Abstract
Hematopoietic stem cell (HSC) gene therapy is a demonstrated effective treatment for X-linked severe combined immunodeficiency (SCID-X1), but B-cell reconstitution and function has been deficient in many of the gene therapy treated patients. Cytoreductive preconditioning is known to improve HSC engraftment, but in general it is not considered for SCID-X1 since the poor health of most of these patients at diagnosis and the risk of toxicity preclude the conditioning used in standard bone marrow stem cell transplantation. We hypothesized that mobilization of HSC by granulocyte colony-stimulating factor (G-CSF) should create temporary space in bone marrow niches to improve engraftment and thereby B-cell reconstitution. In the present pilot study supplementing our earlier preclinical evaluation (Huston et al., 2011), Il2rg−/− mice pretreated with G-CSF were transplanted with wild-type lineage negative (Lin−) cells or Il2rg−/− Lin− cells transduced with therapeutic IL2RG lentiviral vectors. Mice were monitored for reconstitution of lymphocyte populations, level of donor cell chimerism, and antibody responses as compared to 2 Gy total body irradiation (TBI), previously found effective in promoting B-cell reconstitution. The results demonstrate that G-CSF promotes B-cell reconstitution similar to low-dose TBI and provides proof of principle for an alternative approach to improve efficacy of gene therapy in SCID patients without adverse effects associated with cytoreductive conditioning.
Introduction
X-linked severe combined immunodeficiency (SCID-X1), caused by a mutation in the interleukin-2 (IL-2) receptor gamma gene (IL2RG) leading to a nonfunctional common gamma chain protein (Noguchi et al., 1993), is the most common form of SCID, affecting approximately half of SCID patients. While bone marrow (BM) transplantation from healthy human leukocyte antigen (HLA)-identical siblings is effective and curative (Fischer, 1999), HLA-matched sibling donors are available to only ∼10% of patients (Antoine et al., 2003). Transplantation from mismatched or unrelated donors has a significantly higher risk of morbidity and mortality (Gennery et al., 2010). In order to treat SCID-X1 patients lacking a suitable HLA-matched donor, alternative methods such as hematopoietic stem cell (HSC) gene therapy have been developed.
The efficacy of gammaretroviral gene therapy to successfully treat SCID-X1 was demonstrated in seminal clinical trials (Gaspar et al., 2004; Hacein-Bey-Abina et al., 2010). This approach restored T-cell immunity in 18 patients and resulted in long-term survival for 17 patients out of 20, a survival rate similar to HLA-identical BM transplantation (Rocha et al., 2000). T and NK cell counts and serum Ig levels have been sustained for up to 12 years, and at the time of the last report, 6 of the 17 patients are no longer in need of intravenous immunoglobulin (Cavazzana-Calvo et al., 2012). However, the success of these SCID-X1 clinical trials was tempered by the development of leukemia-like symptoms in five patients (Hacein-Bey-Abina et al., 2008; Howe et al., 2008). Further investigation revealed that the uncontrolled monoclonal proliferation of T cells in these patients was because of the gammaretroviral vector integrating near the proto-oncogenes LMO2 or CCND2, resulting in vector-derived insertional mutagenesis (Bushman, 2007; Deichmann et al., 2011). Four of the five affected patients were successfully treated by chemotherapy without reducing the effectiveness of the gene therapy, while one patient succumbed in spite of chemotherapy treatment (Fischer and Cavazzana-Calvo, 2008).
Possibly because of the lack of myelosuppressive conditioning, patients in these SCID-X1 clinical trials generally had poor B-cell reconstitution and low levels of gene marking in B cells. Similarly, in our experiments, high levels of circulating T cells, but not B cells, could be seen in some Il2rg−/− mice after wild-type (WT) lineage-negative (Lin−) cell transplantation without conditioning (Huston et al., 2011). The donor HSCs and B-cell progenitors may have engrafted poorly in the BM because of the limited accessibility of occupied HSC niches. The relative advantage in reconstitution and marking of T-cell progenitors might be attributed to the higher selective advantage of these cell types, the lack of competing T-cell progenitor cells in the host (Buckley, 2004), or the ability of multipotent progenitors T cells to home directly to the thymus (Weerkamp et al., 2006). Recently initiated gene therapy trials to treat SCID-X1 with self-inactivating (SIN) gammaretroviral vectors adhere to similar protocols without preconditioning (Mukherjee and Thrasher, 2013). In contrast, pretransplant myelosuppressive conditioning regimens to suppress or eliminate endogenous BM cells such as those used in the ADA-SCID (Aiuti et al., 2009; Gaspar et al., 2011) and Wiskott–Aldrich syndrome trials (Aiuti et al., 2013) clearly improved B-cell reconstitution, consistent with the improved ability of donor HSC in clinically feasible numbers to contribute to hematopoietic reconstitution (Tomita et al., 1994; Giri et al., 2001).
Granulocyte colony-stimulating factor (G-CSF) suppresses osteoblast lineage cells in the endosteal BM niches, leading to reduced levels of signaling molecules such as CXCL12, VLA-4, and c-Kit, which are essential for HSC retention (Winkler and Levesque, 2006; Winkler et al., 2010; Greenbaum and Link, 2011), resulting in the release of immature HSCs into circulation (Aiuti et al., 1997; Whetton and Graham, 1999). Repeated treatment results in the release of a considerable number of CD34+ cells into the peripheral blood, which can be harvested and subsequently used for patients requiring stem cell transplants (Bensinger et al., 1996; Elfenbein and Sackstein, 2004). The option of using stem cell mobilization to improve donor cell engraftment in HSC transplant recipients has been postulated before (Chen et al., 2006), and has been attempted in conjunction with low-dose total body irradiation (TBI) (Mardiney and Malech, 1996; Barese et al., 2007), but its use as a single conditioning regimen before stem cell gene therapy has not been investigated as yet.
To test whether mobilizing agents can be used to improve donor HSC engraftment as a potentially less toxic and clinically applicable method, we compared preconditioning by G-CSF-induced HSC mobilization (Petit et al., 2002; Shier et al., 2004; Chen et al., 2006) to 2 Gy TBI, identified as the minimum radiation dose required for engraftment of clinically feasible stem cell numbers (Wagemaker et al., 1986) and applied as described previously (Huston et al., 2011) as conditioning for transplantation of gene-modified Il2rg−/− stem cells into Il2rg−/− mice, which have a CD4lowCD8−B220−NK− phenotype. We hypothesized that mobilization of recipient HSCs would open a window for engraftment of gene therapy-treated donor HSCs in the BM and thereby promote B-cell reconstitution.
