concussion (data not shown). It was implicated that concussion-induced depressive-like behavior was not mediated by acute neuronal cell death during the period when concussion caused OS. Thus, we consider that the established concussed mouse model is an adequate tool to explore novel therapeutic approaches to post-TBI injury. On the other hand, edaravone can be neuroprotective in animal models of cerebral ischemia and is currently used in Japan to treat patients with stroke.56 Moreover, edaravone has no known serious side effects.57,58 Common clinical experience suggests that individuals with TBI may be more susceptible to the side effects of many psychiatric medications.52 Additionally, the majority of patients with post-traumatic depression do not respond to typical antidepressants.59 In this study, edaravone protected mice from depressive-like behavior after concussion with a therapeutic window, indicating that edaravone may be a promising treatment in the case of concussion with depression.
In summary, administration of edaravone immediately after concussion suppressed OS and 5-LOX translocation to the nuclear envelope, and these mechanisms prevented mice from developing concussion-induced depressive-like behavior. Further, these findings suggest that edaravone treatment may be effective in preventing depression after concussion.
Acknowledgments
This work was supported, in part, by Grants-in-Aid for Scientific Research ([C], No. 24592133) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and The General Insurance Association of Japan. The authors thank Mitsubishi Tanabe Pharma for providing the edaravone used in this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Whitnall L., McMillan T.M., Murray G.D., and Teasdale G.M. (2006). Disability in young people and adults after head injury: 5–7 year follow up of a prospective cohort study. J. Neurol. Neurosurg. Psychiatry 77, 640–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandey D.K., Yadav S.K., Mahesh R., and Rajkumar R. (2009). Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: a model of comorbid depression and anxiety? Behav. Brain Res. 205, 436–442 [DOI] [PubMed] [Google Scholar]
- 3.Silver J.M., McAllister T.W., and Arciniegas D.B. (2009). Depression and cognitive complaints following mild traumatic brain injury. Am. J. Psychiatry. 166, 653–661 [DOI] [PubMed] [Google Scholar]
- 4.Kibby M.Y., and Long C.J. (1996). Minor head injury: attempts at clarifying the confusion. Brain Inj. 10, 159–186 [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Nievas B.G., García-Bueno B., Caso J.R., Menchén L., and Leza J.C. (2007). Corticosterone as a marker of susceptibility to oxidative/nitrosative cerebral damage after stress exposure in rats. Psychoneuroendocrinology 32, 703–711 [DOI] [PubMed] [Google Scholar]
- 6.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., and Freeman B.A. (1990). Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 87, 1620–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avila M.A., Sell S.L., Kadoi Y., Prough D.S., Hellmich H.L., Velasco M., and Dewitt D.S. (2008). L-Arginine decreases fluid-percussion injury-induced neuronal nitrotyrosine immunoreactivity in rats. J. Cereb. Blood Flow Metab. 28, 1733–1741 [DOI] [PubMed] [Google Scholar]
- 8.Wada K., Chatzipanteli K., Kraydieh S., Busto R., and Dietrich W.D. (1998). Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery 43, 1427–1436 [DOI] [PubMed] [Google Scholar]
- 9.Cherian L., Goodman J.C., and Robertson C.S. (2000). Brain nitric oxide changes after controlled cortical impact injury in rats. J. Neurophysiol. 83, 2171–2178 [DOI] [PubMed] [Google Scholar]
- 10.Ahn M.J., Sherwood E.R., Prough D.S., Lin C.Y., and DeWitt D.S. (2004). The effects of traumatic brain injury on cerebral blood flow and brain tissue nitric oxide levels and cytokine expression. J. Neurotrauma 21, 1431–1442 [DOI] [PubMed] [Google Scholar]
