Abstract
Matrix assisted laser desorption ionization imaging mass spectrometry (MALDI IMS) has the ability to provide an enormous amount of information on the abundances and spatial distributions of molecules within biological tissues. The rapid progress in the development of this technology significantly improves our ability to analyze smaller and smaller areas and features within tissues. The mammalian eye has evolved over millions of years to become an essential asset for survival, providing important sensory input of an organism’s surroundings. The highly complex sensory retina of the eye is comprised of numerous cell types organized into specific layers with varying dimensions, the thinnest of which is the 10 μm retinal pigment epithelium (RPE). This single cell layer and the photoreceptor layer contain the complex biochemical machinery required to convert photons of light into electrical signals that are transported to the brain by axons of retinal ganglion cells. Diseases of the retina including age related macular degeneration (AMD), retinitis pigmentosa, and diabetic retinopathy occur when the functions of these cells are interrupted by molecular processes that are not fully understood. In this report, we demonstrate the use of high spatial resolution MALDI IMS and FT-ICR tandem mass spectrometry in the Abca4−/− knockout mouse model of Stargardt disease, a juvenile onset form of macular degeneration. The spatial distributions and identity of lipid and retinoid metabolites are shown to be unique to specific retinal cell layers.
Introduction
The mammalian retina is a highly complex tissue capable of converting light of various wavelengths into signals that can be interpreted by the brain to produce images. The complex anatomy of the retina is formed from a rich diversity of cells including light sensing neurons not found anywhere else in the body [1]. The layered structure of the mammalian retina was first studied in depth more than 100 years ago, although Leonardo Da Vinci depicted layers in the eye in his anatomical studies dating back as early as the Fifteenth century [2]. Within these layers, the distinct cell types of the retina have well-defined physiological functions, the loss of which can result in eye diseases that cause loss of vision such as age-related macular degeneration (AMD) [3], diabetic retinopathy [4,5], Stargardt’s disease [6-8], and retinitis pigmentosa [9].
MALDI IMS technology has rapidly progressed over the past decade, with significant improvements seen in instrumentation [10-12], laser technology [13-15], and sample preparation [16-24] techniques. These advances provide for increased sensitivity, reduced acquisition time, and greater spatial resolution. Furthermore, these improvements have broadened the application of MALDI IMS to biological tissues of smaller sample size and feature sizes than was previously possible [25-27].
Previous studies of lipid distributions in cross sections of retinal tissue using MALDI IMS included mouse [28], salamander [29], and pig tissues [30]. Hayasaka et al. utilized a MALDI QIT-TOF instrument and observed a number of phospholipid species distributed in the varying layers of a mouse retina at a spatial resolution of 50 μm [28]. The identities of the lipid species were confirmed using MS/MS analysis on the same sections. Roy et al. utilized atmospheric pressure MALDI IMS to observe phospholipid species in a salamander retina at high spatial resolution (8 μm). Images generated from signals unique to the outer and inner plexiform layer and signals originating from the inner and outer segments of the photoreceptors and RPE region were observed. Lipid species present in the salamander retina were identified using LCESI MS/MS analysis [29]. Previously published work using negative ion mode analysis of rat and human retinal tissue had been performed using chloroform/methanol extraction of homogenized tissue followed by LC-MS [31, 32] to identify retinal lipids. However, since the whole tissue was homogenized the spatial distribution of these lipids in relation to the cell types of the retina was lost.
The Abca4 gene (also known as abcr) was identified to be mutated in patients with Stargardt’s disease, a juvenile form of macular degeneration [6-8]. Functionally, the protein is thought to be a retinoid flippase [33]. Mice lacking the Abca4 gene have been shown to have elevated levels of phosphatidylethanolamine (PE) in the photoreceptor cell outer segments and accumulate retinoid metabolites such as N-retinylidene-N-retinylethanolamine (A2E) in the retinal pigment epithelium (RPE) following photoreceptor phagocytosis [34]. These highly lipophilic side products of the retinoid visual cycle are major components of lipofuscin and can be toxic to cells [35-38]. Therefore, the processes involving retinoid regeneration are of great interest for the understanding of retinal degenerative diseases [39-42]. For the purpose of the present work, the high abundance of A2E in the RPE layer provides definition of this single cell layer in MALDI IMS experiments.
Here we present data from Abca4−/− mouse retina imaged at high spatial resolution (10 μm) using sagittal sections of whole mouse eyes in both positive and negative ion mode. The images display numerous lipid species present in the various cell layers and bis-retinoid molecules observed in the RPE layer. Lipid identification was performed on adjacent sections using high mass accuracy measurements and tandem mass spectrometry (MS/MS) data in an FT-ICR instrument. Two of the basic processes of vision, the retinoid visual cycle and photoreceptor outer segment phagocytosis by the RPE, involve the transport and processing of a large amount of lipids and lipophilic retinoids between the photoreceptors and the single layer of RPE cells. Many of these molecules, which play important biological roles in the normal function of retinal tissue, can be altered in the Abca4−/− mouse.
These experiments provide new evidence on the distributions of these crucial molecules in single cell layers, demonstrating that high spatial resolution MALDI IMS can now be successfully utilized to gain valuable information on details of the types of molecules and retinal metabolites present under conditions that lead to macular degeneration in humans.
Experimental
Abca4−/− mice were from established colonies at the Medical University of South Carolina that originated from breeding pairs generously provided by Dr. G.H. Travis. The background strain was Sv129. Animals were reared in cyclic light with a 12-h light cycle (06:00-18:00). All animal procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and were consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. 3-5 month old animals were dark-adapted overnight, sacrificed under dim red light and the eyes were removed. Frozen eyes were shipped from Charleston to Nashville on dry ice for experiments.
