Abstract
Catheter devices allow physicians to access the inside of the human body easily and painlessly through natural orifices and vessels. Although catheters allow for the delivery of fluids and drugs, the deployment of devices, and the acquisition of the measurements, they do not allow clinicians to assess the physical properties of tissue inside the body due to the tissue motion and transmission limitations of the catheter devices, including compliance, friction, and backlash. The goal of this research is to increase the tactile information available to physicians during catheter procedures by providing haptic feedback during palpation procedures. To accomplish this goal, we have developed the first motion compensated actuated catheter system that enables haptic perception of fast moving tissue structures. The actuated catheter is instrumented with a distal tip force sensor and a force feedback interface that allows users to adjust the position of the catheter while experiencing the forces on the catheter tip. The efficacy of this device and interface is evaluated through a psychophyisical study comparing how accurately users can differentiate various materials attached to a cardiac motion simulator using the haptic device and a conventional manual catheter. The results demonstrate that haptics improves a user's ability to differentiate material properties and decreases the total number of errors by 50% over the manual catheter system.
Keywords: Haptics, Medical Robotics, Catheter guidance, Psychophysics
1 Introduction
Catheters are thin, flexible wires and tubes that give clinicians access to the inside of the human body via natural conduits like blood vessels or the urethra. Cardiac catheters are used to provide a large range of diagnostic and interventional procedures inside the heart, including measuring intracardiac pressures, electrophysiology, deploying stents, and dilating stenotic valves [1]. A new class of robotic cardiac catheters, such as the Artisan Control Catheter (Hansen Medical, Mountain View CA, USA) and the CorPath Vascular Robotic System (Corindus Vascular Robotics, Natick MA, USA), permit a human operator to control the positioning of a catheter in the lateral direction and advance it through the vasculature [2],[3]. While these devices do enable position control of catheters inside they heart, they do not increase the physician's perception of the cardiac tissue properties or the environmental conditions inside the heart. It is the objective of our work to evaluate how haptics can improve human perception of tissue properties during catheter procedures.
The long, flexible nature of cardiac catheters that makes them easy to snake into the body also makes them poor at transferring force feedback information to the operator. As a catheter tool makes contact with the tissue, the contact force is balanced by the catheter compliance and frictional losses from seals and viscous fluids. By removing these limitations and giving the clinicians tactile information about the forces at the tip of the catheter, a range of new diagnostic and interventional procedures might become possible with catheters. Haptic feedback would also increase the information available to the clinician beyond what is currently provided by x-ray or ultrasound imaging (Figure 1). For example, a catheter could be used to palpate and examine tissue around a valve in the heart to determine if it is calcified or stenotic and if further procedures are required [4]. Another application where haptic feedback would be useful is in percutaneous device deployment. When inserting a cardiac defect closure device, haptics could inform the cardiologist if the device is positioned correctly and what is the condition of the tissue around the defect.
Figure 1.

Ultrasound image showing the mitral valve annulus, mitral valve leaflets, and the catheter device.
To evaluate the efficacy of the haptic catheter system presented here, a psychophysical study was conducted to determine the stiffness of various materials using the haptic system and a manual device. The human perception of stimuli for all of the senses has been studied extensively [5]. The perception of material stiffness, which combines the tactile perception of both force and displacement, has been investigated with a number of approaches. Jones and Hunter examined a user's perception of stiffness by allowing subjects to adjust the amount of stiffness they experienced until the value matched a reference stiffness. This experiment found the substantial Weber fraction of 0.23 for human perception of stiffness [6]. Srinivasan and LaMotte investigated the tactile discrimination of material stiffness by examining the physiology of the human fingerpad [7]. This research explored how the mechanics of the fingerpad and the tactile approach affects the human perception of different stiffness materials. LaMotte conducted further research to examine how using a stylus effected the sensations on the fingerpad and the ability to discriminate material stiffness [8].
