Abstract
Objective:
We investigated an array of possible markers of early dementia in Parkinson disease.
Methods:
We performed a comprehensive assessment of autonomic, sleep, psychiatric, visual, olfactory, and motor manifestations in 80 patients with Parkinson disease who were dementia-free at baseline. After 4.4 years’ follow-up, patients were evaluated for dementia. Predictive variables were assessed using logistic regression adjusting for disease duration, follow-up duration, age, and sex.
Results:
Of 80 patients, 27 (34%) developed dementia. Patients destined to develop dementia were older and more often male (odds ratio [OR] = 3.64, p = 0.023). Those with baseline mild cognitive impairment had increased dementia risk (OR = 22.5, p < 0.001). REM sleep behavior disorder at baseline dramatically increased dementia risk (OR = 49.7, p = 0.001); however, neither daytime sleepiness nor insomnia predicted dementia. Higher baseline blood pressure increased dementia risk (OR = 1.37 per 10 mm Hg, p = 0.032). Orthostatic blood pressure drop was strongly associated with dementia risk (OR = 1.84 per 10 mm Hg, p < 0.001); having a systolic drop of >10 mm Hg increased dementia odds 7-fold (OR = 7.3, p = 0.002). Abnormal color vision increased dementia risk (OR = 3.3, p = 0.014), but olfactory dysfunction did not. Among baseline motor variables, proportion of gait involvement (OR = 1.12, p = 0.023), falls (OR = 3.02, p = 0.042), and freezing (OR = 2.63, p = 0.013), as well as the Purdue Pegboard Test (OR = 0.67, p = 0.049) and alternate tap test (OR = 0.97, p = 0.033) predicted dementia.
Conclusion:
Cardiovascular autonomic dysfunction, REM sleep behavior disorder, color discrimination ability, and gait dysfunction strongly predict development of dementia in Parkinson disease.
Dementia is among the most devastating nonmotor features of Parkinson disease (PD), causing severe decline in quality of life, increased caregiver burden, increased mortality, and often institutionalization.1–3 The prevalence of dementia in PD ranges between 24% and 50% and accounts for about 3% to 4% of all dementia in the population.4–6 An incidence rate of approximately 100 per 1,000 patient-years, more than 5 times that of age-matched controls, has been estimated.7
Identifying the factors predictive of PD dementia is not only important in identifying high-risk patients for appropriate counseling and planning future care, but also in recognizing early cognitive impairment for targeted therapeutic interventions (e.g., clinical trials). Advanced age is the most established risk factor for dementia. Some studies also find that motor features, such as the akinetic-rigid Parkinson subtype,8 are associated with increased risk. By contrast, nonmotor features have not been extensively studied as possible dementia predictors. The only nonmotor variables found in prospective studies to predict dementia in PD are REM sleep behavior disorder (RBD),9,10 baseline cognitive impairment,11 and olfaction.12 To address this deficit, we assessed a broad array of potential clinical risk factors for dementia in a 4.5-year prospective cohort study, focusing especially on nonmotor features such as sleep disorders, autonomic dysfunction, and special sensory dysfunction.
METHODS
Patient selection and enrollment.
Patients were recruited from the Movement Disorders Clinics of the McGill University Health Centre and the Centre Hospitalier de l’Université de Montréal (Montreal, Canada). Patients with UK Bank Criteria–defined parkinsonism,13 with idiopathic PD determined as the most likely cause, were included. Patients were excluded if they had baseline dementia according to Movement Disorder Society (MDS) criteria14 or if an alternative cause of parkinsonism was diagnosed after comprehensive assessment.
Standard protocol approvals, registrations, and patient consents.
The hospital research ethics boards approved this study, and informed written consent was obtained from all participants.
Diagnosis of dementia.
All patients had a baseline neuropsychological examination. Assessments included measures of 3 main cognitive domains, described below.
Executive function/attention.
For these assessments, we used Digit Span from Wechsler Adult Intelligence Scale III (scaled score), the Trail Making Test, part B (times), and a modified version of the Stroop Color Word Test (part III—part I for times or errors).9
Memory.
The following memory assessments were conducted: Semantic and Letter verbal fluency tests (animals, fruits/vegetables, P, F, L—number of items in 1 minute) and episodic verbal learning and memory (Rey Auditory-Verbal Learning Test [sum of trials 1 to 5, List B, immediate recall, delayed recall, and recognition]).9
Visuospatial.
Copy of the Rey-O figure, Block Design from Wechsler Adult Intelligence Scale III (scaled score), and Bells Test assessed visuospatial abilities.9
Subsequently, follow-up neuropsychological evaluations were offered to all patients. Dementia was diagnosed according to Level 2 MDS criteria for PD dementia,14 defined as impairment on ≥2 cognitive domains on neuropsychological testing with significant functional impairment. If patients declined a neuropsychological examination, MDS Level 1 criteria were used (except that depression was not considered an exclusion criterion, unless pseudodementia was diagnosed by the evaluating neurologist15). The Level 1 evaluation included a systematic questionnaire regarding cognitive symptoms and their effect on an array of daily activities, including the pill questionnaire from the MDS dementia criteria,9,14 the Mini-Mental State Examination,9 and the Montreal Cognitive Assessment.9 If patients had died or were unable to participate in person (i.e., usually because of severe dementia), telephone conversation with caregivers supplemented by clinical chart review were conducted to confirm dementia diagnosis.
