Abstract
Objectives:
To determine the sensitivity and specificity of B-mode ultrasound in the diagnosis of neuromuscular diaphragmatic dysfunction, including phrenic neuropathy.
Methods:
A prospective study of patients with dyspnea referred to the EMG laboratory over a 2-year time frame for evaluation of neuromuscular respiratory failure who were recruited consecutively and examined with ultrasound for possible diaphragm dysfunction. Sonographic outcome measures were absolute thickness of the diaphragm and degree of increased thickness with maximal inspiration. The comparison standard for diagnosis of diaphragm dysfunction was the final clinical diagnosis of clinicians blinded to the diaphragm ultrasound results, but taking into account other diagnostic workup, including chest radiographs, fluoroscopy, phrenic nerve conduction studies, diaphragm EMG, and/or pulmonary function tests.
Results:
Of 82 patients recruited over a 2-year period, 66 were enrolled in the study. Sixteen patients were excluded because of inconclusive or insufficient reference testing. One hemidiaphragm could not be adequately visualized; therefore, hemidiaphragm assessment was conducted in a total of 131 hemidiaphragms in 66 patients. Of the 82 abnormal hemidiaphragms, 76 had abnormal sonographic findings (atrophy or decreased contractility). Of the 49 normal hemidiaphragms, none had a false-positive ultrasound. Diaphragmatic ultrasound was 93% sensitive and 100% specific for the diagnosis of neuromuscular diaphragmatic dysfunction.
Conclusion:
B-mode ultrasound imaging of the diaphragm is a highly sensitive and specific tool for diagnosis of neuromuscular diaphragm dysfunction.
Classification of evidence:
This study provides Class II evidence that diaphragmatic ultrasound performed by well-trained individuals accurately identifies patients with neuromuscular diaphragmatic respiratory failure (sensitivity 93%; specificity 100%).
Diaphragm dysfunction can be difficult to diagnose, particularly when diaphragm paralysis is bilateral. The usual workup of patients presenting with unexplained dyspnea may include chest radiographs, fluoroscopy, phrenic nerve conduction studies (NCS), needle EMG of the diaphragm, pulmonary function testing, and transdiaphragmatic pressure measurements; all of these diagnostic tests can produce false-positive and false-negative findings, and some tests are invasive or uncomfortable for the patient.1–9
Ultrasound of the diaphragm is an imaging technique that has recently become more accessible to clinicians, and it can improve the technical quality and safety of phrenic NCS and needle EMG.10,11 When used in isolation, diagnostic ultrasound can identify atrophy and impaired motion or contractility of the diaphragm.12–17 Normal values for diaphragm muscle thickness and diaphragm thickening ratio imaged with B-mode ultrasound have been published,11 but limited data assess the diagnostic utility of ultrasound in patients with neuromuscular diaphragm dysfunction, including phrenic neuropathy. Most studies to date have been case series, often with no reference standard, and have been limited by small numbers of patients.12–17 M-mode ultrasound appears superior to fluoroscopy, and several authors have concluded that ultrasound is a better test for diaphragm dysfunction than fluoroscopy.17,18
We hypothesized that B-mode ultrasound imaging of the diaphragm would be more sensitive and specific than fluoroscopy, chest radiographs, or electrodiagnostic studies for diagnosis of neuromuscular diaphragm dysfunction.
METHODS
Primary research question and classification of level of evidence.
What is the sensitivity and specificity of B-mode ultrasound measures of diaphragm thickness and/or thickening ratio in identifying neuromuscular diaphragm dysfunction in patients presenting with dyspnea to an EMG lab?—Class II level of evidence.
Subjects.
This prospective, observational study was conducted at our tertiary care academic medical center. Patients presenting to the outpatient or inpatient EMG laboratories for assessment of neuromuscular respiratory function were recruited during the 2-year study period (July 1, 2011 to June 30, 2013).
Ultrasound imaging.
