Abstract
Objective:
To assess the relationships among ABO group, factor VIII (FVIII), and incident cognitive impairment in a large, prospective cohort study of black and white adults in the United States using a nested case-control design.
Methods:
Incident cognitive impairment was defined using cognitive domain tests over a mean follow-up of 3.4 years. ABO blood group was measured by genotyping in a nested case-control sample of 495 cases with cognitive impairment and 587 controls.
Results:
Those with blood group AB and those with higher FVIII had an increased risk of cognitive impairment, adjusting for age, race, region, and sex (respective odds ratios 1.82, 95% confidence interval [CI] 1.15–2.90; and 1.24, 95% CI 1.10–1.38 for 40 IU/dL higher FVIII). Mean FVIII was higher in those with blood type AB (142 IU/dL; 95% CI 119–165) compared with O (104 IU/dL; 95% CI 101–107), and FVIII mediated 18% of the association between AB group and incident cognitive impairment (95% CI for mediation −30% to 68%).
Conclusions:
Blood group AB and higher FVIII were associated with increased incidence of cognitive impairment in this prospective study. The association of blood group AB with incident cognitive impairment was not significantly mediated by FVIII levels.
There is a growing understanding that cardiovascular disease (CVD) and cognitive impairment share many common risk factors. Hypertension,1,2 elevated cholesterol,3 hyperglycemia,2,4 and obesity4,5 are all associated with longitudinal declines in cognitive function and dementia. Higher levels of the hemostatic markers von Willebrand factor (vWF), coagulation factor VIII (FVIII), and d-dimer have also been related to risk of cognitive impairment and dementia.6–9
ABO blood group is associated with many forms of CVD, including coronary heart disease (CHD), stroke, and venous thromboembolism.10–12 In general, individuals with blood group O have a reduced risk of CVD.13,14 Non-O blood types are associated with higher levels of vWF and FVIII, procoagulant proteins that circulate as a complex in blood,11,12 because the ABO antigen affects clearance of vWF.15 Levels of both vWF and FVIII are associated with thrombosis,16 and were recently linked to dementia risk.17 A recent report demonstrating an association between blood type AB and stroke risk found that 60% of that association was mediated by differences in FVIII level.18
Although ABO blood group is a CVD risk factor, we are not aware of studies on its relationship with cognitive impairment. In this study, we examined the relationships among ABO group, FVIII, and incident cognitive impairment in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. We hypothesized that blood group A, B, or AB would be associated with an increased risk of cognitive impairment relative to group O.
METHODS
Cohort.
The REGARDS cohort consists of 30,239 individuals throughout the contiguous United States, aged 45 years and older when recruited between 2003 and 2007. The primary purpose of the REGARDS Study is to investigate the reasons for regional and racial differences in stroke mortality. All participants were self-identified as black or white, with 55% of participants female, 41% black, and 56% living in the southeastern Stroke Belt (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana). Detailed information on the recruitment and demographics of this group has been previously published.19 Demographic and medical history information was collected by telephone interview and in subsequent in-home visits; anthropomorphic data, blood samples, and medication inventory were collected.
Standard protocol approvals, registrations, and patient consents.
All subjects gave informed consent to study participation. All study procedures were approved by the institutional review boards of the collaborating institutions.
Cognitive assessments.
Incident cognitive impairment was defined based on 4 cognitive tests administered during phone interviews: a baseline 6-item screener and a follow up 3-test measure including an animal fluency test, a word list learning test, and word list recall. The 6-item screener consists of 3 immediate recall and 3 temporal orientation items, and was introduced 11 months after the study began and performed annually thereafter. Animal fluency is a verbal fluency test scored as the number of animals that a participant can name in 60 seconds, and word list learning and word list recall measure ability to learn and recall a 10-item list.