Baseline patient evaluation.
A single specialist movement disorder neurologist (R.B.P.) performed a complete evaluation of each participant at baseline. All evaluations were performed in the “on” state. Information on demographics, comorbid diseases, and medication history was obtained. PD variables of interest included the following:
-
Motor variables
a. Unified Parkinson's Disease Rating Scale (UPDRS)—total, and subscales I–IV9
b. Hoehn and Yahr9
c. Mean levodopa dose
d. Symmetry of onset: based on history of unilateral vs bilateral onset of symptoms16
e. Purdue Pegboard Test)17
f. Timed Up and Go17
g. Alternate tap test17
h. Proportion of UPDRS accounted for by tremor, rigidity, bradykinesia, and gait dysfunction17
i. Motor subtype (defined according to Schiess classification17)
j. Axial-limb ratio (sum of UPDRS part III items 18, 19, 22, and 27–30 divided by UPDRS III items 20–26)17
k. History of falls and freezing
-
Special sensory variables
-
Autonomic variables
-
Sleep disorders
-
Cognition
a. Mild cognitive impairment (MCI) at baseline—defined according to the 2012 MDS Task Force Guidelines for MCI in PD23 as subjective cognitive complaint, objective evidence of cognitive decline (impairment in at least 2 neuropsychological tests with scores between 1 to 2 SDs below standardized mean), and preserved activities of daily living
b. Visual hallucinations—assessed by semistructured clinical interview and the UPDRS part I15
c. Depression and apathy (UPDRS 1.3 and 1.4)15
Statistical analysis.
The primary outcome was the occurrence of dementia. All statistical analyses were performed using IBM-SPSS version 22.0 (IBM Corp., Armonk, NY). Factors associated with dementia were assessed using logistic regression adjusting for baseline age, sex, baseline disease duration, and duration of follow-up. Odds ratios (ORs) and 90% and 95% confidence intervals were determined; p values (i.e., 2-sided, 95% confidence interval) less than 0.05 were considered significant.
RESULTS
Between May 2005 and July 2011, 94 patients were enrolled in the cohort. Data were incomplete in 14 cases for the following reasons: 2 patients declined follow-up, 7 patients died before follow-up could be arranged, and 5 were lost to follow-up. Therefore, ultimate dementia outcome was available for 80 patients. The mean follow-up duration was 4.4 years and the average disease duration from onset was 10 years. Of 80 patients, 27 patients (34%) developed dementia. This corresponds to an incidence rate of 76.7 per 1,000 patient-years (figure 1).