Whole Abca4−/− 3-5 month old mouse eyes were rapidly frozen in 1% carboxymethylcellulose [43] (Sigma Aldrich, St. Louis, MO, USA) by placing the eye into a well, fashioned from parafilm, that was placed in a polystyrene container containing dry ice. 12 μm sections were obtained (see supplementary figure 1 for orientation and region of the eye sectioned) with a cryostat (Leica CM3050S, IL, USA) at −20 °C and thaw mounted onto a gold coated MALDI target plate (AB Sciex, Ontario, Canada). The sections were dehydrated in a vacuum desiccator. 2, 6-Dihydroxyacetophenone (DHA) (Sigma Aldrich, St. Louis, MO, USA) was sublimated [23] onto the samples using an in-house sublimation apparatus for 10 minutes at approximately 56 mTorr at 110 °C for positive mode analysis. An age matched Sv129 control retinal section was prepared using the same method. 1, 5-Diaminonaphthalene (DAN) (Sigma Aldrich, St. Louis, MO, USA) was sublimated under the same conditions for negative mode analysis.
Imaging experiments were performed using a Bruker UltrafleXtreme II (Bruker Daltonics, Bremen, Germany) equipped with a 355 nm Smartbeam II laser operated at 500 Hz. The laser was set to the minimum spot size, measured to be 9-10 um using a Zeta 20 profilometer (Zeta instruments, CA, USA.) (data not shown), and to 8% laser power corresponding to 3.1 μJ/pulse. Images were acquired with an average of 100 laser shots/pixel for positive mode analysis and 50 shot/pixel for negative ion mode analysis. Images were generated using FlexImaging 3.0 (Bruker Daltonics, Billerica, MA, USA) and data were normalized to the total ion current.
Lipid identification from adjacent sections was performed using a 9.4T Bruker SolariX FTICR mass spectrometer (Bruker Daltonics, Billerica, MA, USA) to provide both accurate mass measurements and tandem mass spectrometry. Calibration was performed with a series of phosphorus clusters prior to imaging data acquisition [44]. 0.2μL of lithium chloride (100mM) was pipetted onto the sublimated DHA surface of one section for positive ion MS/MS analysis [45]. Precursor ions were mass selected in the source region of the instrument using a linear quadrupole (m/z window: 1.0 Da). The selected ions were fragmented by SORI CID [46] (pulsed argon, 0.25s 500Hz irradiation). Isolation and fragmentation of glycerophosphoethanolamine (A2-GPE) was performed on a Thermo LTQ XL instrument (Thermo Fisher Scientific Inc., MA, USA.) equipped with a MALDI source (337 nm N2 laser) with an average of 5 laser shots and a collision energy of 30. Spectral interpretation was accomplished by using LIPID MAPS to match the accurate mass of the precursor ion and manually by interpretation of fragmentation patterns. MS/MS spectra were processed and figures generated using mMass freeware (www.mmass.org) [47] and structures were produced using Chemdraw (PerkinElmer Inc).
Results and Discussion
The quality of retina sections obtained from cryostat sectioning was found to be crucial in generating high quality MALDI images because of the low density exiguous nature of this tissue lending to disruption of the tissue morphology. Embedding the tissue in 1% carboxymethylcellulose assisted the sectioning process by providing a support material around the tissue and also by preventing compression or stretching of the retinal tissue as denser areas of the tissue such as the sclera or lens passed over the blade.
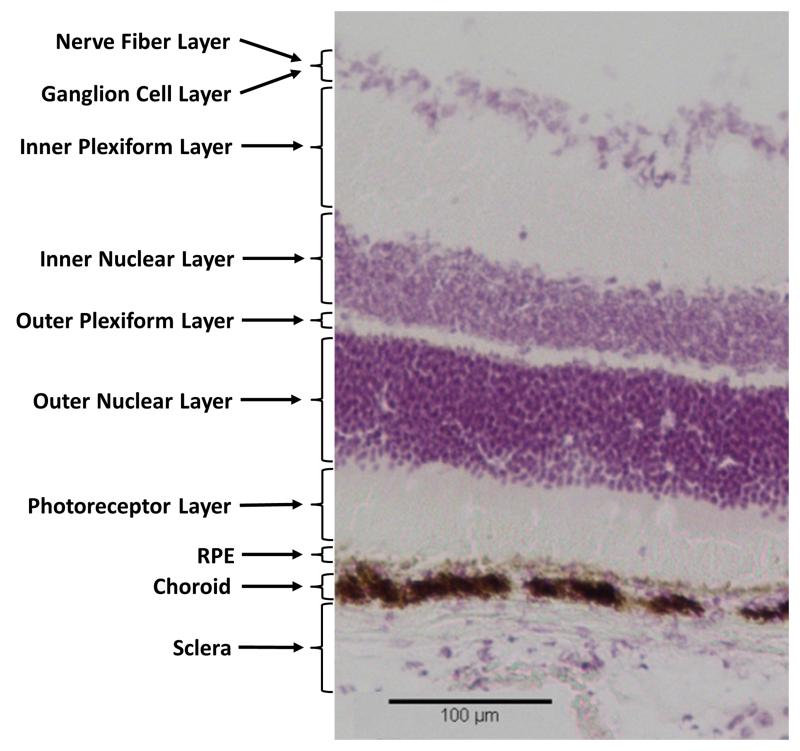
Figure 1 displays an image of H&E stained embedded C57 mouse retinal tissue section indicating the numerous cell layers and their size relative to one another. The nuclei present in the ganglion cell layer, inner nuclear layer, and outer nuclear layer are clearly visible as stained by hematoxylin. The RPE can be seen towards the bottom of the image as a thin brown line above a larger darker region of the choroid. These layers appear brown due to the pigmented cells producing melanin in this region.
Figure 1.
H&E stain of C57 mouse retinal tissue showing the multi layered structure of mouse retinal tissue.
