Abstract
Neurons communicate primarily via action potentials that transmit information on the timescale of milliseconds. Neurons also integrate information via alterations in gene transcription and protein translation that are sustained for hours to days after initiation. Positioned between these two signaling timescales are the minute-by-minute actions of neuromodulators. Over the course of minutes, the classical neuromodulators (such as serotonin, dopamine, octopamine, and norepinephrine) can alter and/or stabilize neural circuit patterning as well as behavioral states. Neuromodulators allow many flexible outputs from neural circuits and can encode information content into the firing state of neural networks. The idea that steroid molecules can operate as genuine behavioral neuromodulators - synthesized by and acting within brain circuits on a minute-by-minute timescale - has gained traction in recent years. Evidence for brain steroid synthesis at synaptic terminals has converged with evidence for the rapid actions of brain-derived steroids on neural circuits and behavior. The general principle emerging from this work is that the production of steroid hormones within brain circuits can alter their functional connectivity and shift sensory representations by enhancing their information coding. Steroids produced in the brain can therefore change the information content of neuronal networks to rapidly modulate sensory experience and sensorimotor functions.
1. What is neuromodulation and why is it so important?
The field of ‘connectomics’ has generated a great deal of interest and enthusiasm in the past few years. Connectomics has already begun to map out large-scale neural circuit diagrams, including ultrastructural analysis of the human brain (e.g., Amunts et al., 2013). These efforts include the US BRAIN Initiative, the Human Connectome Project, and the European Union Flagship Human Brain Project. The push to map brain circuits wholescale is essential for progress in neuroscience as it will provide unprecedented resources to neuroscientists. All the same, a complete map alone will not provide a comprehensive understanding of how brains function and which treatments may ameliorate neurological dysfunction (Bargmann and Newsome, 2014; Oh et al., 2014). In concert with the connectome, small molecules called neuromodulators provide a means to dynamically alter the functional connectivity of circuits in response to external and internal cues (Harris-Warrick, 2011; Marder, 2012). Understanding how circuits are modulated by small diffusible molecules remains a challenge that is particularly well-suited to neuroendocrinologists, given our interest in studying the interplay between neuroanatomy and hormones. Below, I summarize four key features of the classically-identified neuromodulators. I then evaluate how these properties can apply to our emergent understanding of steroid signaling in brain circuits.
a. Neuromodulators occupy an important temporal niche for signaling
Several decades of work on the nematode C. elegans have demonstrated both the explanatory power and the limitations of a completed brain wiring diagram. The neural connectome of C. elegans has been solved (Jarrell et al., 2012; Towlson et al., 2013). Each adult employs a maximum of 302 neurons to direct a variety of behaviors. Each neuron has an established identity across individuals, and the connectivity pattern of each identified neuron is also known. However, on ‘top’ of this map there are more than 200 distinct neuropeptides encoded by the C. elegans genome that can influence the nervous system and behavior (Bargmann, 2012). Thus, the extraordinary layer of signaling interactions via neuromodulation belies the relatively simple ‘solution’ of having the established wiring diagram in hand.
Unpacking how modulation works in the nervous system of C. elegans and other species with so-called ‘simple’ brains has solidified the view that neuromodulators are essential for temporally-flexible brain functions and behaviors. Because neuromodulators generally alter circuit function on the timescale of seconds-to-minutes, they help fill the ‘signaling gap’ between events that can be encoded by fast neurotransmission (i.e., on the timescale of milliseconds) and gene transcription and protein translation (i.e., on the timescale of hours/days). In other words, neuromodulators occupy an important temporal niche for signaling in the nervous system, nestled between fast neuronal action potentials and slower genomic action potentials (for thorough discussions of neuroendocrine and genomic action potentials see: Clayton, 2000; Hofmann, 2010).
b. Neuromodulators provide circuit flexibility and dynamic functional connectivity
The study of neuromodulation has relied heavily on understanding the inner workings of central pattern generators (CPGs). The clearest examples of CPGs in animals are the discrete, identified neuronal assemblages that are both necessary and sufficient for the control of rhythmic behaviors like feeding, locomotion, heart rate, vocalization, and respiration (Bass and Zakon, 2005; Feldman and Del Negro, 2006; Grillner, 2003; Marder and Bucher, 2001; Marder and Calabrese, 1996; Rhodes et al., 2007). The most comprehensive understanding to date of a CPG that exhibits circuit flexibility in response to neuromodulation comes from more than 40 years of investigations in the crustacean stomatogastric ganglion (STG). The STG is a small network of neurons that are identifiable across individuals, and for which the ‘connectome’ circuit diagram has also been solved. The handful of neurons within the STG constitute a central pattern generator (CPG) that drives the motor movements associated with digestion. These rhythmic behaviors are complex, highly variable, and sensitive to environmental cues in crustaceans (Harris-Warrick and Johnson, 2010; Marder and Bucher, 2007; Marder et al., 2005).
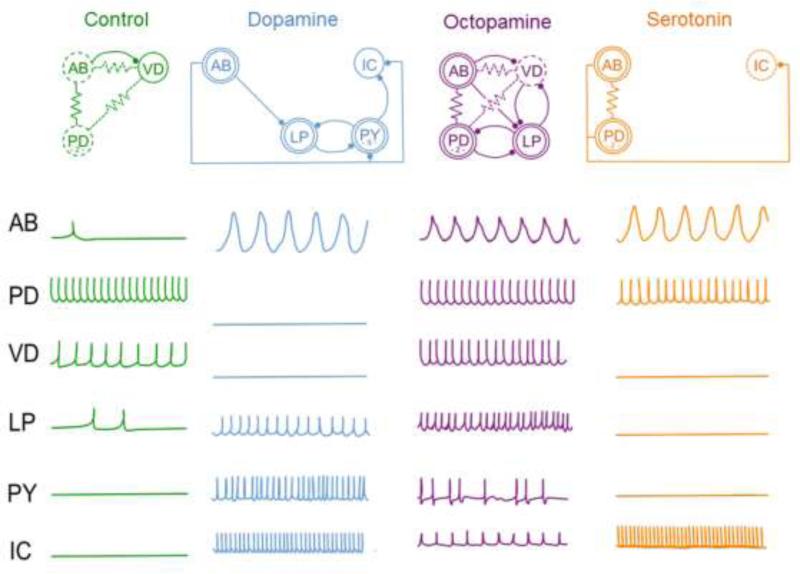
At any one moment the current CPG activation pattern (and hence digestion motor rhythms) can shift depending upon the relative concentrations of neuropeptides and other modulators that are released into the STG (Kiehn and Katz, 1999; Kvarta et al., 2012; Marder, 2012). The shifts in the CPG activation pattern are due to changes in the strength and sign of individual synaptic connections in the STG network. For example, as shown in Fig. 1, in the absence of dopamine stimulation the ‘AB’ neuron drives rhythmic activity in both the ‘VD’ and ‘PD’ neurons (each neuron in the network is named, as each neuron is readily identifiable across animals). In the presence of 10−4 M dopamine the AB neuron switches on strong synaptic input to the LP neuron, and the synaptic inputs from the AB neuron to the PD and VD neurons are attenuated (Flamm and Harris-Warrick, 1986). The pattern of functional connectivity is yet again different in the presence of other neuromodulators like serotonin and octopamine (Fig. 1). Therefore, neuromodulators provide extraordinary flexibility in CPG network properties (Harris-Warrick, 2011; Harris-Warrick and Johnson, 2010).
Figure 1.
The functional rewiring of a neural circuit. Biological amines have multifaceted actions on a central pattern generator (CPG) in the crustacean gut. The top row shows the functional circuit of the primary neurons in the crustacean stomatogastric ganglion in control conditions (left; green) and during application of either dopamine (blue), octopamine (purple), or serotonin (orange). The effects of each amine on the functional circuit can be to strengthen or weaken synaptic connections, as indicated by weak interactions (broken circle), moderate interactions (single circle) or strong activity (double circle). For simplicity, neurons that are not known to participate in a given interaction are not depicted, emphasizing the functional rewiring of the circuit. The firing state changes in the waveforms of individual, identified neurons of the network are shown below each circuit for each modulatory state. This figure is adapted from earlier work of Flamm and Harris-Warrick (1986).
