Abstract
Objective:
In the present study, we tested the hypothesis that having migraine in middle age is related to late-life parkinsonism and a related disorder, restless legs syndrome (RLS), also known as Willis-Ekbom disease (WED).
Methods:
The AGES-Reykjavik cohort (born 1907–1935) has been followed since 1967. Headaches were classified based on symptoms assessed in middle age. From 2002 to 2006, 5,764 participants were reexamined to assess symptoms of parkinsonism, diagnosis of Parkinson disease (PD), family history of PD, and RLS/WED.
Results:
Subjects with midlife migraine, particularly migraine with aura (MA), were in later life more likely than others to report parkinsonian symptoms (odds ratio [OR]MA = 3.6 [95% CI 2.7–4.8]) and diagnosed PD (ORMA = 2.5 [95% CI 1.2–5.2]). Women with MA were more likely than others to have a parent (ORMA = 2.26 [95% CI 1.3–4.0]) or sibling (ORMA = 1.78 [95% CI 1.1–2.9]) with PD. Late-life RLS/WED was increased for headache generally. Associations were independent of cardiovascular disease and MRI-evident presumed ischemic lesions.
Conclusions:
These findings suggest there may be a common vulnerability to, or consequences of, migraine and multiple indicators of parkinsonism. Additional genetic and longitudinal observational studies are needed to identify candidate pathways that may account for the comorbid constellation of symptoms.
Clinical and epidemiologic studies have suggested that some movement disorders are overrepresented in individuals who experience migraine.1 Plausible mechanisms linking migraine with movement disorders include damage to relevant brain structures previously associated with migraine,2 dopamine (DA) dysfunction (which has been hypothesized as a causal factor in migraine pathogenesis),3 parkinsonism due to head injury or ischemic vascular disease (both of which are linked to migraine),4–6 or damage related to iron deposition in the basal ganglia and related structures (also previously linked to migraine).7–10 However, with the exception of restless legs syndrome (RLS), also known as Willis-Ekbom disease (WED),11,12 the relationship between migraine and movement disorders has only been examined in samples specifically selected for presence of clinically diagnosed migraine or movement disorders, or in older cohorts when headache symptoms are retrospectively assessed. Herein, we consider, in a population-based cohort, whether older adults with symptoms of migraine assessed in middle age are more likely than others to manifest in late-life symptoms of movement disorders, specifically parkinsonism, Parkinson disease (PD), and RLS/WED.
METHODS
Overview of study design.
Detailed descriptions of the Reykjavik Study (RS) and its follow-on, the Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-Reykjavik Study) have been published previously.13,14 In brief, the RS is a population-based cohort study established in 1967 by the Icelandic Heart Association to prospectively study cardiovascular disease (CVD) in Iceland.14 The cohort included a random sample of men and women, born 1907 to 1935 and living in Reykjavik at baseline. From 2002 to 2006, the AGES-Reykjavik Study examined in 5,764 RS survivors (58% women) risk factors, genetic susceptibility, and gene/environment interactions in relation to disease and disability in old age.13 Recruitment details and comparisons of the AGES-Reykjavik Study to the original cohort have been described.13
Headache (midlife).
Participants were asked about current headache symptoms in the RS (conducted at mean age 51 years, range 33–65) as previously described.15 Those reporting headache once or more per month were asked follow-on questions to determine whether headaches were accompanied by (1) nausea or vomiting, (2) unilateral location, (3) photophobia, (4) visual disturbance during/just before headache, and (5) unilateral numbness before headache.15 Subjects were classified into 4 mutually exclusive groups approximating International Classification of Headache Disorders, first edition, criteria16 (no headache, nonmigraine headache [NMH], migraine without aura [MO], and migraine with aura [MA]). MA was defined as reporting visual symptoms and/or numbness. MO was defined as 2+ nonaura symptoms (and no aura symptoms). NMH was defined as headaches with at most one nonaura symptom.
All other assessments, below, are from the late-life interview (mean age 77 years, range 66–96).
Parkinsonism.
Six screening questions addressed changes in motor function suggestive of parkinsonism. Questions were a modification of a screening questionnaire developed by Tanner.17,18 Subjects reporting ≥4 symptoms (approximately 9% of subjects) were classified as having high parkinsonian symptoms (PS). Subjects were also asked whether they had been diagnosed with PD. Current medication use was determined from prescription bottles brought to the study visit. We identified medications taken to treat PD using Anatomical Therapeutic Chemical code N04 (anti-Parkinson drugs). Records of inpatient medical encounters were available for the years 1997 through 2010. Medical encounters with ICD-10 diagnostic code G20 (PD) were identified. Finally, we asked about PD history in first-degree relatives.