Our goal was to identify clinically significant cognitive impairment at the individual subject level. To do this, we started by selecting a performance cutoff that reflects a truly rare and poor test performance, i.e., ≥1.5 SD below the mean of the population. This is a frequently used performance threshold in the diagnostic criteria for mild cognitive impairment.20,21 Scores on these tests were considered impaired if they were below 1.57 SDs from the age-, race-, sex-, and education-adjusted mean scores; this cutpoint was selected to provide approximately 500 cases with substantial impairment in this cohort. Participants were considered to have incident cognitive impairment if, over the course of the study, they had no cognitive impairment at the baseline 6-item screener and an impaired score on at least 2 of the other 3 tests during follow-up.22 Requiring impaired scores in at least 2 of the tests was intended to further select for significant cognitive impairment. The mean follow-up time was 3.4 years.
Case-control study design.
A case-control study design was used to allow efficient study of genetic and biomarker relationships with cognitive decline and provide results that would approximate those that might be obtained by measuring biomarkers in the entire cohort. Among the 30,239 REGARDS participants, we excluded those with prebaseline stroke, baseline cognitive impairment on the 6-item screener, insufficient cognitive testing, or anomalous data. This yielded 17,630 participants eligible for the study, with 495 cases of incident cognitive impairment. Unmatched controls consisted of the 587 participants without cognitive impairment who met these eligibility criteria and were also part of a 1,100-person cohort random sample of REGARDS participants selected for a case-cohort study of stroke risk factors. The cohort random sample was created using stratified sampling to ensure representation of black and white subjects, men and women, and age groups 45 to 54 years (20%), 55 to 64 years (20%), 65 to 74 years (25%), 75 to 84 years (25%), and 85 years or older (10%), in an otherwise randomly selected sample.23
Laboratory.
Fasting blood samples for REGARDS were drawn during in-home visits. Processing, which included centrifugation for 10 minutes and transfer of serum, plasma, or cells into mailer tubes, was required within 120 minutes of the sample collection (mean [SD]: 97 [127] minutes). The samples were then shipped on ice overnight to the study laboratory at the University of Vermont, where they were recentrifuged (30,000g) at 4°C for 15 minutes, aliquoted into cryovials, and stored at −80°C. To determine the validity of FVIII antigen measurements and other biomarkers assessed by REGARDS, given this sample collection and processing protocol, a comparison was done between simulated REGARDS sample processing and ideal processing (performed immediately after sample collection) using 20 split samples. In these experiments, and among cognitive impairment cases and controls, FVIII antigen was measured using an ELISA with a coefficient of variability range of 4% to 7% (Enzyme Research Laboratories, South Bend, IN). The Spearman rank correlation for FVIII processed using ideal methods compared with REGARDS methods was 0.92. Six single nucleotide polymorphisms (SNPs), rs507666, rs687289, rs8176704, rs8176720, rs7853989, and rs8176749, determined using TaqMan probes (Life Technologies, Grand Island, NY), were used to construct ABO haplotypes.
Covariates.
Race, either black or white, was established by participant self-report. Diabetes was defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, self-reported physician diagnosis of diabetes, or self-reported use of diabetes medication. Dyslipidemia was defined as total cholesterol ≥240 mg/dL, low-density lipoprotein ≥160 mg/dL, high-density lipoprotein ≤40 mg/dL, or use of cholesterol-lowering medications. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or self-reported use of antihypertensive medications. Baseline CHD included ECG evidence of myocardial infarction or self-reports of myocardial infarction, coronary artery bypass, stent, or angioplasty. Baseline CVD was defined as baseline CHD or peripheral artery disease.
Statistical analysis.
Diplotype probabilities determined by the 6 ABO SNP identities were calculated using PHASE 2.1 (University of Washington, Seattle). These diplotypes were then classified into blood group A, B, AB, or O, based on the genotype of 4 informative SNPs (rs507666, rs687289, rs8176704, and rs8176749).24 This genotyping predicted ABO type with >90% certainty in 1,015 of 1,082 participants (94%), and these individuals were assigned to a single blood group. Those with uncertain or incomplete haplotype information were assigned diplotype probabilities by the software, based on their available SNP data and population frequencies stratified by race. All analyses were weighted for these blood type probabilities.
Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). All analyses were weighted to account for blood type probabilities and control sample stratification. This was done by creating 4 individual records for every participant, each corresponding to a blood group (O, A, B, and AB), and weighted with the probability for that blood type. For example, if an individual had a 60% probability of blood type A, a 30% probability of type O, and a 10% probability of type B, they would be represented as 4 individuals with weights of 0.6, 0.3, 0.1, and 0. Categorical correlates of blood type were determined using weighted logistic regression or the Rao-Scott χ2 with a 95% confidence interval (CI) calculated by Taylor series using finite population correction. For continuous variables, differences in means among blood groups were analyzed using weighted linear regression.
The relationships of blood group, FVIII, and incident cognitive impairment were evaluated with weighted logistic regression models. Blood group O was used as the reference group because it is associated with lower CVD risk than non-O groups. Interaction between race and blood group was tested by including a race × blood group term in the models. Model 1 was adjusted for age, race, region of residence, and sex. The role of FVIII as a potential mediator of the association between blood group and cognitive impairment was assessed by adding it to age, sex, and race in model 2. Model 3 was adjusted for all previous variables and also baseline CVD. Model 4 was additionally adjusted for CVD risk factors. The mediating effect of FVIII on the blood group association in model 2 was investigated by bootstrapping with 1,000 replicate samples, and a CI for the change in the OR for blood type and incident cognitive impairment was estimated.
