Abstract
Introduction
To improve outcomes of patients with Richter's syndrome (RS) and relapsed/refractory chronic lymphocytic leukemia (CLL), we modified the oxaliplatin and cytarabine doses of the oxaliplatin, fludarabine, cytarabine, and rituximab (OFAR1) regimen for this phase I-II study (OFAR2).
Patients and Methods
OFAR2 consisted of oxaliplatin at 30 mg/m2 on days (D) 1–4, fludarabine at 30 mg/m2, cytarabine at 0.5 g/m2, rituximab at 375 mg/m2 (D3), and pelfigrastim at 6 mg (D6). Fludarabine and cytarabine were given on D2–3 (cohort 1), D2–4 (cohort 2), or D2–5 (cohort 3) every 4 weeks. Phase II followed the "3+3" design of phase I.
Results
The 102 patients (CLL, 67; RS, 35) treated had heavily pretreated, high-risk disease. Twelve patients were treated in phase I; cohort 2 was the phase II recommended dose. The most common toxicities were hematologic. Response rates (phase II) were 38.7% for RS (CR, 6.5%) and 50.8% for relapsed/refractory CLL (CR, 4.6%). The median survival durations were 6.6 (RS) and 20.6 (CLL) months. Among 9 patients who underwent allogeneic stem cell transplantation (SCT) as post-remission therapy, none has died (median follow-up, 15.9 months).
Conclusion
OFAR2 had significant antileukemic activity in RS and relapsed/refractory CLL. Patients undergoing SCT as post-remission therapy had favorable outcomes
Keywords: Refractory CLL, OFAR, Richter syndrome, therapy, SCT
Introduction
The clinical outcomes of patients with Richter’s syndrome (RS) and aggressive, relapsed chronic lymphocytic leukemia (CLL) are generally poor. Even if these diseases respond to initial therapy, they eventually relapse and patients die from their disease.(1, 2) However, the recent identification of prognostic factors and the development of newer therapies promise to improve treatment outcomes for patients with these diseases.(3) Platinum compounds, rituximab, fludarabine, and cytarabine are active agents against lymphoproliferative disorders, and platinum-based chemotherapy has shown significant activity in relapsed/refractory lymphoproliferative disorders.(4–10) Oxaliplatin is a third-generation platinum compound that is comprised of an organoplatinum complex in which the platinum atom is complexed with a 1,2diaminocyclohexane carrier ligand and an oxalate ligand.(11, 12) It is associated with more favorable renal and auditory toxicity profiles than cisplatin. (12, 13)The rationale for combining oxaliplatin with fludarabine and cytarabine is based on preclinical data demonstrating synergistic cytotoxicity between these nucleoside analogs and cisplatin.(14–17) In the clinic, sequential administration of fludarabine followed by cytarabine increased the leukemic cellular concentrations of the active cytarabine triphosphate by 40% to 200%(18). The rationale for the design of the OFAR regimens was based on the hypothesis that fludarabine and cytarabine combined would inhibit DNA excision repair of the oxaliplatin adducts, resulting in synergistic cytotoxicity in RS and CLL.(19)
The results of our first phase I-II clinical trial of oxaliplatin, fludarabine, cytarabine, and rituximab (OFAR1) were encouraging.(19) The OFAR1 regimen (phase II) consisted of oxaliplatin at 25 mg/m2/day (days 1 to 4), fludarabine at 30 mg/m2 (days 2–3), cytarabine at 1 g/m2 (days 2–3), and rituximab at 375 mg/m2 (day 3). OFAR1 resulted in response rates of 50% and 36% in patients with RS and fludarabine-refractory CLL, respectively, but it was associated with myelosuppression.(19)
The OFAR1 Phase I-II trial evaluated three doses of oxaliplatin. While the combination was effective, the myelosuppression that was observed was felt to be due to cytarabine. Thus, in OFAR2, the cytarabine dose was reduced from 1 to 0.5 g/m2. Because of this decrease, it was judged that increasing the dose of oxaliplatin from 25 to 30 mg/m2 would be tolerable and could contribute to additional antileukemic activity. The rationale for using oxaliplatin with fludarabine in CLL was based on previously published data demonstrating that fludarabine increases oxaliplatin cytotoxicity in normal and CLL lymphocyties by suppressing interstrand DNA crosslink removal (20)
To increase the antileukemic activity and decrease the myelosuppression of this regimen, we designed a modified phase I-II study of OFAR1 (OFAR2). In OFAR2, the starting dose of oxaliplatin was increased from 25 mg/m2 to 30 mg/m2 and the daily dose of cytarabine was decreased from 1 g/m2 to 0.5 g/m2. In the phase 1 portion of OFAR2, three dose-escalation cohorts were evaluated (3+3 design) byincreasing the number of days of cytarabine and fludarabine treatment (2, 3, or 4 days).