Materials and Methods
Mice
Il2rg−/− mice and syngenic BALB/c WT mice (Huston et al., 2011) were bred in the Experimental Animal Center of Erasmus MC. All mice were used at 6–10 weeks of age and were maintained in specified pathogen-free conditions. Experiments were approved by the institutional Animal Ethical Committee of Erasmus MC in accordance with legislation in the Netherlands.
G-CSF mobilization
Female Il2rg−/− mice were given subcutaneous injections of 6 μg of Filgrastim (recombinant methionylated human G-CSF) (Sandoz) in 50 μl of saline, or saline only, daily for four consecutive days. Peripheral blood was collected at 5 hr after the last injection, at what is considered to be the peak mobilization time. The percentages of CD11b+, Sca-1+, and c-Kit+ cells were measured via flow cytometry. Absolute numbers of these cells were calculated based on total white blood cell (WBC) counts and compared with the values in those animals 1 day before administration of G-CSF. Other cohorts of female Il2rg−/− mice were given one of the aforementioned mobilization protocols and transplanted with WT or LV vector-treated Lin− BM cells 5 hr after the last G-CSF injection.
Lentiviral vectors
Third-generation SIN LV vectors incorporating codon-optimized IL2RG cDNA driven by either the SFFV viral promoter or a 1.1 kb section of the native IL2RG promoter were previously described (Huston et al., 2011). LVs were produced by standard calcium phosphate transfection of HEK 293T cells (Follenzi and Naldini, 2002) with the packaging plasmids pMDL-g/pRRE, pMD2-VSVg, and pRSV-Rev. Vector particle concentration and titration were carried out as previously described.
Transduction and transplantation of lineage-negative BM cells
BM cells from male Il2rg−/− and congenic WT BALB/c mice were purified by lineage depletion (BD Biosciences). Lin− BM cells were transduced overnight at 106 cells/ml in serum-free modified Dulbecco's medium with supplements (Wognum et al., 2000) in the presence of murine stem cell factor (mSCF) 100 ng/ml, human FMS-like tyrosine kinase 3-ligand (hFlt3-L) 50 ng/ml, and murine thrombopoietin (mTPO) 10 ng/ml at HTU multiplicity of infection (MOI) 10 (SF vector) or MOI 3 (γcPr vector). Subsequently, 3×105 or 1×105 cells were injected into the tail vein of female 2 Gy-irradiated or G-CSF-treated Il2rg−/− recipients. Mice were monitored monthly for 6 months by peripheral blood collection and analysis of hematopoietic cell counts and flow cytometry. Mice were sacrificed 6–7 months posttransplant, and BM and spleen cells isolated for further analysis.
Immunophenotyping by flow cytometry
Flow cytometric analyses were performed on cells obtained from blood, BM, and spleen. Peripheral blood was collected monthly in EDTA tubes by retro-orbital puncture under isoflurane anesthesia. Complete blood cell counts were measured using a Vet ABC hematology analyzer (Scil Animal Care Company GmbH). Blood was lysed and leukocytes were washed three times with Hank's balanced salt solution (Invitrogen) containing 0.5% (wt/vol) bovine serum albumin and 0.05% (wt/vol) sodium azide (HBN). Cells were incubated for 30 min at 4°C in HBN containing 2% heat-inactivated normal mouse serum and antibodies against CD3, CD4, CD8, B220, IgM, IgD, CD11b, Sca-1, and c-Kit directly conjugated to R-phycoerythrin (PE), peridinin chlorophyll protein (PerCP), or allophycocyanin (APC; all antibodies BD Biosciences). Subsequently, cells were washed and measured on a FACSCanto (BD Biosciences). BM and spleen cells were evaluated similarly.
Real-time quantitative polymerase chain reactions
Quantitative polymerase chain reaction (qPCR) to quantify the integrated proviral copy number was performed as described before (Huston et al., 2011). qPCRs were performed on spleen and BM DNA from Il2rg−/− mice transplanted with male donor cells transduced with LV vectors to amplify a region of the Y chromosome as described by Pujal and Gallardo (2008), using 100 ng of genomic DNA and primers 5′-TCATCGGAGGGCTAAAGTGTCAC-3′ and 5′-TGGCATGTGGGTTCCTGTCC-3′. A standard curve was generated using spleen and BM DNA from male BALB/c mice. All PCRs were performed in triplicate and analyzed with SDS 2.2.2 software.
Ligation-mediated PCR and LAM-PCR
High-resolution insertion-site analysis by LAM-PCR (Schmidt et al., 2007) was performed on BM DNA from Il2rg−/− mice transduced with LV vectors. Restriction enzyme Tsp509I was used with the lentiviral (HIV) primer set. High-throughput sequencing of LAM-PCR products was performed at GATC Biotech (Konstanz). Following sequencing, the barcode and LTR sequences were trimmed and the remaining genomic sequences uploaded to the MAVRIC analysis tool (Huston et al., 2012) for alignment to the mouse genome and annotation of nearby genes.
Immunization and antibodies measured by ELISA
T-cell-dependent specific antibody responses were determined by intraperitoneal immunization with 16 IU of tetanus toxoid. This process was repeated three times at 2-week intervals, with plasma collected 2 weeks after the last immunization and just before the initial immunization. An ELISA was performed on plates coated with tetanus toxoid, and HRP goat-anti-mouse IgG1 (Invitrogen) was used to measure signal. Anti-tetanus antibody TetE3 (AbCam) was used for calibration. The specific ELISA protocol, using Covalink 96-well plates (Nunc A/S), was as previously described (Huston et al., 2011).
Statistics
p-Values for differences between groups were calculated via two-tailed Mann–Whitney U-test using GraphPad software.
Results
Evaluation of mobilization by G-CSF in Il2rg−/− mice
To assess the efficacy of G-CSF to mobilize HSCs, Il2rg−/− mice were treated with 4 consecutive daily injections of G-CSF, or 4 injections of saline (n=5 in both groups). Peripheral blood was collected from these mice 1 day before the first and 5 hr after the last G-CSF injection to determine the increase in WBC, CD11b+, Sca-1+, and c-Kit+ cells.
The mobilization protocol resulted in a large increase in the number of circulating WBC, composed mainly of CD11b+ myeloid cells in Il2rg−/− mice (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/hum). Of the progenitor/stem cell markers measured, the most dramatic mobilization effect was seen in the c-Kit+Sca-1− subtype, which had a 36-fold increase. The more stem cell-enriched Sca-1+c-Kit+ population had a fold increase of 6. In control mice injected with saline, significant changes were not observed. The absolute numbers of these cell types postmobilization are shown in Supplementary Fig. S1B.