RESULTS
Table 1 presents the distribution of cardiovascular risk factors in the incident cognitive impairment cases (495) and controls (587 weighted to 17,122). Missing data represented no more than 5% for any variables. Cases were more likely than controls to live in the Stroke Belt region, be smokers, and have hypertension, hyperlipidemia, diabetes, and CVD. There were no significant differences in age, race, and sex, because these factors were accounted for in the definition of cognitive impairment.
Table 1.
Distribution of CVD risk factors in cases with incident cognitive impairment and controls
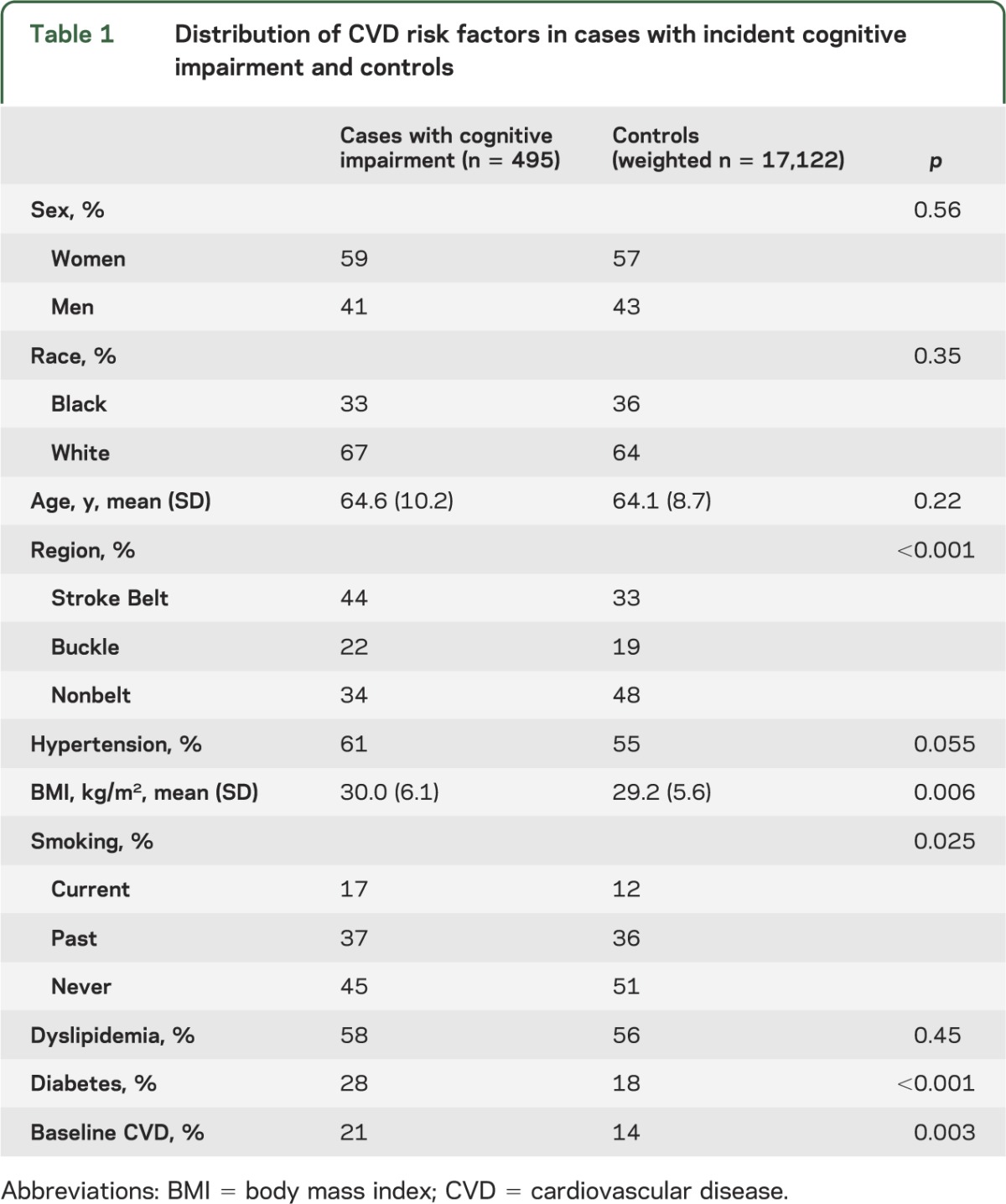
The distribution of blood types in cases and controls is presented in table 2. Cases were more likely to be blood type AB compared with controls. In both cases and controls, black individuals were more likely than white individuals to have blood type B and blood type AB.
Table 2.
Prevalence of blood types by race and incident cognitive impairment status in the REGARDS cohort
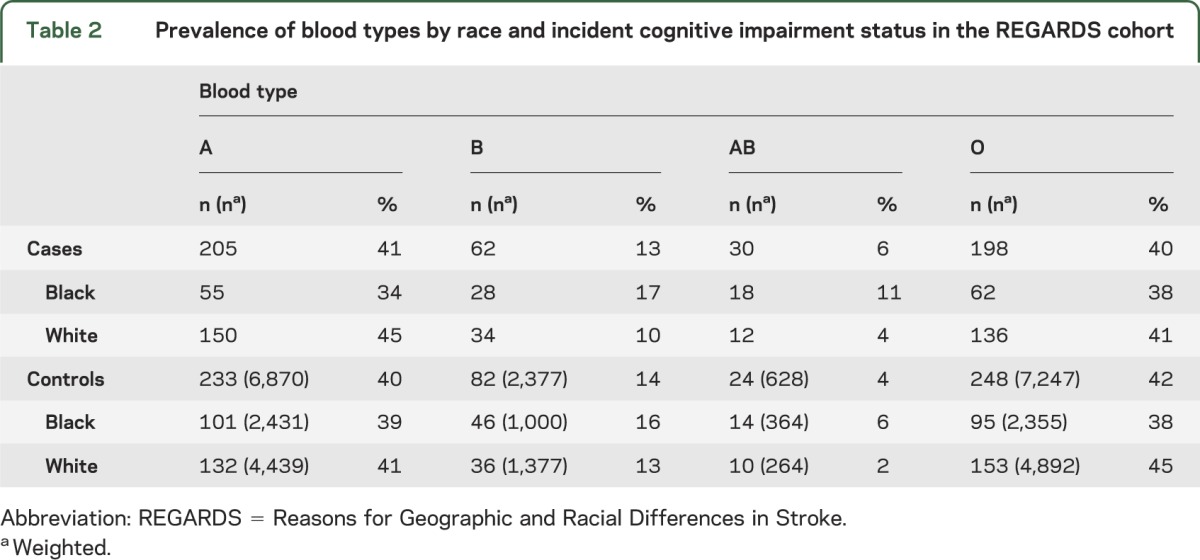
FVIII differed significantly by ABO blood group. The O group had the lowest level, 104 IU/dL (95% CI 101–107), followed by group A with 121 IU/dL (95% CI 116–127), group B with 134 IU/dL (95% CI 126–142), and group AB having the highest level of 142 IU/dL (95% CI 119–165).