Other researchers have used haptics to enhance the functionality of catheters. For example, the HapCath presented in [9] is a unactuated device designed to allow clinicians to perform catheterization procedures with a reduced amount of x-ray imaging by feeling the forces at the tip of the catheter. The Hansen Medical catheter also provides some amount of force feedback. The system provides the clinician with force measurements on a visual display and vibrotactile feedback though the user interface during cardiac electrophysiology procedures [10]. Haptics have also been applied to catheter training simulators to provide force feedback, which clinicians say is important for successful training [11],[12]. An example of a commercial catheter training simulator is the CathSimVR simulator (CAE, Montreal, Quebec, Canada). However, no prior work has investigated how haptics improves a user's psychophysical perception of the moving tissues inside the heart.
In previous work we showed that motion compensation and haptic noise cancelation increase force sensitivity when interacting with moving tissue [13]. Motion compensation virtually freezes the target tissue relative to the actuated tool by commanding the end effector to match the target trajectory. This previous study used a rigid actuated instrument to investigate how motion compensation improves a user's ability to feel a moving target by removing the haptic noise caused by the motion [13]. We have extended these benefits to catheters, which introduces a number of new technical challenges, including the need for a miniature catheter tip force sensor and the catheter performance limitations of friction and backlash [14].
This study presents an actuated catheter system that enables haptic perception of fast-moving intracardiac structures and demonstrates the importance of haptic feedback and motion compensation in order to perceive moving tissue properties. To evaluate this system, we conducted a series of material stiffness discrimination experiments that simulate palpating moving tissue around the mitral valve. The following paper describes the motion compensated catheter system, the haptic feedback interface and the experimental evaluation procedure. To the authors' best knowledge, this catheter system is the first device to allow haptic perception of beating cardiac tissue through the use of motion compensation. The evaluation results show that while some limitations exist, haptics and motion compensation improve a user's ability to discern material stiffness using a catheter.
2 Methods
2.1 Device Design
The haptic system presented here transmits force feedback from the tip of an actuated catheter tracking the fast motion of intracardiac tissue structures. The haptic interface adjusts the position offset of the motion compensation controller as it commands the actuated catheter system to compensate for the cardiac motion. Figure 2 presents a diagram of the catheter system design, described in detail in [14]. In the full clinical system, the cardiologist points the catheter at the cardiac structure of interest and the 3D ultrasound (3DUS) data is sent to the real time visual servoing system that tracks the target tissue in front of the catheter tip (Figure 2). This trajectory is then sent to catheter controller that compensates for the performance limitations in the catheter module to drive the catheter tip to track the tissue or apply a near-constant contact force on the moving intracardiac surface despite motions of 1-2 cm in under 100 ms [15]-[17].
Figure 2.

The catheter system consists of a drive system, a catheter module, and a 3DUS visual servoing system. The system compensates for the fast motion of the cardiac tissue while the surgeon performs the repair procedure.
In this study, a sensor on the motion simulator target provided the position information to the catheter control system to enable motion compensation instead of 3DUS imaging, which has been demonstrated in previous experiments [15],[16].
2.1.1 Actuated Catheter System
The catheter drive system, shown in Figure 3, consists of a linear voice coil actuator with 50.8 mm of travel and a peak force of 26.7 N (NCC20-18-02-1X, H2W Technologies Inc, Valencia CA, USA), a linear ball bearing slide (BX3-3, Tusk Direct, Inc., Bethel CT, USA), and a linear potentiometer position sensor (CLP13, P3America Inc., San Diego, CA, USA) [14]. The catheter module (Figure 3) is composed of a 70 cm long nylon sheath with a 2.70 mm inner diameter and an uncoated stainless steel coil guidewire with a 2.39 mm outer diameter. The catheter sheath can be flexed and bent as required by the vascular geometry. The sheath is fixed relative to the drive system while the guidewire is servoed in and out of the sheath by the drive system. The position of the catheter tip is controlled via a control system that compensated for the performance limitations of backlash and friction [14],[16].
Figure 3.

(Top) The catheter guidewire emerging from the sheath. (Bottom) The catheter drive system consists of a linear actuator, slide, and a potentiometer. The system servos the guidewire inside the fixed sheath.