In addition to the simple presence or absence of a given neuromodulator, the relative concentrations and mixture of neuromodulators at any one moment can provide yet another layer of dynamic functional connections within a circuit. For example, in the spinal CPG for locomotion, dopamine modulates locomotor activity in a dose-dependent manner, by directing opposite rhythms at low vs. high concentrations on the same CPG network (Clemens et al., 2012). Similarly, dopamine and other modulators can alter information transfer in basal ganglia circuits (i.e., between the cortex and thalamus) via dose-dependent actions in the striatum, illustrating how biogenic amines can dynamically shift functional connections in the vertebrate forebrain (Leblois et al., 2010).
Therefore, as a general principle, neural circuits do not have fixed connective properties. The relative weights of synaptic connections between neurons in a network are continually reconfigured by the presence and the relative, momentary concentration of neuromodulators.
c. Neuromoduluators can change the information content of networks to shift sensory and motor representations
It is well-established that neuromodulators like dopamine are responsive to environmental cues. There has been particular interest in neuromodulators because they can provide learning or error-related signals to brain circuits. The vast literature on ‘reward prediction error’ and midbrain dopamine projections illustrates how one neuromodulator can dynamically influence the information contained in neural circuits regarding external/internal conditions, and thereby guide behavioral decisions (e.g., Glimcher, 2011; Schultz, 2013). This role for dopamine is not exclusively a vertebrate phenomenon. Many invertebrate organisms like Aplysia exhibit environmentally-contingent dopamine release that is involved in learning-induced plasticity via specific actions on a motor CPG (Bedecarrats et al., 2013). Therefore, one key feature of neuromodulators is that they can change the information content of a network, providing instructive signals and/or feedback inputs to influence ongoing circuit computations.
Sensory representations are particularly subject to neuromodulation. Many biogenic amines like dopamine, norepinephrine and serotonin can directly shift sensory representations through modulatory actions in the forebrain, midbrain, and thalamus (Devilbiss et al., 2006; Hoke and Pitts, 2012; Hurley and Sullivan, 2012; Jacob et al., 2013; Ramsey et al., 2010). In addition to sensory modulation, in the case of motor networks, the information content of ongoing motor patterns for behaviors is also sensitive to neuromodulation. These include the firing state of locomotor CPGs that can change in the presence of biogenic amines (Clemens et al., 2012; Kiehn and Katz, 1999).
Therefore, because neuromodulators can dynamically shift the information content of neural circuits, they can directly modify not only how the outside world is perceived but also how motor programs unfold to pattern behaviors.
d. Neuromodulators can smooth the selection of behavioral states and transitions
As a result of the three properties above, neuromodulators exert key actions in neural circuits to shift the probability of a single behavioral output from array of possibilities (Katz and Edwards, 1999; Kiehn and Katz, 1999). Norepinephrine in particular is associated with transitions in behavioral state (Bouret and Sara, 2005), and neuromodulators in general can help stabilize ongoing behaviors and smooth transitions between behaviors (Harris-Warrick, 2011). For example, the actions of two modulators (serotonin and the neuropeptide PDF) can stabilize the transitions between dwelling and roaming behaviors in C. elegans (Flavell et al., 2013). Using optogenetic manipulations in behaving animals, Flavell et al. (2013) showed that transient ablation of serotonin and PDF signaling resulted in bouts of sedentary vs. active behaviors that were too short and frenetic for optimal foraging. Since neuromodulators are adept at coordinating neural and behavioral events over the course of minutes, they can help stabilize oscillating circuits and thereby smooth behavioral transitions.
Modulators can operate at multiple timescales to guide behavioral transitions. The transition to behavioral molting of the exoskeleton in crustaceans requires coordination of peripheral structures and a key CPG in crustaceans, and a single neuropeptide appears to stabilize and coordinate both peripheral and CPG elements in concert (Chung et al., 1999). A smooth transition to active flight in the moth, manduca sexta, is enabled by combinatorial actions of the biogenic amine octopamine and its precursor, tyramine on the CPG for flight (Vierk et al., 2009).
In Xenopus, the transition from a silent to a vocally-active state is driven by serotonergic input to a brainstem CPG (Rhodes et al., 2007; Yu and Yamaguchi, 2009). In this system, the actions of serotonin are sex-specific within the same CPG; the serotonin-elicited vocal motor output differs between males and females, despite the involvement of the same serotonin receptor in the CPG of both sexes.
In summary, a predominant role of neuromodulators is to help stabilize behavioral state transitions that occur over the course of minutes by actions within specific circuits. The plasticity that neuromodulators allow a neural network ultimately provides behavioral stability and flexibility to coordinate transitions in the basic oscillatory outputs of neural circuits (Grillner, 2003; Marder, 2012; Prinz et al., 2004; Zhao et al., 2011).
2. Do steroids behave like neuromodulators?
The general principles outlined above for neuromodulation may be useful to guide our thinking about steroid signaling in brain circuits. Does steroid signaling resemble neuromodulation within some brain areas? Do neurosteroids occupy a temporal signaling niche, change functional connectivity of circuits, alter information represented in networks, and guide behavioral transitions? The answers to these questions are far from clear at present. Below I highlight recent work that, as a whole, supports the idea that steroids, in particular estrogens, can act as neuromodulators consistent with the general principles outlined above.
The idea that circulating steroids can interact with CPGs to cause both short- and longer-term changes in circuit output and behavior has received substantial empirical support in the past 10 years. One brain region that has been particularly useful to understand how steroids can interact with CPGs is a conserved hindbrain/spinal compartment that patterns the duration and frequency of vocalizations in amphibians and teleost fishes (Bass and Remage-Healey, 2008; Bass and Zakon, 2005; Kelley and Bass, 2010; Zornik and Kelley, 2011). The vocal CPGs of both Xenopus and midshipman are sensitive to the acute (minute-by-minute) as well as the longer-term (hrs, days) actions of steroids, including androgens and estrogens. In particular, a recent collection of studies have shown that vocal CPGs can pattern sexually-differentiated vocalizations in males and females, and that these CPG outputs are modulated by steroid hormones derived from the circulation (Remage-Healey and Bass, 2007; Zornik and Kelley, 2011). Thus, varying circulating steroids can rapidly alter the firing states of CPGs much in the way of classical neuromodulators. Several other CPGs are also modulated by acute and long-term actions of steroid hormones from the circulation. Adrenal glucocorticoids exert powerful and rapid actions on reproductive clasping in roughskin newts. This suppression occurs even in spinally transected newts (Lewis and Rose, 2003), consistent with an interaction with a CPG (the exact neural locus is currently undefined). Second, a CPG that governs oxytocin release from the hypothalamus is responsive to long-term steroid actions, and these actions appear to drive sex differences in the bursting membrane properties of oxytocinergic neurons (Israel et al., 2014). Circulating androgens can also exert long-term actions on the electric organ that controls social signals in electric fishes, although the involvement of a CPG is not yet clear (Few and Zakon, 2001; Liu et al., 2008).
In summary, it is now evident that circulating steroids can have short and long term actions on CPGs that govern rhythmic outputs and behaviors. More recent evidence, summarized below, has considered the role of brain-synthesized steroids (i.e., steroids not diffusing from the general circulation) in modulating neural circuits. This shift toward thinking about steroids as intrinsic neuromodulators of behavior relies on combining neuroanatomical observations with predictions from the established work on classical neuromodulators.
3. Steroids as neuromodulators intrinsic to the CNS
The rapid, minute-by-minute actions of steroids on neurons are not new. However, only recently has it become clear that steroids can be synthesized within the brain itself to rapidly control local neural circuit function and behavior (Balthazart and Ball, 2006; Cornil, 2009; Cornil et al., 2012a). For example, brain-derived ‘neuroestrogens’ can act with high spatial and temporal specificity in the forebrain, often by interacting with nonclassical receptors (Mukai et al., 2006; Remage-Healey, 2012; Srivastava et al., 2011; Woolley, 2007). Thus, a greater appreciation of rapid neuroestrogen signaling within the brain has become extremely important for understanding how estrogens can govern acute behavioral transitions. Not surprisingly, meticulous neuroanatomical work has led the way toward this new perspective. The enzyme that synthesizes estrogens (aromatase) is found not only in neuronal cell bodies but also within presynaptic terminals in the forebrain of birds and mammals (Naftolin et al., 1996; Peterson et al., 2005). In principle, this anatomical compartmentalization allows for direct delivery of endogenous estrogens to precise neuronal synaptic targets, which has been recently called ‘synaptocrine’ actions of estrogens (Remage-Healey et al., 2011; Saldanha et al., 2011). Therefore, locally-produced steroids like estrogens can contribute to the acute modulation and functional output of neural circuits. In support of this perspective, estrogens can have acute, minute-by-minute effects on sensory processing by acting within discrete forebrain circuits (Kis et al., 2001; Remage-Healey et al., 2010; Remage-Healey et al., 2012; Tremere et al., 2009; Tremere and Pinaud, 2011).
