Abstract
Firemaster® 550 (FM 550) is a mixture of brominated and triarylphosphate flame retardants used in polyurethane foam-based products. The primary components are also used in numerous other applications and are thus common household and industrial contaminants. Our previous animal studies suggested that FM 550 exposure may alter metabolism and cause weight gain. Employing human nuclear receptor (NR) luciferase reporter assays, the goal of this study was to evaluate the agonist actions of FM 550 and its constituent compounds at NRs with known roles in establishing or regulating energy balance. FM 550 was found to have significant agonist activity only at the master regulator of adipocyte differentiation PPARγ. As a result, the concentration response relationships and relative activities of FM 550 at PPARγ were investigated in more detail with the contribution of each chemical component defined and compared to the activities of the prototypical PPARγ environmental ligands triphenyltin and tributylytin. The resulting data indicated that the primary metabolic disruptive effects of FM 550 were likely mediated by the activity of the triarylphosphates at PPARγ, and have identified TPP as a candidate metabolic disruptor that also acts as a cytotoxicant.
Keywords: apoptosis, endocrine disruptor, flame retardant, nuclear receptors, obesity, peroxisome proliferator-activated receptor
1. Introduction
Large quantities of brominated and organophosphate chemical flame retardants are used in the manufacture of plastics, polymers, resins, and numerous other consumer goods including textiles, furniture, and electronics. The phase out of polybrominated diphenyl ethers (PBDEs) has led to the development and increasing use of alternative chemical flame retardants. A flame retardant mixture known as Firemaster® 550 (FM 550) is now commonly used in polyurethane foams found in many baby products and residential furniture from which it migrates to contaminate household dust (Stapleton et al., 2008; Stapleton et al., 2011; Stapleton et al., 2012b). Many formulations of commercial flame retardants (including FM 550) are proprietary. Recent analysis has revealed that FM 550 is a combination of triphenyl phosphate (TPP), a mixture of isopropylated triphenylphosphate isomers (ITPs), 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB), and bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate (TBPH; Figure 1) (Stapleton et al., 2012b). In large part through inhalation and ingestion of household dust, humans and children in particular, are chronically exposed to a diverse array of chemical flame retardants including the constituents of FM 550 (Stapleton et al., 2012a; Stapleton et al., 2014). As replacements for PBDEs, the brominated components of the FM 550 mixture are relatively new flame retardants that have been used increasingly over the past decade. In contrast, the triarylphosphate components of FM550 have been used individually as plasticizers, flame retardants and in a variety of industrial applications for several decades. Thus exposure to both the brominated and organophosphate components in FM 550 is ubiquitous and likely rising due to increase use in consumer goods (Stapleton et al., 2012b).
Figure 1. Chemical structures of the FM 550 components and organotin compounds used in the study.
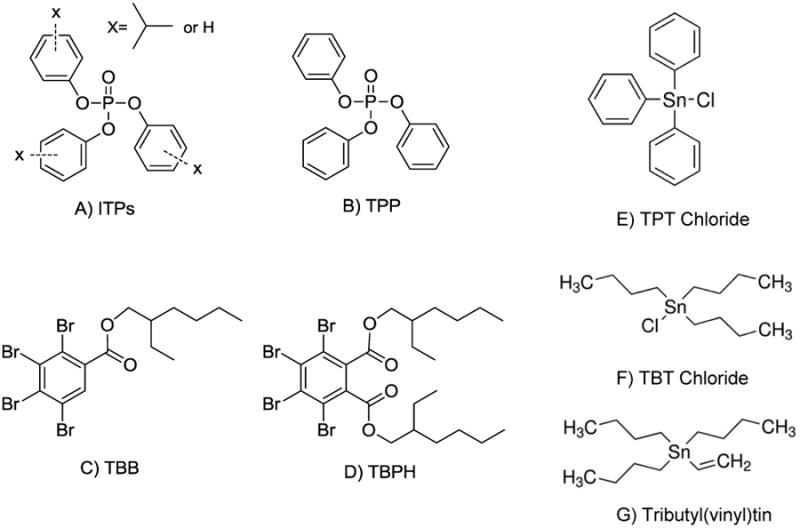
(A) isopropylated triphenylphosphate isomers (ITPs), (B) triphenyl phosphate (TPP), (C) 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB), (D) bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate (TBPH), (E) triphenyl tin chloride (TPT) (F) tributyltin chloride (TBT), (G) tributyl(vinyl)tin.
The developmental toxicity of FM 550 has been inferred from guideline reproductive, fertility, and developmental toxicity studies on a chemical mixture (CN-2065/BZ-54) containing only the brominated components TBB and TBPH. As a result, an exploratory study investigating the effects of perinatal exposure to the complete FM 550 mixture at levels below the no observable adverse effects level (NOAEL) of 50 mg/kg/day for CN-2065/BZ-54 was performed (Patisaul et al., 2013). That study found that pups born to Wistar rat dams exposed daily to oral FM 550 (3 mg/kg/day) from gestational day 6 to weaning (postnatal day 21) developed obesity, insulin resistance, elevated fasting glucose, and left ventricular (LV) hypertrophy. Those phenotypes are consistent with the hallmark features of human metabolic syndrome (Prasad et al., 2012). Because some brominated flame retardants, including PBDEs, are known to disrupt thyroid hormone signaling, the effects of FM 550 exposure on Type 1 deiodinase (Dio1) activity and circulating levels of thyroid hormones were also investigated. No effects on Dio1 activity were found, however modest effects on thyroxine (T4) levels in exposed dams and pups were observed, suggesting that the thyroid hormone axis was modified by exposure to FM 550 (Patisaul et al., 2013). Together, those findings were interpreted to indicate that early developmental exposures to FM 550 may have endocrine and metabolic disrupting actions leading to lifelong adverse health consequences related to metabolic disease (Patisaul et al., 2013).
The altered metabolic phenotypes resulting from early life FM 550 exposures are reminiscent of the actions of some organotin chemicals that act as endocrine disruptors to cause imposex and reproductive failure in aquatic and marine gastropods, and to alter adipocyte differentiation by acting as agonists of the nuclear receptor NR1C3 (also known as peroxisome proliferator-activated receptor gamma or PPARγ) in mammals (Grun and Blumberg, 2009; Iguchi et al., 2008; Janesick and Blumberg, 2011; Nakanishi, 2008). The similarity between the phenotypes resulting from FM 550 exposure, and the effects of organotin environmental pollutants suggests that early in life FM 550 may act as metabolic disruptor and contribute to obesity by inducing metabolic reprogramming (Ismail-Beigi et al., 2006). Further, the triarylphosphate components of FM 550 share chemical structure similarities with the well-characterized PPARγ agonist and metabolic disruptor triphenyl tin (TPT; compare Figure 1B and 1E). It is possible that TPP might contribute to the obesogenic effects resulting from early FM 550 exposure through a similar mechanism involving developmental dysregulation of PPARγ activity. If these triarylphosphates are endocrine disruptive, this could have broad human health ramifications because they have been used for decades as plasticizers and in many other commercial and industrial applications. TPP in particular is a high production volume chemical (10-50 million lbs/year) commonly used in polyvinyl chloride (PVC), circuit boards, hydraulic fluids, adhesives, rubbers, etc., and is a ubiquitous contaminant of residential house dust with concentrations as high as 1.8 mg/g dust (Stapleton et al., 2009).