The objective of the phase I portion of OFAR2 was to determine the Phase II recommended dose of fludarabine and cytarabine in combination with oxaliplatin and rituximab. The objectives of phase II were to assess the response rates, to define the safety and toxicity profile, and to determine the failure-free and overall survival durations.
Patients and Methods
Patients
Eligible patients were at least 18 years old and had histologically or cytologically confirmed Richter’s transformation, prolymphocytic leukemia, or aggressive, relapsed, or refractory B-cell chronic lymphocytic leukemia. Other eligibility criteria were performance status 0–2 (Zubrod scale); serum creatinine ≤2 mg/dL or calculated creatinine clearance >50 mL/min; bilirubin ≤ 2 mg/dL; and alanine aminotransferase or aspartate aminotransferase < 2.5 × the upper limit of normal (ULN) for the reference laboratory, unless due to leukemia or congenital hemolytic disorder (for bilirubin); and platelet counts > 20 × 109/L, unless due to disease involvement or autoimmune disorders. Patients were excluded if they were pregnant; had a history of oxaliplatin, fludarabine, cytarabine, or rituximab intolerance; had received chemotherapy and/or radiation therapy in the preceding 4 weeks; or had any other medical condition deemed by the investigator to be likely to interfere with their ability to give informed consent or cooperate/participate in the study or to interfere with the interpretation of the results. Signed informed consent forms explaining the investigational nature of the trial were obtained from all patients in accordance with institutional policy. The phase I portion of the trial was conducted at MD Anderson and the phase II portion included MD Anderson and other members of the CLL Research Consortium: Moore’s Cancer Center, the University of California, San Diego and Ohio State University Medical Center. The protocol was approved by the institutional review boards of the participating centers. The trial was conducted in accordance with the Declaration of Helsinki.
Data were analyzed by biostastician (Sijin Wen, PhD) and all authors had access to primary clinical trial data. The study was registered in www.clinicaltrials.gov (registration number, NCT00472849).
Treatment
In the phase I portion of this study, oxaliplatin was administered intravenously (IV) at 30 mg/m2 daily on days 1 through 4 (2-hour infusion). Rituximab at 375 mg/m2 IV (4- to 6-hour infusion) was administered on day 3 of the first cycle and day 1 of subsequent cycles. Pegfilgrastim at 6 mg subcutaneously was administered on day 6. Three dose-escalation cohorts were evaluated using a “3+3” design that increased the number of days of cytarabine and fludarabine treatment from 2 days (days 2 to 3; cohort 1) to 3 days (days 2 to 4; cohort 2) to 4 days (days 2 to 5; cohort 3). The daily dose of fludarabine was 30 mg/m2 IV 30-minute infusion, starting after oxaliplatin completion), and the daily dose of cytarabine was 0.5 g/m2 IV (2-hour infusion, starting approximately 4 hours after fludarabine was started).
Patients were to receive a subsequent cycle of treatment no less than 4 weeks from the initiation of the previous cycle if no drug-related grade 3 or 4 non-hematologic life-threatening adverse events had occurred, and drug-related non-hematologic toxicity had resolved to baseline or < grade 2.
Cycles were repeated every 4 weeks until unacceptable toxicity, progressive disease, or a maximum of 6 cycles. Patients received antiemetic medications and oral prophylaxis for tumor lysis syndrome (allopurinol, 300 mg/day), Pneumonocystisjiroveci (trimethoprim, 160 mg/sulfamethoxazole, 800 mg, three times/week), herpes zoster virus (valacyclovir, 500 mg/day), and fungal (fluconazole, 200 mg/day, or itraconazole or voriconazole, 200 mg twice a day) and bacterial (levofloxacin, 500 mg/day) infections.
Patient monitoring
Patients were monitored with a physical examination (including vital signs and performance status) prior to initiation of therapy, weekly, and on day 1 of each cycle. Complete blood counts, differential, platelets, chemistry and electrolytes were monitored at least weekly. Response was assessed approximately four weeks after the start of the third cycle, after the sixth cycle, and afterwards every three to six cycles.
