Abstract
Background
There is limited research on factors that influence the rate of progression in Alzheimer’s Disease (AD). A history of traumatic brain injury (TBI) is associated with an increased risk for AD, but its role on the rate of dementia progression after the onset of AD has not been examined.
Method
A population-based cohort of 325 persons with incident AD were followed for up to 11 years. The sample was 65% female with mean (sd) age of dementia onset = 84.4 (6.4) years. History of TBI was categorized as: number, severity (with or without loss of consciousness), and timing in relation to dementia onset (within 10 years or more than 10 years). Cognition was assessed by the Consortium to Establish a Registry of AD (CERAD) battery and functional ability was assessed by the Clinical Dementia Rating Sum of Boxes (CDR-sb).
Results
In linear mixed models, a history of TBI within 10 years of onset showed faster progression of functional impairment (LR x2 = 10.27, p = 0.006), while those with TBI more than 10 years before dementia onset had higher scores on a measure of list learning (β = 1.61, p = 0.003) and semantic memory (β = 0.75, p = 0.0035).
Conclusion
History of TBI and its recency may be a useful factor to predict functional progression in the course of AD.
Keywords: Traumatic Brain Injury, Dementia Progression, Functional Ability, Alzheimer’s
INTRODUCTION
Individuals over the age of 65 are among the fastest growing segments of the population (Werner, 2011). As a result, rates of AD are expected to increase exponentially. The prevalence of AD in the United States is currently estimated at 5.4 million, with projections to reach nearly 14 million by 2050 (Hebert, et al., 2013). A major concern of the impending rise in the cases of AD is the associated cost of formal and informal care (Coduras, et al., 2010). Estimates for the total cost of care by all payers for individuals with AD increased from 203 billion dollars in 2013 to over 1 trillion dollars in the year 2050 (Alzheimer’s Association, 2013). As expected, highest costs were incurred by those with greater severity of dementia (Herrmann, et al., 2010).
The rate of cognitive and functional decline varies substantially in AD, which likely impacts the number of patients that reach a state of severe disability. An estimate from the population-based Cache County Study reported that approximately 30 to 58% of those with AD decline slowly (Tschanz, et al., 2011). Factors that predict the rate of decline after the onset of AD are not well understood, but if modifiable factors are identified, this may provide a means to reduce the burden and costs of the condition. Several health-related conditions have been examined as predictors of decline in AD including but not limited to cardiovascular (Helzner et al., 2009) or cerebrovascular disease (Helzner et al., 2009; Mielke, et al., 2011). For example, Helzner et al. (2009), followed 156 community dwelling persons with incident AD for a mean of 3.5 years. They reported that higher pre-dementia diagnosis of cholesterol levels (LDL) was associated with greater rates of cognitive decline after controlling for diabetes, HDL cholesterol, age, and education (Helzner, et al., 2009), although there was moderation by Apolipoprotein (APOE) E4 status. Similarly, Mielke and colleagues (2011) found that those with an APOE E4 allele who also had a history of stroke showed greater initial decline than did those with history of stroke and no APOE E4 allele. The rate of progression in the non-APOE E4 carriers, who had a history of stroke, also increased over time.
Traumatic brain injury (TBI) has been examined as a risk factor for AD, but not as a factor that may predict the rate of progression after the onset of dementia. A history of TBI has been associated with a higher risk of developing AD (Jellinger, et al., 2001; Van Den Heuvel, et al., 2007), with greater severity associated with higher risk (Guo, et al., 2000; Jellinger, et al., 2001; Plassman, et al., 2000). In a study of 548 World War II veterans with a history of TBI and 1228 without TBI, Plassman and colleagues (2000) reported that a severe TBI (loss of consciousness or post-traumatic amnesia for more than 24 hours), was associated with 4.5-fold increase in risk for AD, whereas a moderate TBI (loss of consciousness or post-traumatic amnesia between 30 minutes to 24 hours), was associated with a 2-fold increase in risk. A history of TBI has also been associated with an earlier onset of AD (Luukinen et al., 2005). This is illustrated in a study that followed 1283 persons with TBI and that among those who developed AD, more than twice as many people developed AD at a younger age than was expected (Nemetz, et al., 1999). In addition, the age at which a TBI occurs appears to modify the risk of subsequent AD such that the older the age at which TBI occurs, the greater the risk of developing AD (Graves, et al., 1990; Van Duijn, et al., 1992; Mortimer, et al., 1991). In one study, when a TBI occurred later in life (within 30 years of AD diagnosis or the last follow-up visit if not diagnosed) the risk of developing AD was about 5.5 times greater when compared to those who had a TBI 30 or more years before diagnosis or the last follow-up visit (Schofield, et al., 1997). The APOE E4 allele may modify the risk of TBI and AD. One study reported those with a history of TBI who were carriers of the APOE E4 allele had a10-fold increase in risk for AD compared to those with no APOE E4 allele and no history of TBI (Mayeux, et al., 1995).