In response to ligand binding, nuclear receptors (NRs) act primarily as homo- or heterodimers to mediate extracellular signals into specific transcriptional responses. As sensors of a variety of endogenous, nutrient or xenobiotic ligands, nuclear receptors, such as the PPARs, thyroid hormone receptors (TR), and liver X receptor (LXR), can heterodimerize with the retinoid X receptors (RXR), and act in concert to orchestrate diverse developmental and metabolic functions that regulate energy balance (Lefebvre et al., 2010; Liu and Brent, 2010). As a result, these NRs stand as possible mediators of the metabolic and obesogenic effects associated with early-life FM 550 exposure. Here we report the results of concentration response analysis of FM 550 for agonist activities at the metabolically related human nuclear receptors NR1A1 (TRα), NR1A2 (TRβ), NR1C1-C3 (PPARα,β/δ,γ), NR2B1 (RXRα), and NR1H3 (LXRα). Initially focusing on the hypothesis that FM 550 was modifying metabolically related NR agonist activity, FM 550 was found to have significant activity at PPARγ. As a result, the concentration response relationships and relative activities of FM 550 at PPARγ were investigated in more detail with the contribution of each individual chemical component assessed and compared to activities of prototypical organotin PPARγ ligands.
2. Materials and Methods
2.1 Chemicals
Firemaster® 550 (FM 550) was a gift from Chemtura Corporation (Middlebury, CT). The average molecular weight for this batch of FM 550 was estimated from the manufacture's Material Safety Data Sheet and independent analysis of FM 550 solutions (Patisaul et al., 2013; Stapleton et al., 2008). Stock solutions of FM 550 were prepared based on the estimate that FM 550 consisted of approximately: 14% bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate (TBPH); 36% 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB); 32% isopropylated triaryl phosphate isomers (ITP); 18% triphenyl phosphate (TPP). Tributyltin chloride (TBT Cl; 96%), Tributyl(vinyl)tin (TBVT; 97%) and TPP (≥99%) were from Sigma-Aldrich (St. Louis, MO). Dimethyl sulfoxide (DMSO; >99.9%) which was used as the solvent for all test chemicals, and triphenyltin chloride (TPT Cl; 95%) were from Alfa Aesar (Ward Hill, MA), TBPH (>95%) was from Ark Pharm, Inc (Libertyville, IL), ITP was from Jinan Great Chemical Industry Co., Ltd (Commercial Grade, Jinan, PRC), and TBB was purchased from Wellington Laboratories (Guelph, Ontario). The positive control agonists GW0742 (PPARβ/δ), GW590735 (PPARα), 9-cis retinoic acid (RA; RXRα), rosiglitazone (ROSI; PPARγ), TO901317 (LXRα), L-triiodothyronine (T3; TRα/TRβ), and staurosporine (STP) were supplied as DMSO stocks solutions (Indigo Biosciences, State College, PA).
2.2 Nuclear Receptor Transactivation Reporter Assays
Validated Chinese Hamster Ovary (CHO) cell line based human nuclear receptor luciferase reporter assay systems from Indigo Biosciences (State College, PA) were used for determination of the transactivation activity of FM 550, each component brominated or organophosphate flame retardant, and each organotin compound. All assays were performed according to vendor's optimized protocols. Test materials were serially diluted from DMSO stock solutions into compound screening media to yield 2× desired final concentration solutions immediately prior to each experiment. For screening of FM 550 at different NRs, concentration response relationships were analyzed over a typical concentration range of 10-12 to 10-5 M with intermediate concentrations one order of magnitude apart; intermediate concentrations of focused concentration response analysis were in increments of either 2- or 3-fold. Matching vehicle (DMSO) and positive control agonists were included with each experiment. Equal volumes of reporter cells (100 μl) were seeded into wells of opaque white 96-well tissue culture plates (Costar 3917; Corning, NY) to which an equal volume of 2× concentration test compound in compound screening media was added. Following incubation at 37°C in 5% CO2 and 100% humidity for 24 hours, treatment media was removed and cells were processed for viability determination and/or detection of luciferase activity. Luciferase reporter gene activity was measured using a multifunctional microplate reader (SpectraFluor Plus; Tecan, San Jose, CA) controlled with Magellan3 software in luminescence measurement mode (integration time set as maximum dynamic range; gain: 200).
Cytotoxicity was assessed by determining the relative number of live cells resident in each well of the assay plate at the end of treatment using a calcein-AM uptake assay optimized for use in-line with the luciferase-based transactivation reporter assays (Live Cell Multiplex Assay, Indigo Biosciences, State College, PA). In all cases, DMSO vehicle treated samples and vehicle containing media-only (3-4 replicates) controls were included for standardization of 100% viability and background levels of fluorescence. Additionally, cells treated with staurosporine (4 μM) served as a positive control for cytotoxicity. Uptake of calcein-AM (2-[[7′-[[bis(carboxymethyl)amino]methyl]-3′,6′-dihydroxy-3-oxospiro[2-benzofuran-1,9′-xanthene]-2′-yl]methyl-(carboxymethyl)amino]acetic acid) and intracellular conversion to fluorescent calcein was measured using a multifunctional microplate reader (SpectraFluor Plus; Tecan, San Jose, CA) controlled with Magellan3 software in fluorescence top mode (integration time 20μS; gain 30) with a 485nMEx/535Em filter combination.
2.3 MTS viability assay and caspase 3 activity assay
Methods for culturing D283 Med cells, assessment of viability with MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) uptake/reduction assay and the analysis of caspase 3 activity were described previously (Belcher et al., 2009; Wong et al., 2001; Wong et al., 2003). The MTS assay and caspase 3 assays were performed independent of one another. D283 Med cells were incubated for 24 hours with desired concentrations of test compound, positive (4 μM staurosporine) or negative (DMSO) controls. For each assay condition there were typically 6 replicates. For viability measurements, the MTS tetrazolium reagent was added to the media and cultures were incubated in a humidified incubator at 37°C and in 5% CO2 for an additional 2 hours. For caspase 3 measurements treated cell-lysates were prepared and caspase 3 activity was determined from 10 μg of lysate based on the liberation of a colored p-nitroaniline (pNA) from the caspase-3 substrate Ac-DEVD-pNA (1 mM) following incubation in 100 mM HEPES, 0.5 mM EDTA, 0.35% 2-mercaptoethanol and 20% Glycerol (v/v). Specific caspase 3 activity (nmol pNA/hr/mg protein) of each sample was calculated from a standard pNA curve generated from a dilution series of known concentrations of pNA. For both assays the quantity of reaction product was determined by measuring absorbance at 405 nm (caspase 3) or 492 (MTS) using a multifunctional microplate reader (SpectraFluor Plus; Tecan, San Jose, CA) controlled with Magellan3 software.