Endpoints and Statistical Considerations
In the phase I portion of this study, toxicity was measured in the dose-escalation cohorts (cohorts 1–3) to determine the phase II recommended dose. Toxicities were graded according to the National Cancer Institute (NCI) Common Terminology Criteria of Adverse Events, version 3.0. Dose-limiting toxicity (DLT) was defined as any treatment-related non-hematological toxicity involving a major organ system (brain, heart, kidney, liver, lung) ≥ grade 3 on the NCI toxicity scale or ≥ grade 3 neutropenia or thrombocytopenia lasting for ≥6 weeks after the last dose of therapy.
For patients with RS, the response and endpoint assessments conformed to the published International Workshop response criteria for non-Hodgkin’s lymphoma.(21) In fludarabine-refractory CLL, the response criteria were those defined by the NCI–sponsored Working Group. Computed tomography (CT) scans were not required for the evaluation of response in patients with CLL.(22) Response was evaluated on an intent-to-treat basis. Survival was measured from the start of treatment until death from any cause or last follow-up. Failure-free survival (FFS) was measured from the start of treatment on protocol until progression, relapse, or death, whichever came first. In the FFS analysis, patients who underwent stem cell transplantation (SCT) as post-remission therapy or treatment for the elimination of minimal residual disease were censored at that time. Survival for patients who underwent SCT was calculated from the time of SCT. The χ2 test was used to assess the independence of two categoric variables. Survival and FFS were estimated using the Kaplan-Meier method. To test the association between variables and survival or FFS, the two-sided log-rank test (or the Breslow-Gehan-Wilcoxon test, if the survival curves overlapped) was used. A multivariate Cox regression model was used to assess simultaneously the effect of two or more variables on survival and to examine the association between treatment and survival after adjusting for other factors.
To put the results for RS in perspective, OFAR2 was compared with OFAR1. OFAR1 and OFAR2 results were compared with results for Hyper-CVAD (fractionated cyclophosphamide, vincristine, liposomal daunorubicin, and dexamethasone)(23) plus rituximab and R-CHOP(24) (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) from the database of the Department of Leukemia at MD Anderson. Similarly, in relapsed/refractory CLL, OFAR2 was compared with CFAR(25) (cyclophosphamide, fludarabine, alemtuzumab, and rituximab) and FCR(26) (fludarabine, cyclophosphamide, and rituximab. P-values were derived from two-sided tests. P<0.05 was considered to be statistically significant. Statistical analyses were performed using SAS 8.0 (SAS Institute, Cary, NC) and S-Plus 2000 (Statistical Sciences, Seattle, WA).
RESULTS
Patient Characteristics
From June 2007 to June 2010, 102 patients were enrolled in this phase I–II clinical trial (phase I, n=12; phase II, n=90). Thirty-five patients had RS and 67 had relapsed/refractory CLL. Eighty-nine patients were treated at MD Anderson, eight were treated at UCSD, and five at OSU. The pretreatment characteristics according to diagnosis are shown in Table 1. The median age of the patients was 63 years (range, 40 – 81); 73% had Rai stage III–IV disease, 72% had β2-microglobulin ≥4 mg/L, and 17% had a performance status of 2. The median number of prior therapies were 3 (range, 0–9) for RS and 3 (range, 1 to 6). Many patients had adverse biologic and genetic features, including unmutatedIgVH (84%, 62/74), ZAP-70 expression (76%, 66/87), and 17p (37%, 31/84) or 11q (13%, 11/84) deletion.
Table 1.