On the day of enrollment, patients underwent the index test (ultrasound imaging of the diaphragm) either before or after phrenic NCS and/or diaphragm EMG. A high-resolution portable ultrasound machine (Logiq E; GE Healthcare, Waukesha, WI) was used, with a 7- to 13-MHz linear array transducer. Patients were examined in the supine position unless they could not tolerate lying flat because of their dyspnea, in which case they were examined in a semireclined supine position. The diaphragm was identified as a 3-layered structure lying deep to the intercostal muscles and subcutaneous tissue.10
Imaging was performed by either a neurologist or a technician formally trained in ultrasound of the diaphragm. The ultrasound protocol has been described in detail in an earlier study of healthy patients, with 3 images captured at end-expiration and 3 images captured after the patient is asked to inhale as deeply as possible.19 The transducer is positioned in a sagittal oblique plane, spanning 2 ribs, at approximately the anterior axillary line, overlying one of the most caudal intercostal spaces (figure 1). Measurements of diaphragm thickness are made using electronic calipers, and the 3 images for each position are then averaged to give a thickness at resting end-expiration (TMIN) and at maximal inspiration (TMAX), from which a diaphragm thickening ratio is derived: TMAX/TMIN. On the basis of this previous study, normal diaphragm thickness is defined as >0.14 cm and the normal diaphragm thickening ratio is defined as >1.2.19 This technique is reliable, with intraclass correlation coefficients ranging from 0.89 to 0.98 for intrarater and interrater reliability.19
Figure 1. Transducer position.
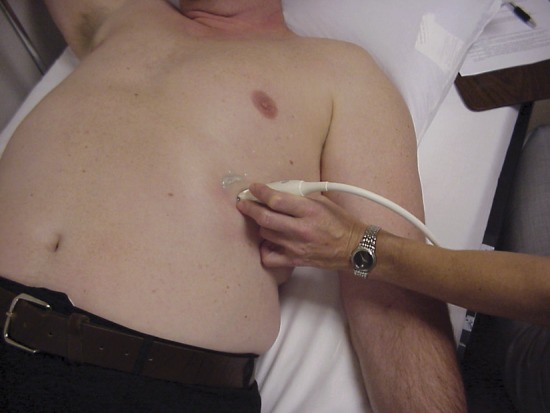
The ultrasound transducer is positioned so that a linear probe can be placed over one of the most caudal intercostal spaces in approximately the anterior axillary line, spanning 2 ribs.
Reference standard.
Clinical data (history and physical examination findings) and available reference testing (phrenic NCS, diaphragm EMG, chest radiographs, fluoroscopy, and pulmonary function tests) were collected by reviewing the electronic health record, and the final clinical diagnosis was arrived at by 1 of 2 clinicians (a board-certified pulmonologist and a board-certified physiatrist), without review of ultrasound findings. Phrenic NCS were performed with the patient supine, or as close to supine as tolerable. The active surface electrode was placed over the sixth intercostal space in the anterior axillary line and the reference electrode was placed over the lower border of the rib cage, in the anterior axillary line. A second set of recording electrodes was placed with the active electrode just above the xiphoid process and the reference electrode 16 cm away, over the anterior costal margin. A ground electrode was placed over the upper third of the sternum. Thus, a 2-channel recording was obtained, and the largest response was chosen from the 2 techniques. The phrenic nerve was stimulated using bipolar surface prongs placed just posterior to the sternocleidomastoid muscle, over the scalene muscles at the level of the thyroid cartilage, with the anode cephalad to the cathode. The stimulator was slid medially, if necessary, to achieve isolated and supramaximal stimulation of the phrenic nerve without brachial plexus stimulation. Phrenic compound muscle action potentials (CMAPs) were recorded during the same phase of the respiratory cycle for each patient, typically after the patient had exhaled. We used the normal values of our EMG laboratory (normal CMAP amplitude ≥3 mV), which are derived from a group of 96 normal subjects.
Patients had to have a minimum of 3 different reference tests available to review in making the final clinical diagnosis; otherwise, their data were excluded. If there was a discrepancy in the final diagnosis, then the case was reviewed again and discussed by the 2 clinicians until a diagnosis was agreed upon. Ultrasound findings (diaphragm thickness and diaphragm thickening ratio) were graded as abnormal or normal and were compared with the final clinical diagnosis arrived at by the blinded clinicians.
Standard protocol approvals, registrations, and patient consents.