RLS/WED.
RLS/WED classification was based on 4 screening questions covering the minimal diagnostic criteria of the International Restless Legs Syndrome Study Group.19,20
Walking speed.
We used the 6-m walking speed test as a secondary outcome variable and objective measure of functional impact. We asked subjects to walk 6 m at their normal pace. We recorded the number of seconds and number of steps taken to walk 6 m over 2 trials and averaged the results.
Confounders or mediators.
Because secondary parkinsonism can be related to cerebrovascular diseases (e.g., “vascular parkinsonism”) or head injury, we included in our analysis whether subjects had a history of head injury and ischemic vascular disease. Lifetime history of moderate to severe head injury was based on a question about whether they had ever had a head trauma associated with loss of consciousness lasting more than 1 hour. CVD measures in this population have been described.15 Briefly, hypertension was defined by the average of 2 blood pressure measures, use of antihypertensive medications, and health history. Diabetes history was defined according to self-reported history of diabetes, use of blood glucose–lowering drugs, or fasting blood glucose level ≥7.0 mmol/L. We assessed smoking history (never, former, current) and history of stroke or TIA. History of coronary artery disease (possible or definite) was defined based on self-reported history of angina, self-report of myocardial infarction, ECG evidence of myocardial infarction, use of nitrates, and self-report of coronary artery bypass graft. We identified subjects (n = 68) with a current prescription for a DA antagonist for use in a sensitivity analysis described below.
Brain infarcts and white matter lesion load.
The brain MRI protocol for the AGES-Reykjavik Study has been described.15 For the present analysis, presence of any brain infarct–like lesions (yes/no) and total white matter lesion load (mL) were used as summary measures of vascular lesions.
Statistical analyses.
Of the 5,764 AGES-Reykjavik participants, we excluded 144 subjects who were older than 65 years at the time of the first examination, leaving 5,620 study participants (57% female) for most analyses. Of these, 83%/82% had MRI data on brain infarct-like lesions/white matter lesion load, respectively. Reasons for nonparticipation in the MRI examination have been described.15
We compared risk of parkinsonism/RLS/WED in later life among subjects without and with migraine in midlife. Our primary outcome variables were as follows: (1) diagnosis of PD, (2) diagnosis of PD in a parent or sibling, (3) parkinsonism (defined as 4 or more of the 6 PS), and (4) RLS (defined as all 4 RLS/WED symptoms).
All analyses were performed with Stata software (version 12; StataCorp, College Station, TX). We used logistic or multinomial logistic regression to calculate odds ratios (ORs) as an estimate of the relative risk of each outcome in subjects with headache (NMH, MO, MA) relative to the comparison group (no headache). Because headache is strongly related to demographic factors, all models were minimally adjusted for sex, age-1 (at baseline), age-2 (at follow-up), and educational level (primary, secondary, college, university). In addition, we adjusted for possible explanatory factors including vascular risk factors, CVD, and history of head injury. Our fully adjusted model additionally adjusted for the presence of infarct-like lesions and white matter lesion load, data that were available for the approximately 80% of subjects who underwent brain MRI. We report results for men and women combined, adjusted for demographic factors only, and note when results were significantly different for men and women using a p value of 0.1 for interaction or if results changed after adjustment for the factors described above. Missing values for categorical independent and dependent variables were coded as “unknown” in order to maximize sample size.
We used quantile regression to compare demographically adjusted medians of continuous variables (walking test variables and some CVD risk factors) by headache categories. Walking speed variables were additionally adjusted for height and diagnosed arthritis. We calculated sex-specific cutpoints for the slowest 10% of subjects and for the top 10% number of steps and used multinomial logistic regression to calculate odds of being in these categories by headache groups. This 10% cutpoint corresponded to approximately 3 seconds/3 steps more than the median for the entire group.
Standard protocol approvals, registrations, and patient consents.
The AGES-Reykjavik Study was approved by the Icelandic National Bioethics Committee (VSN-00-063), the Institutional Review Board for the Icelandic Heart Association, and by the Institutional Review Board for the US National Institute on Aging, NIH. Written informed consent was obtained from all participants.