Although few studies have examined whether a history of TBI affects the rate of progression of dementia, one study from the Cache County population reported an association of history of TBI and neuropsychiatric symptoms in AD. Specifically, those who had a TBI prior to the onset of dementia were almost three times more likely to display the symptom of disinhibition, than those who did not have a history of TBI (Rao, et al., 2010). Data from animal models also suggest a plausible association of TBI with AD progression. For example, in a study of transgenic mice that express Aβ precursor protein, mice receiving either a single or multiple TBIs had increased levels of Aβ compared to the sham control group (Uryu, et al., 2002). Also, mice that experienced multiple TBIs had more cognitive impairment at 16 weeks than did the sham mice and the mice that received a single TBI. Another study reported that among Aβ transgenic mice, those in the TBI group showed a significant reduction in the number of neurons at the site of head trauma at 2, 5, and 8 months after injury (Nakagawa, et al., 1999), suggesting ongoing effects even after the cessation of trauma.
Given the sparse literature on the potential impact of TBI in the clinical expression of dementia after onset, we examined whether a history of TBI predicted the rate of cognitive and functional decline after the onset of AD in the population-based Cache County Study. The information from this study adds to the growing literature on medical comorbidity and the rate of dementia progression, and may be useful for patients, caregivers and health care providers for predicting patient prognosis and planning patient care.
METHOD
Data for this study were from the Cache County Dementia Progression Study (DPS), a longitudinal population-based study that examined factors associated with the development and course of dementia in individuals. The study followed persons with dementia and their caregivers after the individual was identified as having dementia through the Cache County Study on Memory in Aging (CCSMA). The procedures used to identify persons with dementia in the CCSMA will be described briefly below.
Residents of Cache County, Utah, who met the age requirement (>=65 years old) and were residents of the county, were asked to participate in the first wave of the CCSMA. Nearly 90% (5,092) of the 5,657 residents who met these requirements chose to participate. During each of four waves of the study, a screening and assessment protocol was used to assess for dementia status (Breitner, et al., 1999). Brief cognitive screening was conducted using an adapted version of the 100-point 3MS (Tschanz, et al., 2002). Individuals who scored below 60 had a proxy interview to gather more information using the Informant Report of Cognitive Decline in the Elderly (IQCODE; Jorm and Jacomb, 1989). Individuals who screened positive using the 3MS or IQCODE or who were 90 years old or greater completed the Dementia Questionnaire (DQ), which was scored by at least two clinicians (geropsychiatrist and neuropsychologist) who rated whether individuals met criteria for questionable or probable dementia. These individuals were then asked to complete a Clinical Assessment (CA), which was a battery that included a physical examination, neuropsychological testing, and clinical and health interview with a caregiver informant. The information from the CA was reviewed by a geropsychiatrist and neuropsychologist, and preliminary diagnoses of dementia or other cognitive disorders were assigned. Individuals diagnosed with dementia or its prodrome were asked to complete neuroimaging (MRI) and laboratory tests, and a physician examined those individuals with suspected dementia. Diagnoses of dementia were made using the DSM-III-R (APA, 1987) criteria by a panel of experts in neurology, geropsychiatry, neuropsychology, and cognitive neuroscience. AD was diagnosed using NINCDS-ADRDA criteria (McKhann, et al., 1984). The age of dementia onset was estimated by the panel based on a review of the chronology of cognitive and functional impairment as the age when the subject met DSM-III-R criteria for dementia (Breitner, et al., 1999).
Individuals with dementia and their caregivers were asked to participate in the DPS. Home visits were conducted semi-annually by a trained research nurse and neuropsychological technician. Each visit involved assessments of the individual with dementia as well as an interview with the caregiver to obtain additional information about the person with dementia. Relevant information obtained regarding the persons with dementia included current functional status, update of medical and health conditions. All participants with dementia also underwent neuropsychological testing. Study procedures were approved by the Institutional Review Boards of Utah State and the Johns Hopkins Universities. The measures used in the present analyses are described below.