After approval from the Mayo Clinic Institutional Review Board, eligible patients were enrolled consecutively if they gave verbal consent and signed HIPAA (Health Insurance Portability and Accountability Act of 1996) authorization.
Statistical analysis.
Data were analyzed using JMP statistical software (version 9; SAS Institute Inc., Cary, NC). Sensitivity and specificity were calculated for diagnostic ultrasound, with the final clinical diagnosis as the reference standard.
RESULTS
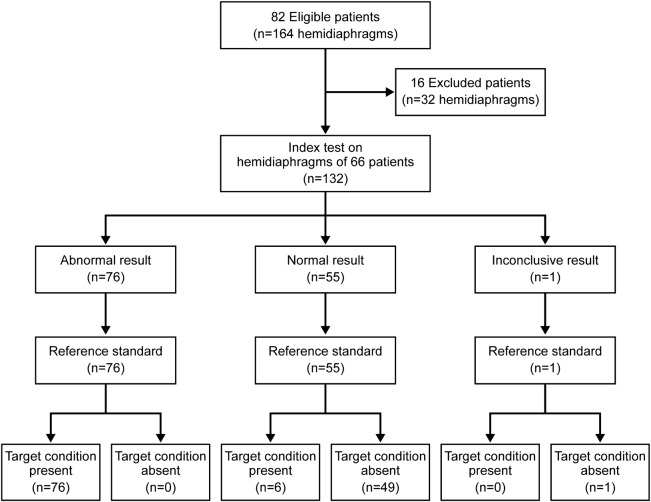
During the 2-year study period, we prospectively recruited 82 patients who were referred to the Mayo Clinic EMG laboratory for evaluation of diaphragm function (figure 2). Sixteen patients were excluded because of nondiagnostic or insufficient reference testing. Thus, 66 patients (mean ± SD age, 62.2 ± 12.0 years [range, 33–84 years]) were subsequently enrolled for sensitivity and specificity analysis, of whom 36 (55%) were men. Fourteen patients were inpatients at the time of the ultrasound testing, and 3 of those were on a ventilator. The other patients (n = 68) were outpatients.
Figure 2. Recruitment flow diagram.
Flow diagram shows patient recruitment and hemidiaphragm ultrasound results.
A total of 131 hemidiaphragms were assessed in 66 individual patients (there was one morbidly obese [body mass index >40 kg/m2], critically ill patient in the intensive care unit, in whom the diaphragm could be adequately visualized on only one side). Fifty patients were ultimately diagnosed with unilateral or bilateral phrenic neuropathy (n = 47) or anterior horn cell disease affecting the phrenic nerve (n = 3); 5 had myopathy involving both hemidiaphragms; one had congenital central hypoventilation syndrome, with normal hemidiaphragms; and 10 were believed to have no evidence of neuromuscular disease after completion of their diagnostic workup.
Of the 50 patients diagnosed with lower motor neuron involvement of the phrenic nerve, 22 had bilateral involvement, 19 had isolated right-sided involvement, and 9 had isolated left-sided involvement. Of these 50 patients, 31 were ultimately diagnosed with inflammatory phrenic neuropathy (Parsonage-Turner syndrome); the remainder were diagnosed with idiopathic (n = 11), iatrogenic (n = 3), paraneoplastic (n = 1), or traumatic phrenic neuropathy (n = 1); amyotrophic lateral sclerosis with diaphragm involvement (n = 2); or cervical spinal cord infarct with anterior horn cell damage (n = 1).
Ultrasound findings for the 66 patients (131 hemidiaphragms) are presented in table 1; an illustrative ultrasound is shown in figure 3. Table 2 summarizes the results of each available test for the entire group of 82 abnormal hemidiaphragms (72 with lower motor neuron involvement or phrenic neuropathy and 10 with myopathy) and for the 10 patients who had no evidence of neuromuscular respiratory disease.
Table 1.
Ultrasound findings in 131 hemidiaphragmsa

Figure 3. Ultrasound of the diaphragm.