In the logistic regression models (table 3), compared with group O, only blood group AB, but not the other blood groups, was associated with incident cognitive impairment in an unadjusted model (OR 1.74, 95% CI 1.12–2.72) (model 0) and the model that adjusted for age, race, region, and sex (OR 1.82, 95% CI 1.15–2.90) (model 1). There was no significant difference in the association of blood group with cognitive impairment by race (p interaction = 0.17). FVIII was also associated with incident cognitive impairment after adjustment for age, race, region, and sex (OR 1.24, 95% CI 1.10–1.38 per 40 IU/dL higher FVIII). When FVIII was added to the ABO model (model 2), the OR for group AB was attenuated (OR 1.64, 95% CI 0.95–2.85), and each 40 IU/dL increase in FVIII remained associated with cognitive impairment (OR 1.23, 95% CI 1.09–1.38). In the 1,000 bootstrapped models, adding FVIII caused a mean change in the AB OR of −0.15 (95% CI −0.56 to 0.25).
Table 3.
Odds ratios (95% confidence intervals) for incident cognitive impairment by ABO blood group
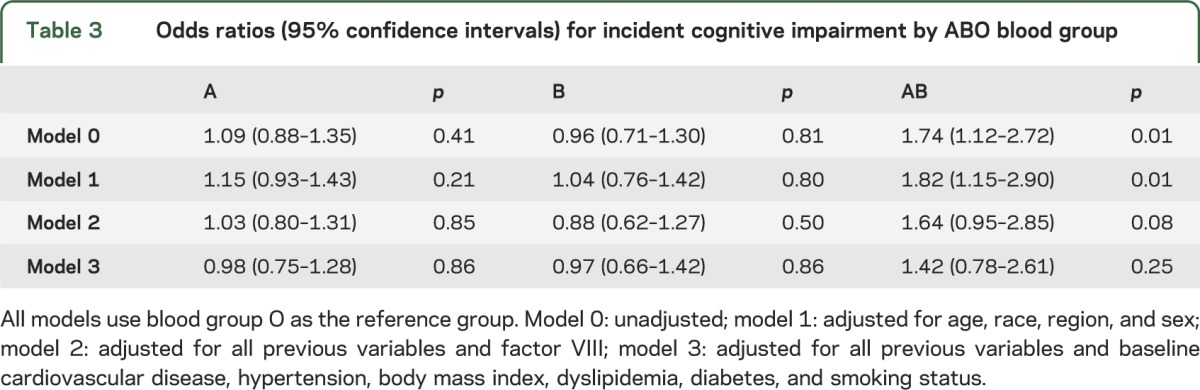
We also examined the effect of adding CVD variables to the model that included FVIII. The addition of baseline CVD and the traditional cardiovascular risk factors hypertension, body mass index, dyslipidemia, diabetes, and smoking status decreased the ORs for blood group AB to 1.42 (95% CI 0.78–2.61) (model 3) and FVIII (per 40 IU/dL) to 1.15 (95% CI 1.01–1.32). Current smoking (p = 0.005), living in the Stroke Belt (p = 0.003), diabetes (p < 0.001), history of CVD (p = 0.02), and white race (p = 0.04) were significantly associated with incident cognitive impairment in this model. Diabetes and was the main factor responsible for the change in the AB OR; however, diabetes was not significantly associated with blood group after adjustment for age and race. To further explore the role of diabetes as a potential mediator in the relationship between blood group and cognitive decline, we performed a bootstrapping analysis, similar to that done for FVIII. Adding diabetes to a model adjusted for age, race, region, and sex resulted in a nonsignificant median change in the AB OR of −0.16 (95% CI −0.54 to 0.13).