Traumatic Brain Injury Status
Lifetime history of traumatic brain injury was ascertained before the onset of dementia by the subject in the initial CCMS or a knowledgeable informant of the subject. For each incident of head trauma, the participant or informant was queried whether there was a loss of consciousness and if so, its duration, whether the individual sought medical attention, and if there was any period of post-traumatic amnesia. TBI status was represented in three variables: 1) number of TBIs (none, one, two or more), 2) TBI with or without loss of consciousness (LOC; no TBI, TBI without LOC, and TBI with LOC), and 3) TBI history in relation to proximity of the age of onset of dementia (no TBI, TBI that occurred less than or equal (LTE) to 10 years before dementia onset, and TBI that occurred greater than (GT) 10 years before dementia onset). The cut-off of 10 years was selected as a reasonable period of time to include the prodromal period of AD (Amieva, et al., 2008) and therefore to possibly affect the development of dementia and/or its progression.
Cognitive Status
The cognitive abilities of individuals with dementia were assessed using the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) neuropsychological battery (Welsh, et al., 1994) which included measures of episodic and semantic memory, verbal fluency, and constructional praxis. The specific tests included the Mini-Mental State Exam (MMSE), Animal Fluency, Boston Naming Test (BNT, replaced 15-item version with 30-item version), Word List Memory (WLM), Word List Recognition and Recall of Verbal List, and Constructional Praxis. The MMSE is a measure of global cognition including items assessing orientation, memory, language, and constructional ability. The maximum score for this measure is 30 points. The Animal Fluency test assesses initiation, speed, and flexibility in verbal output (no maximum score). The WLM assesses learning of 10 words presented individually on cards over three trials for a total score of 30 points. The individual is also asked to recall these words after a delay (10 point maximum) followed by a recognition trial (20 points maximum). The Constructional Praxis test assesses visual motor integration (11 points maximum). The BNT is a measure of semantic memory and assesses ability to name 30-line drawn objects (30 point maximum). Because the majority of AD participants scored near the floor (0) of the delayed WLM task at baseline, performance on this measure was not examined.
Functional Status
Functional status was assessed using the Clinical Dementia Rating (CDR), a scale that was developed to stage the severity of dementia (Morris, 1993). The CDR assesses six domains of functioning: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. The six categories on the CDR are linked to validated diagnostic criteria for Alzheimer’s disease (Morris, et al., 1988). Each of the six domains is scored individually on a 5-point scale with higher numbers representing greater impairment. For this study, the sub scores on the domains were summed to create a total score across all six domains (Tschanz, et al., 2011).
APOE Genotype
APOE genotype was determined from buccal DNA collected from subjects during the first wave of the CCMSA (Breitner, et al., 1999) and represented in the analyses as presence or absence of the APOE E4 allele.
Analyses
Linear mixed models were used to examine the association of history of TBI (represented by number of TBI, TBI with LOC, and timing of TBI; each modeled separately due to multicollinearity between variables) on the rate of dementia progression on each of the neuropsychological and functional measures over time. This statistical approach allows flexibility in analyses related to varying intervals of follow-up and dropouts in that each participant contributes information prior to being lost to follow-up (Singer and Willett, 2003). Non-linear trajectories of each neuropsychological and functional measure were tested by incorporating a quadratic term for time. Covariates in all models tested were the presence or absence of the APOE E4 allele, family history of dementia and demographic factors of education, gender, and age at dementia onset. We examined the associations between these variables and in the models retained only those that had the strongest association with the outcome. Additionally, interactions between APOE E4 and TBI history were examined if there was sufficient sample size to do so. Each predictor or covariate (and interactions) was examined for statistical significance examining the individual Wald type test and retained if there was significant improvement in model fit between a model with the predictor and a model without the predictor, as determined by -2 loglikelihood chisquare test. Because of the number of statistical analyses, we corrected our experiment-wise p value with a Bonferroni adjustment (.05/7) or p <0.007. Statistical analyses were run using SAS software, version 9.2.