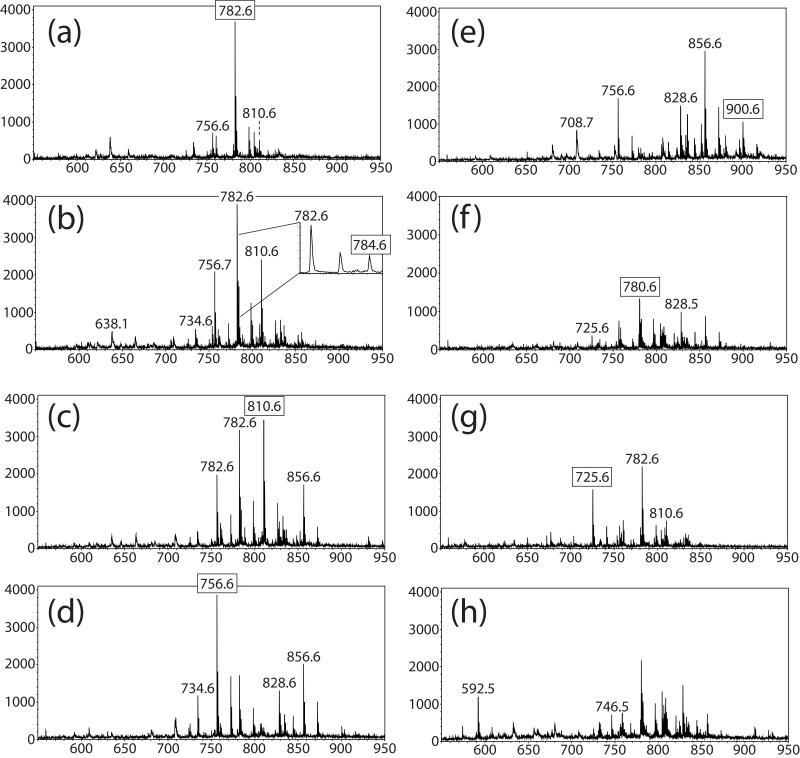
Figure 2a-i displays eight positive ion mass spectra from a retinal section from a three month old Abca4−/− mouse, each generated from a single pixel in different regions of the imaged tissue in Figure 3. These spectra show cell specific ions in the lipid mass range with varying intensities across each retinal layer with some base peaks in the mass spectra reaching intensities as high as 3500 counts. Starting from the interior of the retinal tissue the nerve fiber/ganglion cell layer towards the sclera at the outside of the eye, individual mass spectra display a number of intense signals that are unique to that layer and some which are present in several layers demonstrating the high complexity of lipid species present in this extremely organized and complex tissue. A mass spectrum from the inner nuclear layer was not displayed as the signals from the nerve fiber/ganglion cell layer and the inner nuclear layer displayed very similar molecular signatures. An expanded region in Figure 2b was included to highlight the peak observed at m/z 784.6 adjacent to the abundant peak at 782.6. The intensity of m/z 784.6 is higher than the predicted second 13C isotope of m/z 782.6 and the signals display different distributions within the tissue. The peaks labeled with boxes correspond to identified lipid species used to generate the images shown in Figure 4. The majority of peaks observed in positive ion mode correspond to sodium adducts of phosphatidylcholine lipid species since the tissue section was unwashed leaving biological salt in the tissue.
Figure 2.
Positive ion mode mass spectra from distinct cell layers observed in positive ion MALDI-TOF images (Figure 3 a-i). (a) Mass spectrum from nerve fiber/ganglion cell layer. (b) Mass spectrum from inner plexiform layer. (c) Mass spectrum from outer plexiform layer. (d) Mass spectrum from outer nuclear layer (e) mass spectrum from photoreceptor layer. (f) Mass spectrum from the choroid layer. (g) mass spectrum from the sclera. (h) mass spectrum from retinal pigment epithelium.
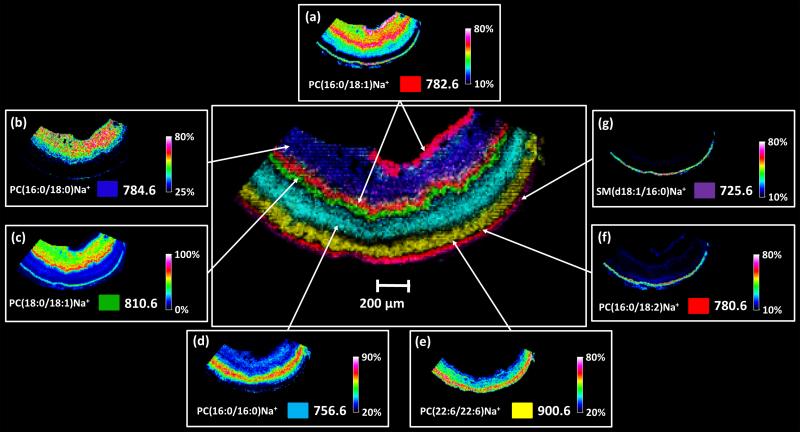
Figure 3.
Positive MALDI-TOF images of mouse Abca4−/− retina tissue displaying eight out of nine distinct layers distinguished by unique lipid signals in an overlaid image (center) along with individual images (a) m/z 782.6, (b) m/z 784.6, (c) 810.6, (d) m/z 756.6, (e) m/z 900.6, (f) m/z 780.6, and (g) m/z 725.6 which form the overlaid image.
Figure 4.
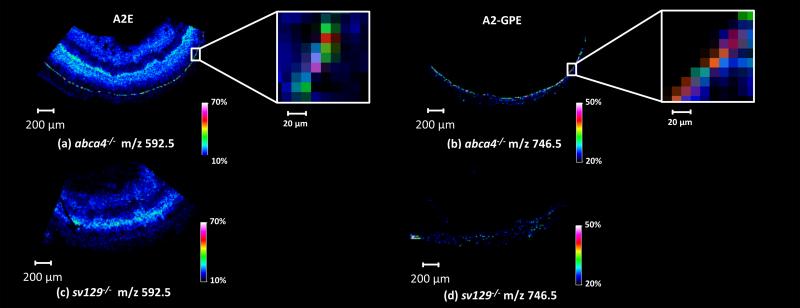
MALDI-TOF images of m/z 592.5 and 746.5 in Abca4-/- (a,b) and Sv129 (c,d) retinal tissue along with zoomed in regions (inserts) showing signals originating from the retinal pigment epithelium (RPE) region of the Abca4-/- retinal tissue.