2.1.2 Catheter Tip Force Sensor
The distal tip of the actuated guidewire is instrumented with a miniature force sensor that measures the compression forces applied along the length of the catheter. The sensor, described in [17], operates by converting the deformation of flexures into force signals by measuring the changes in light reflected off a surface attached to the flexures. The reflected light is transmitted via fiber optic cables down the length of the guidewire to an optical sensor (Figure 4). The sensor structure is fabricated using high-precision 3D printing (Connex500, Objet Geometries Ltd, Billerica, MA, USA). The sensor has a maximum force range of 10 N and an accuracy of 2%. Figure 4 shows a solid model of the sensor and an image of the final product.
Figure 4.

The catheter tip force sensor design and prototype.
2.1.3 Haptic Interface
The haptic device used in this study is a single degree of freedom mechanism that allows the user to input the desired catheter position set point while reflecting the tip forces back to the user. The device, shown in Figure 5, consists of a linear voice coil actuator (NCC20-18-020-1X, H2W Technologies, Inc., Valencia, CA, USA), a linear potentiometer (CLP13, P3 America, San Diego, CA, USA), a linear ball slide bearing (BX4-3, Tusk Direct, Inc., Bethel, CT, USA), and a handle fabricated from a 1.4 cm diameter plastic tube.
Figure 5.

The actuated catheter haptic interface.
The haptic interface operates as a bilateral force reflection interface with no delay: position feedforward and force feedback. The position input from the potentiometer on the interface is added to the motion compensation trajectory to adjust the position of the catheter end effector. In this way, the device functions like a joystick that adjusts the static position offset of the catheter. The forces measured by the catheter tip force sensor are reflected back to the user's hand through signals sent to the voice coil actuator in the haptic device. See [19] for a more detailed explanation of bilateral teleoperation.
The forces displayed by the haptic interface are linearly increased by a gain of two to improve the user's ability to differentiate softer forces from the intrinsic friction in the haptic device. The inertial effects on the interface are not addressed here because they contribute small forces relative to the force feedback at the velocities experienced in this study. Custom C++ code is used to control both the catheter drive system and the haptic interface and make measurements via a data acquisition card at 1 kHz. Commands to the actuators are amplified by a linear current amplifier (AMPAQ, Quanser Inc., Markham, Ontario, Canada). The systems are able to provide sufficient forces (>10 N) and bandwidth (>20 Hz) for this study [15],[16].
The haptic interface was evaluated and shown to accurately display the forces sensed by the tip force sensor. Figure 6 shows the force output of the haptic interface measured by a load cell as a function of a sinusoidal catheter force sensor input. The limitations of the haptic interface output are primarily due to friction and stiction effects in the voice coil and potentiometer. These effects are most pronounced when the force signals change direction, resulting in the sawtooth-like profile in the force output trajectory (Figure 6). The interface's force output is also affected by the limitations of the force sensor and the motion compensation controller. These errors introduce haptic noise to the users and may confuse the material discrimination process.
Figure 6.

The haptic interface force output produced by a sinusoidal input to the catheter tip force sensor.
2.2 Validation: User Study
The psychophysical research technique employed in this work was the method of constant stimuli utilizing difference thresholds [5]. This method evaluates the subject's ability to differentiate between various stimuli with a series of randomized comparisons.
Five polyurethane foams were selected as material stimuli. The foams were characterized using a load cell (LCFD-1KG, Omega Engineering, Stamford CT; range: 10 N, accuracy: +/-0.015 N) and an indentation tool instrumented with a linear potentiometer (CLP13, P3 America, San Diego, CA, USA) and an indentation tip approximately the same dimensions as the catheters. Because the stiffness of the foam is nonlinear, the stiffness of each material was approximated by measuring the indentation depth caused by a 1 N force, which serves as a linear approximation of the stiffness near the average force applied by users during the experiment. The foam stiffness values range from 0.17 – 0.42 N/mm, which we have found encompasses the stiffness range of the majority of tissues in the human heart.
2.2.1 Manual Catheter
The manual catheter system in this experiment consisted of a clinical fixed-core straight wire guide (0.9 mm outer diameter) and a plastic sheath (4.3mm outer diameter, 3.8 mm inner diameter). The proximal end of the catheter is shown in Figure 7. The sheath has two fluid seals to allow the catheter to be pressurized with saline and to prevent blood from flowing out of the vasculature. The difference in the diameters and the seals introduced both friction and backlash to the manual catheter system. These behaviors are common in clinical catheter systems. We have shown in previous work how these limitations impact catheter performance [14].