RESULTS
The analysis included 325 participants with incident (recent onset) AD (see Table 1). A majority of the sample was female (65%) and Caucasian (99%). At least one APOE E4 allele was present in 46% of the sample. The number of participants with a family history of dementia was 113 (34.8%). Mean (SD) age of dementia onset was 84.4 (6.4) years. At entry into the study, the mean (SD) MMSE and CDR global score were 21.90 (4.58) and 1.0 (0.57), respectively. Median follow-up time was 1.54 years, and maximum was 11.18 years after the diagnosis of dementia. Follow-up evaluations occurred between every six to 24 months. Table 2 displays follow-up visits by TBI groups. The majority of cases lost to follow-up were due to death and there were no significant differences in follow-up rates between TBI groups (all χ2 > 8.33; all p > 0.988; see Table 2).
Table 1.
Subject Demographic and Baseline Characteristics
| All Participants | No TBI | TBI Number Groups | LOC Groups | TBI Timing Groups | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2+ | No LOC | LOC | < 10 Years | ≥ 10 Years | |||
| N (%) | 325 | 232 (71%) | 70 (22%) | 23 (7%) | 29 (9%) | 62(19%) | 29 (9%) | 64 (20%) |
| APOE E4 (%) | 149 (46%) | 110 (47%) | 26 (37%) | 13 (57%) | 15 (51%) | 24 (38%) | 18 (62%) | 21 (33%) |
| Gender (male %) | 113 (35%) | 73 (31%) | 25 (36%) | 15 (65%) | 8 (21%) | 30 (48%) | 5 (17%) | 35 (55%) |
| Education Mean (SD) | 13.27 (3.01) | 13.31 (2.96) | 13.14 (3.22) | 13.22 (2.92) | 13.14 (2.59) | 13.13 (3.36) | 13.03 (2.60) | 13.22 (3.37) |
| Baseline MMSE Mean (SD) | 21.90 (4.58) | 22.05 (4.55) | 21.32 (4.57) | 22.23 (5.07) | 21.56 (3.87) | 21.47 (5.14) | 19.54 (4.60) | 22.4 (4.48) |
| Baseline CDR-sb Mean (SD) | 5.90 (3.30) | 6.03 (3.37) | 5.60 (3.32) | 5.46 (2.43) | 5.74 (2.54) | 5.48(3.41) | 6.69 (3.29) | 5.05 (2.91) |
| Dementia Onset Age Mean (SD) | 84.40 (6.41) | 84.23 (6.40) | 85.50 (6.54) | 82.83 (5.77) | 85.59 (6.64) | 84.68 (6.34) | 85.66 (6.73) | 84.47 (6.31) |
| Time from Dementia Onset to Baseline Mean (SD) | 1.66 (1.23) | 1.71 (1.25) | 1.50 (1.22) | 1.59 (1.04) | 1.59 (.91) | 1.44 (1.23) | 1.57 (0.84) | 1.50 (1.30) |
Bold numbers are significant at p < 0.05
Table 2.
Completion of Visits by TBI Grouping
| Number of TBI | LOC Group | Timing Group | |||||
|---|---|---|---|---|---|---|---|
| Visit Number |
No TBI | 1 TBI | 2+ TBI | No LOC | LOC | 10 or Fewer | More Than 10 |
| 1 (Baseline) | 232 | 70 | 23 | 31 | 62 | 27 | 66 |
| 2 | 159 (68.5%) | 47 (67.4%) | 16 (69.6%) | 17 (54.8%) | 46 (74.2%) | 14 (51.9%) | 49 (74.2%) |
| 3 | 106 (45.7%) | 32 (45.7%) | 15 (93.8%) | 14 (82.4%) | 33 (71.7%) | 9 (64.3%) | 38 (77.6%) |
| 4 | 89 (38.4%) | 26 (37.1%) | 11 (73.3%) | 11 (78.6%) | 26 (78.8%) | 7 (77.8%) | 30 (78.9%) |
| 5 | 65 (28.0%) | 21 (30.0%) | 8 (72.7%) | 10 (90.9%) | 19 (73.1%) | 6 (85.7%) | 23 (76.7%) |
| 6 | 51 (22.0%) | 18 (25.7%) | 6 (75.0%) | 9 (90.0%) | 15 (78.9%) | 6 (100.0%) | 18 (78.3%) |
| 7 | 41 (17.7%) | 14 (20.0%) | 2 (33.3%) | 6 (66.7%) | 10 (66.7%) | 4 (66.7%) | 12 (66.7%) |