A primary model system to investigate estrogens as neuromodulators is the Australian zebra finch. Male zebra finches rely predominantly on the central nervous system for the production of estrogens (Remage-Healey et al., 2008; Schlinger and Arnold, 1992), and, as in humans and other primates, systemic estrogen levels can be persistent following gonadectomy (Adkins-Regan et al., 1990). Available evidence indicates that brain estrogen levels can be precisely and acutely controlled at the level of neuronal synaptic terminals in zebra finches (Cornil et al., 2012b; Peterson et al., 2005; Remage-Healey et al., 2011). Interest has therefore grown in understanding the functional significance of acute estrogen production in the brain of zebra finches.
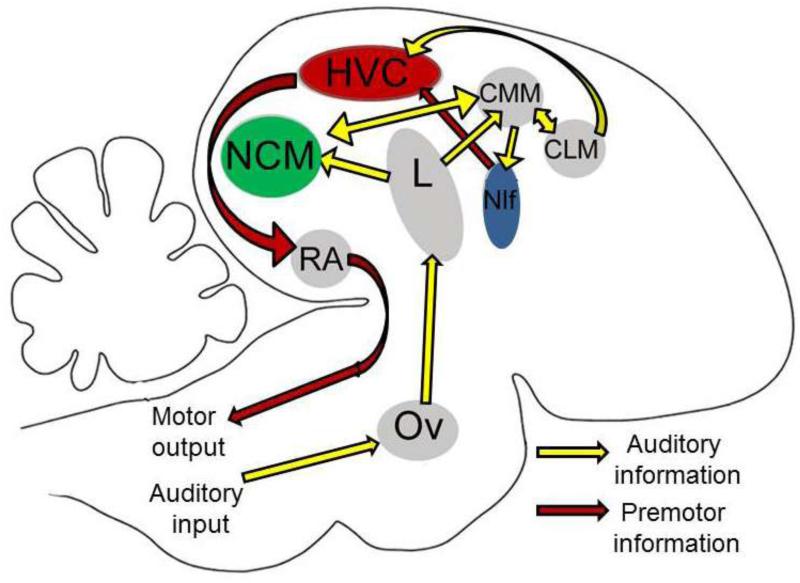
Zebra finches are also an attractive system because of the detailed understanding of their neurobiology. Over three decades of study have illuminated a network of forebrain circuits critical to the regulation of complex behaviors like singing and auditory processing (see Fig. 2). This behaviorally-defined network is essential for audition, auditory-motor integration, and motor patterning of complex vocalizations (Bolhuis and Gahr, 2006; Brainard and Doupe, 2000; Mooney, 2009). The songbird network also shares many features with vocal/ sensorimotor regions in mammalian cortex, including ontogeny, interconnectivity, and laminar/columnar organization (Goldberg and Fee, 2012; Naie and Hahnloser, 2011; Wang et al., 2010). The network consists of a primary motor pathway (Fig. 2; dark red arrows; NIf, HVC, RA), which is responsible for generating a complex motor pattern for learned vocalizations (Leonardo and Fee, 2005; Yu and Margoliash, 1996), and an auditory network (light yellow arrows), which encodes and processes auditory input (Ov, Field L, NCM, CMM, CLM), and sends integrated sensory information into the motor pathway via sensorimotor HVC (Coleman et al., 2007; Keller and Hahnloser, 2009; London and Clayton, 2008). For clarity, a separate basal ganglia pathway that is also involved in song learning and song variability is not depicted in Fig. 2 (for more information on this pathway, consult: Goldberg and Fee, 2012; Heinrich et al., 2005; Olveczky et al., 2011; Sizemore and Perkel, 2011).
Figure 2.
A sagittal schematic of the zebra finch brain. An auditory network is shown connected by yellow arrows, while a sensorimotor/premotor network is shown connected by red arrows. Highlighted in green is the caudomedial nidopallium (NCM), a region of secondary auditory cortex that is enriched with aromatase, the enzyme that synthesizes estrogens. Highlighted in blue is nucleus ‘interface’(NIf) that provides a conduit between auditory and sensorimotor regions in the forebrain. Ov, ovoidalis; L, primary thalamorecipient Field L; NCM, caudomedial nidopallium; HVC (proper name); CMM, caudomedial mesopallium; CLM, caudolateral mesopallium; NIf, nucleus interface; RA, robust nucleus of the arcopallium.
One region that is particularly important for the production of estrogens in zebra finches is the caudomedial nidopallium (NCM; Fig. 2). The NCM is enriched in expression of the estrogen-synthesis enzyme aromatase (Jeong et al., 2011; Saldanha et al., 2000), and it is considered analogous to mammalian secondary auditory cortex (e.g., Bolhuis and Gahr, 2006; Wang et al., 2010). NCM is key for song recognition and discrimination (Boumans et al., 2008; Gobes and Bolhuis, 2007; Sanford et al., 2010; Yoder et al., 2012) and NCM sends information into sensorimotor HVC indirectly via two distinct pathways (NIf and CLM; see Fig. 2). The sensorimotor HVC is essential for both the production and perception of song (Brenowitz, 1991; Gentner et al., 2000; Long and Fee, 2008), and HVC neurons are active during both singing and hearing song (Prather et al., 2008; Yu and Margoliash, 1996). In summary, zebra finches offer an experimental system to combine the study of neurosteroid actions with a precise map of the interconnected forebrain circuits that are devoted to learned vocalizations, akin to human speech (Bolhuis and Gahr, 2006; Hahnloser and Kotowicz, 2010; Jarvis, 2004).
Initial efforts to understand steroids as modulators in the zebra finch utilized an experimental approach to detect and quantify acute changes in brain estrogens, via in vivo microdialysis in the auditory NCM (Remage-Healey et al., 2008). These experiments showed that estrogens fluctuate rapidly in NCM, and not in nearby regions, in response to auditory stimulation and/or social interactions. Follow-up experiments then tested the functional significance of this capacity for neuroestrogen synthesis. Locally-produced estrogens were shown to rapidly regulate auditory processing of single neurons in the NCM (Remage-Healey et al., 2010; Tremere et al., 2009). This enhancement of auditory processing appears to improve the information coding of behaviorally-relevant sounds (Tremere and Pinaud, 2011), indicating a direct effect on the information contained within the NCM. Moreover, acute estrogen-dependent neuromodulation occurs in both males and females in this species, emphasizing its importance to basic sensory processing (Remage-Healey et al., 2012). In summary, this work has demonstrated that the NCM is a critical locus for rapid and localized estrogen neuromodulation in the adult zebra finch brain.
More recent experiments have found that rapid estrogen signaling within auditory NCM enhances processing both within NCM and also downstream in the sensorimotor region HVC. Specifically, in vivo dual electrophysiology recordings have documented that rapid delivery of estrogens to NCM leads to an enhancement of the response selectivity of downstream single HVC neurons (Remage-Healey and Joshi, 2012). In other words, estrogens acting in NCM (but not in adjacent control regions of the auditory forebrain) cause the neurons in downstream HVC to be more selective for sensorimotor-relevant stimuli (Remage-Healey and Joshi, 2012). These findings are especially intriguing in light of prior lesion studies that have shown that NCM and HVC are each critical for perceptual discrimination among complex vocalizations (e.g., Brenowitz, 1991; Gobes and Bolhuis, 2007). Rapid estrogen signaling events are therefore not locally restricted to one brain region and can be transmitted to downstream brain circuits to enhance the representation of complex behaviors.