Pretreatment characteristics by diagnosis (n=102)
| RS (%) | Relapsed/refractor y CLL (%) |
Total number of patients (%) |
|
|---|---|---|---|
| n=35 | n=67 | n=102 | |
| Age ≥ 60 yrs | 25 (71) | 41 (61) | 66 (65) |
| Zubrod PS = 2 | 9 (26) | 8 (12) | 17 (17) |
| Rai stage > II | 26 (74) | 48 (72) | 74 (73) |
| No. of prior therapies ≥ 3 | 19 (54) | 48 (72) | 67 (66) |
| B2-microglobulin ≥ 4 mg/L | 22 (30) | 51 (70) | 73 (72) |
| ALC > 30 × 109/L | 4 (13) | 26 (87) | 30 (29) |
| PLT < 100 × 109/L | 16 (46) | 41 (61) | 57 (56) |
| Albumin < 3.5 g/dL | 15 (43) | 6 (9) | 21 (21) |
| LDH > 1.5 × ULN | 11 (31) | 17 (27) | 28 (27) |
| Unmutated IgVH | 17 (85) | 45 (83) | 62 (61) |
| ZAP-70 positive* | 19 (73) | 47 (77) | 66 (65) |
| Fludarabine-refractory | 12 (34) | 32 (48) | 44 (43) |
| FISH(†) | |||
| 17p del | 6 (23) | 25 (44) | 31 (30) |
| 11q del | 3 (12) | 8 (14) | 11 (11) |
| Trisomy 12 | 5 (19) | 10 (18) | 15 (15) |
| del 13 | 5 (19) | 10 (18) | 15 (15) |
| Negative | 7 (27) | 4 (7) | 11 (11) |
Abbreviations: RS, Richter’s syndrome; CLL, chronic lymphocytic leukemia; PS, performance status; ULN, upper limit of normal; LDH, lactate dehydrogenase; ALC, absolute lymphocyte count; IgVH, immunoglobulin heavy-chain variable region gene rearrangement; FISH, fluorescence in situ hybridization; del, deletion.
By immunohistochemistry.36
No. of patients/total no. of patients with available data.
The interval in days between cycles of OFAR2 in patients with relapsed/refractory CLL and RS and treatment delays are shown in Supplemental file.
Toxicity
Adverse events are described in Table 2. In the phase I portion of the study, toxicity results were as follows: In the first cohort (2-day schedule), one of three patients developed grade 4 neutropenia and all three patients developed grade 3 thrombocytopenia, but no DLT was noted. In the second cohort, three of three patients developed grade 4 neutropenia and grade 4 thrombocytopenia; no DLT was noted. In the third cohort (4-day schedule), three of three patients developed grade 4 neutropenia and grade 4 thrombocytopenia; of these three patients, one developed grade 4 diarrhea and another developed grade 4 neutropenic sepsis. Therefore, the 4-day schedule was considered “dose-limiting”. Subsequently, three additional patients were treated on the 3-day schedule, and one patient experienced grade 3 diarrhea (DLT) (all three patients experienced grade 4 neutropenia and grade 4 thrombocytopenia). None of the patients treated on the 3-day schedule had prolonged (> 28 days) neutropenia or thrombocytopenia. Because one of six patients treated on the 3-day schedule developed a DLT, the 3-day schedule was chosen as the phase II recommended dose.
Table 2.
Grade 3–4 adverse effects
| Phase I | Phase II | ||||
|---|---|---|---|---|---|
| Dose level 1 |
Dose level 2 |
Dose level 3 |
RS | CLL | |
| No. of patients (%) | 3 (25) | 6 (50) | 3 (25) | 30 (33) | 60 (67) |
| Hematologic | |||||
| Neutropenia | 2 (67) | 5 (83) | 3 (100) | 25 (89) | 46 (77) |
| Anemia | 1 (33) | 3 (50) | 1 (33) | 15 (50) | 29 (48) |
| Thrombocytopenia | 3 (100) | 6 (100) | 3 (100) | 23 (77) | 49 (82) |
| Non-hematologic | |||||
| Neutropenic fever | 2(33) | 1(33) | 6 (20) | 6 (10) | |
| Non-neutropenic fever | 3 (5) | ||||
| Dehydration | 1 (3) | 6 (10) | |||
| Fatigue | 1 (2) | ||||
| Infection | 3(50) | 1(33) | 5 (17) | 12 (20) |
|
| Diarrhea | 1(17) | 1 (3) | 2 (3) | ||
| Nausea/vomiting | 2 (6) | 4 (6) | |||
| Hemorrhage | 1 (3) | 2 (3) | |||
| Dyspnea | 3 (9) | ||||
| ARDS | 1 (2) | ||||
| Airway obstruction | 1 (3) | ||||
| Atrial fibrillation | 1 (3) | ||||
| Hypotension | 1 (3) | 1 (2) | |||
| Acute renal failure | 1 (3) | ||||
| Altered mental status | 1 (3) | ||||
| Syncope | 1 (3) | ||||
| Tumor lysis syndrome | 2 (3) | ||||
| Multiorgan failure | 1 (2) | ||||
| Hyperglycemia | 1 (2) | ||||
Abbreviations: ARDS = Adult Respiratory Distress Syndrome
In phase II, the median number of cycles administered was two for RS and three for relapsed/refractory CLL. Grade 3–4 neutropenia, thrombocytopenia, and anemia were noted in79%, 78%, and 49% of patients, respectively, and grade 3–4 infections occurred in 19% of patients.