DISCUSSION
Blood type AB was associated with an increased risk of cognitive impairment, independent of age, race, region, and sex, with an OR of 1.82 (95% CI 1.15–2.90). FVIII was also associated with incident cognitive impairment in this analysis, with an OR of 1.24 (95% CI 1.10–1.38 per each 40 IU/dL higher FVIII), and FVIII mediated approximately 20% of the association between type AB and cognitive impairment, but this mediation was not statistically significant. While black subjects had a higher prevalence of blood type AB than white subjects, there was no significant interaction between race and blood group or FVIII. Cardiovascular risk factors, especially diabetes, attenuated the association of blood type with incident cognitive impairment.
Although we are not aware of any other studies of ABO group and cognitive impairment or dementia, ABO influences CVD risk and many studies have demonstrated a role for CVD risk factors in cognitive impairment and dementia,2,5,25,26 so common etiologies for these diseases are likely. Indeed, like others4,5 we found that cases with cognitive impairment were more likely to have many traditional CVD risk factors present, such as diabetes and obesity. The current finding of an association between blood type AB and incident cognitive impairment may represent another connection between these diseases.
Similarly, little is currently known about the relationship between FVIII and cognitive impairment, although it was prospectively associated with risk of vascular dementia in one study of men.6 Other hemostatic factors, including FVII, fibrinogen, d-dimer, and vWF, are associated with dementia and cognitive impairment,8 highlighting the role of coagulation in the pathogenesis of these disorders.
One of the main mechanisms by which ABO blood group is thought to alter vascular risk is through its effects on vWF and FVIII.11,12 In our analyses, FVIII did not significantly mediate the association between blood group and incident cognitive impairment, although it did attenuate the association and was itself associated with cognitive impairment. This supports the hypothesis that blood group affects risk of cognitive impairment through alternative pathways, possibly related to effects of the ABO glycosyltransferase. Other known correlates of ABO that are candidate mediators include soluble intercellular adhesion molecule-1 and P-selectin. Levels of these molecules appear to be altered in some types of dementia, but their role is unclear.27–29 ABO blood group also influences susceptibility to certain infectious diseases, including norovirus, cholera, and Helicobacter pylori infection,30 through expression of the A and B antigens on gastrointestinal epithelium. It is possible that ABO type could alter CVD risk through its effects on nonblood tissues.
A limitation of this study is that we were unable to measure levels of vWF, which is also influenced by ABO blood type. To compensate for this, we used measurements of FVIII, which circulates bound to vWF and is highly correlated with vWF.31 However, we may have underestimated the effect of coagulation factors in the association between ABO type and incident cognitive impairment by using only FVIII. We were also unable to measure intercellular adhesion molecule-1 or P-selectin, so cannot assess their potential contribution. Another limitation was the use of inferred ABO group based on ABO genotype information and not clinical antigen testing, a limitation shared by most epidemiologic studies of ABO. Because blood type O results from a nonfunctional ABO product, it is possible that rare variants with this phenotype were misclassified. However, this would be expected to bias our results toward the null hypothesis. Our study relied on test-based determination of cognitive impairment, and we lack the information that a clinical evaluation would provide to detect dementia. Finally, because the definition of incident cognitive impairment was based on age-, race-, sex-, and education-adjusted mean scores on cognitive tests (a strength of our design), we could not examine potential mediation of racial disparities in incident cognitive impairment by ABO status.
The strengths of this study include the use of data from a large, prospective cohort study designed to investigate cardiovascular risk factors and outcomes, which provided a large number of 495 cases with incident cognitive impairment over a short follow-up. This study design allowed longitudinal assessment of cognitive impairment in relation to baseline risk factors. The REGARDS cohort is also geographically diverse within the United States, and includes a large proportion of black subjects. Our definition of incident cognitive impairment considered the effects of age, race, sex, and education, utilized testing in multiple cognitive domains, and used a cutpoint to define impairment that has clinical relevance, strengthening our ability to identify those with significant impairment.