Figure 1. Patient flowchart.
Dementia outcomes according to baseline motor features.
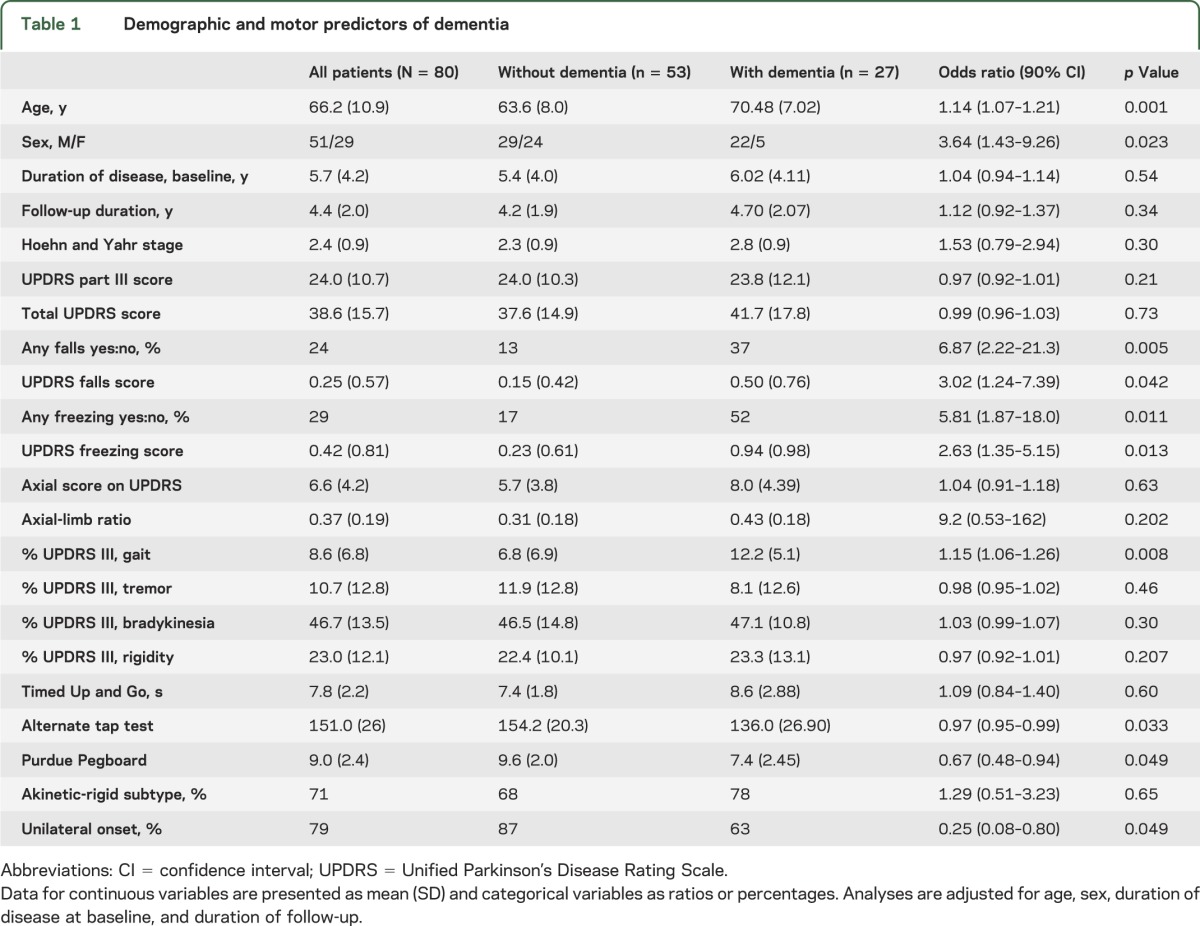
Among motor variables (table 1), the proportion of gait involvement at baseline (OR = 1.15, p = 0.008), falls (OR = 3.02, p = 0.042), and freezing (OR = 2.63, p = 0.013) predicted dementia. Poor performance on the Purdue Pegboard Test (OR = 0.67, p = 0.049) and alternate tap test (OR = 0.97, p = 0.033), but not the UPDRS predicted dementia. Patients developing dementia were more likely to report bilateral onset of motor symptoms (37% vs 13%, p = 0.049). There was no association between dementia status and PD subtype, although the dementia-converted group had slightly more patients with akinetic-rigid Schiess scores than the group without dementia (77.8% vs 67.9%, p = 0.654). Baseline UPDRS part III scores, total UPDRS scores, Timed Up and Go, Hoehn and Yahr stage, and axial-limb ratio were not predictive of dementia status.
Table 1.
Demographic and motor predictors of dementia
Dementia outcomes according to baseline sleep disorders.
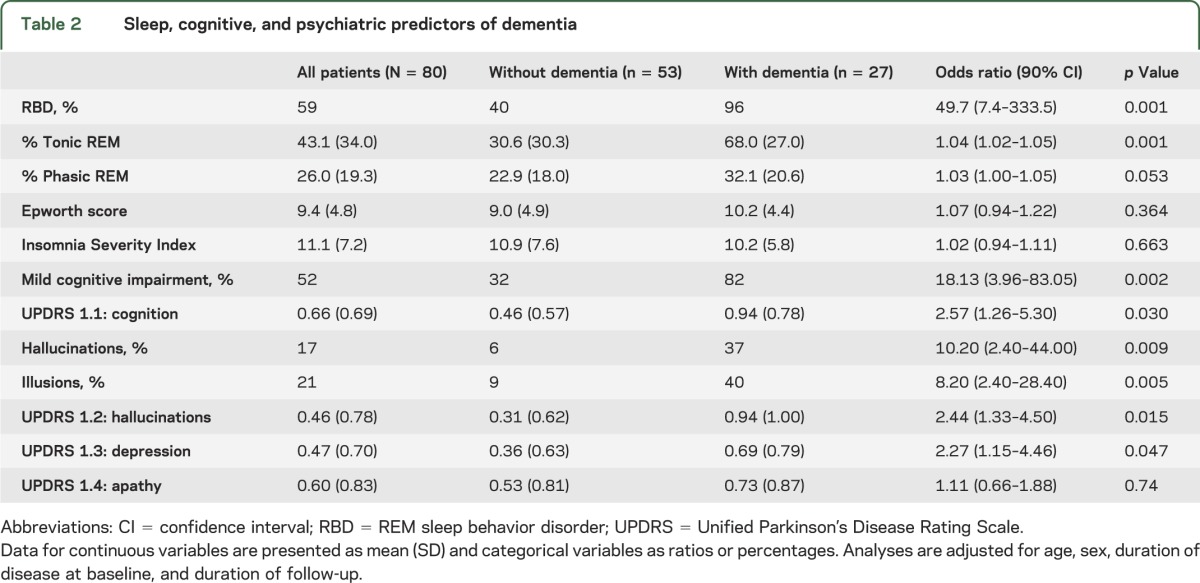
Among sleep disorders (table 2), RBD at baseline dramatically increased the risk of developing dementia (OR = 49.7, p = 0.001). All but one of the converted patients had RBD at baseline (96.3%); the only exception was 87 years old with a 22-year history of PD. Of those with RBD, the risk of developing dementia over the 4.4 years’ follow-up was 43%, compared with 2.5% in those without RBD. Accordingly, the mean percentage of tonic REM sleep EMG activity on baseline polysomnography (i.e., the principal measure of REM sleep atonia loss) was higher in dementia-converted participants (68% compared with 30.6%; OR = 1.4 per 10%, p = 0.001) than in the nonconverted group. The percentage of phasic REM sleep EMG activity was higher in the dementia-converted group, but this was not statistically different when compared with the nonconverted group (OR = 1.3 per 10%, p = 0.053). By contrast, neither daytime sleepiness, as assessed with the Epworth Sleepiness Scale, nor insomnia, as assessed with the Insomnia Severity Index, was significantly associated with dementia risk in our cohort.