Figure 7.

The proximal end of the manual catheter system. The manual guidewire is inside the fluid seal attached to the sheath.
While the passive mechanics of the actuated and manual catheter are not identical, both systems exhibit backlash and friction. However, the actuated catheter compensation controller greatly reduces the effects of these limitations as shown in [14]. Therefore, the passive mechanics of the actuated catheter system do not significantly impact the system performance, regardless of the amount of friction or backlash.
2.2.2 Study Method
The study employed the method of constant stimuli to examine how subjects are able to discriminate between materials of varying stiffness using the manual catheter and the haptic interface [5]. The subject group consisted of 7 subjects (6 male, 1 female), ages 24-30. None of the subjects had previous experience manipulating cardiac catheters or interacting with the haptic interface used in this study.
First, the subjects were briefed on the motivation and background of the study. After the introduction, they were shown examples of the foam materials, instructed to practice palpating them with a rigid stylus, and then trained on the two catheter devices. Training consisted of palpating the foam target with the manual and actuated guidewire with and without visual feedback. The users were then asked to compare two materials that represented the extremes of the stiffness range used in the study. If they were able to differentiate the extremes from the central control material with both catheter systems, it was determined that they were ready to proceed. Because of the subjects' unfamiliarity with both the interfaces and the palpation task, additional training was conducted on a static target using both the manual and haptic interfaces without visual feedback.
The results presented below are from the more realistic configurations where the target simulates the motion of the beating heart. The trajectory of the target, shown in Figure 8, is generated by a cam mechanism that reproduces the motion of a human mitral valve annulus taken from 3D ultrasound data [18]. The evaluation materials were attached to the moving target and translated along the motion trajectory during the study.
Figure 8.

The mitral valve motion simulator trajectory.
In each trial, the subjects were presented with two materials in a randomized order to sequentially palpate: a comparison material that was varied for each trial and a control material that was held constant. Based on the interactions with the materials, the subject was then asked to state which of the two samples they perceived to be softer. They also had the option to repeat the materials if they were uncertain or could not decide which was softer. For each interface, the subjects evaluated ten pairs of materials. The forces applied to the target were recorded for the first five comparisons of each configuration. The order of the five materials and whether the control material was first or second was randomized for each user for each configuration to reduce the impact of time errors, the effect of the subject's fading mental image of the previous stimulus [5]. The five material samples were aligned so that they were the same height and the target was designed to allow the materials to be quickly repositioned (<2 s) to minimize the time delays between each material evaluation trial. After all of the trials were completed, the users were asked for their feedback and evaluation confidence for both of the interfaces on a 1-10 scale. The entire experiment took approximately 1 hour per subject and was approved by the university institutional review board (IRB).
3 Results
All of the subjects employed similar techniques to conduct the discrimination tasks. In each trial, the users slowly pushed the tool into the material and then retracted it. An example input trajectory is seen in the haptic device position plot in Figure 10. In addition to haptic feedback, the actuated catheter system also automatically compensates for the motion of the target to maintain a constant distance between the catheter tip and the target [14]. In the manual catheter case, the users moved the guidewire into contact with the moving target, resulting in the force profile seen in Figure 9. In this configuration, the target's motion caused the guidewire to buckle and apply unintended forces against the target in a cyclic manner. The subjects did not directly perceive all of the forces experienced at the guidewire tip due to the friction in the catheter and the buckling compliance of the guidewire.
Figure 10.

Example results using the haptic interface and motion compensation catheter. (Top) The force applied to the moving target by the actuated catheter. (Bottom) The target trajectory and haptic interface position input.
Figure 9.

Example results using the manual catheter. (Top) The force applied to the moving target by the manual catheter. (Bottom) The target trajectory (the haptic device is not used in the manual case.
The motion compensation system greatly reduces the effects of the target motion on the user's perception. As shown in Figure 10, the motion compensation removed the effects of the target motion and allowed the user to apply a compression force via the user-inputted displacement of the guidewire. While the users were able to apply a more controlled force on the target, they also experienced more haptic feedback from the interface. The interface transmitted both the intentionally applied contact forces and haptic noise forces created by imperfect tracking of the target trajectory. The catheter position control system is able track the target motion with less than 1 mm RMS errors [14], but these small tracking errors introduce the periodic force spikes seen in Figure 10.