WT Lin− cell transplantation after stem cell mobilization in Il2rg−/− mice
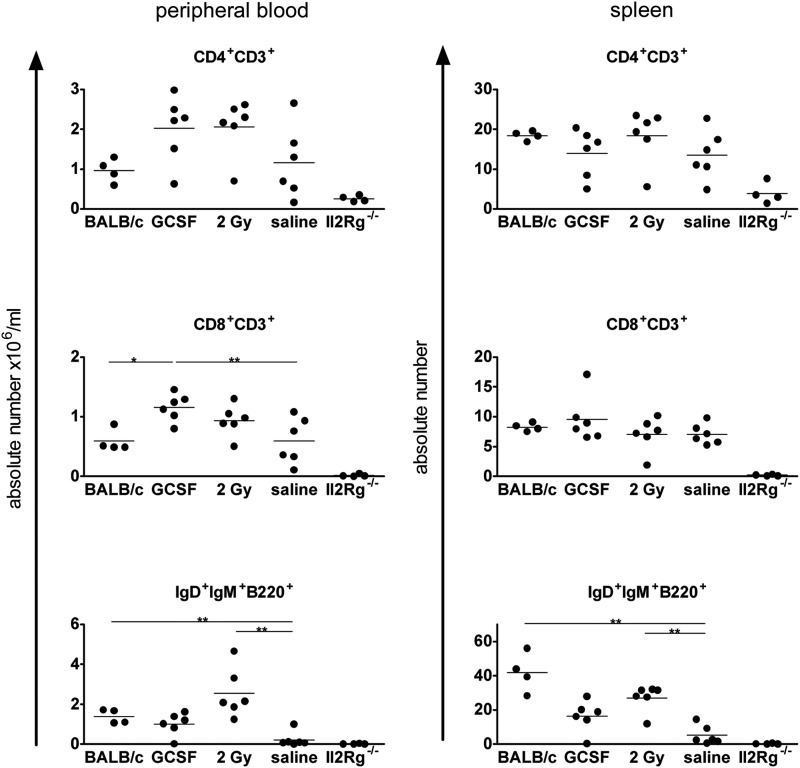
Female Il2rg−/− mice were subjected to the G-CSF mobilization protocol described above, 2 Gy TBI or saline injections, and subsequently transplanted with 3×105 male Lin− WT BALB/c cells (n=3 per group, experiment repeated to a total of n=6 animals per group). Absolute lymphocyte numbers in peripheral blood and spleen (Fig. 1) were determined at 6 months after transplantation and showed high levels of T- and B-cell reconstitution in both G-CSF and 2 Gy irradiation groups. Similar results were found in the BM (Table 1a). Overall, Il2rg−/− mice transplanted with WT Lin− cells after G-CSF or 2 Gy TBI conditioning had lymphocyte levels similar to untreated age-matched WT BALB/c mice (n=4). Mice transplanted with WT cells after saline injections had high levels of circulating T cells but significantly lower B-cell counts compared with the 2 Gy-treated group. Saline control mice (n=4) also had lower levels of Y-chromosome chimerism (i.e., donor cell chimerism) in BM and spleen (Table 1a). The prominent effect of both G-CSF and 2 Gy conditioning on B-cell reconstitution, without a significant effect on T cells, is clearly demonstrated in the splenic differential analysis (Table 1b). The number of B cells reveals that G-CSF and 2 Gy conditioning promotes B-cell reconstitution to levels intermediate to those of the Il2rg−/− and WT phenotypes (Table 1b), also apparent in the CD19+ cells in BM (Table 1c), which in other cell types does not display major differences between both phenotypes.
FIG. 1.
Absolute lymphocyte numbers in Il2rg−/− mice transplanted with wild-type Lin− cells after conditioning regimens. Flow cytometry profiles and cell counts were used to calculate mature lymphocyte numbers in peripheral blood (left column, absolute number per ml) and in spleen (right column, absolute number) of Il2rg−/− mice transplanted with 3×105 Lin− BM cells after G-CSF mobilization or total body irradiation. 2 Gy=2 Gy TBI. Measurements were taken 6 months posttransplant. Untreated age-matched wild-type (BALB/c) and Il2rg−/− mice are included for comparison (horizontal bars indicate statistically significant differences between two groups: *p<0.05, **p<0.01). BM, bone marrow; G-CSF, granulocyte colony-stimulating factor; TBI, total body irradiation.
Table 1.
Spleen and Bone Marrow Parameters in Il2rg−/− Mice Transplanted with BALB/c Lin− Cells After Conditioning
| Table 1A. Y-Chimerism Levels (Mean±SEM) | ||
|---|---|---|
| Conditioning regimen | Spleen cell chimerism (%) | BM cell chimerism (%) |
| G-CSF (n=6) | 50.5±12.3 | 33.3±14.6 |
| 2 Gy irradiation (n=6) | 50.1±10.5 | 42.7±9.0 |
| Saline (n=6) | 28.0±7.7 | 14.3±5.8 |
| BM, bone marrow; G-CSF, granulocyte colony-stimulating factor. | ||
| Table 1B. Differential Counts (×106, Mean±SEM) in Spleen | ||||||
|---|---|---|---|---|---|---|
| Conditioning/phenotype | CD4CD3 | CD8CD3 | IgDIgMB220 | CD19 | CD11bGr1 | Cells/spleen |
| G-CSF (n=6) | 14.0±2.5 | 9.5±1.6 | 16.2±3.7 | 34.5±7.6 | 1.4±0.7 | 101±12 |
| 2 Gy (n=6) | 18.4±2.7 | 7.1±1.1 | 27.1±3.1 | 42.7±6.2 | 1.0±0.4 | 104±12 |
| Saline (n=6) | 13.5±2.5 | 7.0±0.7 | 5.1±2.3 | 10.8±3.2 | 3.3±1.5 | 100±7 |
| Controls | ||||||
| BALB/c WT (n=4) | 18.4±0.6 | 8.3±0.3 | 41.9±5.7 | 77.3±6.5 | 2.0±0.3 | 155±11 |
| BALB/c Il2rg−/− (n=4) | 3.8±1.3 | 0.2±0.1 | 0.2±0.1 | 2.5±0.8 | 2.5±0.9 | 39±18 |
| WT, wild type. | ||||||