Of 35 patients with RS, patients came off-study for the following reasons: progressive disease/no response (n=21), treatment for minimal residual disease (n=2), SCT (n=3), death (n=3), received treatment at home (n=1), prolonged cytopenia (n=1), consent withdrawal (n=1), unknown (n=3).
Response
Response by diagnosis and cohort is shown in Table 2. When all cohorts were included in the analysis, the overall response rate (ORR) was 42.9% for RS and 50.7% in patients with relapsed/refractory CLL (Table 3). When the analysis was limited to the 96 patients (RS, n=31; CLL, n=65) who were treated at the phase II recommended dose, the ORRs were 38.7% in RS [complete response (CR), 6.5%] and 50.8% (CR, 4.6%) in relapsed/refractory CLL (Table 4). In patients with 17p deletion or 11q deletion, the ORRs were 31.0% (9/29) and 54.5% (6/11), respectively (Table 5).
Table 3.
Response in patients with Richter’s syndrome and refractory/relapsed CLL
| Richter’s syndrome | Refractory/Relapsed CLL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | No. of pts. |
CR (%) | nPR | PR | OR | No. of pts. |
CR | nPR | PR | OR |
| 1 | 3 | 1 (33.3) | 1 (33.3) | - | 2 (66.7) | - | - | - | - | - |
| 2 | 31 | 2 (6.5) | - | 10 (32.2) | 12 (38.7) | 65 | 3 (4.6) | 9 (13.9) | 21 (32.3) | 33 (50.8) |
| 3 | 1 | - | - | 1 (100) | 1 (100) | 2 | 1 (50) | - | - | 1 (50) |
| Total | 35 | 3 (8.6) | 1 (2.9) | 11 (31.4) | 15 (42.9) | 67 | 4 (6.0) | 9 (13.4) | 21 (31.3) | 34 (50.7) |
Abbreviations: CLL, chronic lymphocytic leukemia; CR, complete remission; nPR, nodular partial remission; PR, partial remission; OR, overall response.
Table 4.
Response in 96 patients treated at the Phase II recommended dose
| RS (N=31) | Refractory CLL (N= 65) | |||
|---|---|---|---|---|
| No. of patients | % | No. of patients | % | |
| CR | 2 | 6.5 | 3 | 4.6 |
| nPR | 0 | 0 | 9 | 13.9 |
| PR | 10 | 32.2 | 21 | 32.3 |
| Overall response | 12 | 38.7 | 33 | 50.8 |
Abbreviations: CLL= chronic lymphocytic leukemia; CR = complete response; nPR = nodular partial response; PR = partial response; RS = Richter syndrome
Table 5.
Response by molecular aberrations* and diagnosis
| Molecular aberrations |
RS | CLL | Total | |||
|---|---|---|---|---|---|---|
| Patients tested |
OR | Patients tested |
OR | Patients tested |
OR (%) | |
| 17p deletion | 5 | 1 | 24 | 8 | 29 | 9 (31.0) |
| 11q deletion | 3 | 1 | 8 | 5 | 11 | 6 (54.5) |
| 13q deletion | 5 | 2 | 10 | 5 | 15 | 7 (46.7) |
| Trisomy 12 | 4 | 2 | 10 | 8 | 14 | 10 (71.4) |
| No findings | 6 | 3 | 3 | 1 | 9 | 4 (44.4) |
| Not done | 8 | 3 | 10 | 6 | 18 | 9 (50.0) |
As measured by fluorescence in situ hybridization analysis
The response (CR/PR/CRn) rate in patients with fludarabine-refractory CLL was 43% vs. 46% in patients who were not fludarabine-refractory (p = 0.84, Fisher’s test).