| 8 | 33 (14.2%) | 10 (14.3%) | 2 (100.0%) | 5 (83.3%) | 7 (70.0%) | 3 (75.0%) | 9 (75.0%) |
| 9 | 25 (10.8%) | 6 (8.6%) | 2 (100.0%) | 3 (60.0%) | 5 (71.4%) | 2 (66.7%) | 6 (66.7%) |
| 10 | 14 (6.0%) | 2 (2.9%) | 1 (50.0%) | 1 (33.3%) | 2 (40.0%) | 1 (50.0%) | 2 (33.3%) |
| 11 | 8 (3.4%) | 1 (1.4%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) |
| 12 | 7 (3.0%) | 1 (1.4%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) |
| 13 | 2 (0.9%) | 1 (1.4%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) |
Table 1 also displays participant characteristics for those with TBI. Among the TBI groups, a higher percentage of those with multiple TBIs were males (65%) than those with one TBI (36%; χ2= 6.15, df = 1, p = .013) and no TBIs (31%; χ2= 10.55, df = 1, p = .001). Similarly, TBI with LOC had higher percentage of males (48%; χ2= 6.16, df = 1, p = .013) than those with no TBI. Persons who had TBIs GT10 years before dementia onset were mostly males (55%) than those without a TBI (31%; χ2 = 11.673, df = 1, p = .001) and those who had TBIs LTE 10 years before dementia onset (17%; χ2 = 11.417, df = 1, p = .001). Additionally, fewer persons had an APOE E4 allele among those who had TBIs GT 10 years before dementia onset (33%) than those with no TBI (47%; χ2 = 4.355, df = 1, p = .037) and those who had TBIs LTE 10 years before dementia onset (62%; χ2 = 7.015, df = 1, p = .008). Persons with a history of TBI LTE 10 years of dementia onset scored on average, significantly worse on the MMSE. There were no other significant differences between groups.
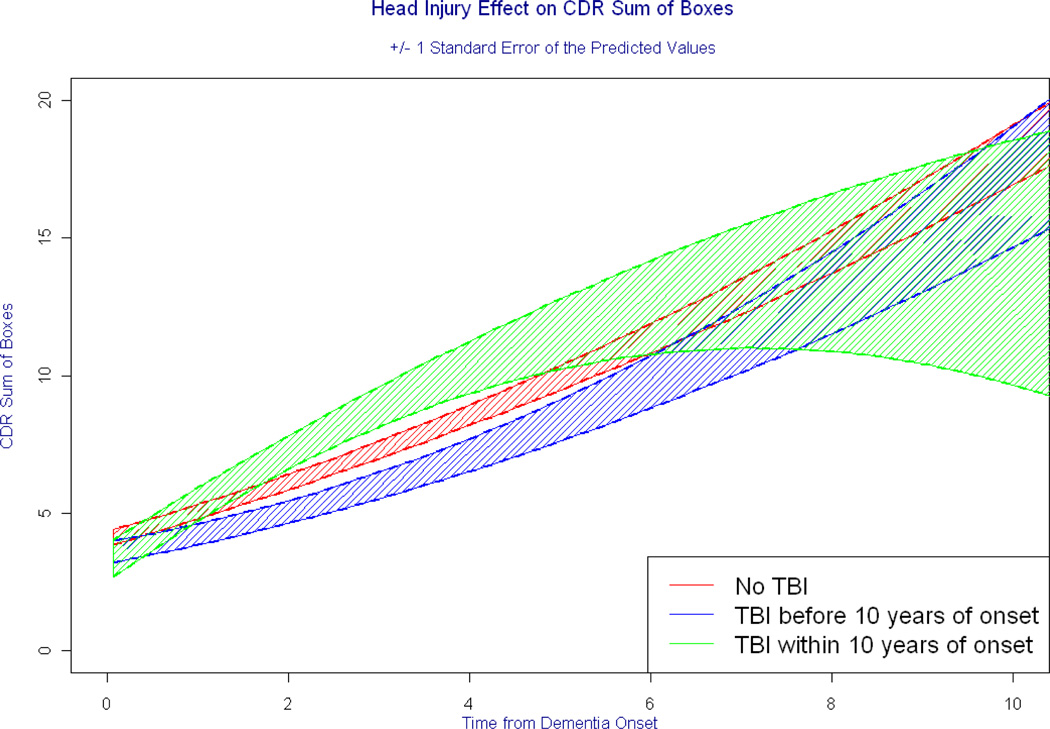
Neither the number of TBI nor history of TBI with LOC was associated with any of the measures of functional or neuropsychological outcomes in AD (all p-values > 0.0075; see Table 3). Due to the sparse numbers of individuals with APOE E4 among the TBI groups, interactions between TBI history and APOE genotype in these analyses were not examined. Timing of TBI was significantly associated with rate of functional impairment, specifically, persons who had experienced a TBI LTE 10 years of dementia onset showed more rapid increase in CDR-sb (LR x2= 10.27, df = 2, p = 0.006) compared to those without a history of TBI (see Table 4). Figure 1 depicts the differences in CDR-sb over time among the TBI groups, and the estimated mean CDR-sb provided in Table 5 increased over time. Notably, as shown in Figure 1, approximately six years after dementia onset, there was much greater variability in the rates of progression. Model estimates at these time points may not be reliable, owing to the sparse surviving participants in the TBI groups. In models predicting neuropsychological tests, persons with a TBI GT 10 years before dementia onset had higher baseline scores on the BNT (β = 0.75, p = 0.0035) and WLM learning (β = 1.61, p = 0.003). Interactions between the timing of TBI and the APOE E4 allele were not significant in predicting any of the neuropsychological or functional outcomes.