One line of reasoning when trying to understand how steroid modulation could propagate between identified circuits is to determine whether these actions can be mimicked by simple alterations in the excitatory state of afferent neurons. Recent in vivo experiments have tested this hypothesis. In male zebra finches, in vivo dual recording experiments reveal that brief electrical microstimulation in NCM (parameters: 15 μA, 100 Hz, 300 ms pulses, 14x; similar to: Lei and Mooney, 2010; Remage-Healey and Bass, 2010) does not mimic the specific modulatory actions of rapid estrogen signaling in NCM (L. Remage-Healey, unpubl. obs.). In four separate in vivo preparations, baseline firing rate was elevated immediately post stimulation in NCM (475 Hz relative to pre stimulation firing rate @ 130 Hz) as expected for brief local current injection. However, song selectivity in downstream HVC was unchanged (avg d’ pre = 0.74, post 0.78, p = 0.97) following this same NCM microstimulation. Therefore, general increases in excitation of NCM neurons had no apparent effect on HVC baseline firing rate or HVC stimulus selectivity, in contrast to our findings that elevated E2 exerts a specific pattern of modulation in NCM that leads to parallel local (NCM) and far-reaching (HVC) effects on how sensory signals are represented by the brain (Remage-Healey and Joshi, 2012). The nature of how such rapid and selective steroid modulation occurs and is communicated between brain circuits is not well understood for any system. It is likely to involve dynamic temporal filtering of sensory signals such as has been observed in other model systems and behavioral networks for the propagation of modulated sensory representations (e.g., Kato et al., 2014). One interpretation of our recent findings is that estrogens are shifting the functional connectivity of the sensory and sensorimotor circuits in zebra finches. This possibility has been most recently explored in experiments outlined below.
A critical node in the pathway between auditory NCM and sensorimotor HVC is an ‘interface’ nucleus called nucleus interfacialis or ‘NIf’ (see Fig. 2). NIf is likely to be key to how modulated signals propagate in this circuit for two reasons. First, NIf provides the primary stimulus-selective sensory input into HVC (Coleman and Mooney, 2004; Janata and Margoliash, 1999), and NIf initiates a premotor signal through HVC that governs the production of complex vocalizations (Naie and Hahnloser, 2011). Second, catecholamines such as dopamine and norepinephrine act in NIf to shift response properties and selectivity in HVC (Cardin and Schmidt, 2004). Thus, NIf occupies a key neurochemical and neuroanatomical position as a conduit for changes in functional connectivity between sensory (NCM) and sensorimotor (HVC) circuits. We have recently collected evidence showing that NIf acts as an ‘interface’ nucleus for how rapid actions of estradiol in auditory NCM are delivered into sensorimotor regions (B.A. Pawlisch and L. Remage-Healey, in submission). These experiments show that single neurons in NIf exhibit changes in response properties during estrogen manipulations in NCM, while neurons in the immediate regions surrounding NIf do not.
In summary, although these studies are a long way off from understanding how brain-derived steroids can shift the functional connectivity of circuits , (akin to how biogenic amines act within the STG; e.g., Fig. 1), they provide evidence for common themes that emerge from considering steroids as potent and intrinsic neuromodulators of behavioral circuits.
4. Broadening the scope
A role for estrogens in neuromodulation may be ancient in the animal kingdom. In fact, a study with C. elegans indicated that estrogen-dependent signaling may be involved in thermotaxis learning (Sugi et al., 2011). Moreover, steroids like estrogens can act over longer timescales to change functional connectivity of behaviorally-relevant circuits in the context of audition and motor output in other model systems (e.g., Caras, 2013; Meitzen et al., 2007). With a current growing interest in the role of steroids as modulators of CPGs and behaviors, there is now increasing evidence that steroids and neuropeptides can interact to co-modulate circuits. For example, neuropeptides and steroids interact at multiple timescales to control the patterning of social signals like electric organ discharges in electric fish (Markham and Stoddard, 2013). There is also good evidence that neuropeptides and glucocorticoids interact in the spinal control to modulate clasping behavior in roughskin newts (Coddington and Moore, 2003; Rose et al., 1995). Perhaps the clearest examples of steroid/peptide co-modulation comes from insect species that show clear co-modulation effects of ecdysteroids to prime the effects of biogenic amines in the control of behaviors like swimming (Mesce, 2002). In this view, steroids can be considered one of the many neuromodulators available in the brain to act as signaling molecules in combination with other signaling molecules.
Because steroids were first characterized as acting via nuclear hormone receptors, their ‘classical’ mode of action was identified as altering gene transcription over extended periods (hrs-weeks). The so-called ‘nonclassical’ steroid actions have been well-described in nearly every tissue in the body, including neurons. Nonclassical steroid actions are typically considered too fast to be accounted for by altered gene transcription/protein translation, since they can occur within seconds to minutes of elevated local steroid concentration. Steroids can therefore influence neuronal membrane excitability and cell signaling cascades via ‘nonclassical’ mechanisms (Laredo et al., 2014; Vasudevan and Pfaff, 2008; Woolley, 2007). The parallels here with neuromodulation are clear. Modulators like dopamine can influence CPG functional connectivity via a whole host of cellular mechanisms, including g-protein cascades and ionic conductances. Many classical neuromodulators alter network properties by modulating multiple cellular properties at once, such as the influence of serotonin on both N- and P/Q-type calcium currents in the mouse spinal locomotion CPG (Abbinanti and Harris-Warrick, 2012). It is especially interesting to note that many of the classical neuromodulators (such as serotonin, octopamine, and dopamine) influence CPGs via specific regulation of metabotropic glutamate receptors. The rapid effects of steroids like estrogens have been thoroughly characterized in the hypothalamus, and appear to be dependent on membrane estrogen receptors coupled to mGluR1 and Gq (Kelly and Ronnekleiv, 2009; Mermelstein, 2009; Micevych and Mermelstein, 2008; Roepke et al., 2011). These findings indicate that steroids are able to modulate neurons using the same form of ‘neuromodulator currency’ (i.e., g-protein coupled receptors) as the classical neuromodulators to control neural excitability and the output of neural circuits. This expanding view reinforces the idea that steroids are one class of signaling molecules put to use in the nervous system for modulating circuits.
5. Conclusions and Future directions
Do steroids generated in brain circuits act as modulators of those circuits? This essay presents some recent evidence that brain-derived estrogens can: 1) alter sensory representations by changing the information content of circuits, and 2) change the functional connectivity of circuits involved in sensorimotor integration. Two clear threads are missing from this story thus far, when compared with the wealth of knowledge about the ‘classical’ neuromodulators. First, it is not clear yet that steroids can guide behavioral transitions by acting as stabilizing agents over acute timescales in the way of classical modulators. Second, steroid modulation within forebrain circuits likely does not occur via interaction with specific CPGs. Instead, it appears that steroid neuromodulation can change the intrinsic rhythmicity of forebrain circuits, perhaps via similar mechanisms. For example, estrogens can shape and sculpt the rhythms of circadian circuits, including the suprachiasmatic nucleus (Kow and Pfaff, 1984). Estrogens can also rapidly increase the synchrony of neurons within identified circuits and the synchrony with external activity rhythms such as the theta rhythm in the septo-hippocampal circuit (Steffensen 2006; see also Scullin and Partridge 2012 for similar findings with pregnenolone). The estrogen-dependent enhanced synchronization of microcircuits (such as the hippocampal CA1) with the rhythmic activity of external cortical inputs could provide a proximate mechanism for the cognitive effects of estrogens, such as improving acquisition and/or consolidation of memories. In this view, estrogens do not act on the activity of ‘CPGs’ in forebrain circuits per se, but they could perform similarly acute coordinating functions by modulating the intrinsic rhythmic activity of circuits (e.g., within the CA1, NCM, POA, or SCN). Further exploration of how neurosteroids change the rhythmic firing patterns of forebrain/cortical circuits are now warranted.