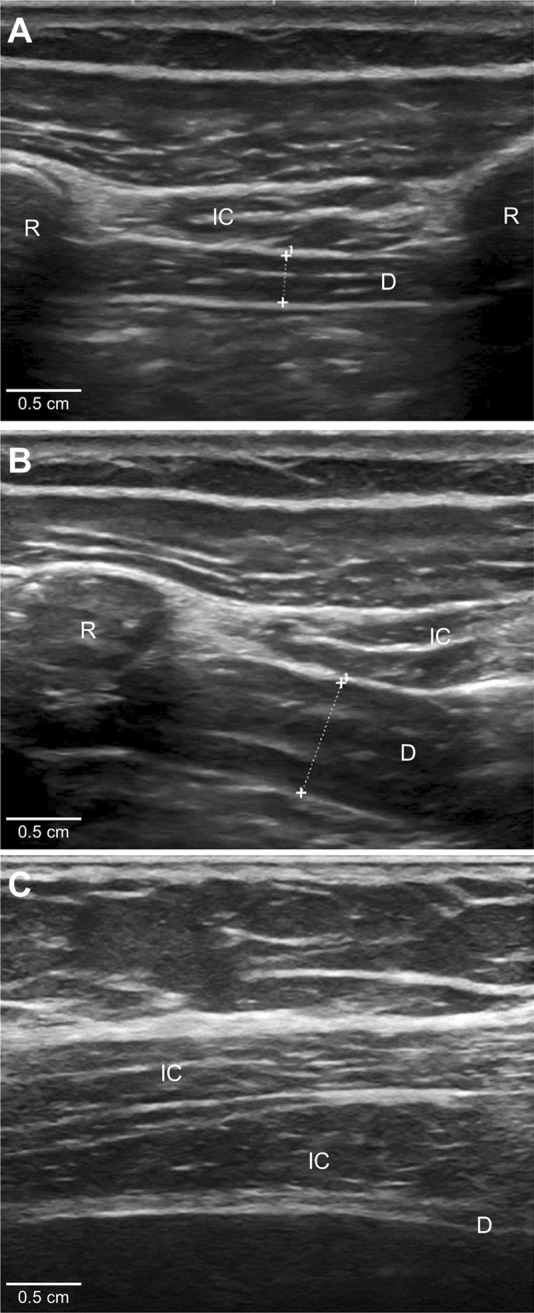
The diaphragm (D) is the 3-layered structure situated deep to the intercostal (IC) muscles that span the 2 ribs. The diaphragm muscle tissue is hypoechoic (dark) on ultrasound, and the 2 layers of connective tissue encasing the muscle (peritoneum and parietal pleura) are hyperechoic (bright) on ultrasound. (A) A normal diaphragm at end-expiration (0.29 cm thick). (B) A normal diaphragm, in the same patient, at maximal inspiration (0.6 cm thick, giving a diaphragm thickening ratio of 2.1). (C) An atrophic diaphragm (0.05 cm thick) in a patient with phrenic neuropathy. R = rib.
Table 2.
Test results

Of the 6 patients with normal ultrasound findings but abnormal hemidiaphragms based on their final clinical diagnosis (false-negatives), 3 had bilateral phrenic neuropathy in which the more affected diaphragm had abnormal ultrasound findings of phrenic neuropathy but the less affected diaphragm was of normal thickness and thickened more than 20% at maximal inspiration, suggesting relatively mild phrenic neuropathy on the less affected side. Two patients had the residua of an old, unilateral phrenic neuropathy in which there was mild diaphragm dysfunction evident as an elevated hemidiaphragm on chest radiographs, decreased excursion of that hemidiaphragm on fluoroscopic sniff testing, and mild neurogenic motor unit changes on needle EMG. Finally, one patient with myopathy had one normal hemidiaphragm on ultrasound found to be myopathic on needle EMG. In that patient, phrenic NCS results were normal bilaterally as were chest radiographs, whereas fluoroscopy showed mildly decreased excursion.
Of the 72 hemidiaphragms with phrenic neuropathy diagnosed clinically, 67 had an abnormally low diaphragm thickening ratio (<1.2), but only 28 were atrophic, below the lower limit of normal of ≤0.14 cm. In addition to the 28 hemidiaphragms that were unequivocally atrophic, 3 patients with unilateral involvement had relative atrophy in the affected hemidiaphragm compared with the unaffected hemidiaphragm (>0.33 cm thinner).19 There were no cases with abnormal diaphragm thickness but normal thickening ratio, but there were 36 of 67 cases (53%) of abnormal thickening ratio but normal diaphragm thickness.