Table 3.
Model Results of TBI Group on Neuropsychological and Functional Outcomes
| Grouping | MMSE | CDR | AF | WLM L | WLM R | BNT | Praxis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | β | p | β | p | β | p | ||
| Number of TBI | None | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1 | .731 | .371 | −.428 | .442 | 1.16 | .912 | .479 | .585 | .082 | .912 | 1.363 | .326 | .437 | .171 | |
| 2+ | .110 | .827 | −.437 | .212 | 1.63 | .067 | .586 | .296 | .858 | .067 | .371 | .682 | −.083 | .677 | |
| TBI & LOC | No TBI | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| TBI;no LOC | .003 | .240 | −.402 | .265 | .784 | .105 | .636 | .273 | .784 | .105 | .014 | .811 | .050 | .240 | |
| TBI;yes LOC | .027 | .452 | −.560 | .329 | .527 | .479 | .411 | .637 | .527 | .479 | .653 | .652 | .145 | .452 | |
| TBI Timing | None | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| TBI 10 or fewer | .239 | .754 | * | * | .891 | .098 | * | * | .742 | .095 | * | * | .069 | .725 | |
| TBI 11 or more | 1.005 | .465 | * | * | .766 | .316 | * | * | .550 | .146 | * | * | .021 | .945 | |
(- represent the reference group. * represents a significant finding that is presented in the manuscript. AF = Animal Fluency, WLM L = Word List Memory Learning, WLM R = Word List Memory Recognition, BNT = Boston Naming Test)
Table 4.
Linear Mixed Models for CDR-sb
| Variable | Estimate | Standard Error |
P Value |
|---|---|---|---|
| Intercept | −1.89 | 1.94 | 0.33 |
| Time | 0.79 | 0.16 | <.0001 |
| TBI GT 10 | −0.69 | 0.6 | 0.25 |
| TBI LTE 10 | −1.24 | 1.01 | 0.22 |
| TBI None | |||
| Time * TBI GT 10 | −0.23 | 0.36 | 0.53 |
| Time * TBI LTE 10 | 1.47 | 0.63 | 0.02 |
| Time * TBI None | |||
| Time2 | 0.08 | 0.02 | <.0001 |
| Time2 * TBI GT 10 | 0.01 | 0.04 | 0.82 |
| Time2 * TBI LTE 10 | −0.18 | 0.08 | 0.03 |
| Time2 * TBI None | |||
| ApoE4 Absent | 0.2 | 0.28 | 0.49 |
| ApoE4 Present | |||
| Onset Age | 0.07 | 0.02 | 0.002 |
Figure 1. Timing of TBI and Progression of Functional Impairment in AD.
Each curve represents the predicted rate of progression on a measure of functional impairment. The shaded regions represent variability in the predicted scores that are a result of error. Note that approximately six years after dementia onset, rate of progression is substantially more variable among those with TBI LTE 10 years of dementia onset. Model results at these latter years are of uncertain reliability owing to the limited numbers of surviving participants among those with a TBI history.
Table 5.