Table 2.
Sleep, cognitive, and psychiatric predictors of dementia
Dementia outcomes according to baseline cognitive and psychiatric variables.
Of those diagnosed with dementia, 11 were diagnosed according to Level 2 MDS criteria, and 16 according to Level 1 criteria. Patients destined to develop dementia were older at baseline (71 vs 64 years, p = 0.001) and more often male (81% vs 55%) (table 1). As expected, those with baseline MCI had increased dementia risk (OR = 18.13, p = 0.002). The prevalence of MCI at baseline in the dementia-converted group was 81.5% compared with 32% in the nondementia group. A baseline history of visual hallucinations (OR = 10.2, p = 0.009), visual illusions (OR = 8.2, p = 0.005), subjective cognitive complaints (OR = 2.6, p = 0.03), thought disorders (UPDRS 1–2) (OR = 2.4, p = 0.015), and depression (OR = 2.27, p = 0.047) also predicted dementia development. Higher apathy scores were observed in the dementia group than in the nonconverted group (OR = 1.11, p = 0.74).
Dementia outcomes according to baseline autonomic variables.
Orthostatic systolic blood pressure drop was strongly associated with dementia risk (OR = 1.84 per 10 mm Hg, p < 0.001). Having a baseline systolic decrease >10 mm Hg increased dementia odds 7-fold (OR = 7.3, p = 0.002). In addition, the mean baseline systolic blood pressure in the group with dementia was higher than in the group without dementia (OR = 1.38 per 10 mm Hg, p = 0.032). Baseline occurrence of urinary symptoms (OR = 1.84, p = 0.095), erectile (sexual) dysfunction (OR = 3.33, p = 0.18), and bowel dysfunction (OR = 1.44, p = 0.54) was higher in those with dementia but not statistically different compared with the group without dementia.
Dementia outcomes according to baseline sensory variables.
Among special sensory variables (table 3), abnormal color vision (Farnsworth-Munsell 100 Test error scores >125% expected values) tripled dementia odds (OR = 3.3, p = 0.014), consistent with other studies suggesting that abnormal color vision is a sign of posterior cortical dysfunction.24 In contrast to a previous study,18 we did not find that olfactory dysfunction predicted dementia (figure 2).
Table 3.
Autonomic, color vision, and olfaction as predictors of dementia
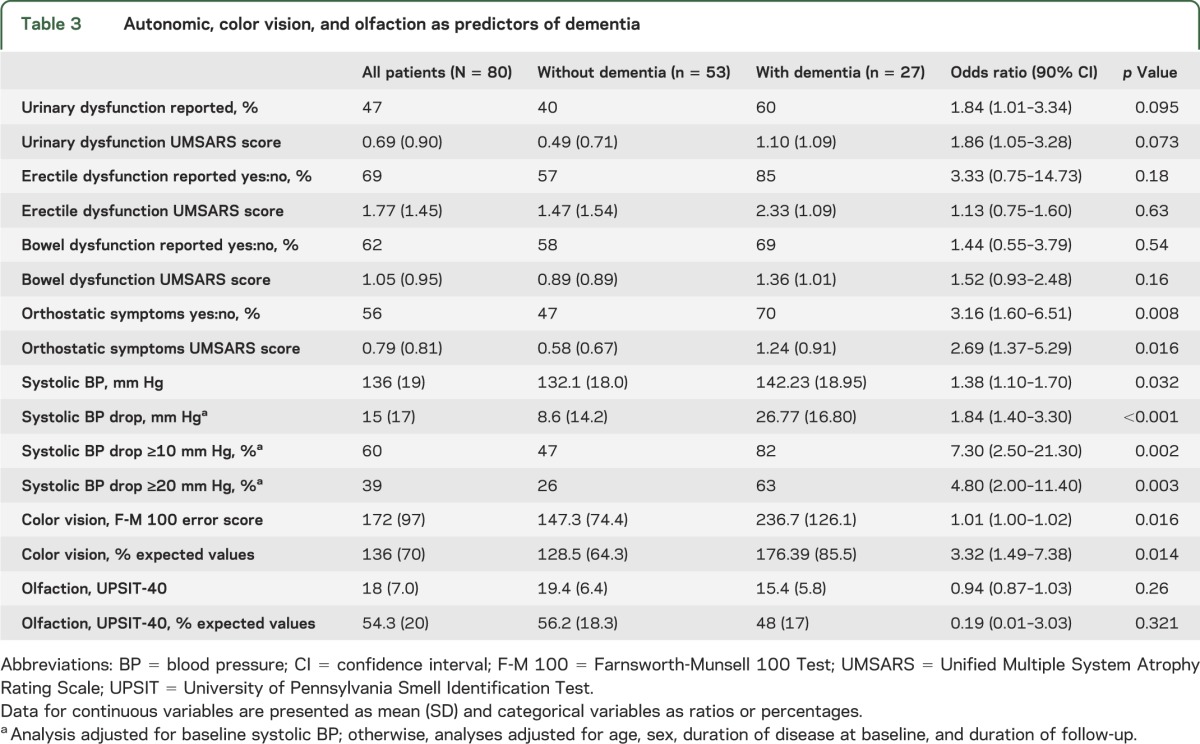
Figure 2. Dementia-free survival based on baseline variables.