The results for all of the subjects are plotted in Figure 11 and Figure 12 The data is expressed as the fraction of subjects who found the comparison material to be stiffer than the control material. The stiffness of control stimulus is 0.31 N/mm. For the case where the control material was compared against itself (the control-control comparison), the data is displayed as the percent of subjects that perceived the first exposure to the control material to be stiffer than the second exposure. A perfect differentiation of the materials, which is possible when directly palpating the materials with one's finger, is 0% stiffer for the two softer materials, 50% stiffer for the control-control material comparison case, and 100% stiffer for the two stiffer materials.
Figure 11.
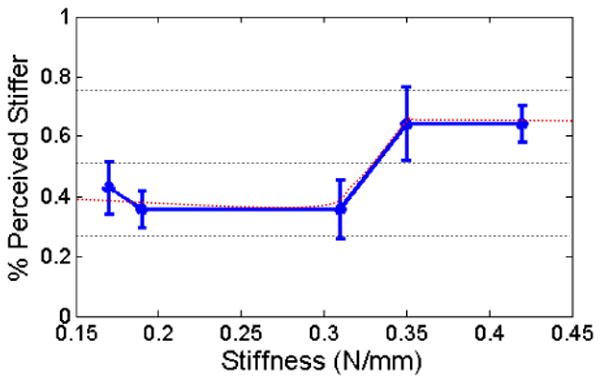
The averaged results for the manual catheter and the moving target. The error bars are the standard error.
Figure 12.

The averaged results for the actuated catheter system and the moving target with the difference threshold values indicated. The error bars are the standard error.
The cumulative results for the manual and haptic catheter configurations, presented with standard error bars in Figure 11 and Figure 12, include a number of interesting results. In the manual tool case, the subjects were unable to differentiate between soft materials and the control any better than the control-control material comparisons. However, the subjects were able to detect the two stiffer materials slightly more than 60% of the time. The haptic interface, on the other hand, allowed the subjects to consistently identify the softer materials and recognize the stiffest material in 80% of the trials. For all of the configurations, the control-control material comparison was less than 50%, indicating that the users perceived their second interaction with the control material to be stiffer than the first exposure.
A method for evaluating the sensitivity of subjects to different stimuli is to determine for the lower and upper difference thresholds, the distance from the 50% stimulus value (the point of subjective equality) to the 25% and 75% stimulus values, respectively [5]. For the manual tool case, the continuous approximation of the subject data never reaches a discrimination accuracy of 25% or 75%. Thus, the upper and lower difference thresholds are both infinite (Figure 11). For the haptic interface results, a lower difference threshold of 0.06 N/mm and an upper difference threshold of 0.07 N/mm were approximated from the results, as shown in Figure 12.
The statistical significance of the results was analyzed by performing a two-sample t-test on the results in Figure 11 and Figure 12 comparing the control-control probability for each interface with the probability of the other materials. For the manual catheter (Figure 11), the majority of the results with were not statistically significant (p-values < 0.05). The p-values from softest to hardest materials were 0.59, 1.00, 0.079, and 0.02. Only the stiffest material was distinct. The haptic interface results (Figure 12), on the other hand, produced almost all statistically significant p-values with the exception of the softest material: 0.13, 0.007, 0.03, and 0.005.
4 Discussion and Conclusions
This work confirms that haptic feedback and motion compensation improve a user's ability to distinguish material properties with a catheter tool. The results presented above show that the motion compensated catheter with a haptic interface enabled users to more accurately discriminate between different materials on a moving target than with a manual catheter. The upper and lower difference thresholds found for the haptic device were approximately 20% of the control material stiffness. No difference thresholds could be found for the manual catheter because the subjects were not accurate enough to achieve the 25% and 75% threshold values.
Another metric to evaluate the efficacy of the haptic system is to examine the average number of errors per user and the user confidence. Out of a total ten trials for each interface, the subjects average three incorrect selections with the manual catheter and 1.5 with the haptic catheter, a 50% improvement. Users were also more confident with the haptic system. The average confidence value for the manual system was 50% while the average for the haptic system was 75%.