- 11.Whalen M.J., Clark R.S., Dixon C.E., Robichaud P., Marion D.W., Vagni V., Graham S.H., Virag L., Hasko G., Stachlewitz R., Szabo C., and Kochanek P.M. (1999). Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab. 19, 835–842 [DOI] [PubMed] [Google Scholar]
- 12.Besson V.C., Croci N., Boulu R.G., Plotkine M., and Marchand-Verrecchia C. (2003). Deleterious poly(ADP-ribose)polymerase-1 pathway activation in traumatic brain injury in rat. Brain Res. 989, 58–66 [DOI] [PubMed] [Google Scholar]
- 13.Forster C., Clark H.B., Ross M.E., and Iadecola C. (1999). Inducible nitric oxide synthase expression in human cerebral infarcts. Acta Neuropathol. 97, 215–220 [DOI] [PubMed] [Google Scholar]
- 14.Allam F., Dao A.T., Chugh G., Bohat R., Jafri F., Patki G., Mowrey C., Asghar M., Alkadhi K.A., and Salim S. (2013). Grape powder supplementation prevents oxidative stress-induced anxiety-like behavior, memory impairment, and high blood pressure in rats. J. Nutr. 143, 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L.F., Yang J., Ma S.P., and Qu R. (2013). Magnolol treatment reversed the glial pathology in an unpredictable chronic mild stress-induced rat model of depression. Eur. J. Pharmacol. 711, 42–49 [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q.G., Zhu L.J., Chen C., Wu H.Y., Luo C.X., Chang L., and Zhu D.Y. (2011). Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. J. Neurosci. 31, 7579–7590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh T., Satou T., Nishida S., Tsubaki M., Imano M., Hashimoto. S., and Ito H. (2010). Edaravone protects against apoptotic neuronal cell death and improves cerebral function after traumatic brain injury in rats. Neurochem, Res. 35, 348–355 [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T., Tahara M., and Todo S. (2008). The novel antioxidant edaravone: from bench to bedside. Cardiovasc, Ther. 26, 101–114 [DOI] [PubMed] [Google Scholar]
- 19.Kamida T., Abe E., Abe T., Ooba H., Fujiki M., and Kobayashi H. (2009). Edaravone, a free radical scavenger, retards the development of amygdala kindling in rats. Neurosci, Lett. 461, 298–301 [DOI] [PubMed] [Google Scholar]
- 20.Ohta M., Higashi Y., Yawata T., Kitahara M., Nobumoto A., Ishida E., Tsuda M., Fujimoto Y., and Shimizu K. (2013). Attenuation of axonal injury and oxidative stress by edaravone protects against cognitive impairments after traumatic brain injury. Brain Res. 1490, 184–192 [DOI] [PubMed] [Google Scholar]
- 21.Zhang L., Zhang W.P., Hu H., Wang M.L., Sheng W.W., Yao H.T., Ding W., Chen Z., and Wei E.Q. (2006). Expression patterns of 5-lipoxygenase in human brain with traumatic injury and astrocytoma. Neuropathology 26, 99–106 [DOI] [PubMed] [Google Scholar]
- 22.Uz T., Dimitrijevic N., Imbesi M., Manev H., and Manev R. (2008). Effects of MK-886, a 5-lipoxygenase activating protein (FLAP) inhibitor, and 5-lipoxygenase deficiency on the forced swimming behavior of mice. Neurosci. Lett. 436, 269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C.T., Zhang W.P., Lu Y.B., Fang S.H., Yuan Y.M., Qi L.L., Zhang L.H., Huang X.J., and Zhang L., Chen Z., and Wei E.Q. (2009). Oxygen-glucose deprivation activates 5-lipoxygenase mediated by oxidative stress through the p38 mitogen-activated protein kinase pathway in PC12 cells. J. Neurosci. Res. 87, 991–1001 [DOI] [PubMed] [Google Scholar]
- 24.Zohar O., Schreiber S., Getslev V., Schwartz J.P., Mullins P.G., and Pick C.G. (2003). Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience 118, 949–955 [DOI] [PubMed] [Google Scholar]
- 25.Milman A., Rosenberg A., Weizman R., and Pick C.G. (2005). Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J. Neurotrauma 22, 1003–1010 [DOI] [PubMed] [Google Scholar]
- 26.Royle S.J., Collins F.C., Rupniak H.T., Barnes J.C., and Anderson R. (1999). Behavioural analysis and susceptibility to CNS injury of four inbred strains of mice. Brain Res. 816, 337–349 [DOI] [PubMed] [Google Scholar]