Based on available evidence reviewed here and elsewhere, it appears that brain-derived steroids can act like classical modulators by using similar molecular mechanisms to influence neural circuit function on a minute-by-minute timescale. This perspective opens up a host of new questions regarding how these signaling systems are integrated by neural circuits, as outlined below.
a. Nonclassical modulators
Our view of what defines the properties of a neuromodulator has recently undergone a major expansion to include molecules like oxide gases, endocanabinoids, hydrogen sulfides, thyroxine, and steroids (Balthazart and Ball, 2006; Caria et al., 2009; Saldanha et al., 2011; Snyder, 2009). For example, nitric oxide appears to act as a stabilizing neuromodulator in the control of multiple movements patterned by a gut CPG in a mollusc (Dyakonova and Dyakonova, 2010). Owing to their somewhat peculiar chemistry, most of these nonclassical modulators are not packaged into vesicles for conventional release by neurons, and their release properties and interactions can be comparatively complex (e.g., Boychuk et al., 2013; Huang and Woolley, 2012). Thyroxine itself may share similarities with steroids in terms of ‘synaptocrine’ signaling in the brain, including nongenomic effects (Caria et al., 2009; Davis et al., 2007), a distribution of bouton-like beads along fibers coursing through cortex (Rozanov and Dratman, 1996), and a hypothesized role as neurotransmitters (Dratman and Gordon, 1996; Greenberg et al., 2006). Our understanding of the group of molecules that can modulate neural circuits is now expanding, which illuminates the flexibility afforded to neural circuits to respond to varying internal and external states and generate many flexible outputs.
b. Co-release of modulators
Most neuropeptides are co-released with ordinary fast-acting neurotransmitters, which means that their downstream effects are very likely inter-dependent (Dabrowska et al., 2013; Dicken et al., 2012; Whittaker, 2010). In fact the co-release of neuropeptide Y and GABA in hypothalamic neurons is necessary for the postsynaptic effects of either to take place (van den Pol, 2012). The predominant mode of action for many neuropeptides is to change the balance of excitation and inhibition on target neurons. The co-release of modulators by individual neurons has now become an active area of interest. For example, three different modulatory neurons project into the crustacean STG that contain the peptide proctolin. These three neurons elicit three different CPG rhythms in the STG when activated, but only because they have three separate neurotransmitter pools that are co-released with proctolin (Blitz et al., 1999). In fact, the release of procotolin + GABA as co-transmitters can influence downstream CPG neurons independently to produce a coordinated rhythmic output in the STG (Stein et al., 2007). In vertebrate CPGs, such as those for locomotion in newts, the actions of peptides (e.g., CRF) and biogenic amines (e.g., 5HT) are coordinated in a way that reflects co-modulation (Lowry et al., 2009). Therefore our understanding is growing rapidly about the co-release of neurotransmitters and peptides, and their coordinated modulation of CPGs and behavior. By contrast, we currently know next to nothing about how the neuromodulatory actions of locally-released steroids in the brain may depend on co-transmission with classical neurotransmitters and/or neuropeptides.
c. Neuromodulation of sensory perception
The role of neuromodulators in setting sensory thresholds and guiding behaviors via regulation of sensory processing continues to be an important area of investigation in behavioral neuroscience. As an example, the classical modulator serotonin alone has received considerable attention for its role in regulating sensory processing in a variety of model systems (e.g., Deemyad et al., 2013; Hurley et al., 2004; Hurley and Pollak, 2005; Petzold et al., 2009). The role of estrogens in guiding sensory perception as a neuromodulator has now become an active area of interest to songbird researchers, as summarized here and elsewhere (Maney and Pinaud, 2011). Steroids can modulate peripheral sensory encoding in several vertebrate groups (Caras, 2013; Sisneros, 2009) and estrogen synthesis enzymes are enriched in the sensory cortex especially in primates (Azcoitia et al., 2011; Biegon et al., 2010). Therefore, it is likely that the estrogen-dependent modulation of sensory representations in the CNS is not unique to songbirds, and will be an important area for future exploration.
d. A balancing act of peripheral and central modulation
In the crustacean STG, peripheral endocrine glands (such as the pericardial organs) secrete the same modulators into the hemolymph that can activate CPG rhythms (i.e., biogenic amines) as are secreted by the neuromodulator neurons that are contained within the STG itself (Marder, 2012). This raises an issue that is pertinent to our current focus on steroids as neuromodulators. Namely, how do substances that are secreted by peripheral glands (e.g., estrogens secreted by the ovary) synergize with the same substances secreted within brain circuits (e.g., neuroestrogens) to act as neuromodulators of those circuits? It appears that, once again, invertebrate models have begun to shed light on this complex issue. The concentration of dopamine in the hemolymph of spiny lobsters is at a low basal tone that changes slowly over time, whereas the concentration thought to be released within the STG is considerably higher and more transient (Rodgers et al., 2011). It remains to be determined whether the differential concentration of circulating steroids vs. neurosteroids can produce a similar basal tonic vs. elevated phasic modulation of neural circuits relevant to behavior (see Cornil, 2009; Cornil et al., 2012a for further discussion).
e. Evolution of neuromodulation
Since neuromodulators offer a means to achieve network stability and flexibility, it is perhaps not surprising that natural selection has reached the ‘solution’ of the regulation of similar behaviors via remarkably convergent actions of modulators. In fact, in several cases there appears to have been a convergent evolution of neuromodulation for the control of behavior in distantly-related species (Katz, 2011). For example, in two sea slug species (Tritonia and Pleurobranchaea) swimming behavior via dorsal-ventral body flexion is controlled by CPGs consisting of identified neurons with homologous connectivity and firing properties. The sea slug escape swimming CPG appears to have evolved independently in these two species, as demonstrated by phylogenetic parsimony analyses including the observations that sister taxa do not exhibit this behavior or CPG (Newcomb et al., 2012). Recent work has shown that the initiation of the swimming CPG is controlled by serotonergic input in both species, and swimming is not elicited by serotonin in a closely-related species without the CPG (Lillvis and Katz, 2013). Therefore, similar neuromodulator systems can be coopted independently to solve very similar network/behavioral problems in distantly related species. The role of serotonin in locomotion is also a common theme in vertebrates, and just as in sea slugs, the CPGs for mammalian, amphibian, and lamprey locomotion alike are activated and shaped by serotonergic actions (Biro et al., 2006; Dunbar et al., 2010; Jordan and Slawinska, 2011; Lowry et al., 2009; Schmidt and Jordan, 2000).
The behavioral regulation by neuropeptides appears to be a common mechanism associated with species-level differences in social systems among animals (Goodson and Thompson, 2010). There are many systems in which parallel evolutionary relationships between neural networks and neuromodulators are expected to be found. These include the well-documented vole species clade, in which the actions of neuropeptides like oxytocin and vasopressin are known to regulate social pair bonding behavior via actions in the ventral forebrain (Young and Wang, 2004). This system offers a terrific opportunity to test whether neuropeptides shift the firing properties of ventral forebrain neurons in the species that pair bond (e.g., prairie voles) vs. those that do not (e.g., meadow voles). In a similar vein, there are three apparently independently evolved clades of vocal learning in birds (parrots, hummingbirds, and oscine songbirds), which offer a rich diversity of neural networks that are similar but not identical in their connectivity. Although there has been a good deal of exploration into the neural, neurogenetic and hormonal similarities among these three clades of avian vocal learners (Clayton et al., 2009; Hara et al., 2012; Jarvis et al., 2013; Replogle et al., 2008), it also remains to be tested whether networks of neurons involved in song learning are similarly regulated by the actions of neuromodulators to support a complex behavior that has evolved independently.
The comments in this essay may resonate for behavioral neuroscientists and neuroendocrinologists, since the field has been propelled by considering the neuromodulatory actions of neurochemicals that interact with the ‘social behavior network’ as originally formulated by Sarah Newman (Newman, 1999) and more recently elaborated in the context of neuropeptides (Goodson, 2005). The dynamics of functional connectivity within behaviorally-relevant circuits have continued to drive interest in the field (e.g., Hoke et al., 2005; Maney et al., 2008). The relatively new perspective of steroids as intrinsic modulators of circuits in the control of ethologically-relevant behaviors will hopefully contribute to new directions in understanding behavioral neurobiology and endocrinology.
Highlights.
Classical neuromodulators change functional circuit connectivity.
Classical neuromodulators also enable many flexible behavioral outputs.
Brain generated steroids may act as neuromodulators within brain circuits.
Future directions for this line of research are discussed.