One interesting result from this study is that the users were not able to accurately distinguish the softer materials from the control material with the manual catheter as illustrated by Figure 11 and the high p-values (p > 0.5). One possible explanation for this trend is that the backlash and friction in the manual clinical catheter make it challenging to perceive the point of contact with the target material. Users were instructed to evaluate a material by first determining the point of initial tip contact and then exploring force-displacement relationship after that point. For the soft materials, it is unclear when the manual tool first makes contact because the initial reaction force transmitted down the length of the guidewire is overshadowed by the friction forces in the catheter. Only once significant compression of the target material has been achieved does the user to feel the reaction force from target. While this phenomenon is considerably decreased with the haptic interface, the results in Figure 12 do show that a small fraction of the participant had trouble sensing the contact with the softest material.
Another interesting trend is that the control-control comparison value is approximately 40% for both the manual and haptic experiments. This means that the users perceived the second interaction with the control material to be stiffer than the first interaction. One possible explanation is that the viscoelastic properties of the material altered its mechanical response after the first interaction. This trend can also be explained by user impatience and time errors introduced by pausing between each comparison. To prevent this type of biasing, the order of the comparisons was randomized to reduce time delays and material order from affecting the rest of the data.
The results presented here were shaped by the limitations of the catheter devices and interfaces used in the study. For example, the manual guidewire is able to more clearly transmit information about stiffer materials than softer materials because the friction and backlash in guidewire-sheath system cloud the tactile information created by the light contact forces. Hard materials, on the other hand, apply more substantial reaction forces with smaller deformations, which were easier for users to sense.
The haptic system experienced a different set of limitations. Users were able to perceive the light reaction forces applied by the softer materials due to the distal tip force sensing and lower friction haptic interface. However, stiffer materials were not always accurately perceived due to the compliance of the guidewire. When applying a force to the stiffer materials with the actuated catheter, the guidewire-sheath system slightly deformed and buckled under the compressive loads. This deformation appeared to the user as the deformation of the target material. The catheter and the target material act like two springs in series, thus giving the users the perception that the stiff materials are more compliant. Robotic manipulator compliance in haptic teleoperation tasks affects the user's ability to perceive the environment and is examined in detail in [21].
The catheter compliance effect is not as noticeable for materials that are much softer than the control material because the softer material compliance dominates in those cases. The compression compliance of the catheter system in this experiment is approximately 2 N/mm at 1 N of force. The compliance of the catheter, coupled with the haptic feedback noise caused by imperfect target tracking, reduced the discrimination accuracy for the stiffer materials to approximately 80% (Figure 12).
5 Future Work
In this study we have described the first haptic motion compensated catheter system and demonstrated the importance of motion compensation while sensing the stiffness of moving cardiac tissue. While this study has explored the potential advantages and limitations of using haptics to improve catheter procedures, work is needed to successfully evaluate this concept in vivo. First, the actuated catheter system must be miniaturized to work better with clinical catheter techniques. Second, the motion compensation needs to be improved to reduce haptic noise caused by target tracking errors. Finally, the friction and compliance properties of the actuated catheter and the blood vessels need to be accurately modeled and eliminated from the haptic interface output to give the users a clear perception of the forces and displacements of the catheter tip. We have shown in previous work how catheter configuration and in vivo conditions impact catheter friction and backlash [14],[16]. We hope this system will one day allow clinicians to feel and more accurately interact with the organs and tissue structures inside the body, potentially opening up a new world of diagnostic and interventional procedures that can be achieved with a catheter.