Selected individual ion images shown in Figure 3a-g demonstrate unique distributions in different cell layers of the Abca4−/− mouse retinal tissue. Different colors representing the distribution of individual ions in the combined image (Figure 3 center) were selected to highlight the spatial relationship of each lipid in the retinal cell layers. Eight of the expected ten cell layers can be differentiated by unique lipid signals. Each of these ions were isolated and fragmented directly from tissue using an FT-ICR mass spectrometer to obtain structural identifications (see Supplementary Table 1 for details).
The MALDI IMS images shown in Figure 3 generated in positive ion mode from sodium adducts of phosphatidylcholines and one sphingomyelin species are described below. Additional “layer-specific” images of unidentified lipid signals are included in Supplementary Figures 2 and 3.
Figure 3a displays the ion image generated from m/z 782.6 identified using accurate mass and MS/MS as PC(16:0/18:1)Na+ which was observed in both the nerve fiber/ganglion cell region in high abundance as well as in lower abundance in the inner nuclear layer and the choroid/sclera region. This lipid has been previously reported in a similar location of the inner plexiform layer of the mouse retina [28]. Using the solvent free matrix application method described earlier and a higher spatial resolution acquisition method, we are able to differentiate between the inner plexiform, inner nuclear, and outer plexiform layers and observe a higher abundance in the layers adjacent to the inner plexiform layer with this example. The nerve fiber layer is comprised of axons responsible for transmitting the action potentials generated by the ganglion cells toward the optic nerve disc before joining at the optic nerve and relaying the signals to the visual cortex region of the brain. The inner nuclear layer contains the nuclei for bipolar cells and also synaptic connections leading to the nerve fiber layer. The PC(16:0/18:1) lipid species has previously been observed in mouse optic nerve tissue, spinal cord and mouse brain tissue [48, 49, 50] suggesting its association with axons of neuronal tissue.
PC(18:0/16:0)Na+ at m/z 784.6 (Figure 3b) was observed in high abundance in the inner plexiform layer and in lower abundance in the choroid/retinal pigment epithelium. Signals originating from both the plexiform layers observed in the ion image generated from m/z 810.6 in Figure 3c identified as PC(18:1/18:0)Na+. This lipid was observed at high abundance in the outer plexiform layer with lower abundance in the inner plexiform layer and retinal pigment epithelium/choroid region. The plexiform layers form the synaptic regions in the retina and are comprised of bipolar cells along with horizontal cells and amacrine cells to form synaptic connections from the photoreceptors to the ganglion cells of the nerve fiber layer [1].
Figure 3d displays an ion image generated from m/z 756.6, identified as PC(16:0/16:0)Na+ and observed in the outer nuclear layer. The relatively thick outer nuclear layer contains the cell bodies and nuclei for the numerous photoreceptors cells in the photoreceptor layer.
One signal originating from the photoreceptor layer observed at m/z 900.6 can be seen in Figure 3e was identified as PC(22:6/22:6)Na+. This lipid species contains the polyunsaturated 22:6 fatty acid chain docosahexanoic acid (DHA, omega n-3) in both the sn-1 and sn-2 positions. This unusual fatty acid is well documented to be present in rod photoreceptor membranes. [51, 52, 53]
Figure 3f displays the ion image generated from a signal at m/z 780.6 identified as PC(18:2/16:0)Na+ that was observed in the retinal pigment epithelium/choroid region. Since the retinal pigment epithelium is only 15 μm thick it is difficult to distinguish the retinal pigment epithelium from the choroid. The outermost sclera region of the eye is depicted by a signal observed at m/z 725.6 identified as a sphingomyelin SM(d18:1/16:0).
Figure 4a and b displays images from signals identified as retinoids A2E and (A2-GPE) [54] at m/z 592.5 and 746.5 respectively in the Abca-/- retinal tissue, which clearly define the RPE region from the positive ion mode data. The higher intensities observed in this region are one to two pixels across (10-20 um). This two pixel distribution is dependent on where the laser irradiates the tissue (e.g., if half the laser footprint is on the retinal pigment epithelium then a specific signal would appear in two pixels). The lower intensity signals observed in blue color surrounding the retinal pigment layer are attributed to background noise while the signal observed in the interior of the eye for m/z 592.5 are due to an interfering ion which cannot be resolved from the A2E signal with the TOF instrument. This interfering ion is apparent in figure 4c within the interior retinal layers of a Sv129 control section. A2E has been reported to be present in the neural retina, on the basis of HPLC analysis of organic extracts [33, 55-57], but its distribution across different layers is unknown. Analysis of the retinal tissue using an FT-ICR instrument revealed a number of ions between m/z 592.1 and 592.6 including m/z 592.2982, m/z 592.4510 and m/z 592.4989 which can be seen in Supplementary Figure 4. Fragmentation of these ions from the interior of the retinal tissue did not provide sufficient information for identification. Although each of these ions could not be isolated and fragmented individually, the ion at m/z 592.4510 is within 0.5 ppm of the predicted A2E mass (A2E theoretical mass = m/z 592.4513). In addition, MS/MS data collected for m/z 592.4-592.5 window in the RPE region resulted in a number of fragment ions that are consistent with the structure of A2E (Supplementary Figure 5). It should be noted that unidentified ions in the fragmentation spectrum likely result from the other ions with similar m/z values that cannot be separated from A2E. The signal obtained for A2-GPE (C45H65NO6P+) observed at m/z 746.5 (A2-GPE theoretical mass = m/z 746.4544) from the RPE was isolated and fragmented using a Thermo MALDI Duo LTQ instrument. Based on the fragmentation patterns observed, the signal observed at m/z 746.5 was confirmed to be A2-GPE (Supplementary Figure 6). Figure 4b and c display data obtained from a Sv129 control retinal section at the same m/z values, no strong signals are present in the RPE region of this tissue.