| Table 1C. Differential Counts (×106±SEM) in Bone Marrow | ||||||
|---|---|---|---|---|---|---|
| Conditioning/phenotype | CD4CD3 | CD8CD3 | IgDIgMB220 | CD19 | CD11bGr1 | Cells/femur |
| G-CSF | 0.7±0.1 | 0.8±0.1 | 0.6±0.2 | 2.0±0.5 | 3.5±0.7 | 28±2 |
| 2 Gy | 0.5±0.1 | 0.4±0.1 | 0.9±0.3 | 2.8±0.5 | 2.7±0.4 | 25±3 |
| Saline | 0.2±0.1 | 0.5±0.1 | 0.2±0.1 | 0.5±0.3 | 4.0±0.8 | 17±4 |
| Controls | ||||||
| BALB/c WT | 0.2±0.0 | 0.1±0.0 | 0.4±0.1 | 5.5±1.5 | 3.4±0.6 | 19±4 |
| BALB/c Il2rg−/− | 0.2±0.0 | 0.0±0.0 | 0.0±0.0 | 0.1±0.0 | 4.6±0.4 | 22±4 |
Lentiviral vector gene therapy in Il2rg−/− mice after G-CSF stem cell mobilization
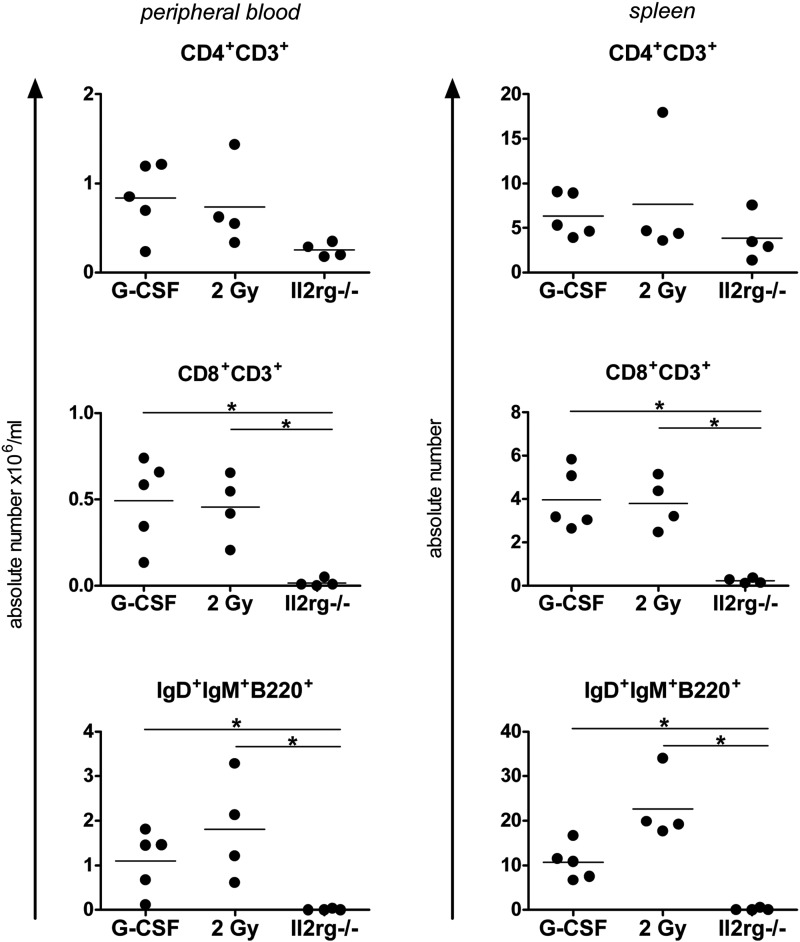
To determine the efficacy of G-CSF-induced HSC mobilization as a pretransplant regimen before lentiviral gene therapy, female Il2rg−/− mice were subjected to G-CSF (n=5) or 2 Gy irradiation (n=4) and subsequently transplanted with 3×105 Lin− Il2rg−/− cells transduced at a HeLa MOI of 3 overnight with SIN lentiviral vectors containing a codon-optimized human IL2RG gene (coIL2RG) driven by the spleen focus forming virus (SF) promoter, as previously described (Huston et al., 2011).
Absolute lymphocyte numbers at 7 months after transplantation in peripheral blood and in spleen cells were compared with those of untreated Il2rg−/− mice and WT BALB/c mice (Fig. 2). Significant differences (p<0.05) compared with untreated Il2rg−/− mice were found in SF.coIL2RG-treated mice in the CD8+ and IgD+IgM+B220+ peripheral blood lymphocyte populations regardless of whether the mice were conditioned with G-CSF or 2 Gy TBI. Spleen vector copy number (VCN) per cell taken from SF.coIL2RG-treated mice revealed average integrations per cell of 1.7 and 1.4 for the G-CSF and 2 Gy-conditioned groups, respectively (Table 2a). BM Y-chimerism levels were significantly higher in the 2 Gy group compared with the G-CSF group (p<0.05), but spleen Y-chimerism levels were similar.
FIG. 2.
Comparison of G-CSF and 2 Gy conditioning regimens in mice treated with SF.coIL2RG lentiviral vectors. Reconstitution of lymphocytes in peripheral blood (left column) at 6 months and spleen (right column) at 7 months after transplantation of 3×105 SF.coIL2RG-transduced Il2rg−/− Lin− BM cells following conditioning regimens of G-CSF or 2 Gy TBI (2 Gy). Untreated age-matched Il2rg−/− mice are included for comparison (horizontal bars indicate statistically significant differences between two groups: *p<0.05).
Table 2.
Y-Chimerism and Viral Integrations per Cell in Il2rg−/− Mice Transplanted with Lentiviral Vector Treated Il2rg−/− Lin− Cells
| Table 2A. Il2rg−/−Mice Transplanted with LV.SF coIL2RG Vector-Treated Cells | ||||
|---|---|---|---|---|
| Conditioning | Spleen cell chimerism (%)a | BM cell chimerism (%)a | Integrations per cell spleenb | Integrations per cell BMb |
| G-CSF (n=5) | 52.6±5.1 | 15.0±1.0 | 1.7±0.2 | 1.1±0.5 |
| 2 Gy irradiation (n=4) | 49.7±13.0 | 53.3±13.8 | 1.4±0.2 | 0.9±0.5 |
| Table 2B. Il2rg−/−Mice Transplanted with SF or γcPr coIL2RG Vector-Treated Cells | ||||
|---|---|---|---|---|
| Vector and conditioning | Spleen cell chimerism (%)a | BM cell chimerism (%)a | Integrations per cell spleenb | Integrations per cell BMb |
| SF.coIL2RG G-CSF (n=5) | 21.6±6.5 | 11.6±2.6 | 2.0±0.3 | 1.3±0.2 |
| SF.coIL2RG 2 Gy (n=4) | 47.9±12.0 | 46.5±12.1 | 1.1±0.2 | 1.1±0.4 |
| γcPr.coIL2RG G-CSF (n=4) | 11.5±4.4 | 8.6±5.5 | 1.6±0.9 | 1.3±0.3 |
| γcPr.coIL2RG 2 Gy (n=4) | 21.8±9.5 | 17.4±3.3 | 1.5±0.1 | 1.0±0.3 |
Data are shown as mean±SEM.