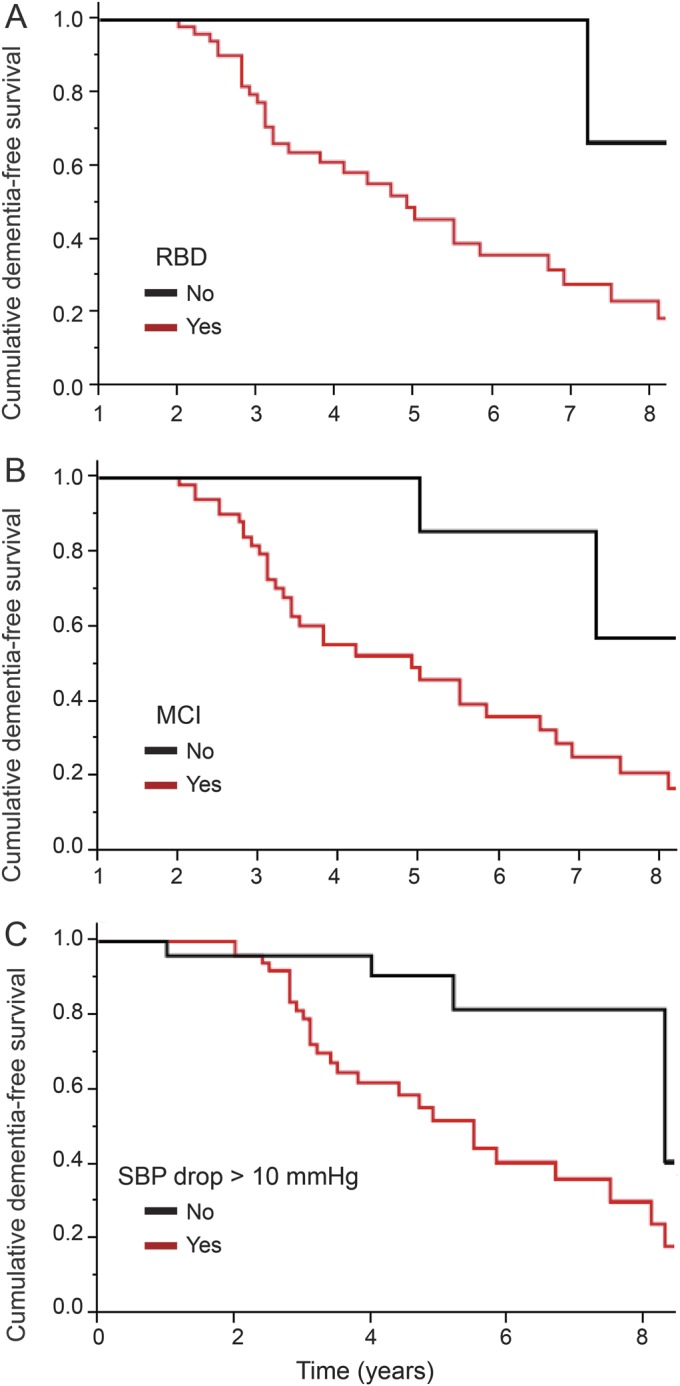
Cumulative dementia-free survival based on baseline variables (A) REM sleep behavior disorder (RBD), (B) mild cognitive impairment (MCI), and (C) systolic blood pressure (SBP) drop.
DISCUSSION
In this prospective cohort study, we found that several nonmotor and “nondopaminergic” motor features, most prominently orthostatic blood pressure drop, RBD, color vision, MCI, and gait dysfunction, are strong predictors of dementia.