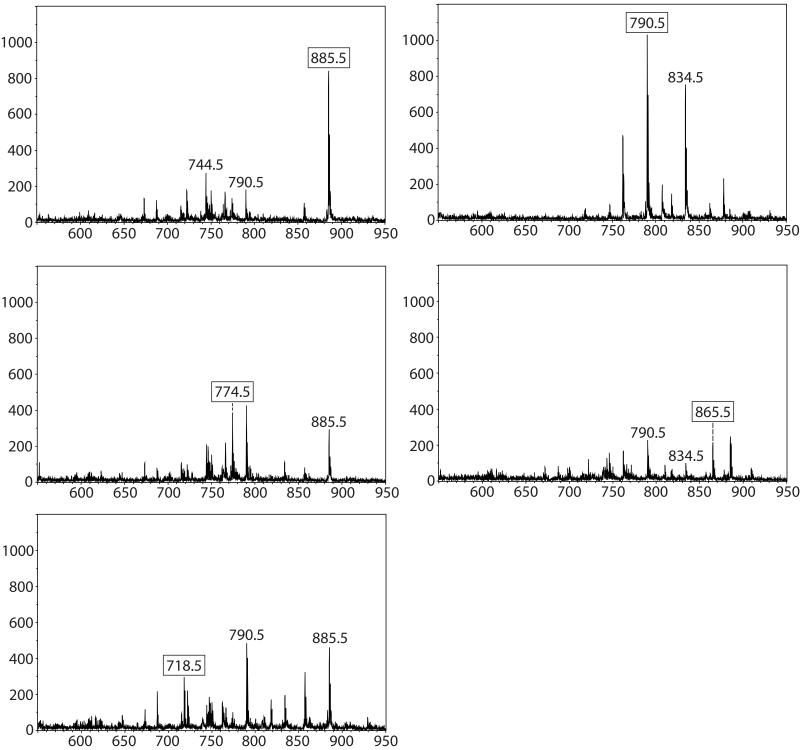
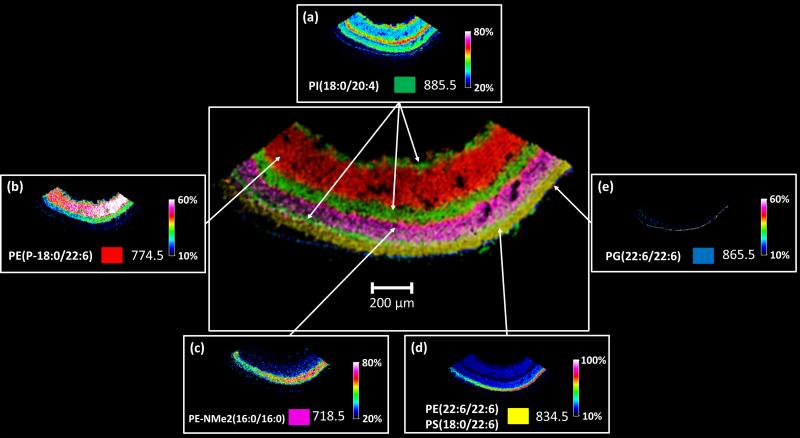
Negative ion MALDI imaging typically reveals complementary data to positive ion mode analysis indicating the presence of phosphatidylethanolamines (PEs), phosphatidylinositols (PIs), phosphatidylglycerols (PGs) and sulfatides (STs) [24]. The negative ion MALDI image data from retina tissue in general yielded similar results to the positive ion images and defined 6 distinct cell layers. Figure 5 a-e displays negative ion mode mass spectra from a five month old acba4−/− mouse, each generated from a single pixel from distinct regions in the imaged tissue in Figure 6. The negative ion mode analysis shows the distributions of a number of deprotonated lipid species in the varying cell types and layers of the retina. Again, a mass spectrum from the inner nuclear layer was not displayed since the signals from the nerve fiber/ganglion cell layer and the inner nuclear layer displayed very similar molecular signatures. No specific mass spectra with clear signals could be generated for the outer plexiform layer or the sclera using the negative ion mode. Figure 6 displays the individual ion images from selected peaks in Figure 5 surrounding a central image of overlaid ion images. Figure 6a displays the distribution of the base peak at m/z 885.5 identified as PI(18:0/20:4). Using MALDI IMS, it was found to be located in the nerve fiber/ganglion cell layer and the inner nuclear layer spanning into the outer plexiform layer of the Abca4−/− mouse retina. A very thin band can also be observed in the region between the photoreceptor layer and the outer nuclear layer. This feature can be seen in the overlay as a thin green layer. A signal observed in the inner plexiform layer with high abundance at m/z 774.5 (Figure 6b) identified as the plasmalogen PE(P-18:0/22:6). No lipid species with specific distributions in the outer plexiform layer were observed in the negative ion data. A signal observed at m/z 718.5 originating from the outer nuclear layer with high abundance can be seen in Figure 5c, identified as PE-NMe2(16:0/16:0). This lipid species is very similar to the lipid observed in positive ion mode (Figure 4d (PC(16:0/16:0)), differing only by CH3 on the head group of the lipid resulting in the PE-NMe2 compared to the PC with the same side chains in the sn-1 sn-2 position. As these lipid species are present in plant and animal tissues as precursors for the biosynthesis of PC lipids and are only observed with trace amounts, we interpret the high intensity signal at m/z 718.5 as a result of in-source fragmentation of PC(16:0/16:0), (Lipid Library, Lipid MAPS).
Figure 5.
Negative ion mode mass spectra from layers observed in negative ion MALDI-TOF image (Figure 6 a-e). (a) Mass spectrum from nerve fiber/ganglion cell layer. (b) Mass spectrum from inner plexiform layer. (c) Mass spectrum from outer nuclear layer. (d) Mass spectrum from photoreceptor layer (e) Mass spectrum from retinal pigment epithelium.