RESULTS
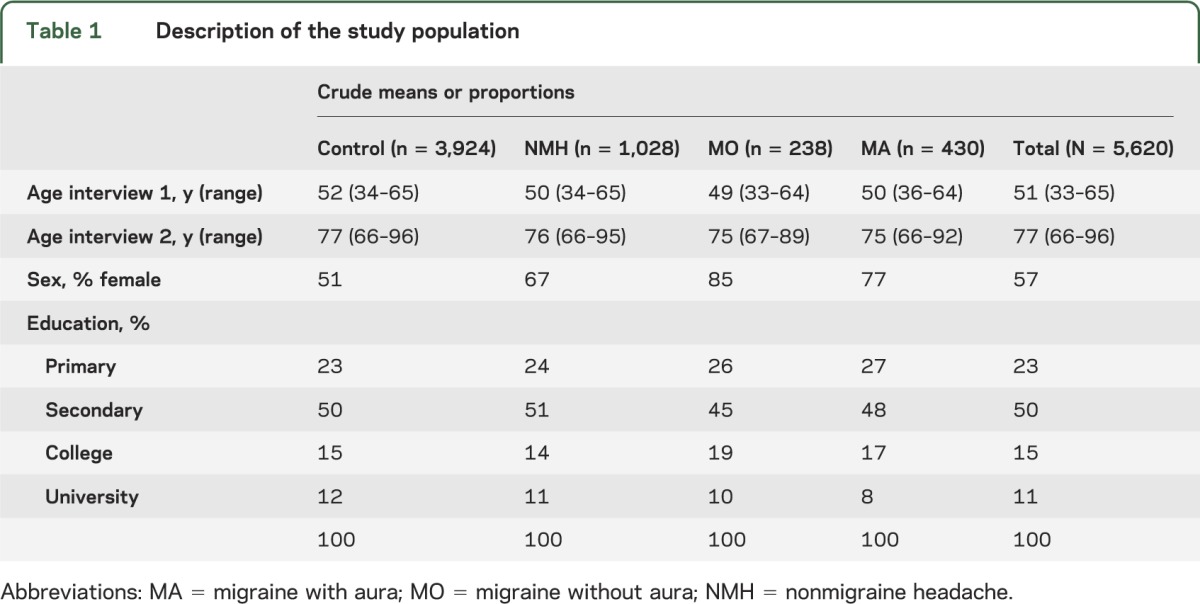
Table 1 shows the demographic characteristics of our study population. Table e-1 on the Neurology® Web site at Neurology.org shows the distribution by headache categories of the factors that we considered as confounders or mediators of the relationship between migraine and late-life parkinsonism/RLS/WED. Migraineurs, particularly women, were more likely than controls to have risk factors or diagnoses of CVD as previously reported. Men and women with MA were about twice as likely as controls to report a history of a moderate to severe head injury (men: 21% vs 10%; women: 12% vs 5%; p < 0.005).
Table 1.
Description of the study population
Parkinsonism.
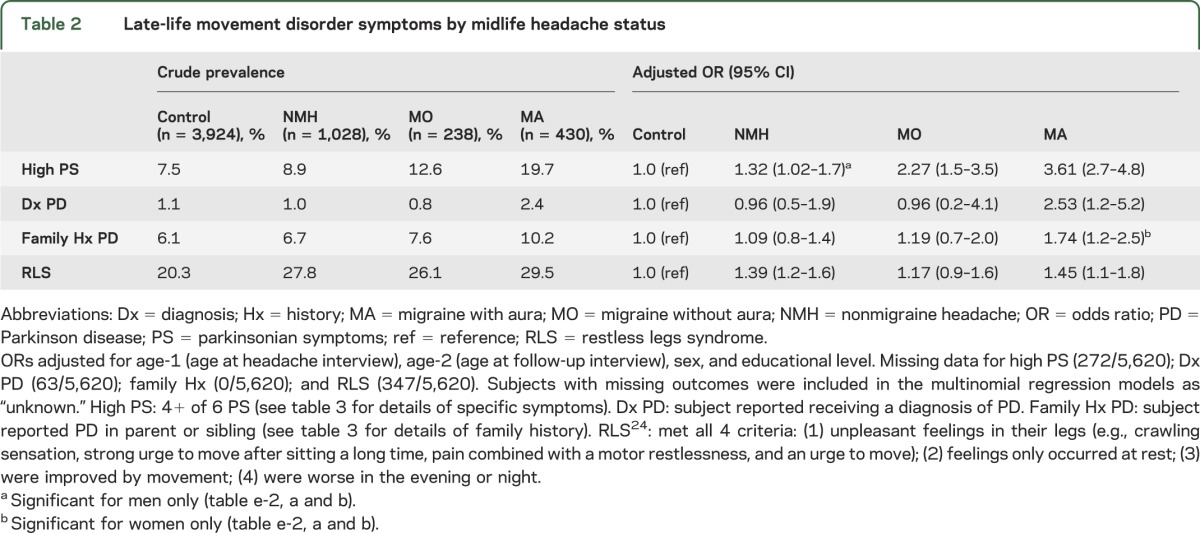
A total of 8.9% of subjects reported 4 or more PS. PS were more common in subjects with midlife headache than controls, particularly for MA (table 2: ORMA = 3.61 [95% CI 2.7–4.8]; ORMO = 2.27 [95% CI 1.5–3.5]; ORNMH = 1.32 [95% CI 1.02–1.7]). Results were similar after excluding the subjects with a current