Survival
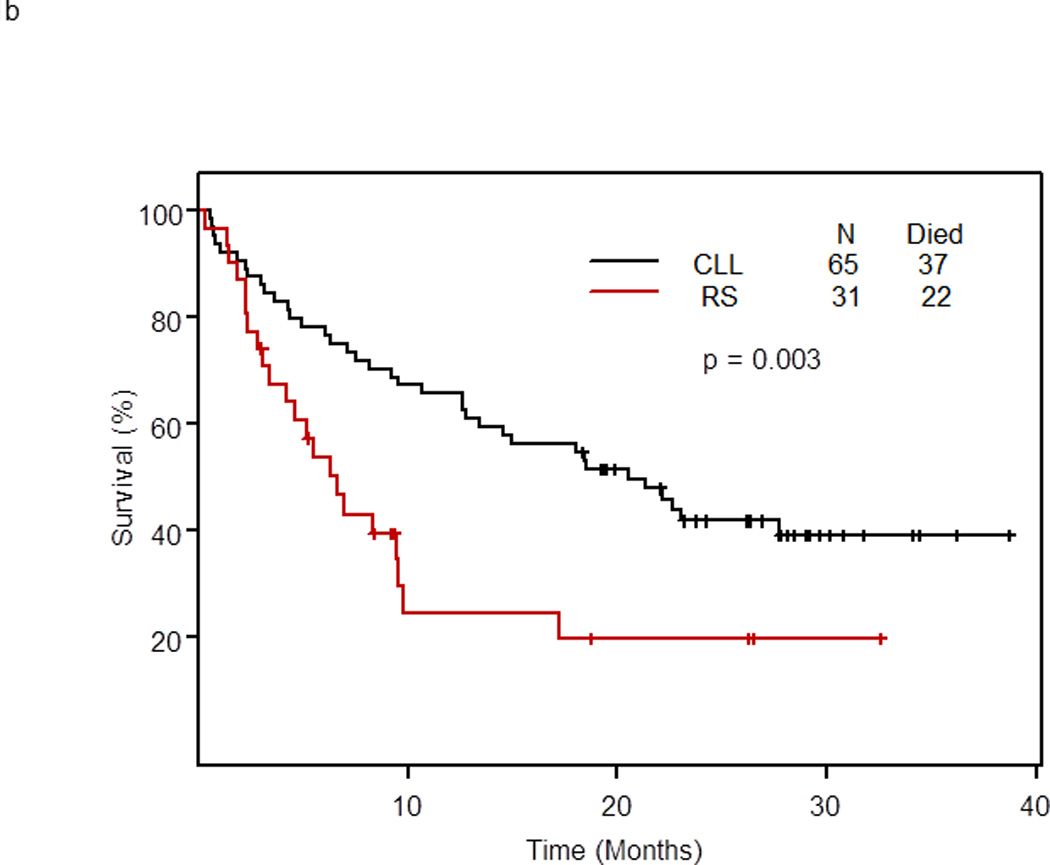
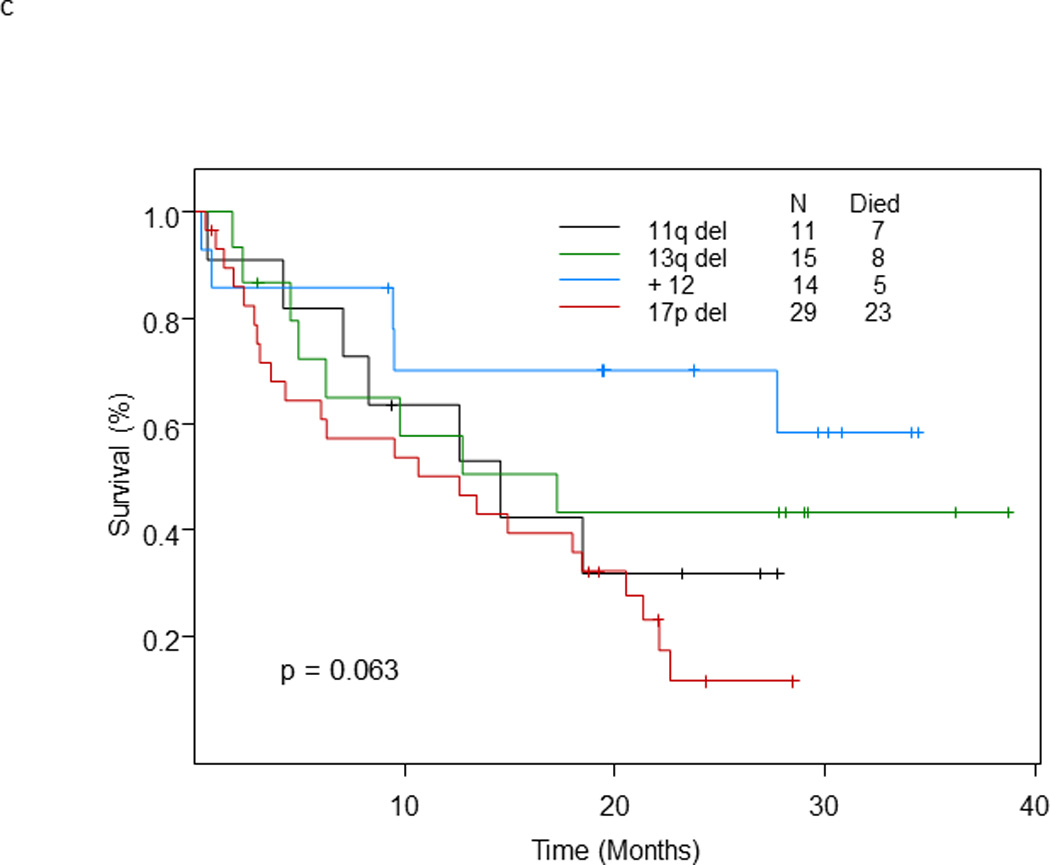
With a median follow-up for surviving patients of 26 months, 59 of 96 patients (61.5%) treated at the phase II recommended dose have died; their median survival was 13.4 months (95% CI: 9.4 – 22.1) (Figure 1a). The median survival of patients with RS was 6.6 months (95% CI: 4.2 – 9.8) and of patients with relapsed/refractory CLL was 20.6 months (95% CI: 13.4 – 40+) (p = 0.003). At 2 years, 19.7% of patients