Vector copy per cell corrected for chimerism.
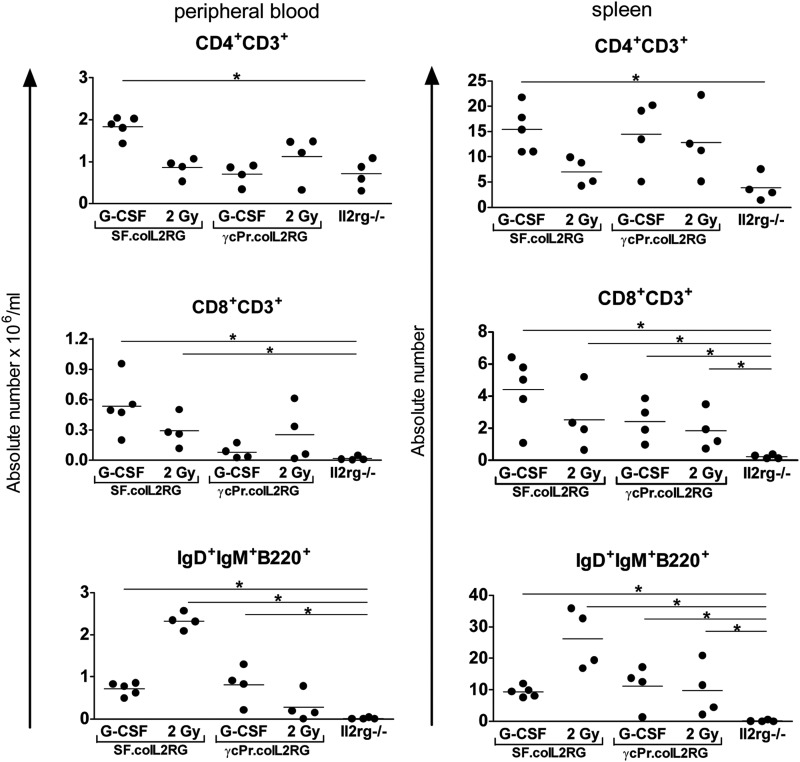
To determine whether G-CSF mobilization was sufficient to allow engraftment of Lin− cells treated with lentiviral vectors containing an eukaryotic promoter of relatively low strength, we repeated the G-CSF protocol using the SF.coIL2RG vector or a vector driven by a 1.1 kb section of the native IL2RG promoter (γcPr). An MOI of 3 was used aiming at 1 VCN per cell. The number of Lin− BM cells transplanted was reduced slightly to 105. The SF group had significantly higher levels of CD8+ and IgM+IgD+B220+ splenic lymphocytes compared with the untreated Il2rg−/− mice (Fig. 3). The γcPr vector was able to increase the T and B populations in most Il2rg−/− mice, particularly in the spleen, but not to WT levels. Analyses of Y-chimerism and vector integration per cell revealed a similar vector copy number but lower levels of donor cell engraftment in spleen and BM (Table 2b) for the γcPr group relative to the SF group.
FIG. 3.
Comparison of various promoter elements in mice given coIL2RG vectors following a G-CSF conditioning regimen. Reconstitution of lymphocytes in peripheral blood (left column) and spleen (right column) after transplantation of 105 Lin− Il2rg−/− cells transduced with coIL2RG lentiviral vectors containing SF or γcPr promoter elements. Measurements calculated by cell counts and flow cytometric analysis 6 months posttransplant. Untreated age-matched Il2rg−/− mice are included for comparison (horizontal bars indicate statistically significant differences between two groups: *p<0.05, **p<0.01).
T-cell-dependent antibody responses
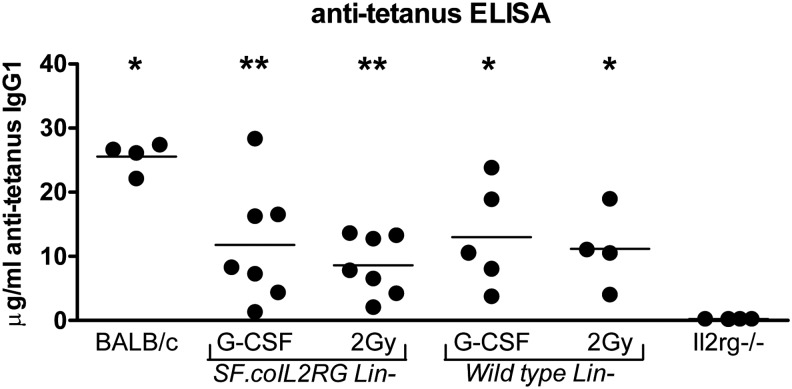
To confirm that a specific T-cell-dependent antibody response was restored in the Il2rg−/− mice transplanted with SF.coIL2RG or WT cells, tetanus toxoid immunization at 5 months after transplantation (Huston et al., 2011) demonstrated specific IgG1 antibody levels in all groups given WT cells or SF.coIL2RG-treated cells (Fig. 4). Antibody titers were highest in the WT group, but the majority of the mice in the G-CSF and 2 Gy-conditioned groups had a strong specific anti-tetanus immune response.
FIG. 4.
Anti-tetanus toxoid IgG1 immune response. Mice transplanted with WT BALB/c Lin− cells or SF.coIL2RG vector-treated Il2rg−/− Lin− cells after 2 Gy TBI or G-CSF conditioning were subjected to tetanus toxoid immunization protocol beginning 4 months posttransplant. Concentrations of plasma anti-IgG1 tetanus toxoid antibodies were determined 10 days after the last tetanus injection and compared with untreated BALB/c and Il2rg−/− mice (*p<0.05, **p<0.01 compared with the Il2rg−/− group).
Adverse effects
One mouse in the 2 Gy irradiation group (WT donor cells) developed a large tumor in the left hind leg and had to be sacrificed 6 months posttransplant; analysis of the spleen, BM, and peripheral blood revealed no hematopoietic abnormalities. One mouse in the G-CSF group (SF.coIL2RG donor cells) developed an extremely high WBC count (258×106 cells per ml) 6 months posttransplant and had to be sacrificed. Analysis of this mouse revealed an enlarged spleen (980 mg), and a high percentage of CD4+CD8+ cells in the BM (60.9%) and spleen. Integration analysis of BM cells revealed a dominant integration site found in 97% of all LAM-PCR sequencing reads for this mouse on chromosome 12, in intron 8–9 of the transcriptional repressor gene Pum2; for the purposes of this study, this leukemic mouse was excluded from group comparison analysis.