Footnotes
Index Terms: H.5.2 [User Interfaces and Presentations]: User Interfaces—Haptics I/O; I.2.9 [Computing Methodologies]: Robotics—Manipulators
References
- 1.Baim DS. Grossman's Cardiac Catheterization, Angiography, and Intervention. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 2.Camarillo DB, Milne CF, Carlson CR, Zinn MR, Salisbury JK. Mechanics Modeling of Tendon-Driven Continuum Manipulators. IEEE Trans Robotics. 2008;24:1262–1273. [Google Scholar]
- 3.Beyar R. Navigation within the heart and vessels in clinical practice. Annals of NY Acad of Sci. 2010;1188:207–218. doi: 10.1111/j.1749-6632.2009.05102.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohn LH. Cardiac Surgery in the Adult. McGraw-Hill; New York: 2007. pp. 973–1012. [Google Scholar]
- 5.Geschieder GA. Psychophysics: Method and Theory. Lawrence Erlbaum Associates, Inc.; Hillsdale, New Jersey: 1976. [Google Scholar]
- 6.Jones LA, Hunter IW. A perceptual analysis of stiffness. Exp Brain Res. 1990;79:150–156. doi: 10.1007/BF00228884. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan MA, LaMotte RH. Tactual Discrimination of Softness. J Neurophysiology. 1995;73:88–101. doi: 10.1152/jn.1995.73.1.88. [DOI] [PubMed] [Google Scholar]
- 8.LaMotte RH. Softness discrimination with a tool. J Neurophysiology. 2000;83:1777–1786. doi: 10.1152/jn.2000.83.4.1777. [DOI] [PubMed] [Google Scholar]
- 9.Meiß T, et al. Proc of World Haptics. Salt Lake City, USA: 2009. Intravascular palpation and haptic feedback during angioplasty. [Google Scholar]
- 10.Okumura Y, Johnson S, Packer D. An analysis of catheter tip/tissue contact force-induced distortion of three-dimensional electroanatomical mapping created using the sensei robotic catheter system. Heart Rhythm. 2007;4 [Google Scholar]
- 11.Gallagher AG, Cates CU. Virtual reality training for the operating room and cardiac catheterisation laboratory. The Lancet. 2004 October;364:1538–1540. doi: 10.1016/S0140-6736(04)17278-4. [DOI] [PubMed] [Google Scholar]
- 12.Dawson SL, et al. Designing a computer-based simulator for interventional cardiology training. Catheterization and Cardiovascular Intervention. 2000;51:522–527. doi: 10.1002/1522-726x(200012)51:4<522::aid-ccd30>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Yuen Shelten G, Dubec Karl-Alex, Howe Robert D. Haptic Noise Cancellation: Restoring Force Perception in Robotically-Assisted Beating Heart Surgery. Proc of IEEE Haptics Symposium; Waltham. March 25-26, 2010; pp. 387–392. [Google Scholar]
- 14.Kesner SB, Howe RD. Design and Control of Motion Compensation Cardiac Catheters. Proc of IEEE Int Conf Robotics and Automation. 2010:1059–1065. doi: 10.1109/ROBOT.2010.5509250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuen SG, Kettler DT, Novotny PM, Plowes RD, Howe RD. Robotic Motion Compensation for Beating Heart Intracardiac Surgery. Int J Robotics Research. 2009;28(no. 10):1355–1372. doi: 10.1177/0278364909104065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesner SB, Yuen SG, Howe RD. Proc of Information Processing and Computer-Aided Intervention. Vol. 6135. LCNS; 2010. Ultrasound Servoing of Catheters for Beating Heart Valve Repair; pp. 168–178. [Google Scholar]
- 17.Kesner SB, Howe RD. Force Control of Flexible Catheter Robots for Beating Heart Surgery. Proc of IEEE Int Conf Robotics and Automation; Shanghai, China. 2011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesner SB, Howe RD. Design Principles for the Rapid Prototyping of Forces Sensors using 3D Printing. pre-print in review, submitted November, 2010. (available: http://biorobotics.harvard.edu/pubs.html)
- 19.Hokayem PF, Spong MW. Bilateral teleoperation: An historical survey. Automatica. 2006;42:2035–2057. [Google Scholar]
- 20.Kettler DT, et al. An active motion compensation instrument for beating heart mitral valve surgery. Proc of IEEE/RSJ Int Conf Intelligent Robots and Systems. 2007:1290–1295. [Google Scholar]
- 21.Tavakoli M, Howe RD. Haptic Effects of Surgical Teleoperator Flexibility. International Journal of Robotics Research, Special Issue on Medical Robotics. 2009;28:1289–1302. [Google Scholar]