Acknowledgments
The author thanks Daniel Vahaba for help in adapting Fig. 1, and L. Michael Romero, Elizabeth Adkins-Regan, Andrew Bass, and Barney Schlinger for mentorship and formative discussions. The author also thanks the Society for Behavioral Neuroendocrinology for its active commitment to trainee mentoring and awards. Preparation of this review was supported in part by NIH grants R00NS066179, R01NS082179 and NSF grant IOS 1354906.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbinanti MD, Harris-Warrick RM. Serotonin modulates multiple calcium current subtypes in commissural interneurons of the neonatal mouse. J Neurophysiol. 2012;107:2212–2219. doi: 10.1152/jn.00768.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex Steroid-Levels in Developing and Adult Male and Female Zebra Finches (Poephila-Guttata) Gen. Comp. Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Amunts K, Lepage C, Borgeat L, Mohlberg H, Dickscheid T, Rousseau ME, Bludau S, Bazin PL, Lewis LB, Oros-Peusquens AM, Shah NJ, Lippert T, Zilles K, Evans AC. BigBrain: an ultrahigh-resolution 3D human brain model. Science. 2013;340:1472–1475. doi: 10.1126/science.1235381. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Newsome WT. The Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative and Neurology. JAMA Neurol. 2014 doi: 10.1001/jamaneurol.2014.411. [DOI] [PubMed] [Google Scholar]
- Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav. 2008;53:659–672. doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Zakon HH. Sonic and electric fish: at the crossroads of neuroethology and behavioral neuroendocrinology. Horm Behav. 2005;48:360–372. doi: 10.1016/j.yhbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Bedecarrats A, Cornet C, Simmers J, Nargeot R. Implication of dopaminergic modulation in operant reward learning and the induction of compulsive-like feeding behavior in Aplysia. Learn Mem. 2013;20:318–327. doi: 10.1101/lm.029140.112. [DOI] [PubMed] [Google Scholar]
- Biegon A, Kim SW, Alexoff DL, Jayne M, Carter P, Hubbard B, King P, Logan J, Muench L, Pareto D, Schlyer D, Shea C, Telang F, Wang GJ, Xu Y, Fowler JS. Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11C]vorozole. Synapse. 2010;64:801–807. doi: 10.1002/syn.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro Z, Hill RH, Grillner S. 5-HT Modulation of identified segmental premotor interneurons in the lamprey spinal cord. J Neurophysiol. 2006;96:931–935. doi: 10.1152/jn.00309.2006. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci. 1999;19:5449–5463. doi: 10.1523/JNEUROSCI.19-13-05449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nature Reviews Neuroscience. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Boumans T, Vignal C, Smolders A, Sijbers J, Verhoye M, Van Audekerke J, Mathevon N, Van der Linden A. Functional magnetic resonance imaging in zebra finch discerns the neural substrate involved in segregation of conspecific song from background noise. J Neurophysiol. 2008;99:931–938. doi: 10.1152/jn.00483.2007. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Boychuk CR, Zsombok A, Tasker JG, Smith BN. Rapid Glucocorticoid-Induced Activation of TRP and CB1 Receptors Causes Biphasic Modulation of Glutamate Release in Gastric-Related Hypothalamic Preautonomic Neurons. Front Neurosci. 2013;7:3. doi: 10.3389/fnins.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nature Reviews Neuroscience. 2000;1:31–40. doi: 10.1038/35036205. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Altered Perception of Species-Specific Song by Female Birds after Lesions of a Forebrain Nucleus. Science. 1991;251:303–305. doi: 10.1126/science.1987645. [DOI] [PubMed] [Google Scholar]
- Caras ML. Estrogenic modulation of auditory processing: a vertebrate comparison. Front Neuroendocrinol. 2013;34:285–299. doi: 10.1016/j.yfrne.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic Inputs Mediate State Dependence of Auditory Responses in the Avian Song System. J. Neurosci. 2004;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caria MA, Dratman MB, Kow LM, Mameli O, Pavlides C. Thyroid Hormone Action: Nongenomic Modulation of Neuronal Excitability in the Hippocampus. J Neuroendocrinol. 2009;21:98–107. doi: 10.1111/j.1365-2826.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- Chung JS, Dircksen H, Webster SG. A remarkable, precisely timed release of hyperglycemic hormone from endocrine cells in the gut is associated with ecdysis in the crab Carcinus maenas. Proc Natl Acad Sci U S A. 1999;96:13103–13107. doi: 10.1073/pnas.96.23.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DF. The genomic action potential. Neurobiol Learn Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- Clayton DF, George JM, Mello CV, Siepka SM. Conservation and expression of IQ-domain-containing calpacitin gene products (neuromodulin/GAP-43, neurogranin/RC3) in the adult and developing oscine song control system. Dev Neurobiol. 2009;69:124–140. doi: 10.1002/dneu.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Belin-Rauscent A, Simmers J, Combes D. Opposing modulatory effects of D1- and D2-like receptor activation on a spinal central pattern generator. J Neurophysiol. 2012;107:2250–2259. doi: 10.1152/jn.00366.2011. [DOI] [PubMed] [Google Scholar]
- Coddington E, Moore FL. Neuroendocrinology of context-dependent stress responses: vasotocin alters the effect of corticosterone on amphibian behaviors. Horm Behav. 2003;43:222–228. doi: 10.1016/s0018-506x(02)00015-6. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Mooney R. Synaptic transformations underlying highly selective auditory representations of learned birdsong. J. Neurosci. 2004;24:7251–7265. doi: 10.1523/JNEUROSCI.0947-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Roy A, Wild JM, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. J. Neurosci. 2007;27:10024–10036. doi: 10.1523/JNEUROSCI.2215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA. Rapid Regulation of Brain Oestrogen Synthesis: The Behavioural Roles of Oestrogens and their Fates. J Neuroendocrinol. 2009;21:217–226. doi: 10.1111/j.1365-2826.2009.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain-derived estrogens. Front Neuroendocrinol. 2012a;33:425–446. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Leung CH, Pletcher ER, Naranjo KC, Blauman SJ, Saldanha CJ. Acute and specific modulation of presynaptic aromatization in the vertebrate brain. Endocrinology. 2012b;153:2562–2567. doi: 10.1210/en.2011-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG. Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci. 2013;7:156. doi: 10.3389/fnins.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P, Leonard J, Davis F. Mechanisms of nongenomic actions of thyroid hormone. Frontiers in Neuroendocrinology. 2007 doi: 10.1016/j.yfrne.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Deemyad T, Metzen MG, Pan Y, Chacron MJ. Serotonin selectively enhances perception and sensory neural responses to stimuli generated by same-sex conspecifics. Proc Natl Acad Sci U S A. 2013;110:19609–19614. doi: 10.1073/pnas.1314008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Page ME, Waterhouse BD. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J Neurosci. 2006;26:9860–9872. doi: 10.1523/JNEUROSCI.1776-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicken MS, Tooker RE, Hentges ST. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J Neurosci. 2012;32:4042–4048. doi: 10.1523/JNEUROSCI.6032-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratman MB, Gordon JT. Thyroid hormones as neurotransmitters. Thyroid. 1996;6:639–647. doi: 10.1089/thy.1996.6.639. [DOI] [PubMed] [Google Scholar]