We found a strong connection between baseline orthostatic blood pressure drop and dementia, assessed both subjectively and objectively, with an 80% increased dementia risk for every 10 mm Hg decrease in systolic blood pressure. Although this finding has never been previously shown in a prospective study, it is consistent with cross-sectional studies that found strong links between cognition and orthostatic changes.25,26 For example, one recent cross-sectional study found an orthostatic decrease of 12 mm Hg in PD with normal cognition, 20 mm Hg in PD-MCI, and 58 mm Hg in PD dementia.25 The mechanism for this striking relationship is unclear. It is possible that autonomic dysfunction (like many markers in this study) is a marker of a “diffuse” disease subtype, characterized by generalized degeneration, including autonomic areas and cortical regions involved in dementia. However, it is also possible that a causative relationship exists. First, we found no clear connection between other autonomic variables and dementia, suggesting that generalized diffuse degeneration may not suffice to explain results. Second, in the general population, declining diastolic blood pressure has been linked to brain atrophy, particularly when associated with reduced cerebral blood flow.27 Among hypertensive patients, associated orthostatic hypotension is linked to overall cognitive decline and impaired executive function, and predicts stroke and all-cause mortality27–29 (we also found hypertension increased dementia risk, although less strikingly than orthostatic drop). Although cerebrovascular autoregulation mechanisms prevent mild orthostatic changes from reducing cerebral blood flow, moderate changes, particularly in PD with its multisystem cardiovascular dysautonomia, might cause substantial cerebral hypoperfusion. Hypoxia and hypotension have been shown in both animal models and human studies to cause neuronal damage and increase accumulation of pathologic proteins such as β-amyloid.30–32 Therefore, it is plausible that repeated episodes of severe hypotension over years can adversely affect cortical neurons already made vulnerable by synucleinopathy. Finally, it is conceivable that orthostatic hypotension may underlie fluctuations in PD dementia; one retrospective observational chart review suggested that treatment of orthostatic hypotension in dementia was associated with dramatic improvements in cognition, particularly fluctuations,33 and a small study in PD showed that tests of attention decline with upright posture in PD dementia.34
We also found that impaired baseline color vision was associated with a 3-fold increased risk of dementia. Deficits in color discrimination are common nonmotor features of PD, but the exact pathophysiology is not well understood. Some of the deficit could be explained by retinopathic changes found in patients with PD, but cortical alterations and cognitive impairment have also been correlated with color discrimination deficits.24,35 We previously reported that color discrimination deficits in PD were strongly associated with cognitive testing variables and posterior white matter degeneration. White matter degeneration in the right posterior cortical areas in patients with PD may disrupt intrahemispheric and interhemispheric networks required for visuoperceptual, visuospatial integration, and executive functioning.24 Therefore, it appears that color vision loss may predict dementia because it is a marker of posterior neurodegeneration.
Another striking predictor of dementia was RBD; all but one of the converted patients had RBD at baseline (96.3%) in keeping with a previously demonstrated relationship between RBD and dementia by our group in a subset of this population15 and confirmatory findings by others.10 It is unclear why this very strong relationship should exist. In general, RBD is not associated with substantial sleep disruption, making a causal relationship relatively unlikely. We previously reported a close link between orthostatic blood pressure drop and RBD in PD; therefore, it is conceivable that RBD reflects autonomic dysfunction. However, adjusting for systolic blood pressure drop in the model did not attenuate the effect of RBD on dementia risk (OR = 31.77, p = 0.006). We were surprised to observe that the Epworth Sleepiness Scale was not associated with eventual dementia risk, because somnolence is a very common feature of synuclein-mediated dementia. In many cases, baseline Epworth scores were reported without input from caregivers, and many patients (perhaps especially those with subtle cognitive impairment) underreport daytime sleep episodes.36
As expected, we found that the presence of MCI at baseline identified a highly increased risk of developing dementia. These findings are consistent with other cohort studies indicating an association between MCI at baseline and risk of developing dementia in PD.37 We also found that other symptoms of cognitive loss, including visual hallucinations, illusions, history of psychotic symptoms, and subjective cognitive complaints on UPDRS, are strong predictors. In other studies, visual hallucinations have been associated with increased risk of developing dementia.38 Patients with PD who have visual hallucinations have reduced activation in ventral/lateral visual association cortices preceding image recognition.39
We did not find a clear association between development of dementia and a specific akinetic-rigid (vs tremor-predominant) subtype of disease. By contrast, we found a relatively strong connection between gait dysfunction and eventual dementia risk. Of importance, gait and postural disturbance are part of the classification of akinetic-rigid vs tremor-predominant disease; our study suggests that it may be the gait component of this classification that is the primary driver of dementia risk. Of the remaining motor variables, we found contrasting results—standard ratings of disease severity (e.g., UPDRS) were not correlated with eventual risk, but quantitative motor testing could predict disease. It is conceivable that some of the quantitative motor testing could reflect visuoperceptual dysfunction (both the alternate tap test and Purdue Pegboard Test require hand–eye coordination) or perhaps motivational factors confound results (the quantitative motor tasks may demand more sustained effort than a motor examination).