Figure 6.
Negative MALDI-TOF images of mouse Abca4−/− retina tissue displaying seven out of nine layers distinguished by unique lipid signals in an overlaid image (center) along with individual images. (a) m/z 885.5, (b) m/z 774.5, (c) m/z 718.6, (d) m/z 834.5, and (e) 865.5 which form the overlaid image.
One signal, at m/z 834.5, observed in the photoreceptor layer observed (Figure 6d) was found to be comprised from two lipids identified as PE(22:6/22:6) and PS(18:0/22:6) using high mass resolution FT-ICR. The theoretical m/z values are 834.5079 and 834.5291, respectively, that with a mass difference of 0.0212 Da are unable to be resolved with the TOF instrument.
An ion image which depicts the RPE layer (Figure 5e) revealed by a signal present in a 1-2 pixel region at m/z 865.5, was identified as PG(22:6/22:6). This lipid also contains two docosahexanoic acid side chains, has not been previously observed in retinal tissue, and could be unique to the RPE region of the Abca4−/− mouse model.
The RPE is the site of complex biochemistry related to regeneration of photopigments and this biochemistry is important in eye diseases, such as age related macular degeneration (AMD). docosahexanoic acid and arachidonic acid are found in high concentration in the retina as structural components of the photoreceptor outer segments [53] and vascular tissue of the choroid. Their purpose is more than just as structural element since docosahexanoic acid released from the membrane phospholipids by phospholipase A2 stimulated by oxidative stress is converted to neuroprotectin D1 (NPD1) by 15-lipoxgenase-1. The bioactive NPD1 acts as a potent mediator for protective, anti-inflammatory processes including induction of anti-apoptotic proteins and inhibition of pro-apoptotic proteins [58].
Conclusion
Embedding of whole eyes in carboxymethyl cellulose was beneficial to obtaining reliable sections and to preserving tissue morphology by preventing wrinkling and folding during sectioning. As MALDI IMS moves to higher spatial resolution, preservation of the original tissue morphology becomes more challenging. Sublimation of the DHA matrix provided excellent sensitivity for a number of sodium adducts of identified phosphochloline lipids and one identified sphingomyelin species in positive ion mode. The spectral quality from pixel to pixel in negative ion mode was greatly improved with the use of 1,5-diaminonaphthalene as a matrix. Negative ion mode imaging provided the spatial distributions of a number of lipid species including phosphoethanolamines, phosphoinositols and phosphoglycerols. The combination of tissue embedding, matrix application, and small laser spot size (10 μm) allowed high spatial resolution imaging and differentiation between retinal cell layers. As the Abca4−/− mouse model has been shown to accumulate high levels of retinoid metabolites in the RPE layer [8, 33, 40, 42, 59] these metabolites can be clearly observed in situ using this method. As an example, a number of signals originating from the RPE were observed with minimal interference from adjacent cell layers or manual removal of the retinal tissue during sample preparation, unlike previous homogenization or flat mounting techniques.
Further studies using this technology to compare healthy donor retinal tissue with AMD, diabetic retinopathy, and retinitis pigmentosa tissues to identify disease related lipidomic changes such as oxidation and age related glycation end products and their spatial distributions will lead to a better understanding of these diseases and identification of new therapeutic targets to slow or prevent disease progression.
Supplementary Material
Acknowledgements
This project was supported by a grant from the National Institute of General Medical Sciences (5P41 GM103391-02), formerly the National Center for Research Resources (5P41RR031461-01). The authors would also like to thank Dr. G. H. Travis for providing the original breading pair of Abca4−/− mice.
References
- 1.Mullins RF, Skeie JM. Essentials of Retinal Morphology Animals Models for Retinal Diseases. Neuromethods New York City (NY) 2010;Vol. 46 [Google Scholar]
- 2.Leonardo Da Vinci . Anatomy of the eye, section of a man’s head. Royal Library; Windsor Castle: [Google Scholar]
- 3.Tang PH, Kono M, Koutalos Y, Ablonczy Z, Crouch RK. New insights into retinoid metabolism and cycling within the retina. Prog. Retin. Eye Res. 2013;32:48–63. doi: 10.1016/j.preteyeres.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyazawa T, Nakagawa K, Shimasaki S, Nagai R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012;42:1163–70. doi: 10.1007/s00726-010-0772-3. [DOI] [PubMed] [Google Scholar]
- 5.Antonetti DA, Klein R, Gardner TW. Diabetic Retinopathy. N. Engl. J. Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 6.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 1997;15:236–46. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 7.Stone EM, Webster AR, Vandenburgh K, Streb LM, Hockey RR, Lotery AJ, Sheffield VC. Allelic variation in ABCR associated with Stargardt disease but not age-related macular degeneration. Nat. Genet. 1998;20:328–9. doi: 10.1038/3798. [DOI] [PubMed] [Google Scholar]
- 8.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 9.Phelan JK, Bok D. A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Mol. Vis. 2000;8:116–24. [PubMed] [Google Scholar]
- 10.Chaurand P, Schriver KE, Caprioli RM. Instrument design and characterization for high resolution MALDI-MS imaging of tissue sections. J. Mass Spectrom. 2007;42:476–489. doi: 10.1002/jms.1180. [DOI] [PubMed] [Google Scholar]