- Dunbar MJ, Tran MA, Whelan PJ. Endogenous extracellular serotonin modulates the spinal locomotor network of the neonatal mouse. J Physiol. 2010;588:139–156. doi: 10.1113/jphysiol.2009.177378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyakonova VE, Dyakonova TL. Coordination of rhythm-generating units via NO and extrasynaptic neurotransmitter release. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196:529–541. doi: 10.1007/s00359-010-0541-5. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Few WP, Zakon HH. Androgens alter electric organ discharge pulse duration despite stability in electric organ discharge frequency. Horm. Behav. 2001;40:434–442. doi: 10.1006/hbeh.2001.1709. [DOI] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM. Aminergic modulation in lobster stomatogastric ganglion. II. Target neurons of dopamine, octopamine, and serotonin within the pyloric circuit. J Neurophysiol. 1986;55:866–881. doi: 10.1152/jn.1986.55.5.866. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell. 2013;154:1023–1035. doi: 10.1016/j.cell.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Bentley GE, Ball GF. Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning, and categorization of conspecific song in adult songbirds. J Neurobiol. 2000;42:117–133. doi: 10.1002/(sici)1097-4695(200001)42:1<117::aid-neu11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Glimcher PW. Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15647–15654. doi: 10.1073/pnas.1014269108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobes SMH, Bolhuis JJ. Birdsong memory: A neural dissociation between song recognition and production. Curr Biol. 2007;17:789–793. doi: 10.1016/j.cub.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Fee MS. A cortical motor nucleus drives the basal ganglia-recipient thalamus in singing birds. Nat Neurosci. 2012;15:620–627. doi: 10.1038/nn.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiol. 2010;20:784–794. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Greenberg JH, Reivich M, Gordon JT, Schoenhoff MB, Patlak CS, Dratman MB. Imaging triiodothyronine binding kinetics in rat brain: A model for studies in human subjects. Synapse. 2006;60:212–222. doi: 10.1002/syn.20293. [DOI] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: From ion channels to neuronal networks. Nature Reviews Neuroscience. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Hahnloser RH, Kotowicz A. Auditory representations and memory in birdsong learning. Curr Opin Neurobiol. 2010;20:332–339. doi: 10.1016/j.conb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Hara E, Rivas MV, Ward JM, Okanoya K, Jarvis ED. Convergent differential regulation of parvalbumin in the brains of vocal learners. PLoS One. 2012;7:e29457. doi: 10.1371/journal.pone.0029457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM. Neuromodulation and flexibility in Central Pattern Generator networks. Curr Opin Neurobiol. 2011;21:685–692. doi: 10.1016/j.conb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Johnson BR. Checks and balances in neuromodulation. Front Behav Neurosci. 2010;4 doi: 10.3389/fnbeh.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich JE, Nordeen KW, Nordeen EJ. Dissociation between extension of the sensitive period for avian vocal learning and dendritic spine loss in the song nucleus IMAN. Neurobiol. Learn. Mem. 2005;83:143–150. doi: 10.1016/j.nlm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Hofmann HA. The neuroendocrine action potential. Winner of the 2008 Frank Beach Award in Behavioral Neuroendocrinology. Horm Behav. 2010;58:555–562. doi: 10.1016/j.yhbeh.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Hoke KL, Pitts NL. Modulation of sensory-motor integration as a general mechanism for context dependence of behavior. Gen Comp Endocrinol. 2012;176:465–471. doi: 10.1016/j.ygcen.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc Natl Acad Sci U S A. 2005;102:10712–10717. doi: 10.1073/pnas.0502361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin shifts first-spike latencies of inferior colliculus neurons. J Neurosci. 2005;25:7876–7886. doi: 10.1523/JNEUROSCI.1178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Sullivan MR. From behavioral context to receptors: serotonergic modulatory pathways in the IC. Front Neural Circuits. 2012;6:58. doi: 10.3389/fncir.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel JM, Cabelguen JM, Le Masson G, Oliet SH, Ciofi P. Neonatal testosterone suppresses a neuroendocrine pulse generator required for reproduction. Nat Commun. 2014;5:3285. doi: 10.1038/ncomms4285. [DOI] [PubMed] [Google Scholar]
- Jacob SN, Ott T, Nieder A. Dopamine regulates two classes of primate prefrontal neurons that represent sensory signals. J Neurosci. 2013;33:13724–13734. doi: 10.1523/JNEUROSCI.0210-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata P, Margoliash D. Gradual emergence of song selectivity in sensorimotor structures of the male zebra finch song system. J Neurosci. 1999;19:5108–5118. doi: 10.1523/JNEUROSCI.19-12-05108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell TA, Wang Y, Bloniarz AE, Brittin CA, Xu M, Thomson JN, Albertson DG, Hall DH, Emmons SW. The connectome of a decision-making neural network. Science. 2012;337:437–444. doi: 10.1126/science.1221762. [DOI] [PubMed] [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Yu J, Rivas MV, Horita H, Feenders G, Whitney O, Jarvis SC, Jarvis ER, Kubikova L, Puck AE, Siang-Bakshi C, Martin S, McElroy M, Hara E, Howard J, Pfenning A, Mouritsen H, Chen CC, Wada K. Global view of the functional molecular organization of the avian cerebrum: mirror images and functional columns. J Comp Neurol. 2013;521:3614–3665. doi: 10.1002/cne.23404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JK, Burrows K, Tremere LA, Pinaud R. Neurochemical organization and experience-dependent activation of estrogen-associated circuits in the songbird auditory forebrain. Eur J Neurosci. 2011;34:283–291. doi: 10.1111/j.1460-9568.2011.07743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan LM, Slawinska U. Chapter 12--modulation of rhythmic movement: control of coordination. Prog Brain Res. 2011;188:181–195. doi: 10.1016/B978-0-444-53825-3.00017-6. [DOI] [PubMed] [Google Scholar]
- Kato S, Xu Y, Cho CE, Abbott LF, Bargmann CI. Temporal responses of C. elegans chemosensory neurons are preserved in behavioral dynamics. Neuron. 2014;81:616–628. doi: 10.1016/j.neuron.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS. Neural mechanisms underlying the evolvability of behaviour. Philos Trans R Soc Lond B Biol Sci. 2011;366:2086–2099. doi: 10.1098/rstb.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Edwards DH. Metamodulation: the control and modulation of neuromodulation. In: Katz PS, editor. Beyond neurotransmission: neuromodulation and its importance for information processing. Oxford: 1999. pp. 349–381. [Google Scholar]
- Keller GB, Hahnloser RHR. Neural processing of auditory feedback during vocal practice in a songbird. Nature. 2009;457:187–190. doi: 10.1038/nature07467. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Bass AH. Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning. Curr Opin Neurobiol. 2010;20:748–753. doi: 10.1016/j.conb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol Cell Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Katz PS. Making circuits dance: neuromodulation of motor systems. In: Katz PS, editor. Beyond neurotransmission: neuromodulation and its importance for information processing. Oxford: 1999. pp. 275–317. [Google Scholar]
- Kis Z, Budai D, Imre G, Farkas T, Horvath S, Toldi J. The modulatory effect of estrogen on the neuronal activity in the barrel cortex of the rat. An electrophysiological study. Neuroreport. 2001;12:2509–2512. doi: 10.1097/00001756-200108080-00044. [DOI] [PubMed] [Google Scholar]
- Kvarta MD, Harris-Warrick RM, Johnson BR. Neuromodulator-evoked synaptic metaplasticity within a central pattern generator network. J Neurophysiol. 2012;108:2846–2856. doi: 10.1152/jn.00586.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo SA, Villalon Landeros R, Trainor BC. Rapid effects of estrogens on behavior: Environmental modulation and molecular mechanisms. Front Neuroendocrinol. 2014 doi: 10.1016/j.yfrne.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Wendel BJ, Perkel DJ. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J Neurosci. 2010;30:5730–5743. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Mooney R. Manipulation of a Central Auditory Representation Shapes Learned Vocal Output. Neuron. 2010;65:122–134. doi: 10.1016/j.neuron.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A, Fee MS. Ensemble coding of vocal control in birdsong. J. Neurosci. 2005;25:652–661. doi: 10.1523/JNEUROSCI.3036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Rose JD. Rapid corticosterone-induced impairment of amplectic clasping occurs in the spinal cord of roughskin newts (Taricha granulosa) Horm. Behav. 2003;43:93–98. doi: 10.1016/s0018-506x(02)00019-3. [DOI] [PubMed] [Google Scholar]