In the 5 patients with myopathy, ultrasound was abnormal in 9 of 10 hemidiaphragms. Chest radiography findings were normal in all 5 patients, phrenic NCS results were abnormal in 6 of 9 hemidiaphragms, EMG was abnormal in 7 of 7 hemidiaphragms, and fluoroscopy was normal or inconclusive in 4 hemidiaphragms (2 patients).
DISCUSSION
Patients with unexplained dyspnea or respiratory failure are often referred to neurologists to rule out neuromuscular causes. Many of the appropriate tests currently available are invasive or technically challenging. Ultrasound has long been available as a tool for diagnosing diaphragm dysfunction but has been little used to date. This study demonstrated high rates of sensitivity and specificity for diaphragm ultrasound, which compared favorably to other diagnostic techniques (phrenic NCS, needle EMG, chest radiographs, and fluoroscopic sniff test).
These patients presenting for evaluation of possible neuromuscular diaphragm dysfunction had no false-positives on ultrasound and only 6 false-negatives (patients with phrenic neuropathy or myopathy but normal findings on ultrasound). In all 6 cases, the other available diagnostic tests indicated relatively mild diaphragm dysfunction; therefore, we hypothesize that, in those cases, the normal ultrasound measurements reflected relative preservation of diaphragm function.
Historically, plain chest films and fluoroscopic sniff testing have been the most frequently utilized imaging studies for diagnosis of diaphragm dysfunction, despite their modest specificity and their ability to provide only indirect information regarding diaphragm function.3,12,20,21 In our study, chest radiographs were abnormal in only 21% (10/48 hemidiaphragms) of patients with bilateral dysfunction but quite helpful in cases of unilateral dysfunction (abnormal findings in 23 of 27 hemidiaphragms [85%]). Fluoroscopy of the diaphragm has poor sensitivity, particularly in bilateral dysfunction.1,22 Our study findings corroborated this, with fluoroscopy abnormal in only 32% (12/38 hemidiaphragms) of the patients with bilateral dysfunction. However, in patients with unilateral dysfunction, fluoroscopy was abnormal in 15 of 17 patients (88%).
Needle EMG of the diaphragm is the best test to differentiate myopathy from phrenic neuropathy or anterior horn cell disease affecting the diaphragm. However, the proximity of vital structures (e.g., lung) and the depth of the diaphragm make many electromyographers uncomfortable examining this muscle. Furthermore, when the muscle is atrophic, it becomes extremely thin (sometimes <1 mm), which makes placing the needle within the muscle difficult, particularly without image guidance. This difficulty was highlighted in the current study, in which diaphragm EMG was definitively abnormal in only 43 of 61 abnormal hemidiaphragms (70%) because of a high number of inconclusive cases in which the muscle could not be located. We found a high rate of bilateral phrenic nerve damage (44%). Other authors have also reported high rates of bilateral phrenic nerve involvement in cases of neuralgic amyotrophy.23,24 For these patients, ultrasound is an excellent diagnostic test, because many electromyographers are not comfortable performing needle EMG of the diaphragm bilaterally on the same day, given the remote but possible risk of bilateral pneumothoraces.
Phrenic NCS is more readily available than EMG of the diaphragm; however, it can also be technically challenging, particularly in patients who are obese. False-positive results can occur because of inaccurate electrode placement or failure to adequately stimulate the phrenic nerve in the supraclavicular fossa. False-negative results can occur when a CMAP is recorded from the overlying chest wall muscles, rather than from the diaphragm.