2.4 Data and Statistical Analysis
All presented NR activity data are representative of multiple experiments each containing 3-5 replicates for each concentration of test compound or positive control agonist. Matching vehicle and media blank controls were included on the same plate for each experiment. Data is reported as mean values ± SEM. Luciferase reporter assay data is reported in relative luciferase units (RLU) without normalization for cell viability unless stated otherwise. Concentration response curves and AC50 estimates were derived from RLU data from experiments using a 4-parameter variable slope model. Estimates were made from multiple experiments that contained at least one concentration resulting in a submaximal response, and the 20 nM Rosi positive control group. Where inverted U-shaped non-monotonic concentration response relationships were observed the maximal responses were assumed to represent an asymptotic maximal response for AC50 calculations. Percentage data was arcsine transformed (arcsine of the square root of the value) for statistical analysis. Statistical analysis was conducted using one-way analysis of variance (ANOVA) with post-test comparison between treatment groups and control using Dunnett's or Tukey-Kramer multiple comparison tests. A minimal level of statistical significance for differences in values was considered to be p <0.05. Data were analyzed with Excel (Microsoft) and GraphPad Prism® version 5.0 (GraphPad Software Inc.).
3. Results
3.1 Concentration response analysis screening of FM 550 for NR activity
Initial concentration response analysis was done using NR-specific response-element luciferase-reporter cell assays to screen FM 550 for agonist activity at human nuclear receptors RXRα,TRα, TRβ, PPARs α, β/δ and γ, and LXRα (Fig. 2). Significant [F(8,18) = 37.40, p < .0001] increases in PPARγ transactivation activity were detected in cells exposed to FM 550 (Fig. 2F). Maximal PPARγ activity was observed at 20 μM with a response 5.18 ± 0.32-fold greater than vehicle control. The mean effect of a 10-fold higher concentration (200 μM) was significantly greater than control, but reduced (2.47 ± 0.47-fold) compared to the effect observed at 20 μM. No significant increases in transactivation activity of RXRα, TRα, TRβ, PPARα, PPARβ/δ or LXRα were observed at any FM 550 concentration tested (Fig. 2).
Figure 2. Screening of FM 550 nuclear receptor activity and concentration response analysis.
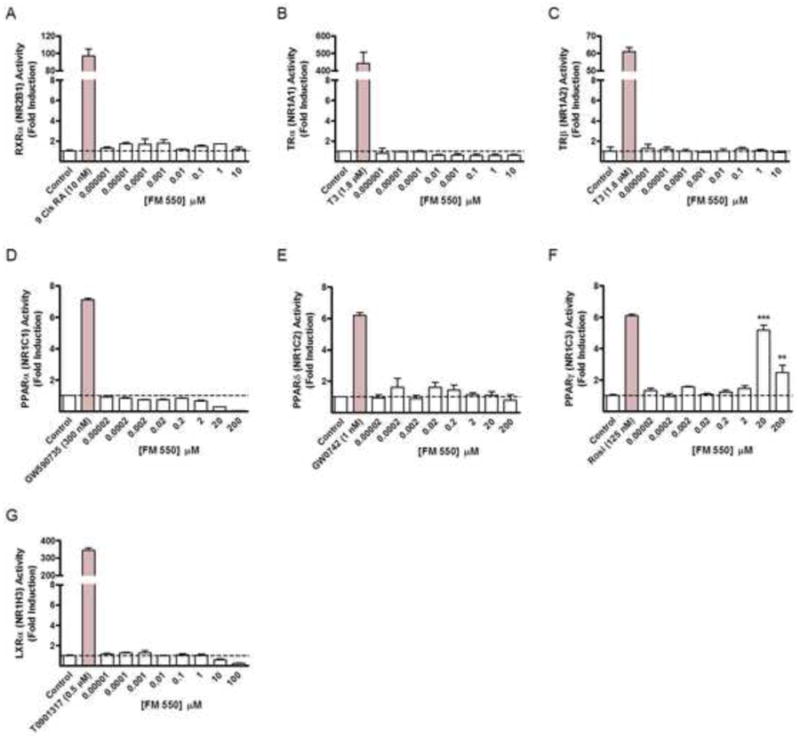
The effects of increasing concentrations of FM 550 were assessed in Chinese Hamster Ovary (CHO) cell line based human nuclear receptor luciferase reporter assays following 24 hr. exposures. Results are indicated as fold increase in activity relative to the mean baseline luciferase activity of vehicle control. No significant agonist activity was observed for FM 550 at human (A) RXRα, (B) TRα, (C) TRβ, (D) PPARα, (E) PPAR β/δ or (G) LXRα. (F) Significant increases in PPARγ activity were observed in response to FM 550. The presence of 20 μM FM 550 resulted in a 5.2 fold increase in reporter activity compared to control. A 2.5 fold increase in activity was observed at 200 μM FM 550. *** indicates P < .001, ** indicates P < .01 vs. control; hatched lines indicates mean control activity.
To understand in more detail the concentration response relationship for FM 550 at PPARγ, experiments were performed to more closely evaluate the agonist effects across the 1 to 200 μM concentration range (Fig. 3A-C). Focused concentration response analysis with an incremental 2-fold increase in FM 550 found significant increases [F(8,18) = 115.9, p < .0001] in activity beginning around 3 μM with a mean maximal increase in activity of 7.04 ± 0.47-fold (p < .001) at 25 μM. At concentrations of FM 550 greater than 25 μM PPARγ activity dose dependently decreased (Fig. 3 B-C). An inverted U-shaped non-monotonic concentration response relationship and a corresponding loss of activity associated with high levels of transactivation activity was not observed for the thiazolidinedione PPARγ agonist rosiglitazone (Fig. 3D).
Figure 3. Concentration response analysis of FM 550 at PPARγ.