prescription for a DA antagonist (ORMA = 3.72 [95% CI 2.8–5.0]; ORMO = 2.38 [95% CI 1.6–3.6]; ORNMH = 1.33 [95% CI 1.03–1.7]). NMH was associated with high PS in men but not women (p = 0.08 for interaction by sex) (see table e-2, a and b, for sex-stratified results). Results were similar after adjusting for CVD and head injury in the fully adjusted model (data not shown).
Table 2.
Late-life movement disorder symptoms by midlife headache status
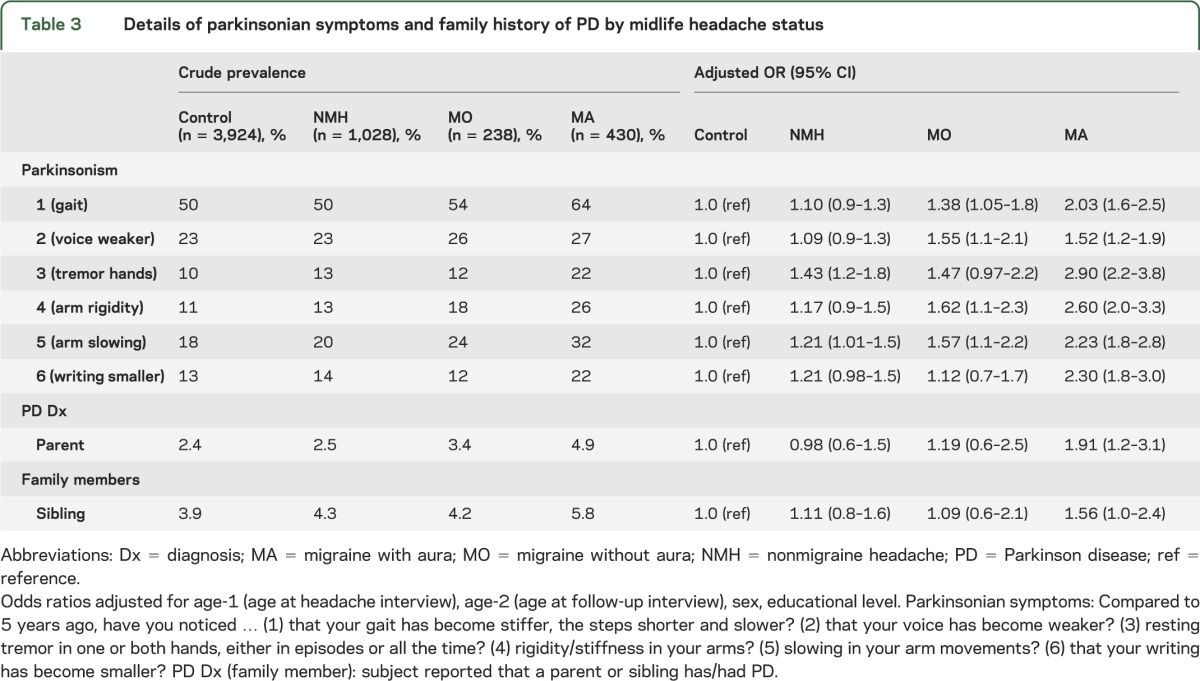
Results for each of the 6 symptoms are shown in table 3 (table e-3, a and b, shows sex-stratified results). All 6 symptoms were more common in subjects with midlife MA compared with controls, with the strongest association for tremor (ORMA = 2.90 [95% CI 2.2–3.8]) followed by arm rigidity (ORMA = 2.60 [95% CI 2.0–3.3]). Most symptoms were also associated with MO although the magnitude of effect was generally smaller (table 3). Subjects with NMH were more likely than controls to report tremor (ORNMH = 1.43 [95% CI 1.2–1.8]) and arm slowing (ORNMH = 1.21 [95% CI 1.01–1.5]).