- 27.Shapira Y., Yadid G., Cotev S., and Shohami E. (1989). Accumulation of calcium in the brain following head trauma. Neurol. Res. 11, 169–172 [DOI] [PubMed] [Google Scholar]
- 28.Elizalde N., Gil-Bea F.J., Ramírez M.J., Aisa B., Lasheras B., Del, Rio J., and Tordera R.M. (2008). Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology 199, 1–14 [DOI] [PubMed] [Google Scholar]
- 29.Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J. and Klenk D.C. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85 [DOI] [PubMed] [Google Scholar]
- 30.Banno M., Mizuno T., Kato H., Zhang G., Kawanokuchi J., Wang J., Kuno R., Jin S., Takeuchi H., and Suzumura A. (2005). The radical scavenger edaravone prevents oxidative neurotoxicity induced by peroxynitrite and activated microglia. Neuropharmacology 48, 283–290 [DOI] [PubMed] [Google Scholar]
- 31.Dzitoyeva S., Imbesi M., Uz T., Dimitrijevic N., Manev H., and Manev R. (2008). Caffeic acid attenuates the decrease of cortical BDNF transcript IV mRNA induced by swim stress in wild-type but not in 5-lipoxygenase-deficient mice. J. Neural Transm. 115, 823–827 [DOI] [PubMed] [Google Scholar]
- 32.Rådmark O., Werz O., Steinhilber D., and Samuelsson B. (2007). 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem. Sci. 32, 332–341 [DOI] [PubMed] [Google Scholar]
- 33.Hammarberg T., Provost P., Persson B., and Rådmark O. (2000). The N-terminal domain of 5-lipoxygenase binds calcium and mediates calcium stimulation of enzyme activity. J. Biol. Chem. 275, 38787–38793 [DOI] [PubMed] [Google Scholar]
- 34.Kulkarni S., Das S., Funk C.D., Murray D., and Cho W. (2002). Molecular basis of the specific subcellular localization of the C2-like domain of 5-lipoxygenase. J. Biol. Chem. 277, 13167–13174 [DOI] [PubMed] [Google Scholar]
- 35.Yeadon M., Dougan F.L., Petrovic A., Beesley J.E., and Payne A.N. (1993). Effect of BW B70C, a novel inhibitor of arachidonic acid 5-lipoxygenase, on allergen-induced bronchoconstriction and late-phase lung eosinophil accumulation in sensitised guinea-pigs. Agents Actions 38, 8–18 [DOI] [PubMed] [Google Scholar]
- 36.Li C.T., Zhang W.P., Fang S.H., Lu Y.B., Zhang L.H., Qi L.L., Huang X.Q., Huang X.J., and Wei E.Q. (2010). Baicalin attenuates oxygen-glucose deprivation-induced injury by inhibiting oxidative stress-mediated 5-lipoxygenase activation in PC12 cells. Acta Pharmacol. Sin. 31, 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S.Y., Lee S.J., Han C., Patkar A.A., Masand P.S., and Pae C.U. (2013). Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry. 46, 224–235 [DOI] [PubMed] [Google Scholar]
- 38.Castrén E., Võikar V., and Rantamäki T. (2007). Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 7, 18–21 [DOI] [PubMed] [Google Scholar]
- 39.Hsieh H.L., and Yang C.M. (2013). Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed. Res. Int. 2013, 484613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh I.N., Sullivan P.G., Deng Y., Mbye L.H., and Hall E.D. (2006). Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 26, 1407–1418 [DOI] [PubMed] [Google Scholar]
- 41.Cooney S.J., Bermudez-Sabogal S.L., and Byrnes K.R. (2013). Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J. Neuroinflammation 10, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi B.Y., Jang B.G., Kim J.H., Lee B.E., Sohn M., Song H.K., and Suh S.W. (2012). Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res. 1481, 49–58 [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q.G., Laird M.D., Han D., Nguyen K., Scott E., Dong Y., Dhandapani K.M., and Brann D.W. (2012). Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One 7, e34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo J.S., Park J.Y., Choi J., Kim T.K., Shin J.H., Lee J.K., and Han P.L. (2012). NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J. Neurosci. 