The results of our study support the hypothesis that ABO blood group represents a link between cardiovascular risk and cognitive function. Blood group AB and higher FVIII were associated with cognitive decline in a large cohort of both black and white Americans, and while higher FVIII levels were seen in those with type AB, mediation of the group AB–cognitive decline association by FVIII was nonsignificant. This indicates that the effect may be the result of other pathways influenced by the ABO glycosyltransferase. Further work is needed to confirm these results and to determine the underlying mechanisms responsible.
ACKNOWLEDGMENT
The authors thank the other investigators, the staff, and the participants of the REGARDS Study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
GLOSSARY
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- FVIII
factor VIII
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
- SNP
single nucleotide polymorphism
- vWF
von Willebrand factor
AUTHOR CONTRIBUTIONS
K.S.A. performed statistical analysis, interpreted the data, and drafted the manuscript. N.A.Z. assisted with statistical analysis. S.G., V.W., M.C., L.A.M., and F.U. developed the definition of incident cognitive impairment. K.S.A., N.A.Z., S.G., and M.C. designed the study. S.G., V.W., M.C., L.A.M., F.U., and N.Z. contributed to and approved the final manuscript.
STUDY FUNDING
Supported by cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS), NIH, Department of Health and Human Service. Additional salary support was provided by the National Heart, Lung and Blood Institute (NHLBI) T32HL007594-27 (K.S.A.), and NHLBI grant K08HL096841 (N.A.Z.) was used to fund FVIII measurements for this study.
DISCLOSURE
K. Alexander receives research support from the NIH. N. Zakai receives research funding from the NIH and serves as a guest editor for Circulation. S. Gillett has received support from the NIH. L. McClure serves on a data monitoring committee for the NIH/NINDS and receives research support from Genzyme Corporation, the NIH (NINDS, NICHD, NHLBI), and NASA. V. Wadley has received funding for travel from the Alzheimer's Association; serves on the editorial board of Current Gerontology and Geriatrics Research and the Journal of Aging Science; and receives research support from Genzyme Corporation and the NIH. F. Unverzagt has served as a consultant to UCB Pharma SA; serves on the editorial boards of the Journal of the International Neuropsychological Association, Current Alzheimer Research, and Neuropsychology; receives research support from the NIH and Posit Science Inc.; and holds stock in Eli Lilly and Company. M. Cushman is an associate editor of Journal of Thrombosis and Haemostasis, senior guest editor of Circulation, serves on the editorial boards of Thrombosis and Thrombolysis and Coronary Artery Disease, has been a consultant for Daiichi Sankyo, and receives research support from diaDexus and the NIH. She has received travel reimbursement for volunteer work with the American Heart Association/American Stroke Association. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology 2011;77:1729–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement 2009;5:207–214 [DOI] [PubMed] [Google Scholar]
- 3.Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology 2001;56:1683–1689 [DOI] [PubMed] [Google Scholar]
- 4.Reijmer YD, van den Berg E, Dekker JM, et al. Development of vascular risk factors over 15 years in relation to cognition: the Hoorn Study. J Am Geriatr Soc 2012;60:1426–1433 [DOI] [PubMed] [Google Scholar]
- 5.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556–1560 [DOI] [PubMed] [Google Scholar]
- 6.Gallacher J, Bayer A, Lowe G, et al. Is sticky blood bad for the brain? Hemostatic and inflammatory systems and dementia in the Caerphilly Prospective Study. Arterioscler Thromb Vasc Biol 2010;30:599–604 [DOI] [PubMed] [Google Scholar]
- 7.Hagnelius NO, Boman K, Nilsson TK. Fibrinolysis and von Willebrand factor in Alzheimer's disease and vascular dementia: a case-referent study. Thromb Res 2010;126:35–38 [DOI] [PubMed] [Google Scholar]