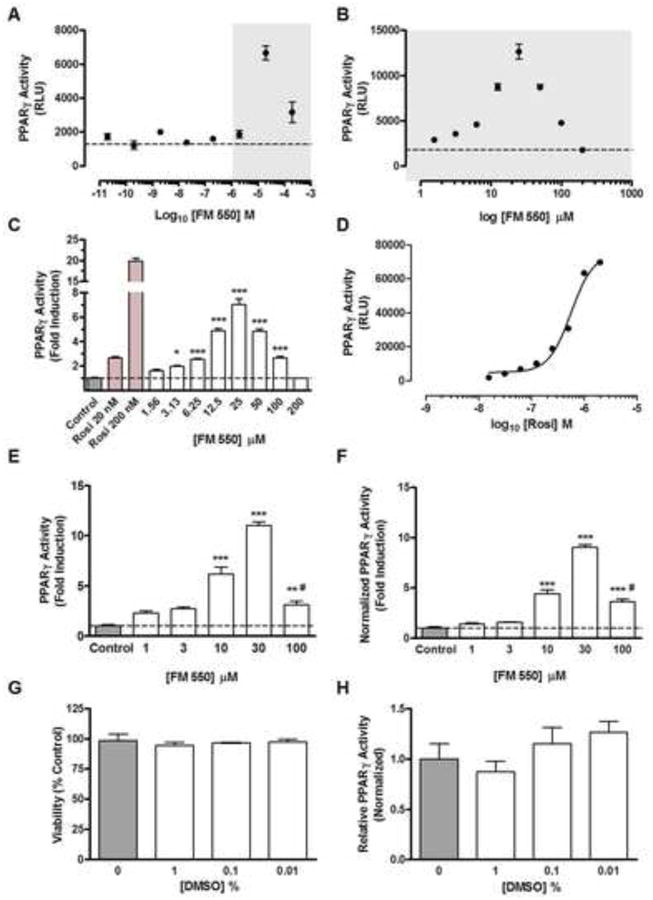
(A) PPARγ transgene activity is indicated in relative luciferase units in response to 10 fold increases in FM 550 concentration suggesting a loss of activity in response to the highest concentration tested. To define in detail the concentration response relationship for FM 550, a detailed analysis was performed spanning the concentration range indicated with gray shading. (B) FM 550 concentration response relationship defined by 2-fold incremental increases in concentration from 1 μM to 200 μM are expressed as transgene activity (RLU). (C) Results of focused FM 550 concentration response analysis expressed as the fold increase in activity relative to baseline vehicle control activity. Two concentrations of rosiglitazone (Rosi), 20 nM and 200 nM were included as positive controls and indicate that transgene expression was proportional to ligand concentration. (D) Rosiglitazone concentration response curve demonstrating that high levels of ligand activity (transgene expression) was not associated with a muting of response as was observed with FM 550. Comparison of results of FM 550 concentration response analysis at PPARγ without normalization for viable cell numbers (E) and following normalization (F) indicated that loss in viability did not fully account for the loss in transgene activity observed at 100 μM FM550. # indicates that Tukey's Multiple Comparison Test found the mean induction values for 30 and 100 μM were significantly different (P < 0.001). Reporter cell (G) viability and (H) constitutive luciferase activity of the PPARγ reporter cells was not decreased by DMSO vehicle. *** indicates P < .001, ** indicates P < .01, and * indicates P < .05 vs. control; hatched line indicates mean control activity.
Additional independent control experiments were performed to determine if the loss of activity observed at higher FM 550 concentrations could be accounted for by decreasing viability or were the result of DMSO vehicle present in the samples. When PPARγ activity was normalized to the number of reporter cells present in each well (Figs. 3E and 3F), only a small proportion of the decrease in activity at the highest concentrations (100 μM) was accounted for by decreased viability. There was no impact on viability by DMSO even when present at 10 times higher concentrations than were present in analyzed samples (Fig. 3G; [F(3,12) = 0.2941, p = .8289]). Increasing amounts of DMSO did not significantly reduce the ability to detect the constitutive baseline levels of LUC-activity expressed in this reporter assay (Fig. 3H; [F(3,13) = 1.791, p = .1986]).
3.2 PPARγ activity of FM550 and each component compound
Increases in PPARγ activity resulted from exposure to TPP [F(8,18) = 36.15, p < .0001] with a 6.23 ± 0.63 and 2.84 ± 0.34-fold increase in activity observed at 10 μM and 100 μM, respectively (Fig. 4A). Likewise a significant [F(8,18) = 62.43, p < .0001] increase in PPARγ activity resulted from exposure to ITP with an observed 6.91 ± 0.49-fold increase in activity at 10 μM (Fig. 4B). No statistical differences in PPARγ activity were observed in response to TBB [F(8,18) = 1.312, p = .2988] (Fig. 4C) or TBPH [F(9,20) = 0.9591, p = .4998] (Fig 4D).
Figure 4. Comparative PPARγ concentration response analysis of each triarylphospate and brominated FM 550 component, triphenyltin, tributyltin and tributyl(vinyl)tin.
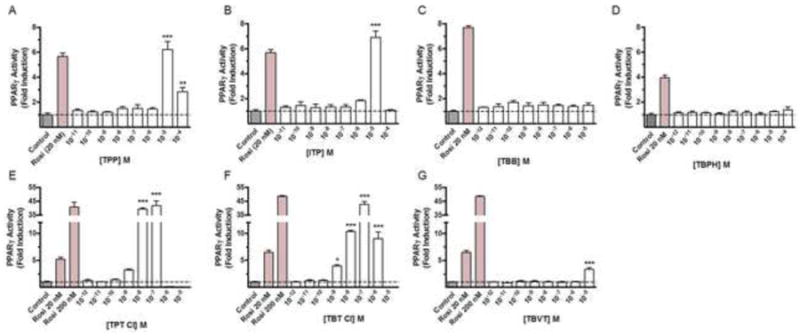
The effects of increasing concentrations of each compound are indicated as fold increase in activity relative to the mean baseline luciferase activity of vehicle control. Significant agonist activity was observed for TPP (A) and ITP (B) at human PPARγ. The presence of 10-5 M TPP or ITP resulted in a mean 6.2 fold and 6.9 fold increases in reporter activity. No activity was observed for TBB (C) or TBPH (D). (E) Concentration response analysis for agonist activity at PPARγ indicated that 10-8 M and 10-7 M TPT Cl significantly increased activity by 39 fold and 42 fold. (F) Significant increases in activity were observed in response to TBT Cl from 10-9 M to 10-6 M with a mean peak induction of 43 fold at 10-7 M. (G) TBVT was much less potent and efficacious with only a modest 3 fold induction observed at 10-5 M. Rosiglitazone (Rosi), 20 nM and/or 200 nM were included as positive controls; * indicates P < .05; ** indicates P < .01; *** indicates P < .001, vs. control; hatched lines indicate mean control activity.