32, 9690–9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Härtig W., Michalski D., Seeger G., Voigt C., Donat C.K., Dulin J., Kacza J, Meixensberger J., Arendt T., and Schuhmann M.U. (2012). Impact of 5-lipoxygenase inhibitors on the spatiotemporal distribution of inflammatory cells and neuronal COX-2 expression following experimental traumatic brain injury in rats. Brain Res. 2012December23 pii: . doi: 10.1016/j.brainres.2012.12.022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Ikonomovic M.D., Abrahamson E.E., Uz T., Manev H., and Dekosky S.T. (2008). Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer's disease. J. Histochem. Cytochem. 56, 1065–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erlemann K.R., Rokach J., and Powell W.S. (2004). Oxidative stress stimulates the synthesis of the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid by inflammatory cells. J. Biol. Chem. 279, 40376–40384 [DOI] [PubMed] [Google Scholar]
- 48.Erlemann K.R., Cossette C., Gravel S., Stamatiou P.B., Lee G.J., Rokach J., and Powell W.S. (2006). Metabolism of 5-hydroxy-6,8,11,14-eicosatetraenoic acid by human endothelial cells. Biochem. Biophys. Res. Commun. 350, 151–156 [DOI] [PubMed] [Google Scholar]
- 49.Lammers C.H., Schweitzer P., Facchinetti P., Arrang J.M., Madamba S.G., Siggins G.R., and Piomelli D. (1996). Arachidonate 5-lipoxygenase and its activating protein: prominent hippocampal expression and role in somatostatin signaling. J. Neurochem. 66, 147–152 [DOI] [PubMed] [Google Scholar]
- 50.Uz T., Pesold C., Longone P., and Manev H. (1998). Aging-associated up-regulation of neuronal 5-lipoxygenase expression: putative role in neuronal vulnerability. FASEB J. 12, 439–449 [DOI] [PubMed] [Google Scholar]
- 51.Peng Y.L., Liu Y.N., Liu L., Wang X., Jiang C.L., and Wang Y.X. (2012). Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J. Neuroinflammation 9, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silver J.M., McAllister T.W., and Arciniegas D.B. (2009). Depression and cognitive complaints following mild traumatic brain injury. Am. J. Psychiatry. 166, 653–661 [DOI] [PubMed] [Google Scholar]
- 53.Schwarzbold M.L., Rial D., De Bem T., Machado D.G., Cunha M.P., dos Santos A.A., dos Santos D.B., Figueiredo C.P., Farina M., Goldfeder E.M., Rodrigues A.L., Prediger R.D., and Walz R. (2010). Effects of traumatic brain injury of different severities on emotional, cognitive, and oxidative stress-related parameters in mice. J. Neurotrauma 27, 1883–1893 [DOI] [PubMed] [Google Scholar]
- 54.Reddy C.C. (2011). Postconcussion syndrome: a physiatrist's approach. PM R. 3(10 Suppl. 2), S396–S405 [DOI] [PubMed] [Google Scholar]
- 55.Zohar O., Rubovitch V., Milman A., Schreiber S., and Pick C.G. (2011). Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol. Exp. 71, 36–45 [DOI] [PubMed] [Google Scholar]
- 56.Naritomi H., Moriwaki H., Metoki N., Nishimura H., Higashi Y., Yamamoto Y., Yuasa H., Oe H., Tanaka K., Saito K., Terayama Y., Oda T., Tanahashi N., and Kondo H. (2010). Effects of edaravone on muscle atrophy and locomotor function in patients with ischemic stroke: a randomized controlled pilot study. Drugs R D. 10, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawai H., Nakai H., Suga M., Yuki S., Watanabe T., and Saito K. (1997). Effects of a novel free radical scavenger, MCl-186, on ischemic brain damage in the rat distal middle cerebral artery occlusion model. J. Pharmacol. Exp. Ther. 281, 921–927 [PubMed] [Google Scholar]
- 58.Watanabe T., Yuki S., Egawa M., and Nishi H. (1994). Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J. Pharmacol. Exp. Ther. 268, 1597–1604 [PubMed] [Google Scholar]
- 59.Maller J.J., Thomson R.H., Lewis P.M., Rose S.E., Pannek K., and Fitzgerald P.B. (2010). Traumatic brain injury, major depression, and diffusion tensor imaging: making connections. Brain Res. Rev. 64, 213–240 [DOI] [PubMed] [Google Scholar]