Discussion
We previously documented the benefit of pretransplant conditioning in improving engraftment of gene therapy-treated donor HSCs in the mouse model of SCID-X1 (Huston et al., 2011). However, the benefit of using current conditioning methods such as irradiation and chemotherapeutic agents to enhance hematopoietic engraftment in SCID patients has been debated (Haddad et al., 2013), as these regimens increase the risks of transplant-related morbidity and mortality (Armitage, 1994), in addition to late adverse effects such as retarded growth. SCID-X1 patients are often very young or in such poor health from recurrent infections by the time they are eligible for gene therapeutic intervention that even mild myelosuppressive regimens could be lethal (Cavazzana-Calvo et al., 2005).
Since engraftment of HSCs in BM is a competitive process (Stewart et al., 1998), we postulated that G-CSF-induced mobilization of recipient HSC would improve donor cell engraftment and thereby B-cell reconstitution in immune-deficient mice. In the present pilot study supplementing our previous preclinical evaluation (Huston et al., 2011), the efficacy of G-CSF as an agent to mobilize immature murine HSCs in Il2rg−/− mice was assessed and found similar to that in WT mice using murine G-CSF (Cynshi et al., 1991; Link, 2000). Subsequently, WT and Il2rg−/− lentiviral vector-transduced Lin− cells were transplanted into G-CSF-treated Il2rg−/− mice, demonstrating significantly improved levels of CD8+ and B220+ lymphocytes as well as T-cell-dependent antibody responses.
We purposely compared the efficacy of G-CSF as a conditioning agent with the radiation dose of 2 Gy that, because of the radiation sensitivity of HSCs (Meijne et al., 1991), eliminates 80–90% of the BM stem cells and was shown effective in promoting B-cell reconstitution in X-linked SCID mice using relatively low numbers of transplanted cells (Huston et al., 2011). In the present study, we also choose to test the efficacy of G-CSF by transplantation of limited numbers of Lin− cells, comparable to 0.4–1.2×108/kg body weight autologous BM cells in humans, which would contain some 106 CD34+ cells/kg. After 2 Gy or G-CSF conditioning, the gene-modified cells need to compete with, respectively, a one or two orders of magnitude excess of recipient stem cells.
Irrespective of conditioning, promoter, WT, or gene-modified Il2rg−/− cells transplanted, T cells increased to normal or near-normal levels, consistent with the selective advantage of the T-cell lineage in these mice and BM-independent homing in the thymus (Weerkamp et al., 2006). In contrast, B-cell reconstitution was strongly dependent on conditioning. A consistent difference between G-CSF and 2 Gy conditioning in B-cell reconstitution was not observed, thus demonstrating the robust efficacy of conditioning by G-CSF mobilization, which is remarkable in view of the large difference in competing recipient stem cells and suggests that conditioning by mobilizing agents might also be effective in disorders in which gene therapy-treated cells do not confer a selective advantage at the stem cell level.
The mice displayed significant T-cell-dependent antibody responses when using the strong viral SF promoter without any difference between G-CSF and 2 Gy TBI conditioning. On average, the γcPr groups had lower vector marking and lower donor cell chimerism relative to mice treated with the SF vector. The modest efficacy of the γcPr promoter is consistent with previous observations in this animal model using 2 Gy conditioning (Huston et al., 2011) and is likely related to the low strength of the native promoter compared with the strong viral SF promoter. We do not exclude that, once sufficient space has been achieved in BM, the gene-corrected B-cell differentiation also displays a selective advantage over deficient cells, and therefore is codependent on the expression level of the IL2RG gene.
A major phenotypic difference in SCID-X1 patients and Il2rg−/− mice is the presence of (nonfunctional) B cells in the former, but not in the latter. The presence of these endogenous B cells might be among the reasons why poor B-cell reconstitution and low levels of B-cell gene marking were observed in the SCID-X clinical trials. Conversely, the lack of B cells seen in Il2rg−/− mice could mean that transplanted B-cell precursors have a lower barrier to engraftment than the SCID-X1 patients and that G-CSF mobilization may therefore be more effective in the laboratory setting than in the clinic. A combination of mobilization agents and B-cell cytoreductive agents, such as anti-CD20 monoclonal antibody Rituximab (Maloney et al., 1994), may be required to substantially improve B-cell engraftment in SCID-X1 patients, as should be further evaluated in clinical trial. However, B cells do not occupy stem cell niches and pre-B cells are located specifically in Galectin-1-expressing niches (Espeli et al., 2009; Mourcin et al., 2011). Successful competition of normal B differentiation with the deficient SCID B cells will also in humans be dependent on sufficient engraftment of the precursor stem cells; therefore, at this stage it should not be excluded that G-CSF conditioning will prove to be sufficient in human patients as well.
Since not all patients respond to G-CSF-induced HSC mobilization and need additional agents such as Plerixafor (AMD3100) to improve mobilization (Broxmeyer et al., 2005; DiPersio et al., 2009), combinations of mobilization agents that mobilize HSC by different mechanisms (Winkler et al., 2012) may further improve engraftment to levels similar to current cytoreductive conditioning. However, similar to Chen et al. (2006), we have been unsuccessful using AMD3100 to obtain significant levels of engraftment following transplantation of clinically feasible stem cell numbers (unpublished results). In retrospect, this failure is not surprising in view of the timing of transplantation (2 hr after AMD3100 administration), which, relative to its pharmacokinetic properties with a circulating half-life of 4–5 hr (Hendrix et al., 2000; MacFarland et al., 2010), means that after 2 hr sufficient AMD3100 is still available to block homing. In other words, even if there is some homing in the BM of the transplanted cells, these will be immediately mobilized by the AMD3100 still present. Other options to improve engraftment may involve temporary modifications of either the BM stem cell niches (Kollet et al., 2006) or the HSC (Campbell et al., 2007; Spiegel et al., 2007; Hoggatt et al., 2009), for example, by ex vivo expansion (Zhang et al., 2006) and upregulation of CXCR4 to provide a selective homing advantage to the gene-modified cells (Peled et al., 1999; Petit et al., 2002; Kahn et al., 2004).
One mouse in the SF.coIL2RG group fell ill with an extremely high WBC count (258×106 cells/liter) at 6 months posttransplant and had to be sacrificed. FACS analysis of spleen and BM cells revealed a high percentage of CD4CD8 double-positive cells, suggesting a T-cell leukemia. LAM-PCR-based integration analysis of BM cells revealed a dominant integration site on chromosome 12, in intron 8–9 of the transcriptional repressor gene Pum2. This leukemia highlights the risks inherent to the viral promoter in the context of the SCID-X1 phenotype as has also been observed by others (Kustikova et al., 2005). The optimal promoter for SCID-X1 gene therapy is likely a eukaryotic promoter, such as the ubiquitous chromatin opening element (UCOE) promoter (Knight et al., 2012), that produces more robust transgene expression than the native γc promoter used in our current experiments and is methylation resistant.