3.3 Comparative analysis of FM 550 components and organotin PPARγ ligands
Comparative concentration response analysis for the highly efficacious organotin compounds TPT Cl and TBT Cl, and TBVT, a structural analogue of TBT (Figure 1) with low efficacy and potency at PPARγ (Hiromori et al., 2009), revealed detectable increases in PPARγ transactivation for each compound (Fig. 4E-G). For TPT Cl, a significant [F(8,18) = 228.8, p < .0001] increase in activity was observed with maximal mean increases about 40-fold greater than baseline at 0.1 and 0.01 μM (Fig. 4E). Similar effects were observed for TBT Cl where significant [F(8,18) = 612.8, p < .0001] increases in activity was observed to reach a maximum of 42 ± 2-fold above baseline at 0.1 μM. For both of these compounds a remarkable decrease in activity was also observed at concentrations greater than the 0.1 μM. In contrast, TBVT was found to induce a modest (3.3 ± 0.3 fold) increase in PPARγ activity only at 10 μM (Fig. 4G).
Focused concentration response analysis of PPARγ activity and concurrent assessment of cell viability were done for FM 550 and each OPFR (Fig. 5). Results of those analysis indicated that TPP [F(7,23) = 61.94, p < .0001] and ITP [F(7,23) = 92.62, p < .0001] increased PPARγ activity. The relative efficacy and potency for FM 550 and its individual components were compared to those of the organotins (Table 1). Of the FM 550 components TPP was the most efficacious PPARγ ligand, with a 20 ± 2.2-fold increase in activity observed at 30 μM (Fig. 5D-E). The relative maximal activity of TPP was 57% ± 6% of the prototypic environmental PPARγ ligand TPT Cl (Table 1). For ITP maximal increases in PPARγ activity were observed at 10 μM, where activity was increased 5.1 ± 0.25 fold above baseline (Fig. 5G-H). As shown in Table 1, FM 550 and the constituent triarylphosphates are less potent PPARγ agonists than the organotins. Compared to TPT Cl and TBT Cl with observed AC50 values of 3 nM and 16 nM, the calculated AC50 values for FM 550 and TPP were around 12 μM, and ITP was slightly more potent with an AC50 of about 4 μM (Table 1). PPARγ agonist activity for either brominated component of FM 550 was not detected.
Figure 5. Focused concentration response analysis of the triarylphosphate components of FM 550 PPARγ activity and cytotoxicity.
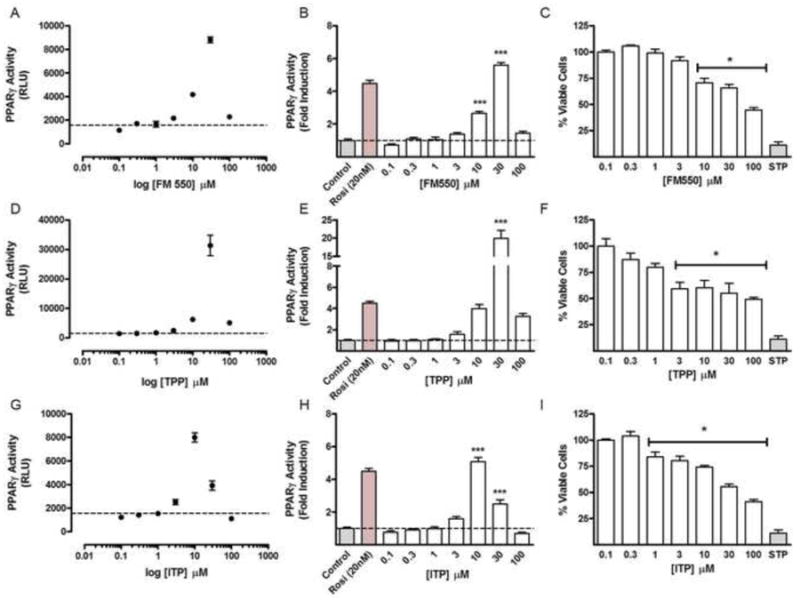
Comparative concentration response relationships for PPARγ agonist activity and calcein AM-uptake as a measure of cell viability were performed for FM 550 (A-C), TPP (D-F) and ITP (G-H) across a concentration range of from 0.1 μM to 100 μM. A half-log10 (3-fold) incremental increase in ligand concentration was used. PPARγ activity was expressed as luciferase transgene activity (RLU) and relative to baseline of vehicle controls. Cell viability is indicated as a percentage of vehicle control viability. Rosiglitazone (Rosi), 20 nM was used as a positive control for PPARγ activity. Staurosporine, 4 μM served as a positive control for cytotoxic effects. *** indicates P < .001, vs. vehicle control; hatched lines indicate mean control activity; for viability analysis all exposure groups covered by the black line * indicates a minimal level of statistical significance of p < .05.
Table 1. Relative Maximal PPARγ Activity Observed.
| CAS RN | Concentration | Relative Activity (SEM) | AC50 | |
|---|---|---|---|---|
|
| ||||
| TPT Cl | 639-58-7 | 0.1 μM | 1.0 (0.04) | 3.3 nM |
| TBT Cl | 1461-22-9 | 0.1 μM | 0.84 (0.04) | 16.0 nM |
| TBVT | 7486-35-3 | 10 μM | 0.07 (0.01) | ND |
| FM550 | - | 25 μM | 0.34 (0.02) | 11.8 μM |
| TPP | 115-86-6 | 30 μM | 0.57 (0.06) | 12.3 μM |
| ITP | 68937-41-7 | 10 μM | 0.15 (0.01) | 3.8 μM |
| TBB | 183658-27-7 | NA | ND | NA |
| TBPH | 26040-51-7 | NA | ND | NA |
NA, no activity; ND, not determinable; SEM, standard error of the mean; AC50, concentration eliciting half-maximal response; CAS RN, CAS registry number
3.4 Assessment of cytotoxicity effects
As observed with FM 550, significant [F(7,44) = 18.52, p < .0001] and dose dependent decreases in cell viability were detected in cultures treated with either TPP [F(7,50) = 21.06, p < .0001], or ITP [F(7,54) = 48.29, p < .0001], (Fig. 5C; 5F; 5I). Calculated IC50 values for loss of viability were 37 μM for TPP, 40 μM ITP, and 90 μM for the FM 550 mixture (Fig. 6A). Because about 50% of the FM 550 mixture is composed of TPP and ITP, this finding suggests that these OPFRs account for the cytotoxic actions resulting from FM 550 exposure.
Figure 6. Cytotoxicity of FM 550 and the triarylphosphate components in human CHO reporter cells and human brain tumor cells.