Table 3.
Details of parkinsonian symptoms and family history of PD by midlife headache status
History of diagnosed PD.
A total of 1.2% of subjects reported receiving a diagnosis of PD, ranging from 0.05% (1/1,923) of those with no PS to 35% (20/58) of subjects reporting all 6 PS. Most (86%) of the self-reported diagnoses were confirmed by either having a current prescription for a medication taken to treat PD or an inpatient medical encounter for PD. Subjects with midlife MA were more likely than controls to report having been diagnosed with PD (ORMA = 2.5 [95% CI 1.2–5.2]). Results were not significantly different for men and women and were similar in the fully adjusted model. Results were unchanged using the stricter case definition (OR = 2.8 [95% CI 1.3–5.9]) or after excluding the subjects with a current prescription for a DA antagonist (ORMA = 2.7 [95% CI 1.3–5.6]).
Subjects had 4.8 siblings on average. A total of 6.6% of subjects reported having at least one parent or sibling with PD. Women, but not men, with midlife MA were more likely to have a family history of PD than controls (men: ORMA = 0.93 [95% CI 0.4–2.2]; women: ORMA = 2.10 [95% CI 1.4–3.1]; p = 0.09 for interaction by sex). Results were similar in the fully adjusted model (data not shown).
More details of PD family history are shown in tables 3 and e-3, a and b. Women with midlife MA were more likely to have a parent with PD (ORMA = 2.26 [95% CI 1.3–4.0]) or a sibling with PD (ORMA = 1.78 [95% CI 1.1–2.9]) compared with female controls.
RLS/WED.
A total of 23% of subjects met screening criteria for RLS/WED. RLS/WED was more common in subjects with headache compared with controls (table 2). Results were similar for men and women and in the fully adjusted model (data not shown).
Walking speed.
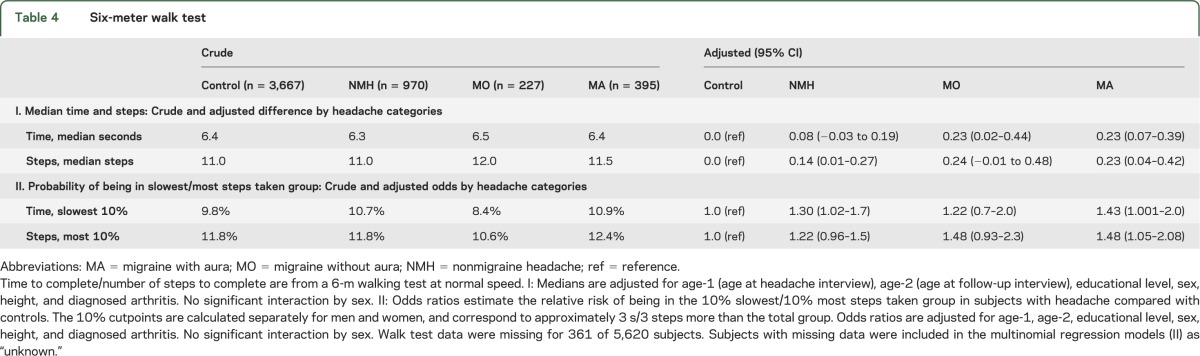
There were significant but small differences in median walking time and number of steps by headache categories. Compared to controls, subjects with midlife headache were more likely to be in the slowest 10% of subjects or take the top 10% of steps (table 4).
Table 4.
Six-meter walk test
DISCUSSION
In the present study, we found that subjects with a midlife history of headache were, about 25 years later, more likely than others to report movement disorder symptoms (parkinsonism and RLS/WED), to have been diagnosed with PD, and to have a family history of PD (women). Some findings differed by headache type. Risk of late-life RLS/WED was increased for all types of headache. In contrast, increased risk of late-life parkinsonism, diagnosed PD, and family history of PD was limited to, or more evident for, those with a history of MA. Regarding possible functional impact, we found a small, but unlikely to be clinically important, difference in walking speed where those with midlife headache were slower than those without headache. To put findings in context, midlife MA was associated with approximately doubled risk of late-life PD (from 1.1% to 2.4%).