- 8.Quinn TJ, Gallacher J, Deary IJ, Lowe GD, Fenton C, Stott DJ. Association between circulating hemostatic measures and dementia or cognitive impairment: systematic review and meta-analyzes. J Thromb Haemost 2011;9:1475–1482 [DOI] [PubMed] [Google Scholar]
- 9.Stott DJ, Spilg E, Campbell AM, Rumley A, Mansoor MA, Lowe GD. Haemostasis in ischaemic stroke and vascular dementia. Blood Coagul Fibrinolysis 2001;12:651–657 [DOI] [PubMed] [Google Scholar]
- 10.He M, Wolpin B, Rexrode K, et al. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol 2012;32:2314–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiggins KL, Smith NL, Glazer NL, et al. ABO genotype and risk of thrombotic events and hemorrhagic stroke. J Thromb Haemost 2009;7:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohira T, Cushman M, Tsai MY, et al. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE). J Thromb Haemost 2007;5:1455–1461 [DOI] [PubMed] [Google Scholar]
- 13.Liumbruno GM, Franchini M. Beyond immunohaematology: the role of the ABO blood group in human diseases. Blood Transfus 2013;11:491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dentali F, Sironi AP, Ageno W, Crestani S, Franchini M. ABO blood group and vascular disease: an update. Semin Thromb Hemost 2014;40:49–59 [DOI] [PubMed] [Google Scholar]
- 15.van Schie MC, van Loon JE, de Maat MP, Leebeek FW. Genetic determinants of von Willebrand factor levels and activity in relation to the risk of cardiovascular disease: a review. J Thromb Haemost 2011;9:899–908 [DOI] [PubMed] [Google Scholar]
- 16.Koster T, Blann AD, Briet E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995;345:152–155 [DOI] [PubMed] [Google Scholar]
- 17.Bath PM, Anderton PR, Ankolekar S. Hemostasis and vascular dementia. Arterioscler Thromb Vasc Biol 2010;30:461–463 [DOI] [PubMed] [Google Scholar]
- 18.Zakai NA, Judd SE, Alexander K, et al. ABO blood type and stroke risk: the Reasons for Geographic and Racial Differences in Stroke Study. J Thromb Haemost 2014;12:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology 2005;25:135–143 [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194 [DOI] [PubMed] [Google Scholar]
- 21.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240–246 [DOI] [PubMed] [Google Scholar]
- 22.Thacker EL, Gillett SR, Wadley VG, et al. The American Heart Association Life's Simple 7 and incident cognitive impairment: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. J Am Heart Assoc 2014;3:e000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cushman M, Judd SE, Howard VJ, et al. N-terminal pro-B-type natriuretic peptide and stroke risk: the Reasons for Geographic and Racial Differences in Stroke Cohort. Stroke 2014;45:1646–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranade K, Chang MS, Ting CT, et al. High-throughput genotyping with single nucleotide polymorphisms. Genome Res 2001;11:1262–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fillit H, Nash DT, Rundek T, Zuckerman A. Cardiovascular risk factors and dementia. Am J Geriatr Pharmacother 2008;6:100–118 [DOI] [PubMed] [Google Scholar]
- 26.Ganguli M, Fu B, Snitz BE, Hughes TF, Chang CC. Mild cognitive impairment: incidence and vascular risk factors in a population-based cohort. Neurology 2013;80:2112–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewers M, Mielke MM, Hampel H. Blood-based biomarkers of microvascular pathology in Alzheimer's disease. Exp Gerontol 2010;45:75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochstrasser T, Weiss E, Marksteiner J, Humpel C. Soluble cell adhesion molecules in monocytes of Alzheimer's disease and mild cognitive impairment. Exp Gerontol 2010;45:70–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaremo P, Milovanovic M, Buller C, Nilsson S, Winblad B. P-selectin paradox and dementia of the Alzheimer type: circulating P-selectin is increased but platelet-bound P-selectin after agonist provocation is compromised. Scand J Clin Lab Invest 2013;73:170–174 [DOI] [PubMed] [Google Scholar]
- 30.Anstee DJ. The relationship between blood groups and disease. Blood 2010;115:4635–4643 [DOI] [PubMed] [Google Scholar]
- 31.Kaufman RJ, Dorner AJ, Fass DN. von Willebrand factor elevates plasma factor VIII without induction of factor VIII messenger RNA in the liver. Blood 1999;93:193–197 [PubMed] [Google Scholar]