There are some limitations to this study. In many cases, we were unable to confirm diagnosis of dementia upon full neuropsychological examination; generally, this was because of severe dementia. Given the clear history, combined with the fact that MDS Level 1 criteria are highly specific (but insensitive),16 it is unlikely that these patients were misdiagnosed. However, some patients without dementia may have ultimately had dementia diagnosed on more intensive testing. The size of our cohort, although larger than many other studies, was still insufficient to examine markers with modest predictive value. Some factors, such as baseline cognitive reserve, history of prior severe head injury, nonparkinsonian medication use, and family history of dementia, were not analyzed and may be of interest in future studies. We studied a large diversity of variables and did not correct for multiple comparisons (to do so would inappropriately underpower the study40); this implies that some significant relationships may have been attributable to chance, and so our findings should be considered exploratory. Although the mean follow-up duration of 4.4 years is relatively long compared with many trials, it is presumed that findings would differ with continued follow-up, at which time more patients would develop dementia. By contrast, the main strengths of the study include a relatively long prospective follow-up period, few patients lost to follow-up, and a thorough baseline evaluation that included full neuropsychological examination and a comprehensive evaluation of nonmotor features.
We have found that nonmotor and “nondopaminergic” features of PD, including autonomic dysfunction, color vision, RBD, and gait impairment, can strongly predict development of dementia in PD.
GLOSSARY
- MCI
mild cognitive impairment
- MDS
Movement Disorder Society
- OR
odds ratio
- PD
Parkinson disease
- RBD
REM sleep behavior disorder
- UPDRS
Unified Parkinson’s Disease Rating Scale
AUTHOR CONTRIBUTIONS
Dr. Julius B.M. Anang: analysis, interpretation, and manuscript drafting and revisions. Dr. Jean-Francois Gagnon: study concept and design, data acquisition, and critical revision of manuscript. Dr. Josie-Anne Bertrand: data acquisition and review of manuscript. Dr. Silvia Rios Romenets: data interpretation and review of manuscript. Veronique Latreille, Dr. Michel Panisset, and Dr. Jacques Montplaisir: data acquisition and review of manuscript. Dr. Ronald B. Postuma: study concept and design, data acquisition, analysis, and critical revision of manuscript.
STUDY FUNDING
Supported by the Fonds de la Recherche en Santé du Québec and the Canadian Institutes of Health Research; the authors have no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years.
DISCLOSURE
J. Anang reports no disclosures relevant to the manuscript. J. Gagnon is funded by grants from the Fonds de la Recherche en Santé du Québec, the W. Garfield Weston Foundation, and by the Canadian Institutes of Health Research. J. Bertrand, S. Rios Romenets, and V. Latreille report no disclosures relevant to the manuscript. M. Panisset received research grants from Teva Neuroscience, Novartis, and Allergan. He was a lecturer for Allergan, Merz, Novartis, and Teva. He participated in advisory boards for Merck, EMD Serono, Allergan, Merz, Novartis, and Teva. J. Montplaisir received personal compensation as consultant (Impax Pharma, Servier, Jazz Pharma, Merck, Valeant) and speaker (Valeant), and received financial support for research activities from Merck and GlaxoSmithKline and grants from the Fonds de la Recherche en Santé du Québec, and by the Canadian Institutes of Health Research. R. Postuma received personal compensation for travel and speaker fees from Novartis Canada and Teva Neurosciences, and is funded by grants from the Fonds de la Recherche en Santé du Québec, the Parkinson Society Canada, the Webster Foundation, and by the Canadian Institutes of Health Research. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson's disease: diagnosis, biomarkers, and treatment. Lancet Neurol 2012;11:697–707 [DOI] [PubMed] [Google Scholar]
- 2.Fosaa EB, Larsen JP, Wentzel-Larsen T, Alves G. What predicts mortality in Parkinson's disease? A prospective population-based long-term study. Neurology 2010;75:1270–1276 [DOI] [PubMed] [Google Scholar]
- 3.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology 2008;70:1423–1430 [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson's disease. Brain Pathol 2010;20:633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayeux R, Denaro J, Hemenegildo N, et al. A population-based investigation of Parkinson's disease with and without dementia: relationship to age and gender. Arch Neurol 1992;49:492–497 [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence of dementia in Parkinson's disease. Mov Disord 2005;20:1255–1263 [DOI] [PubMed] [Google Scholar]
- 7.Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson's disease in the United Kingdom. Mov Disord 2004;19:1043–1049 [DOI] [PubMed] [Google Scholar]
- 8.Burn DJ, Rowan EN, Allan LM, Molloy S, O’Brien JT, McKeith IG. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:585–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postuma RB, Bertrand JA, Montplaisir J, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson's disease: a prospective study. Mov Disord 2012;27:720–726 [DOI] [PubMed] [Google Scholar]