Migraine is the most common neurologic disorder in both men and women.21 Beyond its public health impact, migraine is of scientific interest because of the conditions with which it is comorbid and its varying manifestations and characteristic epidemiologic profile across the lifespan. Migraine symptoms and associated features vary by age, ranging from early childhood migraine equivalents or possible precursors to late-life aura-like symptoms without headache.22,23 Previous population-based studies have found that migraineurs, particularly those with aura, are at increased risk of cerebrovascular disease (subclinical infarct-like lesions, clinically evident stroke, and white matter hyperintensities) and CVD (myocardial infarction, CVD risk factors).24 In both the Women's Health Study and the Physician's Health Study, subjects with migraine were at increased odds of RLS/WED compared to those without headache.11,12 This is consistent with the results herein based on an older population. Clinical studies, but none to our knowledge based on subjects in the general population, have noted potential associations between migraine and other movement disorders (reviewed by d'Onofrio et al.1).
It is plausible that migraine might be linked with the movement disorders and motor symptoms evaluated in the present study. Dopaminergic dysfunction, common to both parkinsonism and RLS/WED, has been hypothesized as a causal factor in migraine pathogenesis for many years.3 Prodromal and accompanying symptoms of migraine, such as excessive yawning, nausea, and vomiting, are thought to be related to DA receptor stimulation, and pharmacologic studies of DA agonists suggest DA hypersensitivity in migraine patients.25 The nature of the relationship is likely complicated because DA may be both therapeutic and pathogenic in migraine,3,25 and in some studies,1,26,27 it has been suggested that PD may have a favorable effect on migraine after diagnosis.
Other studies have suggested that migraineurs more often than nonmigraineurs have increased iron accumulation in deep brain nuclei.7–9 Iron is essential for normal neuronal function including DA regulation.28 Deposition of nonheme iron, particularly in the basal ganglia, increases with normal aging until early middle age after which the rate of increase slows or plateaus.29 Relatively increased or decreased iron accumulation has been observed in a variety of chronic brain disorders including in both PD, where iron is increased in the substantia nigra, and RLS/WED, where there is evidence in some studies of decreased iron stores.30–33 Iron deposition may itself be pathogenic, a marker of tissue damage due to other processes, or a reversible epiphenomenon.7,28,31 If the increased iron deposition observed in migraineurs in these few studies reflects (directly or indirectly) neuronal damage, this would be consistent with the late-life symptoms observed in our study population. Arguing against this is the lack of linkage, in most studies, between iron accumulation and MA specifically.
Finally, cerebrovascular disease and traumatic brain injury (TBI) are both plausible reasons why migraine might be linked with parkinsonism because both have been linked separately with migraine and both are causes of parkinsonism.6,24,34 Given our robust control for cerebrovascular disease and risk factors, including evaluation of infarct-like and white matter lesion load on brain MRI, our data do not support this as a primary mechanism linking migraine with parkinsonism or with RLS/WED in this population. We cannot rule out a contributing role of head injury given the limitations of the way we assessed this. We note that our subjects with MA were twice as likely as others to report a history of a moderate to severe TBI. DA dysfunction has been implicated as a central mechanism of pathology after TBI,35 and posttraumatic headache is among the most common manifestations of TBI.36
Strengths of this study are the population-based design and comprehensive measurement of vascular risk factors and history. Our findings were evident for both diagnosis-based and symptom-based outcomes, increasing confidence that the findings are robust. We assessed migraine symptoms in middle age, which is critically important because migraine attacks often remit or change features in later life. Likewise, parkinsonism and other symptoms were assessed in later life when these symptoms are more likely to manifest. As previously mentioned, earlier reports26,27 have suggested that PD may have a favorable effect on migraine prognosis—perhaps due to changes in nociception or medications taken to treat PD37—making the longitudinal design of the present study a particular strength.