- 11.Jungmann JH, MacAleese L, Buijs R, Giskes F, Snaijer A, Visser J, Visschers J, Vrakking MJJ, Heeren RMA. Fast, High Resolution Mass Spectrometry Imaging Using a Medipix Pixelated Detector. J. Am. Soc. Mass Spectrom. 2010;21:2023–2030. doi: 10.1016/j.jasms.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Klerk LA, Altelaar AFM, Froesch M, McDonnell LA, Heeren RMA. Fast and automated large-area imaging MALDI mass spectrometry in microprobe and microscope mode. Int. J. Mass Spectrom. 2009;285:19–25. [Google Scholar]
- 13.Trim PJ, Djidja MC, Atkinson SJ, Oakes K, Cole LM, Anderson DM, Hart PJ, Francese S, Clench MR. Introduction of a 20 kHz Nd,YVO4 laser into a hybrid quadrupole time-of-flight mass spectrometer for MALDI-MS imaging. Anal. Bioanal. Chem. 2010;397:3409–3419. doi: 10.1007/s00216-010-3874-6. [DOI] [PubMed] [Google Scholar]
- 14.Holle A, Haase A, Kayser M, Höhndorf J. Optimizing UV laser focus profiles for improved MALDI performance. J. Mass Spectrom. 2006;41:705–716. doi: 10.1002/jms.1041. [DOI] [PubMed] [Google Scholar]
- 15.Zavalin A, Yang J, Caprioli RM. Laser beam filtration for high spatial resolution MALDI imaging mass spectrometry. J. Am. Soc. Mass Spectrom. 2013;24:1153–1156. doi: 10.1007/s13361-013-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seeley EH, Oppenheimer SR, Mi D, Chaurand P, Caprioli RM. Enhancement of Protein Sensitivity for MALDI Imaging Mass Spectrometry after Chemical Treatment of Tissue Sections. J. Am. Soc. Mass Spectrom. 2008;19:1069–1077. doi: 10.1016/j.jasms.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angel PM, Spraggins JM, Baldwin HS, Caprioli RM. Enhanced Sensitivity for High Spatial Resolution Lipid Imaging by Negative Ion Mode MALDI Imaging Mass Spectrometry. Anal. Chem. 2012;84:1557–1564. doi: 10.1021/ac202383m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas A, Charbonneau JL, Fournaise E, Chaurand P. Sublimation of New Matrix Candidates for High Spatial Resolution Imaging Mass Spectrometry of Lipids, Enhanced Information in Both Positive and Negative Polarities after 1,5-Diaminonapthalene Deposition. Anal. Chem. 2012;84:2048–2054. doi: 10.1021/ac2033547. [DOI] [PubMed] [Google Scholar]
- 19.Puolitaival SM, Burnum EK, Cornett SC, Caprioli RM. Solvent-free matrix dry-coating for MALDI imaging of phospholipids. J. Am. Soc. Mass Spectrom. 2008;19:882–886. doi: 10.1016/j.jasms.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deutskens F, Junhai Y, Caprioli RM. High spatial resolution imaging mass spectrometry and classical histology on a single tissue section. J Mass Spectrom. 2011;46:568–571. doi: 10.1002/jms.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Caprioli RM. Matrix sublimation/recrystallization for imaging proteins by mass spectrometry at high spatial resolution. Anal. Chem. 2011;83:5728–5734. doi: 10.1021/ac200998a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Caprioli RM. Matrix precoated targets for direct lipid analysis and imaging of tissue. Anal. Chem. 2013;85:2907–12. doi: 10.1021/ac303554e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hankin JA, Barkley RM, Murphy RCJ. Sublimation as a method of matrix application for mass spectrometric imaging. Am. Soc. Mass Spectrom. 2007;18:1646–1652. doi: 10.1016/j.jasms.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry KA, Hankin JA, Barkley RM, Spraggins JM, Caprioli RM, Murphy RC. MALDI imaging of lipid biochemistry in tissues by mass spectrometry. Chem. Rev. 2011;111:6491–6512. doi: 10.1021/cr200280p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zavalin A, Todd EM, Rawhouser PD, Yang J, Norris JL, Caprioli RM. Direct imaging of single cells and tissue at sub-cellular spatial resolution using transmission geometry MALDI MS. J Mass Spectrom. 2012;47:1395–1535. doi: 10.1002/jms.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schober Y, Guenther S, Spengler B, Roempp A. High-resolution matrix-assisted laser desorption/ionization imaging of tryptic peptides from tissue. Rapid Commun. Mass Spectrom. 2012;26:1141–1146. doi: 10.1002/rcm.6192. [DOI] [PubMed] [Google Scholar]
- 27.Altelaar AFM, Taban IM, McDonnell LA, Verhaert PDEM, Lange RPJ, Adan RAH, Mooi WJ, Heeren RMA, Piersma SR. High-resolution MALDI imaging mass spectrometry allows localization of peptide distributions at cellular length scales in pituitary tissue sections. Int. J. Mass Spectrom. 2007;260:203–211. [Google Scholar]
- 28.Hayasaka T, Goto-Inoue N, Sugiura Y, Zaima N, Nakanishi H, Ohishi K, Nakanishi S, Naito T, Taguchi R, Setou M. Matrix-assisted laser desorption/ionization quadrupole ion trap time-of-flight (MALDI-QIT-TOF)-based imaging mass spectrometry reveals a layered distribution of phospholipid molecular species in the mouse retina. Rapid Commun. Mass Spectrom. 2008;22:3415–3426. doi: 10.1002/rcm.3751. [DOI] [PubMed] [Google Scholar]
- 29.Roy MC, Nakanishi H, Takahashi K, Nakanishi S, Kajihara S, Hayasaka T, Setou M, Ogawa K, Taguchi R, Naito T. Salamander retina phospholipids and their localization by MALDI imaging mass spectrometry at cellular size resolution. J. Lipid Res. 2011;52:463–470. doi: 10.1194/jlr.M010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer AD, Griffiths R, Styles I, Claridge E, Calcagni A, Bunch J. Sucrose cryo-protection facilitates imaging of whole eye sections by MALDI mass spectrometry. J. Mass Spectrom. 2012;47:237–41. doi: 10.1002/jms.2049. [DOI] [PubMed] [Google Scholar]