We found a low rate of false-negative results in our study (9% [7 of 79 abnormal hemidiaphragms had normal phrenic NCS]) but a high rate of false-positive results (37% [17 of 46 normal hemidiaphragms had abnormal phrenic NCS]). In the group of 10 patients with no neuromuscular disease, 9 underwent phrenic NCS, and 4 of these patients had absent or low-amplitude responses bilaterally. In analyzing this group further, we identified only one patient who had absent responses bilaterally, and he was obese (body mass index 42 kg/m2). The other 3 patients had bilaterally low-amplitude phrenic CMAPs based on our laboratory's normal cutoff of ≥0.3 mV. One patient had phrenic CMAPs of 0.1 mV bilaterally, and 2 patients had CMAPs of 0.2 mV bilaterally. This suggests that the normal cutoff for our EMG laboratory of ≥0.3 mV may be too high. In reviewing the literature, we found several studies that examined normal ranges of phrenic CMAPs, and the lower limit of normal varied between 0.1 and 0.4 mV.7,8,25–27 One way to decrease the risk of recording a volume-conducted response (a false-negative) is to directly visualize the diaphragm with ultrasound to observe diaphragm contraction while stimulating the phrenic nerve, and this is a technique that we have adopted recently in our practice.10,25
One limitation of this study is that only the role of B-mode sonography (and not M-mode sonography) was evaluated in diagnosing diaphragm dysfunction. M-mode sonography is technically more challenging, particularly in patients who are obese or for imaging the left hemidiaphragm; however, it can identify decreased or paradoxical excursion of the diaphragm.18,20,28,29 We did use this technique in some of the patients; in those cases, our findings were consistent with what we had found using B-mode sonography, but we did not systematically study the usefulness of M-mode imaging in this patient cohort. In addition, we did not attempt to examine the phrenic nerve itself with ultrasound, given how small the nerve is, the resolution of the portable ultrasound machine we were using, and the lack of normal values for the phrenic nerve cross-sectional area. However, with continuing advances in technology, this is a parameter that could be evaluated in future studies, keeping in mind that only some of the nerve is accessible to ultrasound imaging.
Another limitation is that the ultrasound testing was not always performed in a blinded manner. Study subjects were composed of patients referred to the EMG laboratory to rule out neuromuscular respiratory dysfunction, and the study was conducted prospectively in the course of their clinical workup. Also, we should point out that ultrasound cannot unequivocally differentiate myopathy from phrenic neuropathy as a cause of diaphragm dysfunction. As such, ultrasound does not obviate the need for needle EMG of the diaphragm in cases for which the history and physical examination do not help differentiate myopathy from phrenic neuropathy.
This study evaluated mostly ambulatory patients, with only 3 of 62 patients evaluated while on mechanical ventilation. Mechanical ventilation has a profound and almost immediate effect on diaphragm function, and this is an area that requires more research to determine the role of ultrasound.30 We are hopeful that we can use this modality in the future to help predict weaning success in the intensive care unit. At this time, if a patient is alert enough and able to follow commands, we switch the ventilator off temporarily and have the patient attempt to breathe spontaneously to determine whether there is any thickening of the diaphragm with inspiration. Otherwise, the evaluation is limited to measurement of diaphragm thickness and contractile response to phrenic nerve stimulation. In addition, we did not attempt to measure diaphragm thickness or contractility while patients were not supine or semireclined; however, other authors have done so and have found normal values similar to ours regarding diaphragm thickness and thickening ratio when measured with B-mode ultrasound.13–15,31,32
Lastly, we found 6 hemidiaphragms that had normal ultrasound findings but were diagnosed with phrenic neuropathy on the basis of other testing and clinical evaluation. Ultrasound diagnosis was based on a decrease in absolute thickness of the diaphragm and/or a decrease in thickening ratio. It is important to remember that our cutoff for abnormal thickening is at the fifth percentile of 150 normal subjects, and that many people have markedly higher thickening ratios (an increase in diaphragm thickness of up to 4 times); thus, an individual patient may lose much function before dropping below the fifth percentile. Similarly, normal diaphragm thickness can vary between 0.15 and 0.9 cm, so many people will have an atrophic diaphragm that is still thicker than 0.15 cm. The technique used by Johnson et al.25 to evaluate for diaphragm weakness may be more sensitive for partial phrenic neuropathies (evaluating diaphragm excursion using M-mode ultrasound in response to phrenic stimulation and with tidal breathing).