A limitation of our study is that we assessed diagnostic history of PD by self-report. Our observed prevalence of diagnosed PD (1.2%) may be on the low side given the age of our study population.38 Furthermore, surveillance bias is possible when using diagnostic history, whether self-reported or via medical records, as a proxy for disease. If the subjects with midlife MA were more likely than others to be under the care of a neurologist for persistent late-life headaches or other reasons, they might have been diagnosed more promptly if they then developed symptoms suggestive of PD. This type of bias might have exaggerated the association between migraine and diagnosed PD. Nonetheless, we assessed parkinsonism several ways, with symptoms, diagnostic history, and family history, and results were internally consistent. Our assessment of visual aura may have included individuals with nonspecific visual symptoms, a type of bias that is more likely to have attenuated rather than exaggerated our results. Results are based on residents of Reykjavik, Iceland, and may not generalize to other populations. Finally, our assessment of TBI did not allow us to evaluate the role of multiple mild head injuries, which have been reported, separately, to be related to both chronic headaches39 and later parkinsonism.40
Our finding linking migraine with different indicators of parkinsonism suggests shared cerebral vulnerability that could reflect common pathology, genetic or environmental risk factors, or changes in the brain from one condition that increases the likelihood of symptoms reflecting the other conditions. Future longitudinal studies with more targeted brain imaging and neurologic examinations are warranted.
Supplementary Material
GLOSSARY
- AGES-Reykjavik Study
Age, Gene/Environment Susceptibility–Reykjavik Study
- CVD
cardiovascular disease
- DA
dopamine
- ICD-10
International Classification of Diseases, tenth revision
- MA
migraine with aura
- MO
migraine without aura
- NMH
nonmigraine headache
- OR
odds ratio
- PD
Parkinson disease
- PS
parkinsonian symptoms
- RLS
restless legs syndrome
- RS
Reykjavik Study
- TBI
traumatic brain injury
- WED
Willis-Ekbom disease
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Ann I. Scher: drafting/revising the manuscript for content, study concept or design, analysis or interpretation of data. G. Webster Ross: drafting/revising the manuscript for content, analysis or interpretation of data. Sigurdur Sigurdsson: drafting/revising the manuscript for content, acquisition of data. Melissa Garcia: drafting/revising the manuscript for content, study supervision or coordination. Larus S. Gudmundsson, Sigurlaug Sveinbjörnsdóttir, Amy K. Wagner, and Vilmundur Gudnason: drafting/revising the manuscript for content. Lenore J. Launer: drafting/revising the manuscript for content, study concept or design, analysis or interpretation of data.
STUDY FUNDING
The AGES-Reykjavik Study was supported by NIH N01-AG-12100, the National Institute on Aging Intramural Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). The views expressed in this work are those of the authors and do not necessarily reflect the official policy or position of the Department of Defense or the US Government.
DISCLOSURE
A. Scher serves on a scientific advisory board for Allergan, Inc., and is an associate editor for Cephalalgia and Pain Medicine. G. Ross, S. Sigurdsson, M. Garcia, L. Gudmundsson, S. Sveinbjörnsdóttir, A. Wagner, V. Gudnason, and L. Launer report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.d'Onofrio F, Barbanti P, Petretta V, et al. Migraine and movement disorders. Neurol Sci 2012;33(suppl 1):S55–S59 [DOI] [PubMed] [Google Scholar]
- 2.Bashir A, Lipton RB, Ashina S, Ashina M. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology 2013;81:1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charbit AR, Akerman S, Goadsby PJ. Dopamine: what's new in migraine? Curr Opin Neurol 2010;23:275–281 [DOI] [PubMed] [Google Scholar]
- 4.Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol 2012;72:893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colosimo C, Morgante L, Antonini A, et al. Non-motor symptoms in atypical and secondary parkinsonism: the PRIAMO Study. J Neurol 2010;257:5–14 [DOI] [PubMed] [Google Scholar]
- 6.de Laat KF, van Norden AG, Gons RA, et al. Cerebral white matter lesions and lacunar infarcts contribute to the presence of mild parkinsonian signs. Stroke 2012;43:2574–2579 [DOI] [PubMed] [Google Scholar]
- 7.Kruit MC, Launer LJ, Overbosch J, van Buchem MA, Ferrari MD. Iron accumulation in deep brain nuclei in migraine: a population-based magnetic resonance imaging study. Cephalalgia 2009;29:351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tepper SJ, Lowe MJ, Beall E, et al. Iron deposition in pain-regulatory nuclei in episodic migraine and chronic daily headache by MRI. Headache 2012;52:236–243 [DOI] [PubMed] [Google Scholar]