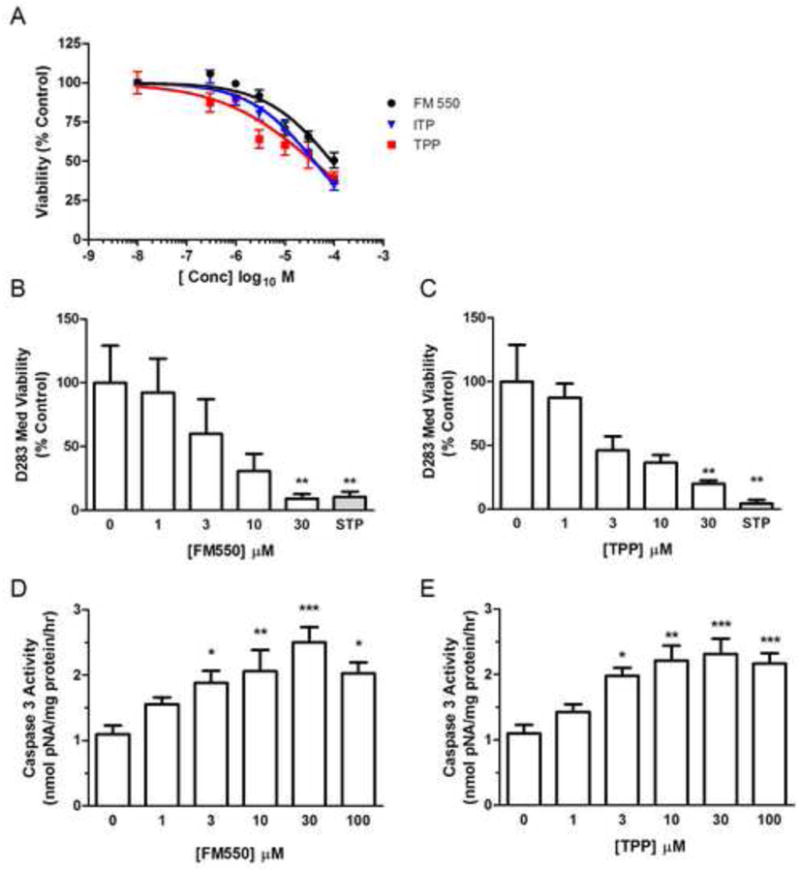
(A) Concentration response curves plotting the log concentration of FM 550, TPP, or ITP vs. normalized calcein AM-uptake as a measure of CHO PPARγ reporter-cell viability relative to vehicle treated control. Best fit curves were calculated with a variable slope Hill model to calculate EC50 values and are indicated with solid lines for each test material. Calcein AM-uptake was assessed following a 24 hr. exposure period. Cell viability is indicated as a percentage of vehicle control viability. Concentration response analysis of the effects of FM 550 (B) or TPP (C) following 48 hour exposures in D283 Med cells. To ensure that effects on viability were not a result of artifacts related to the Calcein AM viability assay, MTS-uptake/reduction assay was used to assess the relative numbers of viable cells. Staurosporine (STP) 4 μM served as a positive control for cytotoxic effects. As a measure of apoptotic cell mechanisms, caspase 3 activity was measured in D283 Med cells following 6 hour exposure to FM 550 (D) or TPP (E). * indicates P < .05; ** indicates P < .01; *** indicates P < .001, vs. control.
To investigate whether the cytotoxic actions observed in these CHO derived cell lines was a more generalizable effect, human neuroectodermal brain tumor-derived D283 Med cells were exposed to increasing concentrations of FM 550 or TPP and viability determined using the MTS viability assay. As observed in the CHO cell based PPARγ reporter system, increasing concentrations of FM 550 [F(4,24) = 4.387, p < .0084], or TPP [F(4,21) = 18.52, p < .0005] from 1 μM to 30 μM decreased viability (Fig.6B-C). As an initial step in understanding the mode of action responsible for the observed loss of cell viability the effects of increasing exposure on caspase 3 activity in D283 Med cells was analyzed to determine whether an apoptotic mechanism of cell death was mediating the observed decreases in viability. In response to a 6 hour exposure with increasing concentrations of FM 550 or TPP significant dose dependent increases in caspase 3 activities were observed in D283 Med cells (Fig. 6D-E). For both FM 550 and TPP significant increases in caspase activity were observed at concentrations above 1 μM.
4. Discussion
The goal of this study was to evaluate the agonist actions of the flame retardant mixture FM 550 and its constituent compounds at NRs with known roles in establishing or regulating energy balance. The foundations for this study were based on our initial in vivo findings in rats where perinatal FM 550 exposure resulted in phenotypes reminiscent of metabolic syndrome. Metabolic syndrome is a clinical term describing a set of obesity-related co-morbidities that together increase the risk for coronary artery disease, stroke and type 2 diabetes in humans (Prasad et al., 2012; Vazzana et al., 2011). In both sexes, the most distinctive physiological outcome resulting from FM 550 exposures was markedly elevated body weight that was evident in juveniles and persisted into adulthood (Patisaul et al., 2013). However, the mechanisms responsible for the obesity-related phenotypes resulting from FM 550 exposure were undefined. This gap in understanding was identified as requiring additional research to “elucidate the mechanisms responsible for the observed changes in weight, anxiety and insulin resistance” (Patisaul et al., 2013).
Building on those initial findings we have screened the FM 550 mixture for agonist activities at candidate NRs with developmental activities involved in establishing metabolic set points, energy metabolism, or development and differentiation of adipose tissue (Janesick and Blumberg, 2011; Lefebvre et al., 2010; Liu and Brent, 2010; Nakanishi, 2008). Agonist activity for FM 550 was observed only at PPARγ prompting a more detailed analysis of the concentration response relationship for FM 550 and its component flame retardant chemicals at PPARγ. These effects were reminiscent of the actions of the organotins TBT and TPT, the most well characterized environmental contaminants implicated as obesogenic endocrine disruptors capable of acting developmentally to alter metabolism and adipocyte differentiation (Grün et al., 2006; Hiromori et al., 2009). Because alterations in PPARγ during early adipocyte development alter adipogenesis and lipolysis later in life, the evidence reported here establish alterations in human PPARγ NR activity as a biologically plausible mechanism through which FM 550 may act and contribute to the obese phenotypes observed in adulthood (Janesick and Blumberg, 2011; Patisaul et al., 2012).
Evidence suggestive of FM 550 impacting the thyroid hormone (TH) axis was also found in exposed rat dams and offspring in our previous analysis (Patisaul et al., 2013). Direct agonist effects of FM 550 on human TR activity were not detected in the transactivation assay experiments performed here. The failure to observe direct agonist effects of FM 550 at either TRα or TRβ suggests that the brominated components, TBB and TBPH are not directly acting through TRs to perturb thyroid hormone axis functionality. While the possibility for antagonist-like effects were not addressed here, it is important to note that even for the more well-studied PBDEs flame retardants, where TH-disruptive actions are established, multiple modes of action including thyroid hormone transport, metabolism and TR activity are impacted (Jugan et al., 2010). Importantly, the involvement of TBB and TBPH biotranformation, and the generation of active metabolites that could influence TH homeostasis remains unaddressed. A number of key metabolites of both TBB and TBPH, and also the triarylphosphate components of FM 550, have been identified (Cooper et al., 2011; Patisaul et al., 2013; Roberts et al., 2012; Van den Eede et al., 2013). Future study is necessary to determine the potential for TBB and TBPH to act as TR antagonists, and to assess whether their metabolites act as ligands at TRs.