with RS and 41.9% of patients with relapsed/refractory CLL remained alive (Figure 1b). The median survival was 12.6 months for patients with 17p deletion, 14.6 months for patients with 11q deletion, and 17.2 months for patients with 13q deletion. In patients with trisomy 12, the median survival has not been reached, and in patients with no genomic aberrations, the median survival was 5 months (p=0.054) (Figure 1c).
Figure 1.
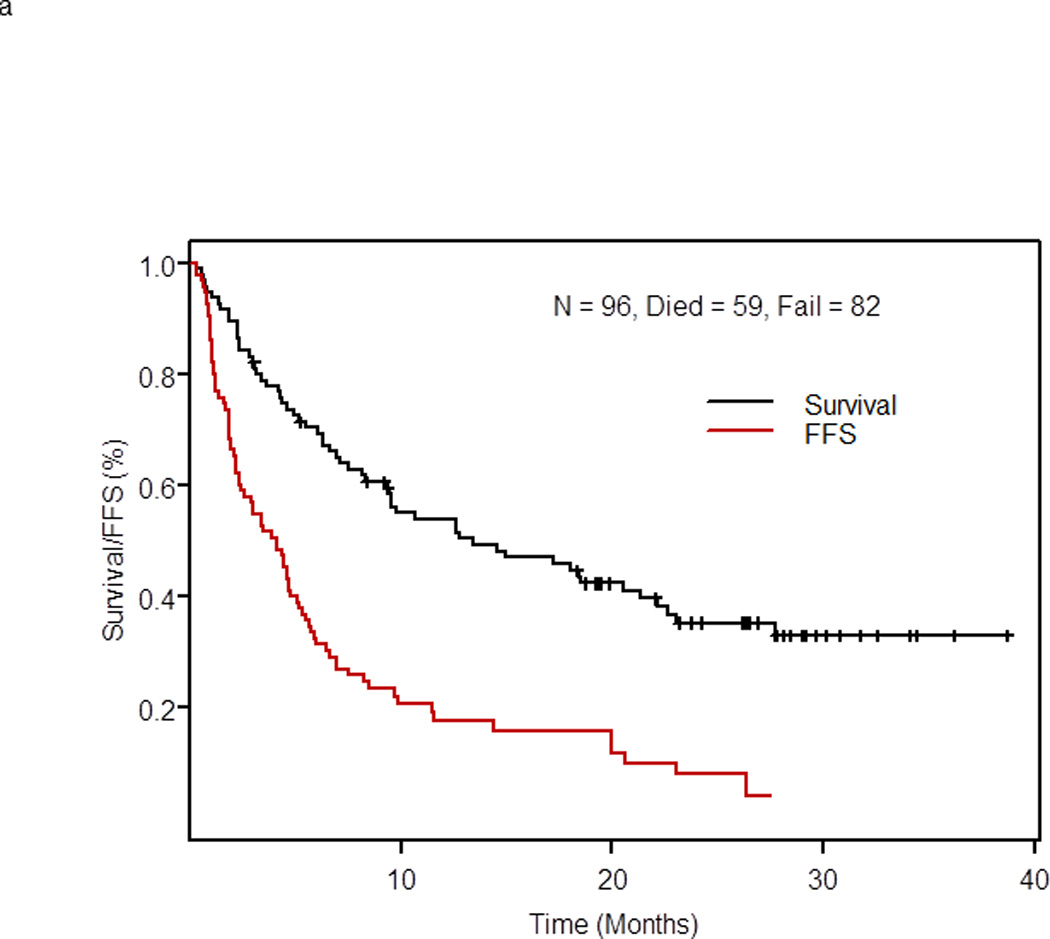