- 9.Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache 2001;41:629–637 [DOI] [PubMed] [Google Scholar]
- 10.Granziera C, Daducci A, Romascano D, et al. Structural abnormalities in the thalamus of migraineurs with aura: a multiparametric study at 3 T. Hum Brain Mapp 2014;35:1461–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schurks M, Winter AC, Berger K, Buring JE, Kurth T. Migraine and restless legs syndrome in women. Cephalalgia 2012;32:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winter AC, Schurks M, Berger K, Buring JE, Gaziano JM, Kurth T. Migraine and restless legs syndrome in men. Cephalalgia 2013;33:130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment susceptibility–Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;65:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med 1995;122:96–102 [DOI] [PubMed] [Google Scholar]
- 15.Scher AI, Gudmundsson LS, Sigurdsson S, et al. Migraine headache in middle age and late-life brain infarcts. JAMA 2009;301:2563–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8(suppl 7):1–96 [PubMed] [Google Scholar]
- 17.Tanner CM. Early intervention in Parkinson's disease: epidemiologic considerations. Ann Epidemiol 1996;6:438–441 [DOI] [PubMed] [Google Scholar]
- 18.Dahodwala N, Siderowf A, Baumgarten M, Abrams A, Karlawish J. Screening questionnaires for parkinsonism: a systematic review. Parkinsonism Relat Disord 2012;18:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walters AS. Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Mov Disord 1995;10:634–642 [DOI] [PubMed] [Google Scholar]
- 20.Silber MH, Becker PM, Earley C, Garcia-Borreguero D, Ondo WG. Willis-Ekbom Disease Foundation revised consensus statement on the management of restless legs syndrome. Mayo Clin Proc 2013;88:977–986 [DOI] [PubMed] [Google Scholar]
- 21.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology 2007;68:326–337 [DOI] [PubMed] [Google Scholar]
- 22.Bigal ME, Liberman JN, Lipton RB. Age-dependent prevalence and clinical features of migraine. Neurology 2006;67:246–251 [DOI] [PubMed] [Google Scholar]
- 23.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(suppl 1):9–160 [DOI] [PubMed] [Google Scholar]
- 24.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 2009;339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbanti P, Fofi L, Aurilia C, Egeo G. Dopaminergic symptoms in migraine. Neurol Sci 2013;34(suppl 1):S67–S70 [DOI] [PubMed] [Google Scholar]
- 26.van Hilten JJ. The migraine-dopamine link: do migraine and Parkinson's disease coexist? Clin Neurol Neurosurg 1992;94(suppl):S168–S170 [DOI] [PubMed] [Google Scholar]
- 27.Barbanti P, Fabbrini G, Vanacore N, et al. Dopamine and migraine: does Parkinson's disease modify migraine course? Cephalalgia 2000;20:720–723 [DOI] [PubMed] [Google Scholar]
- 28.Dusek P, Jankovic J, Le W. Iron dysregulation in movement disorders. Neurobiol Dis 2012;46:1–18 [DOI] [PubMed] [Google Scholar]
- 29.Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem 1958;3:41–51 [DOI] [PubMed] [Google Scholar]
- 30.Desseilles M, Dang-Vu T, Schabus M, Sterpenich V, Maquet P, Schwartz S. Neuroimaging insights into the pathophysiology of sleep disorders. Sleep 2008;31:777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics 2007;4:371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology 2001;56:263–265 [DOI] [PubMed] [Google Scholar]
- 33.Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 2003;61:304–309 [DOI] [PubMed] [Google Scholar]
- 34.Jafari S, Etminan M, Aminzadeh F, Samii A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord 2013;28:1222–1229 [DOI] [PubMed] [Google Scholar]
- 35.Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci Biobehav Rev 2009;33:981–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas S, Hoffman JM, Bell KR, Walker W, Dikmen S. Characterization of headache after traumatic brain injury. Cephalalgia 2012;32:600–606 [DOI] [PubMed] [Google Scholar]
- 37.Barbanti P, Fabbrini G. Migraine and the extrapyramidal system. Cephalalgia 2002;22:2–11 [DOI] [PubMed] [Google Scholar]
- 38.de Rijk MC, Tzourio C, Breteler MM, et al. Prevalence of parkinsonism and Parkinson's disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson's disease. J Neurol Neurosurg Psychiatry 1997;62:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couch JR, Lipton RB, Stewart WF, Scher AI. Head or neck injury increases the risk of chronic daily headache: a population-based study. Neurology 2007;69:1169–1177 [DOI] [PubMed] [Google Scholar]
- 40.Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol 2012;69:1245–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