The obvious and well-defined inverted U-shaped non-monotonic concentration response relationship for FM 550 at PPARγ appears to be mediated exclusively by the triarylphosphate components of this mixture. The observed agonist activity and a small portion of the descending slope of the inverted shaped dose response curve are accounted for by ligand induced transactivation (up-slope) and associated with the loss of cell viability (down-slope), respectively. However, correction for the loss of viability at higher concentrations of FM 550 or the individual OPFRs did not account fully for the observed reductions in transactivation activity. The concentration response curves of the more potent and efficacious organotin ligands TBT and TPT were similarly non-monotonic suggesting that increases of PPARγ activity above a certain level might result in a decrease in reporter transgene expression or activity. A negative-feedback effect resulting from increased PPARγ appears unlikely because similar effects were not observed for the concentration response analysis of the extremely efficacious thiazolidinedione PPARγ agonist rosiglitazone. This conclusion is supported further by the observation when used as a positive control the corresponding responses in transgene activity to 10-fold increases in rosiglitazone (20 nM to 200 nM) were proportional (e.g. Fig. 3C and 3D).
The association of decreasing luciferase activity with increasing cytotoxicity however suggests there was a negative interaction between cytotoxic mechanisms and transactivation activity that might be responsible for the observed diminution of transgene activity. It however, remains a possibility that the inhibitory actions of these chemically similar compounds were mediated by interactions of the ligand at inhibitory binding site(s) on the receptor, and/or through intermolecular interactions that cause repression of transactivation similar to what we observed for some estrogen signaling activities of estrogen receptor β (ER β) (Belcher et al., 2005). Alternatively, assay interference by direct inhibition of luciferase enzyme activity could explain the observed decreases in reporter activity. While there are no obvious similarities between the triarylphospahate or organotin compounds and the chemical structures of compounds that inhibit luciferase, assay interference by direct inhibition of luciferase-activity was not ruled out (Thorne et al., 2012). In theory, it is also possible that assay interference could result from apoptotic mechanisms acting to further destabilize the luciferase enzyme. Further analysis is required to understand the mechanism of the dose dependent loss of functional reporter gene activity observed with these PPARγ ligands.
From a practical perspective, the observed non-monotonic concentration response associated with the triarylphosphate and organotin compounds highlight an important consideration for in vitro and high-throughput toxicity screening efforts (Dix et al., 2007). Specifically, concentration response assessments with one log differences between concentration intervals are likely to underestimate potential maximal efficacy (Emax) for some compounds. The experiments shown in Figures 4 and 5 highlight the limitations of activity assignment based on screening using a 10-fold concentration interval. Particularly notable is the case of TPP where the maximal effect detected was 3.2 times lower than was defined with a slightly more focused half-log (3-fold) concentration interval.
Finally we investigated the impact of FM 550 exposure on cell viability. It was initially found that increasing concentrations of FM 550 resulted in increased cell death of the CHO-based reporter cells. Because this effect was also observed in an unrelated brain tumor cell line of neuroectodermal origin, it seems that the evident cytotoxic effects arising from exposure to FM 550 are not unique to the CHO cells, or a consequence of receptor/reporter gene expression. We further delineated that the triarylphosphate components of FM550 accounted for all of the observed cytotoxic effects, and that exposure to FM550 or TPP increased caspase 3 activities, findings consistent with an apoptotic mechanism of cell death. It is possible that the defined cytotoxic actions of the triarylphosphates could contribute to the adult phenotypes observed in vivo following development exposures to FM550 by impacting the normal balance of apoptosis during critical periods of development (Patisaul, 2012).
The potential for high doses of heterogeneous mixtures of triarylphosphates to act as neurotoxicants causing organophosphate-induced delayed neurotoxicity has been well-established for some triarylphosphate congeners (van der Veen and de Boer, 2012). Recently, interest in the potential for triarylphosphates to act as endocrine disruptors has resulted in an increasing number of studies employing both in vitro and in vivo models. Some of those studies also investigated possible activities of these compounds at NRs, and have reported findings implicating a variety of potentially responsive NR targets including the constitutively active receptor, pregnane X receptor, androgen receptor, estrogen receptors, and aryl hydrocarbon receptors (Honkakoski et al., 2004; Kojima et al., 2013; McGee et al., 2013). While TPP was similarly found inactive at RXR, in contrast to our finding that TPP is an agonist of PPARγ, Kojima et al (2013) concluded that TPP was inactive at PPARγ. Although slight activity at PPARγ was noted by Kojima et al (2013) a criteria of being greater than 20% of the maximal activity of the highly efficacious positive control pioglitazone, was used to define whether or not a compound was active. This criterion led to the incorrect conclusions that TPP was inactive and that there must be important differences imposed by the tin or phosphorus moieties that resulted in TPPs apparent lack of activity. In contrast, our findings suggest that while TPP is less potent than TPT at PPARγ, its structure is similarly compatible with ligand binding at PPARγ and adoption of an active conformation that is compatible with co-activator interactions and transactivation. While the potency of TPP is lower than for TPT, human exposure to TPP is likely much higher. As a result, the potential for TPP to have endocrine or metabolic impacts via alteration of PPARγ activity is of concern and highlights a need for additional hazards and exposure characterization to better define the current potential impact on human populations.
The flame retardant FM 550 activates human PPARγ luciferase reporter assays
A non-monotonic concentration response relationship was identified for FM 550
The triarylphosphate components of FM 550 were responsible for PPARγ activation
Triphenyl phosphate was the most efficacious PPARγ ligand
The triarylphosphate components of FM 550 induced dose dependent apoptosis
Acknowledgments
This study was supported by pilot project research grants from The University of Cincinnati Center for Environmental Genetics (NIEHS P30-ES006096) awarded to SMB and by the NCSU Center for Human Health and the Environment (CHHE) awarded to HBP. FM 550 was provided by Chemtura. The authors acknowledge the dedicated efforts of their labs.