- Lillvis JL, Katz PS. Parallel evolution of serotonergic neuromodulation underlies independent evolution of rhythmic motor behavior. J Neurosci. 2013;33:2709–2717. doi: 10.1523/JNEUROSCI.4196-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wu MM, Zakon HH. A novel Na+ channel splice form contributes to the regulation of an androgen-dependent social signal. J Neurosci. 2008;28:9173–9182. doi: 10.1523/JNEUROSCI.2783-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nature Neuroscience. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Burke KA, Renner KJ, Moore FL. Fluoxetine potentiates the effects of corticotropin-releasing factor on locomotor activity and serotonergic systems in the roughskin newt, Taricha granulosa. Horm Behav. 2009;56:177–184. doi: 10.1016/j.yhbeh.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol Modulates Neural Responses to Song in a Seasonal Songbird. J. Comp. Neurol. 2008;511:173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Maney DL, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrinol. 2011;32:287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol. 2001;11:R986–996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol. 2005;15:R685–699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Markham MR, Stoddard PK. Cellular mechanisms of developmental and sex differences in the rapid hormonal modulation of a social communication signal. Horm Behav. 2013;63:586–597. doi: 10.1016/j.yhbeh.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J. Neurosci. 2007;27:12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG. Membrane-localised oestrogen receptor alpha and beta influence neuronal activity through activation of metabotropic glutamate receptors. J Neuroendocrinol. 2009;21:257–262. doi: 10.1111/j.1365-2826.2009.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesce KA. Metamodulation of the biogenic amines: Second-order modulation by steroid hormones and amine cocktails. Brain Behav. Evol. 2002;60:339–349. doi: 10.1159/000067793. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learning & Memory. 2009;16:655–669. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Mukai H, Takata N, Ishii HT, Tanabe N, Hojo Y, Furukawa A, Kimoto T, Kawato S. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: Synaptocrinology. Neuroscience. 2006;138:757–764. doi: 10.1016/j.neuroscience.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- Naie K, Hahnloser RH. Regulation of learned vocal behavior by an auditory motor cortical nucleus in juvenile zebra finches. J Neurophysiol. 2011;106:291–300. doi: 10.1152/jn.01035.2010. [DOI] [PubMed] [Google Scholar]
- Newcomb JM, Sakurai A, Lillvis JL, Gunaratne CA, Katz PS. Homology and homoplasy of swimming behaviors and neural circuits in the Nudipleura (Mollusca, Gastropoda, Opisthobranchia) Proc Natl Acad Sci U S A. 2012;109(Suppl 1):10669–10676. doi: 10.1073/pnas.1201877109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior - A node in the mammalian social behavior network. Advancing from the Ventral Striatum to the Extended Amygdala. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, Mortrud MT, Ouellette B, Nguyen TN, Sorensen SA, Slaughterbeck CR, Wakeman W, Li Y, Feng D, Ho A, Nicholas E, Hirokawa KE, Bohn P, Joines KM, Peng H, Hawrylycz MJ, Phillips JW, Hohmann JG, Wohnoutka P, Gerfen CR, Koch C, Bernard A, Dang C, Jones AR, Zeng H. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olveczky BP, Otchy TM, Goldberg JH, Aronov D, Fee MS. Changes in the neural control of a complex motor sequence during learning. J Neurophysiol. 2011;106:386–397. doi: 10.1152/jn.00018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings of the Royal Society B-Biological Sciences. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009;12:784–791. doi: 10.1038/nn.2335. [DOI] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory—vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- Ramsey LC, Sinha SR, Hurley LM. 5-HT1A and 5-HT1B receptors differentially modulate rate and timing of auditory responses in the mouse inferior colliculus. Eur J Neurosci. 2010;32:368–379. doi: 10.1111/j.1460-9568.2010.07299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L. Brain estrogen signaling effects acute modulation of acoustic communication behaviors: A working hypothesis. Bioessays. 2012;34:1009–1016. doi: 10.1002/bies.201200081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J. Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Estradiol interacts with an opioidergic network to achieve rapid modulation of a vocal pattern generator. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196:137–146. doi: 10.1007/s00359-009-0500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Joshi NR. Changing Neuroestrogens Within the Auditory Forebrain Rapidly Transform Stimulus Selectivity in a Downstream Sensorimotor Nucleus. J Neurosci. 2012;32:8231–8241. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Saldanha CJ, Schlinger BA. Estradiol synthesis and action at the synapse: Evidence for 'synaptocrine' signaling. Frontiers in Endocrinology. 2011;2:1–13. doi: 10.3389/fendo.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Replogle K, Arnold AP, Ball GF, Band M, Bensch S, Brenowitz EA, Dong S, Drnevich J, Ferris M, George JM, Gong G, Hasselquist D, Hernandez AG, Kim R, Lewin HA, Liu L, Lovell PV, Mello CV, Naurin S, Rodriguez-Zas S, Thimmapuram J, Wade J, Clayton DF. The Songbird Neurogenomics (SoNG) Initiative: Community-based tools and strategies for study of brain gene function and evolution. Bmc Genomics. 2008;9 doi: 10.1186/1471-2164-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes HJ, Yu HJ, Yamaguchi A. Xenopus Vocalizations Are Controlled by a Sexually Differentiated Hindbrain Central Pattern Generator. J. Neurosci. 2007;27:1485–1497. doi: 10.1523/JNEUROSCI.4720-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers EW, Fu JJ, Krenz WD, Baro DJ. Tonic nanomolar dopamine enables an activity-dependent phase recovery mechanism that persistently alters the maximal conductance of the hyperpolarization-activated current in a rhythmically active neuron. J Neurosci. 2011;31:16387–16397. doi: 10.1523/JNEUROSCI.3770-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front Biosci. 2011;16:1560–1573. doi: 10.2741/3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JD, Kinnaird JR, Moore FL. Neurophysiological Effects of Vasotocin and Corticosterone on Medullary Neurons - Implications for Hormonal-Control of Amphibian Courtship Behavior. Neuroendocrinology. 1995;62:406–417. doi: 10.1159/000127030. [DOI] [PubMed] [Google Scholar]
- Rozanov CB, Dratman MB. Immunohistochemical mapping of brain triiodothyronine reveals prominent localization in central noradrenergic systems. Neuroscience. 1996;74:897–915. doi: 10.1016/0306-4522(96)00186-8. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine Signaling: Steroid Synthesis and Action at the Synapse. Endocr Rev. 2011 doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J. Comp. Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sanford SE, Lange HS, Maney DL. Topography of Estradiol-Modulated Genomic Responses in the Songbird Auditory Forebrain. Dev Neurobiol. 2010;70:73–86. doi: 10.1002/dneu.20757. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Circulating Estrogens in a Male Songbird Originate in the Brain. Proc. Natl. Acad. Sci. U. S. A. 1992;89:7650–7653. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA. Steroid-dependent auditory plasticity for the enhancement of acoustic communication: recent insights from a vocal teleost fish. Hear Res. 2009;252:9–14. doi: 10.1016/j.heares.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore M, Perkel DJ. Premotor synaptic plasticity limited to the critical period for song learning. Proc Natl Acad Sci U S A. 2011;108:17492–17497. doi: 10.1073/pnas.1104255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH. Neurotransmitters, receptors, and second messengers galore in 40 years. J Neurosci. 2009;29:12717–12721. doi: 10.1523/JNEUROSCI.3670-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci. 2007;26:1148–1165. doi: 10.1111/j.1460-9568.2007.05744.x. [DOI] [PubMed] [Google Scholar]
- Sugi T, Nishida Y, Mori I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nat Neurosci. 2011;14:984–992. doi: 10.1038/nn.2854. [DOI] [PubMed] [Google Scholar]
- Towlson EK, Vertes PE, Ahnert SE, Schafer WR, Bullmore ET. The rich club of the C. elegans neuronal connectome. J Neurosci. 2013;33:6380–6387. doi: 10.1523/JNEUROSCI.3784-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Vierk R, Pflueger HJ, Duch C. Differential effects of octopamine and tyramine on the central pattern generator for Manduca flight. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:265–277. doi: 10.1007/s00359-008-0404-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proc Natl Acad Sci U S A. 2010;107:12676–12681. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker VP. Some currently neglected aspects of cholinergic function. J Mol Neurosci. 2010;40:7–11. doi: 10.1007/s12031-009-9247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Yoder KM, Lu K, Vicario DS. Blocking estradiol synthesis affects memory for songs in auditory forebrain of male zebra finches. Neuroreport. 2012;23:922–926. doi: 10.1097/WNR.0b013e3283588b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang ZX. The neurobiology of pair bonding. Nature Neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- Yu HJ, Yamaguchi A. 5-HT2C-like receptors in the brain of Xenopus laevis initiate sex-typical fictive vocalizations. J Neurophysiol. 2009;102:752–765. doi: 10.1152/jn.90469.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Sheibanie AF, Oh M, Rabbah P, Nadim F. Peptide neuromodulation of synaptic dynamics in an oscillatory network. J Neurosci. 2011;31:13991–14004. doi: 10.1523/JNEUROSCI.3624-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornik E, Kelley DB. A neuroendocrine basis for the hierarchical control of frog courtship vocalizations. Front Neuroendocrinol. 2011;32:353–366. doi: 10.1016/j.yfrne.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]