- 10.Nomura T, Inoue Y, Kagimura T, Nashima K. Clinical significance of REM sleep behavior disorder in Parkinson's disease. Sleep Med 2013;14:131–135 [DOI] [PubMed] [Google Scholar]
- 11.Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 2007;130:1787–1798 [DOI] [PubMed] [Google Scholar]
- 12.Baba T, Kikuchi A, Hirayama K, et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson's disease: a 3 year longitudinal study. Brain 2012;135:161–169 [DOI] [PubMed] [Google Scholar]
- 13.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology 1992;42:1142–1146 [DOI] [PubMed] [Google Scholar]
- 14.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the Movement Disorder Society Task Force. Mov Disord 2007;22:2314–2324 [DOI] [PubMed] [Google Scholar]
- 15.Barton BR, Grabli D, Bernard B, et al. Clinical validation of diagnostic procedures for Parkinson's disease dementia (PD-D). Neurology 2010;74:A299–A300 [Google Scholar]
- 16.Postuma RB, Gagnon JF, Vendette M, Charland K, Monplaisir J. REM sleep behavior disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry 2008;79:1117–1121 [DOI] [PubMed] [Google Scholar]
- 17.Romenets SR, Gagnon JF, Latreille V, et al. Rapid eye movement sleep behavior disorder and subtypes of Parkinson's disease. Mov Disord 2012;27:996–1003 [DOI] [PubMed] [Google Scholar]
- 18.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord 2008;23:1665–1672 [DOI] [PubMed] [Google Scholar]
- 19.American Sleep Disorders Association. The International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual. Rochester: American Sleep Disorders Association; 1997:177–180 [Google Scholar]
- 20.Montplaisir J, Gagnon JF, Fantini ML, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord 2010;25:2044–2051 [DOI] [PubMed] [Google Scholar]
- 21.Morin CM, Belleville G, Belanger L, Ivers H. Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545 [DOI] [PubMed] [Google Scholar]
- 23.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force Guidelines. Mov Disord 2012;27:349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertrand JA, Bedetti C, Postuma RB, et al. Color discrimination deficits in Parkinson's disease are related to cognitive impairment and white-matter alterations. Mov Disord 2012;27:1781–1788 [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, Oh YS, Lee KS, et al. Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology 2012;79:1323–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilleri M, Facchini S, Gasparoli E, et al. Cognitive and MRI correlates of orthostatic hypotension in Parkinson's disease. J Neurol 2013;260:253–259 [DOI] [PubMed] [Google Scholar]
- 27.Frewen J, Finucane C, Sawa GM, Boyle G, Kenny RA. Orthostatic hypotension is associated with lower cognitive performance in adults aged 50 plus with supine hypertension. J Gerontol A Biol Sci Med Sci 2013;69:878–885 [DOI] [PubMed] [Google Scholar]
- 28.Kario K, Equchi K, Hoshide S, et al. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol 2000;40:133–141 [DOI] [PubMed] [Google Scholar]
- 29.Fedorowski A, Hedblad B, Melander O. Early postural blood pressure response and cause-specific mortality among middle-aged adults. Eur J Epidemiol 2011;26:537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol 2012;2012:367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Xing A, Wang X, Liu G, Li L. Regulation of beta-amyloid level in the brain of rats with cerebrovascular hypoperfusion. Neurobiol Aging 2012;33:826.e31–e42 [DOI] [PubMed] [Google Scholar]
- 32.Pimentel-Coelho PM, Michaud JP, Rivest S. Effects of mild chronic cerebral hypoperfusion and early amyloid pathology on spatial learning and the cellular innate immune response in mice. Neurobiol Aging 2013;34:679–693 [DOI] [PubMed] [Google Scholar]
- 33.Freidenberg DL, Shaffer LE, Macalester S, Fannin EA. Orthostatic hypotension in patients with dementia: clinical features and response to treatment. Cogn Behav Neurol 2013;26:105–120 [DOI] [PubMed] [Google Scholar]
- 34.Peralta C, Stampfer-Kountchev M, Karner E, et al. Orthostatic hypotension and attention in Parkinson's disease with and without dementia. J Neural Transm 2007;114:585–588 [DOI] [PubMed] [Google Scholar]
- 35.Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. Neurol Clin 2003;21:709–728 [DOI] [PubMed] [Google Scholar]
- 36.Merino-Andreu M, Arnulf I, Konofal E, Derenne JP, Agid Y. Unawareness of naps in Parkinson's disease and in disorders with excessive daytime sleepiness. Neurology 2003;60:1553–1554 [DOI] [PubMed] [Google Scholar]
- 37.Pederson KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest Study. JAMA Neurol 2013;70:580–586 [DOI] [PubMed] [Google Scholar]
- 38.Uc EY, McDermott MP, Marder KS, et al. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology 2009;73:1469–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meppelink AM, de Jong BM, Renken R, et al. Impaired visual processing preceding image recognition in Parkinson's disease patients with visual hallucinations. Brain 2009;132:2980–2993 [DOI] [PubMed] [Google Scholar]
- 40.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol 2001;54:343–349 [DOI] [PubMed] [Google Scholar]