To conclude, mobilization of recipient BM HSCs with G-CSF before transplantation of therapeutic gene-modified HSCs promotes BM engraftment and functional B-cell reconstitution in X-linked SCID, providing proof of principle for this approach that lacks the toxicity of myelosuppressive conditioning used in current clinical HSC gene therapy trials (Aiuti et al., 2007, 2013; Biffi et al., 2013) and warrants its further development to benefit genetic correction of a variety of monogenic inherited disorders.
Supplementary Material
Acknowledgments
The authors thank Dr. Orit Kollet and Prof. Tsvee Lapidot at the Weizmann Institute of Science (Rehovot, Israel) for their assistance in study design. Funding was provided by the European Commission's 5th, 6th, and 7th Framework Programs; Contracts QLK3-CT-2001-00427-INHERINET, LSHB-CT-2004-005242-CONSERT, LSHB-CT-2006-19038-Magselectofection, Grant Agreement 222878-PERSIST, and Grant Agreement 261387 CELL-PID; and by the Netherlands Health Research and Development Organization ZonMW (Translational Gene Therapy program grants 43100016 and 43400010).
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Aiuti A., Webb I.J., Bleul C., et al. (1997). The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J. Exp. Med. 185, 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A., Cassani B., Andolfi G., et al. (2007). Multilineage hematopoietic reconstitution without clonal selection in ADA-SCID patients treated with stem cell gene therapy. J. Clin. Invest. 117, 2233–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A., Cattaneo F., Galimberti S., et al. (2009). Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 360, 447–458 [DOI] [PubMed] [Google Scholar]
- Aiuti A., Biasco L., Scaramuzza S., et al. (2013). Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341, 1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine C., Muller S., Cant A., et al. (2003). Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet 361, 553–560 [DOI] [PubMed] [Google Scholar]
- Armitage J.O. (1994). Bone marrow transplantation. N. Engl. J. Med. 330, 827–838 [DOI] [PubMed] [Google Scholar]
- Barese C., Pech N., Dirscherl S., et al. (2007). Granulocyte colony-stimulating factor prior to nonmyeloablative irradiation decreases murine host hematopoietic stem cell function and increases engraftment of donor marrow cells. Stem Cells 25, 1578–1585 [DOI] [PubMed] [Google Scholar]
- Bensinger W.I., Buckner C.D., Rowley S., et al. (1996). Treatment of normal donors with recombinant growth factors for transplantation of allogeneic blood stem cells. Bone Marrow Transplant. 17Suppl 2, S19–S21 [PubMed] [Google Scholar]
- Biffi A., Montini E., Lorioli L., et al. (2013). Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341, 1233158. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Orschell C.M., Clapp D.W., et al. (2005). Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 201, 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley R.H. (2004). Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu. Rev. Immunol. 22, 625–655 [DOI] [PubMed] [Google Scholar]
- Bushman F.D. (2007). Retroviral integration and human gene therapy. J. Clin. Invest. 117, 2083–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T.B., Hangoc G., Liu Y., et al. (2007). Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 16, 347–354 [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M., Lagresle C., Hacein-Bey-Abina S., and Fischer A. (2005). Gene therapy for severe combined immunodeficiency. Annu. Rev. Med. 56, 585–602 [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M., Fischer A., Hacein-Bey-Abina S., and Aiuti A. (2012). Gene therapy for primary immunodeficiencies: part 1. Curr. Opin. Immunol. 24, 580–584 [DOI] [PubMed] [Google Scholar]
- Chen J., Larochelle A., Fricker S., et al. (2006). Mobilization as a preparative regimen for hematopoietic stem cell transplantation. Blood 107, 3764–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynshi O., Satoh K., Shimonaka Y., et al. (1991). Reduced response to granulocyte colony-stimulating factor in W/Wv and Sl/Sld mice. Leukemia 5, 75–77 [PubMed] [Google Scholar]
- Deichmann A., Brugman H.M., Bartholomae C.C., et al. (2011). Insertion sites in engrafted cells cluster within a limited repertoire of genomic areas after gammaretroviral vector gene therapy. Mol. Ther. 19, 2031–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio J.F., Stadtmauer E.A., Nademanee A., et al. (2009). Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 113, 5720–5726 [DOI] [PubMed] [Google Scholar]
- Elfenbein G.J., and Sackstein R. (2004). Primed marrow for autologous and allogeneic transplantation: a review comparing primed marrow to mobilized blood and steady-state marrow. Exp. Hematol. 32, 327–339 [DOI] [PubMed] [Google Scholar]
- Espeli M., Mancini S.J., Breton C., et al. (2009). Impaired B-cell development at the pre-BII-cell stage in galectin-1-deficient mice due to inefficient pre-BII/stromal cell interactions. Blood 113, 5878–5886 [DOI] [PubMed] [Google Scholar]
- Fischer A. (1999). Thirty years of bone marrow transplantation for severe combined immunodeficiency. N. Engl. J. Med. 340, 559–561 [DOI] [PubMed] [Google Scholar]
- Fischer A., and Cavazzana-Calvo M. (2008). Gene therapy of inherited diseases. Lancet 371, 2044–2047 [DOI] [PubMed] [Google Scholar]
- Follenzi A., and Naldini L. (2002). HIV-based vectors. Preparation and use. Methods Mol. Med. 69, 259–274 [PubMed] [Google Scholar]
- Gaspar H.B., Parsley K.L., Howe S., et al. (2004). Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet 364, 2181–2187 [DOI] [PubMed] [Google Scholar]
- Gaspar H.B., Cooray S., Gilmour K.C., et al. (2011). Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 3, 97ra79 [DOI] [PubMed] [Google Scholar]
- Gennery A.R., Slatter M.A., Grandin L., et al. (2010). Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J. Allergy Clin. Immunol. 126, 602–610e1–e11 [DOI] [PubMed] [Google Scholar]
- Giri N., Kaushiva A., Wu T., et al. (2001). The effects of SCF/G-CSF prestimulation on radiation sensitivity and engraftment in nonmyeloablated murine hosts. Exp. Hematol. 29, 779–785 [DOI] [PubMed] [Google Scholar]