Abbreviations
- AC50
concentration of half maximal activity
- Calcein-AM
2-[[7′-[[bis(carboxymethyl)amino]methyl]-3′,6′-dihydroxy-3-oxospiro[2-benzofuran-1,9′-xanthene]-2′-yl]methyl-(carboxymethyl)amino]acetic acid, MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, CHO, Chinese Hamster Ovary
- DMSO
Dimethyl sulfoxide
- FM 550
Firemaster® 550
- ITPs
isopropylated triphenylphosphate isomers
- LXR
liver X receptor
- NR
nuclear receptor
- PBDEs
polybrominated diphenyl ethers
- PPAR
peroxisome proliferator-activated receptor
- PVC
polyvinyl chloride
- RA
9-cis retinoic acid
- RLU
relative luciferase units
- ROSI
rosiglitazone
- RXR
retinoid X receptor
- STP
staurosporine
- T3
L-triiodothyronine
- T4
thyroxine
- TBB
2-ethylhexyl-2,3,4,5-tetrabromobenzoate
- TBPH
bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate
- TBT
tributytin
- TBVT
Tributyl(vinyl)tin
- TPP
triphenyl phosphate
- TPT
triphenyl tin
- TR
thyroid hormone receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belcher SM, Le HH, Spurling L, Wong JK. Rapid Estrogenic Regulation of Extracellular Signal- Regulated Kinase 1/2 Signaling in Cerebellar Granule Cells Involves a G Protein- and Protein Kinase A-Dependent Mechanism and Intracellular Activation of Protein Phosphatase 2A. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Ma X, Le HH. Blockade of Estrogen Receptor Signaling Inhibits Growth and Migration of Medulloblastoma. Endocrinology. 2009;150:1112–1121. doi: 10.1210/en.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EM, Covaci A, Nuijs ALN, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography–tandem mass spectrometry. Analytical and Bioanalytical Chemistry. 2011;401:2123–2132. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ. The ToxCast Program for Prioritizing Toxicity Testing of Environmental Chemicals. Toxicological Sciences. 2007;95:5–12. doi: 10.1093/toxsci/kfl103. [DOI] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Minireview: the case for obesogens. Mol Endocrinol. 2009;23:1127–1134. doi: 10.1210/me.2008-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grün F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. Endocrine-Disrupting Organotin Compounds Are Potent Inducers of Adipogenesis in Vertebrates. Molecular Endocrinology. 2006;20:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- Hiromori Y, Nishikawa Ji, Yoshida I, Nagase H, Nakanishi T. Structure-dependent activation of peroxisome proliferator-activated receptor (PPAR) γ by organotin compounds. Chemico-Biological Interactions. 2009;180:238–244. doi: 10.1016/j.cbi.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Palvimo JJ, Penttilä L, Vepsäläinen J, Auriola S. Effects of triaryl phosphates on mouse and human nuclear receptors. Biochemical Pharmacology. 2004;67:97–106. doi: 10.1016/j.bcp.2003.08.037. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Watanabe H, Ohta Y, Blumberg B. Developmental effects: oestrogen-induced vaginal changes and organotin-induced adipogenesis. International Journal of Andrology. 2008;31:263–268. doi: 10.1111/j.1365-2605.2008.00863.x. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F, Catalano PM, Hanson RW. Metabolic programming: fetal origins of obesity and metabolic syndrome in the adult. Am J Physiol Endocrinol Metab. 2006;291:E439–440. doi: 10.1152/ajpendo.00105.2006. [DOI] [PubMed] [Google Scholar]
- Janesick A, Blumberg B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res C Embryo Today. 2011;93:34–50. doi: 10.1002/bdrc.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugan ML, Levi Y, Blondeau JP. Endocrine disruptors and thyroid hormone physiology. Biochemical Pharmacology. 2010;79:939–947. doi: 10.1016/j.bcp.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology. 2013;314:76–83. doi: 10.1016/j.tox.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Benomar Y, Staels B. Retinoid X receptors: common heterodimerization partners with distinct functions. Trends in Endocrinology & Metabolism. 2010;21:676–683. doi: 10.1016/j.tem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Liu YY, Brent GA. Thyroid hormone crosstalk with nuclear receptor signaling in metabolic regulation. Trends in Endocrinology & Metabolism. 2010;21:166–173. doi: 10.1016/j.tem.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SP, Konstantinov A, Stapleton HM, Volz DC. Aryl Phosphate Esters Within a Major PentaBDE Replacement Product Induce Cardiotoxicity in Developing Zebrafish Embryos: Potential Role of the Aryl Hydrocarbon Receptor. Toxicological Sciences. 2013;133:144–156. doi: 10.1093/toxsci/kft020. [DOI] [PubMed] [Google Scholar]
- Nakanishi T. Endocrine disruption induced by organotin compounds; organotins function as a powerful agonist for nuclear receptors rather than an aromatase inhibitor. The Journal of Toxicological Sciences. 2008;33:269–276. doi: 10.2131/jts.33.269. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and endocrine disrupting effects of the flame retardant mixture firemaster((R)) 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27:124–136. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H, Ryan DA, Celzo MF, Stapleton D. Metabolic syndrome: definition and therapeutic implications. Postgrad Med. 2012;124:21–30. doi: 10.3810/pgm.2012.01.2514. [DOI] [PubMed] [Google Scholar]
- Roberts SC, Macaulay LJ, Stapleton HM. In vitro metabolism of the brominated flame retardants 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in human and rat tissues. Chem Res Toxicol. 2012;25:1435–1441. doi: 10.1021/tx300086x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol. 2008;42:6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina Toddler Cohort: Associations with Hand Wipes, House Dust and Socioeconomic Variables. Environmental Health Perspectives. 2012a doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43:7490–7495. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of Flame Retardants in Polyurethane Foam Collected from Baby Products. Environmental Science & Technology. 2011;45:5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children's handwipes and house dust. Chemosphere. 2014 doi: 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environmental Science & Technology. 2012b;46:13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Shen M, Lea SA, Simeonov A, Lovell S, Auld DS, Inglese J. Firefly Luciferase in Chemical Biology: A Compendium of Inhibitors, Mechanistic Evaluation of Chemotypes, and Suggested Use As a Reporter. Chemistry & Biology. 2012;19:1060–1072. doi: 10.1016/j.chembiol.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicology Letters. 2013;223:9–15. doi: 10.1016/j.toxlet.2013.08.012. [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88:1119–1153. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Vazzana N, Santilli F, Sestili S, Cuccurullo C, Davi G. Determinants of increased cardiovascular disease in obesity and metabolic syndrome. Curr Med Chem. 2011;18:5267–5280. doi: 10.2174/092986711798184299. [DOI] [PubMed] [Google Scholar]
- Wong JK, Kennedy PR, Belcher SM. Simplified serum- and steroid-free culture conditions for high-throughput viability analysis of primary cultures of cerebellar granule neurons. Journal of Neuroscience Methods. 2001;110:45–55. doi: 10.1016/s0165-0270(01)00419-8. [DOI] [PubMed] [Google Scholar]
- Wong JK, Le HH, Zsarnovszky A, Belcher SM. Estrogens and ICI182,780 (Faslodex) Modulate Mitosis and Cell Death in Immature Cerebellar Neurons via Rapid Activation of p44/p42 Mitogen-Activated Protein Kinase. The Journal of Neuroscience. 2003;23:4984–4995. doi: 10.1523/JNEUROSCI.23-12-04984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