Estimates of Mean CDR-sb Scores from Linear Mixed Models at Selected time points
| TBI Groups | Years Post Dementia Onset | ||
|---|---|---|---|
| 1 | 3 | 5 | |
| NO TBI | 5.09 | 7.31 | 10.17 |
| TBI GT 10 | 4.18 | 6.02 | 8.58 |
| TBI LTE 10 | 5.14 | 8.86 | 11.78 |
DISCUSSION
In this study, we report a significant effect of the timing of TBI in relation to the rate of functional impairment in AD, extending our prior work in identifying medical factors that influence the rate of cognitive and functional decline after the onset of dementia (Mielke, et al., 2007; Leoutsakos 2012).
There are very few published studies that report on the association between TBI and rate of progression in AD, with the majority of studies examining TBI and risk of developing AD (Jellinger, et al., 2001; Plassman, et al., 2000; Van Den Heuvel, et al., 2007). A prior report in Cache County found that history of TBI was associated with increased risk of disinhibition after the onset of AD (Rao, et al., 2010). We now add that there is risk of greater functional decline in AD, at least for those with a history of TBI that occurred relatively close in time to the onset of dementia. While the underlying mechanism is unclear, some data from animal studies indicate greater Aβ deposition in transgenic mice exposed to head trauma (Uryu, et al., 2002). We did not see any association of functional or neuropsychological impairment with number or severity of TBI (loss of consciousness). However, limited sample sizes may have affected our statistical power to detect associations. An unexpected finding was the higher overall scores on measures of semantic memory and list learning in those with a TBI history beyond ten years of dementia onset, although there was no association with rate of progression. TBIs that occur earlier in life may have less residual impact and effects for neurodegenerative diseases due to the brain’s ability to recover from injury. One may speculate also that differential survival amongst persons with TBI may underlie this result.
The strengths of this study include the population-based sample, the assessment of TBI with respect to number, indicator of severity (loss of consciousness) and timing with respect to onset of dementia, high participation rates (Tschanz, et al., 2011), and assessment of functional and cognitive outcomes. Limitations include small sample of persons with TBI and large number of statistical tests that may increase Type I error, although results remained significant with the Bonferroni adjustment. Additionally, TBI was based on retrospective recall and TBIs that occurred after the onset of dementia were not examined. Of the latter, only 20 (6.2%) participants had experienced one or more TBI’s after dementia onset, the majority (85%) of which were in the baseline “no-TBI” group. The largely Caucasian sample may limit generalizability to other populations.
In summary, we report that TBIs that occur in late-life are predictive of more rapid progression of functional impairment after the onset of AD. This information may be useful for families and health care providers in predicting prognosis after the onset of dementia, and future work should examine whether TBI history is associated with more rapid onset of severe disability in AD. To the extent that TBIs may be preventable in late life, particularly those associated with falls, preventive measures such as the installment of safety bars and other supports are warranted for older adults with balance or mobility problems.
Acknowledgements
This project was supported by NIA grants R01AG21136 and R01AG11380. The authors are indebted to Dr. Ronald Munger for his unqualified support of the DPS. We also acknowledge the contributions of the following individuals whose activities have helped to ensure the success of the project: John C.S. Breitner, M.D., M.P.H., Cara Brewer, B.A., Tony Calvert, R.N., B.A., Michelle Carlson, Ph.D., Kimberly Graham, B.A., Robert C. Green, M.D., M.P.H., Hochang Ben Lee, M.D., Jeanne-Marie Leoutsakos, Ph.D., Carol Leslie, M.S., Lawrence S. Mayer, Ph.D., Michelle M. Mielke, Ph.D., Chiadi U. Onyike, M.D., Roxane Pfister, M.S., Georgiann Sanborn, M.S., Nancy Sassano, Ph.D., Sarah Schwartz, M.S, Ingmar Skoog, M.D., Martin Steinberg, M.D., Katherine Treiber, Ph.D., Yorghos Tripodis, Ph.D., Kathleen A. Welsh-Bohmer, Ph.D., Heidi Wengreen, Ph.D., RD, James Wyatt, and Peter P. Zandi, Ph.D., M.P.H. Finally, we thank the participants and their families for their participation and support.
Footnotes
Presented in preliminary form at the Alzheimer’s Association International Conference in Vancouver, BC, 2012 and the International Neuropsychological Society Conference in Boston, MA in 2011.
Conflict of Interest
none
Description of Author’s Roles
Mac Gilbert, M.S.: Participated in data collection and analysis and manuscript preparation and submission.