Despite these limitations, this study shows that 2-dimensional B-mode ultrasound of the diaphragm is a feasible, relatively inexpensive, noninvasive diagnostic test with excellent sensitivity and specificity for diagnosis of neuromuscular diaphragm dysfunction. It is best used in conjunction with electrophysiologic testing that can provide additional pathophysiologic information regarding the underlying cause of the diaphragm dysfunction. Ultrasound has significant advantages over fluoroscopy and chest radiographs in the workup of patients with possible phrenic neuropathy, particularly in cases of bilateral dysfunction.
GLOSSARY
- CMAP
compound muscle action potential
- NCS
nerve conduction study
- TMAX
thickness at maximal inspiration
- TMAX/TMIN
diaphragm thickening ratio
- TMIN
thickness at resting end-expiration
AUTHOR CONTRIBUTIONS
Dr. Boon was responsible for design or conceptualization of the study, analysis or interpretation of the data, and drafting or revising the manuscript for intellectual content. Dr. Sekiguchi and Ms. Harper were responsible for analysis or interpretation of the data and drafting or revising the manuscript for intellectual content. Dr. Strommen was responsible for drafting or revising the manuscript for intellectual content. Dr. Shahgholi Ghahfarokhi was responsible for analysis or interpretation of the data and drafting or revising the manuscript for intellectual content. Dr. Watson was responsible for drafting or revising the manuscript for intellectual content. Dr. Sorenson was responsible for analysis or interpretation of the data and drafting or revising the manuscript for intellectual content.
STUDY FUNDING
This publication was made possible by Clinical and Translational Science Award grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH, US Department of Health and Human Services, Bethesda, MD. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCATS or NIH.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Alexander C. Diaphragm movements and the diagnosis of diaphragmatic paralysis. Clin Radiol 1966;17:79–83 [DOI] [PubMed] [Google Scholar]
- 2.Al-Shekhlee A, Shapiro BE, Preston DC. Iatrogenic complications and risks of nerve conduction studies and needle electromyography. Muscle Nerve 2003;27:517–526 [DOI] [PubMed] [Google Scholar]
- 3.Chetta A, Rehman AK, Moxham J, Carr DH, Polkey MI. Chest radiography cannot predict diaphragm function. Respir Med 2005;99:39–44 [DOI] [PubMed] [Google Scholar]
- 4.Kimura J. Facts, fallacies, and fancies of nerve conduction studies: twenty-first annual Edward H. Lambert Lecture. Muscle Nerve 1997;20:777–787 [DOI] [PubMed] [Google Scholar]
- 5.McCool FD, Tzelepis GE. Dysfunction of the diaphragm. N Engl J Med 2012;366:932–942 [DOI] [PubMed] [Google Scholar]
- 6.Miller J. Pneumothorax: complication of needle EMG of thoracic wall. N J Med 1990;87:653. [PubMed] [Google Scholar]
- 7.Resman-Gaspersc A, Podnar S. Phrenic nerve conduction studies: technical aspects and normative data. Muscle Nerve 2008;37:36–41 [DOI] [PubMed] [Google Scholar]
- 8.Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve 2013;47:319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swenson MR, Rubenstein RS. Phrenic nerve conduction studies. Muscle Nerve 1992;15:597–603 [DOI] [PubMed] [Google Scholar]
- 10.Boon AJ, Alsharif KI, Harper CM, Smith J. Ultrasound-guided needle EMG of the diaphragm: technique description and case report. Muscle Nerve 2008;38:1623–1626 [DOI] [PubMed] [Google Scholar]
- 11.Mertens L. Diaphragmatic paralysis after cardiac surgery: how to look at it? Pediatr Crit Care Med 2006;7:491–492 [DOI] [PubMed] [Google Scholar]
- 12.Chavhan GB, Babyn PS, Cohen RA, Langer JC. Multimodality imaging of the pediatric diaphragm: anatomy and pathologic conditions. Radiographics 2010;30:1797–1817 [DOI] [PubMed] [Google Scholar]