- Greenbaum A.M., and Link D.C. (2011). Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia 25, 211–217 [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S., Garrigue A., Wang G.P., et al. (2008). Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 118, 3132–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S., Hauer J., Lim A., et al. (2010). Efficacy of gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 363, 355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad E., Leroy S., and Buckley R.H. (2013). B-cell reconstitution for SCID: should a conditioning regimen be used in SCID treatment? J. Allergy Clin. Immunol. 131, 994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix C.W., Flexner C., MacFarland R.T., et al. (2000). Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob. Agents Chemother. 44, 1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J., Singh P., Sampath J., and Pelus L.M. (2009). Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 113, 5444–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe S.J., Mansour M.R., Schwarzwaelder K., et al. (2008). Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 118, 3143–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston M.W., Van Til N.P., Visser T.P., et al. (2011). Correction of murine SCID-X1 by lentiviral gene therapy using a codon-optimized IL2RG gene and minimal pretransplant conditioning. Mol. Ther. 19, 1867–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston M.W., Brugman M.H., Horsman S., et al. (2012). Comprehensive investigation of parameter choice in viral integration site analysis and its effects on the gene annotations produced. Hum. Gene Ther. 23, 1209–1219 [DOI] [PubMed] [Google Scholar]
- Kahn J., Byk T., Jansson-Sjostrand L., et al. (2004). Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood 103, 2942–2949 [DOI] [PubMed] [Google Scholar]
- Knight S., Zhang F., Mueller-Kuller U., et al. (2012). Safer, silencing-resistant lentiviral vectors: optimization of the ubiquitous chromatin-opening element through elimination of aberrant splicing. J. Virol. 86, 9088–9095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollet O., Dar A., Shivtiel S., et al. (2006). Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 12, 657–664 [DOI] [PubMed] [Google Scholar]
- Kustikova O., Fehse B., Modlich U., et al. (2005). Clonal dominance of hematopoietic stem cells triggered by retroviral gene marking. Science 308, 1171–1174 [DOI] [PubMed] [Google Scholar]
- Link D.C. (2000). Mechanisms of granulocyte colony-stimulating factor-induced hematopoietic progenitor-cell mobilization. Semin. Hematol. 37, 25–32 [DOI] [PubMed] [Google Scholar]
- MacFarland R., Hard M.L., Scarborough R., et al. (2010). A pharmacokinetic study of plerixafor in subjects with varying degrees of renal impairment. Biol. Blood Marrow Transplant. 16, 95–101 [DOI] [PubMed] [Google Scholar]
- Maloney D.G., Liles T.M., Czerwinski D.K., et al. (1994). Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood 84, 2457–2466 [PubMed] [Google Scholar]
- Mardiney M., 3rd, and Malech H.L. (1996). Enhanced engraftment of hematopoietic progenitor cells in mice treated with granulocyte colony-stimulating factor before low-dose irradiation: implications for gene therapy. Blood 87, 4049–4056 [PubMed] [Google Scholar]
- Meijne E.I., Van Der Winden-Van Groenewegen R.J., Ploemacher R.E., et al. (1991). The effects of x-irradiation on hematopoietic stem cell compartments in the mouse. Exp. Hematol. 19, 617–623 [PubMed] [Google Scholar]
- Mourcin F., Breton C., Tellier J., et al. (2011). Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood 117, 6552–6561 [DOI] [PubMed] [Google Scholar]
- Mukherjee S., and Thrasher A.J. (2013). Gene therapy for PIDs: progress, pitfalls and prospects. Gene 525, 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M., Yi H., Rosenblatt H.M., et al. (1993). Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73, 147–157 [DOI] [PubMed] [Google Scholar]
- Peled A., Petit I., Kollet O., et al. (1999). Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283, 845–848 [DOI] [PubMed] [Google Scholar]
- Petit I., Szyper-Kravitz M., Nagler A., et al. (2002). G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 3, 687–694 [DOI] [PubMed] [Google Scholar]
- Pujal J.M., and Gallardo D. (2008). PCR-based methodology for molecular microchimerism detection and quantification. Exp. Biol. Med. (Maywood) 233, 1161–1170 [DOI] [PubMed] [Google Scholar]
- Rocha V., Wagner J.E., Jr., Sobocinski K.A., et al. (2000). Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N. Engl. J. Med. 342, 1846–1854 [DOI] [PubMed] [Google Scholar]
- Schmidt M., Schwarzwaelder K., Bartholomae C., et al. (2007). High-resolution insertion-site analysis by linear amplification-mediated PCR (LAM-PCR). Nat. Methods 4, 1051–1057 [DOI] [PubMed] [Google Scholar]
- Shier L.R., Schultz K.R., Imren S., et al. (2004). Differential effects of granulocyte colony-stimulating factor on marrow- and blood-derived hematopoietic and immune cell populations in healthy human donors. Biol. Blood Marrow Transplant. 10, 624–634 [DOI] [PubMed] [Google Scholar]
- Spiegel A., Shivtiel S., Kalinkovich A., et al. (2007). Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat. Immunol. 8, 1123–1131 [DOI] [PubMed] [Google Scholar]
- Stewart F.M., Zhong S., Wuu J., et al. (1998). Lymphohematopoietic engraftment in minimally myeloablated hosts. Blood 91, 3681–3687 [PubMed] [Google Scholar]
- Tomita Y., Sachs D.H., and Sykes M. (1994). Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood 83, 939–948 [PubMed] [Google Scholar]
- Wagemaker G., Visser T.P., and Van Bekkum D.W. (1986). Cure of murine thalassemia by bone marrow transplantation without eradication of endogenous stem cells. Transplantation 42, 248–251 [DOI] [PubMed] [Google Scholar]
- Weerkamp F., Baert M.R., Brugman M.H., et al. (2006). Human thymus contains multipotent progenitors with T/B lymphoid, myeloid, and erythroid lineage potential. Blood 107, 3131–3137 [DOI] [PubMed] [Google Scholar]
- Whetton A.D., and Graham G.J. (1999). Homing and mobilization in the stem cell niche. Trends Cell Biol 9, 233–238 [DOI] [PubMed] [Google Scholar]
- Winkler I.G., and Levesque J.P. (2006). Mechanisms of hematopoietic stem cell mobilization: when innate immunity assails the cells that make blood and bone. Exp. Hematol. 34, 996–1009 [DOI] [PubMed] [Google Scholar]
- Winkler I.G., Sims A.N., Pettit A.R., et al. (2010). Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116, 4815–4828 [DOI] [PubMed] [Google Scholar]
- Winkler I.G., Pettit A.R., Raggatt L.J., et al. (2012). Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia 26, 1594–1601 [DOI] [PubMed] [Google Scholar]
- Wognum A.W., Visser T.P., Peters K., et al. (2000). Stimulation of mouse bone marrow cells with kit ligand, FLT3 ligand, and thrombopoietin leads to efficient retrovirus-mediated gene transfer to stem cells, whereas interleukin 3 and interleukin 11 reduce transduction of short- and long-term repopulating cells. Hum. Gene Ther. 11, 2129–2141 [DOI] [PubMed] [Google Scholar]
- Zhang C.C., Kaba M., Ge G., et al. (2006). Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat. Med. 12, 240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.