Christine Snyder, M.S.: Participated in data collection and analysis and manuscript preparation.
Chris Corcoran, Sc.D.: Participated in data analysis and manuscript preparation.
Maria C. Norton, Ph.D.: Participated in development of the study and manuscript preparation.
Constantine G. Lyketsos, M.D., MHS.: Participated in development of the study, running of the study, and manuscript preparation.
JoAnn T. Tschanz, Ph.D.: Participated in development of the study, managed and participated in the running of the study, development of research questions, data analysis, and manuscript preparation.
References
- Amieva H, et al. Prodromal Alzheimer’s disease: Successive emergence of the clinical symptoms. Annals of Neurology. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- Breitner JC, et al. APOE-epsilon4 count predicts age when Prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Coduras A, et al. Prospective one-year cost of illness study in a cohort of patients with dementia of Alzheimer’s disease type in Spain: the ECO study. Journal of Alzheimers Disease. 2010;19:601–615. doi: 10.3233/JAD-2010-1258. [DOI] [PubMed] [Google Scholar]
- Graves AB, et al. The association between head trauma and Alzheimer’s disease. American Journal of Epidemiology. 1990;131:491–501. doi: 10.1093/oxfordjournals.aje.a115523. [DOI] [PubMed] [Google Scholar]
- Guo Z, et al. Head injury and the risk fo AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzner EP, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Archives of Neurology. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann N, Tam DY, Balshaw R, Sambrook R, Lesnikova N, Lanctot KL. The relation between disease severity and cost of caring for patients with Alzheimer disease in Canada. Canadian Journal of Psychiatry. 2010;55:768–775. doi: 10.1177/070674371005501204. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Paulus W, Wrocklage C, Litvan I. Traumatic brain injury as a risk factor for Alzheimer disease. Comparison of two retrospective autopsy cohorts with evaluation of ApoE genotype. BioMed Centeal Neurology. 2001;1:3. doi: 10.1186/1471-2377-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The informant questionnaire on cognitive decline in the elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychology and Medicine. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Luukinen H, et al. Fall-related brain injuries and the risk of dementia in elderly people: a population-based study. European Journal of Neurology. 2005;12:86–92. doi: 10.1111/j.1468-1331.2004.00953.x. [DOI] [PubMed] [Google Scholar]
- Mayeux R, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mielke MM, et al. Interaction between vascular factors and the APOE e4 allele in predicting rate of progression in Alzheimer’s disease. Journal of Alzheimers Disease. 2011;26:127–134. doi: 10.3233/JAD-2011-110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules, 1993. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, jr, Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer’s disease. Annals of Neurology. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, et al. Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case control studies. EURODEM risk factors research group. International Journal of Epidemiology. 1991;20:S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, et al. Traumatic brain injury in young, amyloid-beta peptide over expressing transgenic mice induces marked ipsilateral hippocampal atrophy and diminished Abeta deposition during aging. Journal of Comparative Neurology. 1999;411:390–398. [PubMed] [Google Scholar]
- Nemetz PN, et al. Traumatic brain injury and time to onset of Alzheimer’s disease : a population-based study. American journal of Epidemiology. 1999;149:32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- Plassman BL, et al. Documented head injury in early adulthood and risk of Alzhereimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- Rao V, et al. Neuropsychiatric symptoms in dementia patients with and without a history of traumatic brain injury. Journal of Neuropsychiatry and Clinical Neuroscience. 2010;2:166–172. doi: 10.1176/appi.neuropsych.22.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PW, et al. Alzheimer’s disease after remote head injury: an incidence study. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;62:119–124. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Methods for Studying Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC. An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: the Cache County study. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2002;15:28–38. [PubMed] [Google Scholar]
- Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer Dementia: the Cache County dementia progression study. American Journal of Geriatric Psychiatry. 2011;19:532–542. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, et al. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. Journal of Neuroscience. 2002;22:446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: a review. Progress in Brain Research. 2007;161:303–316. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- Van Duijn CM, et al. Head trauma and the risk of Alzheimer’s disease. American Journal of Epidemiology. 1992;135:775–782. doi: 10.1093/oxfordjournals.aje.a116364. [DOI] [PubMed] [Google Scholar]
- Welsh KA, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- Werner CA. The older population: 2010 Census briefs. U.S. Census Bureau; 2011. C2010BR-09. [Google Scholar]