- 13.Cohn D, Benditt JO, Eveloff S, McCool FD. Diaphragm thickening during inspiration. J Appl Physiol 1997;83:291–296 [DOI] [PubMed] [Google Scholar]
- 14.De Bruin PF, Ueki J, Bush A, Khan Y, Watson A, Pride NB. Diaphragm thickness and inspiratory strength in patients with Duchenne muscular dystrophy. Thorax 1997;52:472–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottesman E, McCool FD. Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med 1997;155:1570–1574 [DOI] [PubMed] [Google Scholar]
- 16.Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD. Monitoring recovery from diaphragm paralysis with ultrasound. Chest 2008;133:737–743 [DOI] [PubMed] [Google Scholar]
- 17.Miller SG, Brook MM, Tacy TA. Reliability of two-dimensional echocardiography in the assessment of clinically significant abnormal hemidiaphragm motion in pediatric cardiothoracic patients: comparison with fluoroscopy. Pediatr Crit Care Med 2006;7:441–444 [DOI] [PubMed] [Google Scholar]
- 18.Houston JG, Morris AD, Howie CA, Reid JL, McMillan N. Technical report: quantitative assessment of diaphragmatic movement: a reproducible method using ultrasound. Clin Radiol 1992;46:405–407 [DOI] [PubMed] [Google Scholar]
- 19.Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, Sorenson EJ. Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve 2013;47:884–889 [DOI] [PubMed] [Google Scholar]
- 20.Epelman M, Navarro OM, Daneman A, Miller SF. M-mode sonography of diaphragmatic motion: description of technique and experience in 278 pediatric patients. Pediatr Radiol 2005;35:661–667 [DOI] [PubMed] [Google Scholar]
- 21.Ayoub J, Cohendy R, Prioux J, et al. Diaphragm movement before and after cholecystectomy: a sonographic study. Anesth Analg 2001;92:755–761 [DOI] [PubMed] [Google Scholar]
- 22.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med 2003;168:10–48 [DOI] [PubMed] [Google Scholar]
- 23.Tsao BE, Ostrovskiy DA, Wilbourn AJ, Shields RW., Jr Phrenic neuropathy due to neuralgic amyotrophy. Neurology 2006;66:1582–1584 [DOI] [PubMed] [Google Scholar]
- 24.Mulvey DA, Aquilina RJ, Elliott MW, Moxham J, Green M. Diaphragmatic dysfunction in neuralgic amyotrophy: an electrophysiologic evaluation of 16 patients presenting with dyspnea. Am Rev Respir Dis 1993;147:66–71 [DOI] [PubMed] [Google Scholar]
- 25.Johnson NE, Utz M, Patrick E, et al. Visualization of the diaphragm muscle with ultrasound improves diagnostic accuracy of phrenic nerve conduction studies. Muscle Nerve 2014;49:669–675 [DOI] [PubMed] [Google Scholar]
- 26.MacLean IC, Mattioni TA. Phrenic nerve conduction studies: a new technique and its application in quadriplegic patients. Arch Phys Med Rehabil 1981;62:70–73 [PubMed] [Google Scholar]
- 27.Markand ON, Kincaid JC, Pourmand RA, et al. Electrophysiologic evaluation of diaphragm by transcutaneous phrenic nerve stimulation. Neurology 1984;34:604–614 [DOI] [PubMed] [Google Scholar]
- 28.Gerscovich EO, Cronan M, McGahan JP, Jain K, Jones CD, McDonald C. Ultrasonographic evaluation of diaphragmatic motion. J Ultrasound Med 2001;20:597–604 [DOI] [PubMed] [Google Scholar]
- 29.Harris RS, Giovannetti M, Kim BK. Normal ventilatory movement of the right hemidiaphragm studied by ultrasonography and pneumotachography. Radiology 1983;146:141–144 [DOI] [PubMed] [Google Scholar]
- 30.Matsuoka H. Topographic arrangement of the projection from the anterior thalamic nuclei to the cingulate cortex in the cat. Neurosci Res 1986;4:62–66 [DOI] [PubMed] [Google Scholar]
- 31.Ueki J, De Bruin PF, Pride NB. In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax 1995;50:1157–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wait JL, Nahormek PA, Yost WT, Rochester DP. Diaphragmatic thickness–lung volume relationship in vivo. J Appl Physiol 1989;67:1560–1568 [DOI] [PubMed] [Google Scholar]