- 31.Ford DA, Monda JK, Brush RS, Anderson RE, Richards MJ, Fliesler SJ. Lipidomic analysis of the retina in a rat model of Smith-Lemli-Opitz syndrome: alterations in docosahexaenoic acid content of phospholipid molecular species. J. Neurochem. 2008;105:1032–47. doi: 10.1111/j.1471-4159.2007.05203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acar N, Berdeaux O, Grégoire S, Cabaret S, Martine L, Gain P, Thuret G, Creuzot-Garcher CP, Bron AM, Bretillon L. Lipid composition of the human eye: are red blood cells a good mirror of retinal and optic nerve fatty acids? PLoS One. 7:e35102. doi: 10.1371/journal.pone.0035102. 012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl. Acad. Sci. U S A. 2000;97:7154–9. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–6. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 35.Rózanowska M, Wessels J, Boulton M, Burke JM, Rodgers MA, Truscott TG, Sarna T. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic. Biol. Med. 1998;24:1107–12. doi: 10.1016/s0891-5849(97)00395-x. [DOI] [PubMed] [Google Scholar]
- 36.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest. Ophthalmol Vis. Sci. 1999;40:2988–95. [PubMed] [Google Scholar]
- 37.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest. Ophthalmol. Vis. Sci. 2000;41:1981–9. [PubMed] [Google Scholar]
- 38.Klevering J, Maugeri A, Wagner A, Go SL, Vink C, Cremers FPM, Hoyng CB. Three Families Displaying the Combination of Stargardt’s Disease with Cone–RodDystrophy or Retinitis Pigmentosa. American Academy of Ophthalmology. 2004;111:546–553. doi: 10.1016/j.ophtha.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. PNAS. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grey AC, Crouch RK, Koutalos Y, Schey KL, Ablonczy Z. Spatial localization of A2E in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2011;52:3926–3933. doi: 10.1167/iovs.10-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrett TJ, Menger RF, Dawson WW, Yost RA. Lipid analysis of flat-mounted eye tissue by imaging mass spectrometry with identification of contaminants in preservation. Anal. Bioanal. Chem. 2011;401:103–113. doi: 10.1007/s00216-011-5044-x. [DOI] [PubMed] [Google Scholar]
- 42.Ablonczy Z, Higbee D, Anderson DM, Dahrouj M, Grey AC, Koutalos Y, Schey KL, Gutierrez D, Hanneken A, Crouch RK. Lack of correlation between the spatial distribution of A2E and lipofuscin fluorescence in the human RPE. Invest. Ophth. Vis. Sci. 2013;54:5535–42. doi: 10.1167/iovs.13-12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoeckli M, Staab D, Schweitzer A. Compound and metabolite distribution measured by MALDI mass spectrometric imaging in whole-body tissue sections. Int. J. Mass Spectrom. 2007;260:195–202. [Google Scholar]
- 44.Sládková K, Houška J, Havel J. Laser desorption ionization of red phosphorus clusters and their use for mass calibration in time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2009;23:3114–3118. doi: 10.1002/rcm.4230. [DOI] [PubMed] [Google Scholar]
- 45.Burnum KE, Cornett DS, Puolitaival SM, Milne SB, Myers DS, Tranguch S, Brown HA, Dey SK, Caprioli RM. Spatial and temporal alterations of phospholipids determined by mass spectrometry during mouse embryo implantation. J. Lipid Res. 2009;50:2290–2298. doi: 10.1194/jlr.M900100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrmann KA, Somogyi A, Wysocki VH, Drahos L, Vékey K. Combination of sustained off-resonance excitation in FT-ICR. Anal. Chem. 2005;77:7626–7638. doi: 10.1021/ac050828+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niedermeyer THJ, Strohalm M. mMass as a Software Tool for the Annotation of Cyclic Peptide Tandem Mass Spectra. PLoS ONE. 2012;7(9):e44913. doi: 10.1371/journal.pone.0044913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson DMG, Mills D, Spraggins J, Lambert WS, Calkins DJ, Schey KL. High Resolution MALDI-Imaging Mass Spectrometry of Lipids in Rodent Optic Nerve Tissue. Mol. Vis. 2013;19:581–92. [PMC free article] [PubMed] [Google Scholar]
- 49.Hanada M, Sugiura Y, Shino R, Masaki N, Imagama S, Ishiguro N, Matsuyama Y, Setou M. Spatiotemporal alteration of phospholipids and prostaglandins in a rat model of spinal cord injury. Anal. Bioanal. Chem. 2012;403:1873–1884. doi: 10.1007/s00216-012-5900-3. [DOI] [PubMed] [Google Scholar]
- 50.Hankin JA, Murphy RC. The relationship between MALDI IMS intensity and measured quantity of selected phospholipids in rat brain sections. Anal. Chem. 2010;82:8476–8484. doi: 10.1021/ac101079v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rotstein NP, Politi LE, Aveldaño MI. Docosahexaenoic acid promotes differentiation of developing photoreceptors in culture. Invest. Ophthalmol. Vis. Sci. 1998;39:2750–8. [PubMed] [Google Scholar]
- 52.Agbaga MP, Mandal MN, Anderson RE. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J. Lipid Res. 2010;51:1624–42. doi: 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto K, Yoon KD, Ueda K, Hashimoto M, Sparrow JR. A novel bisretinoid of retina is an adduct on glycerophosphoethanolamine. Invest. Ophthalmol. Vis. Sci. 2011;25:9084–90. doi: 10.1167/iovs.11-8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-Shabat S, Parish CA, Vollmer HR, Itagaki Y, Fishkin N, Nakanishi K, Sparrow JR. Biosynthetic studies of A2E, a major fluorophore of retinal pigment epithelial lipofuscin. J. Biol. Chem. 2002;277:7183–90. doi: 10.1074/jbc.M108981200. [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow JR. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J. Biol. Chem. 2000;275:29354–60. doi: 10.1074/jbc.M910191199. [DOI] [PubMed] [Google Scholar]
- 57.Boyer NP, Higbee D, Currin MB, Blakeley LR, Chen C, Ablonczy Z, Crouch RK, Koutalos Y. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: their origin is 11-cis-retinal. J. Biol. Chem. 2012;287:22276–86. doi: 10.1074/jbc.M111.329235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J. Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012;31:121–35. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.