a. Overall and failure-free survival of 96 patients with RS or relapsed/refractory CLL treated with OFAR2
b. Survival by diagnosis of patients with RS (n=31) or relapsed/refractory CLL (n=65) treated with OFAR2
c. Survival by genomic aberrations (as determined by fluorescence in situ hybridization)
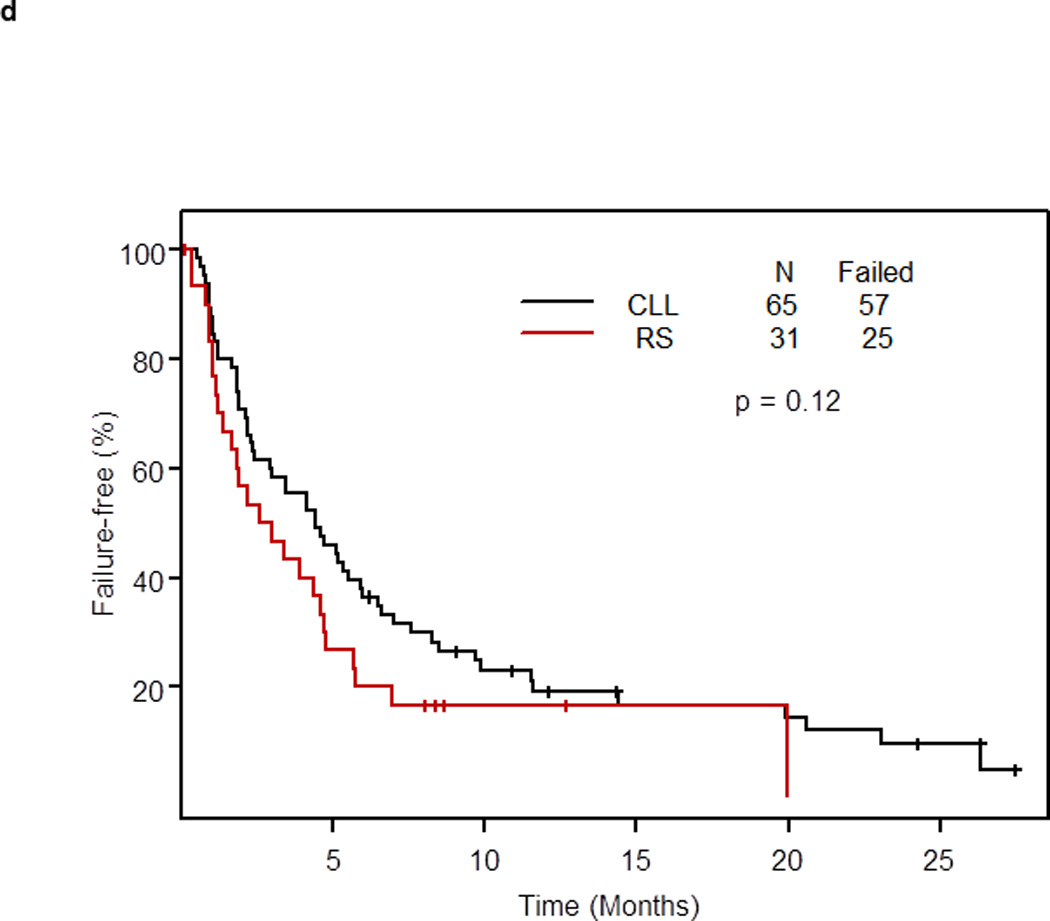
d. Failure-free survival in patients treated with OFAR2 by diagnosis (patients who received SCT as post-remission therapy or to eliminate minimal residual disease were censored at the time of SCT)
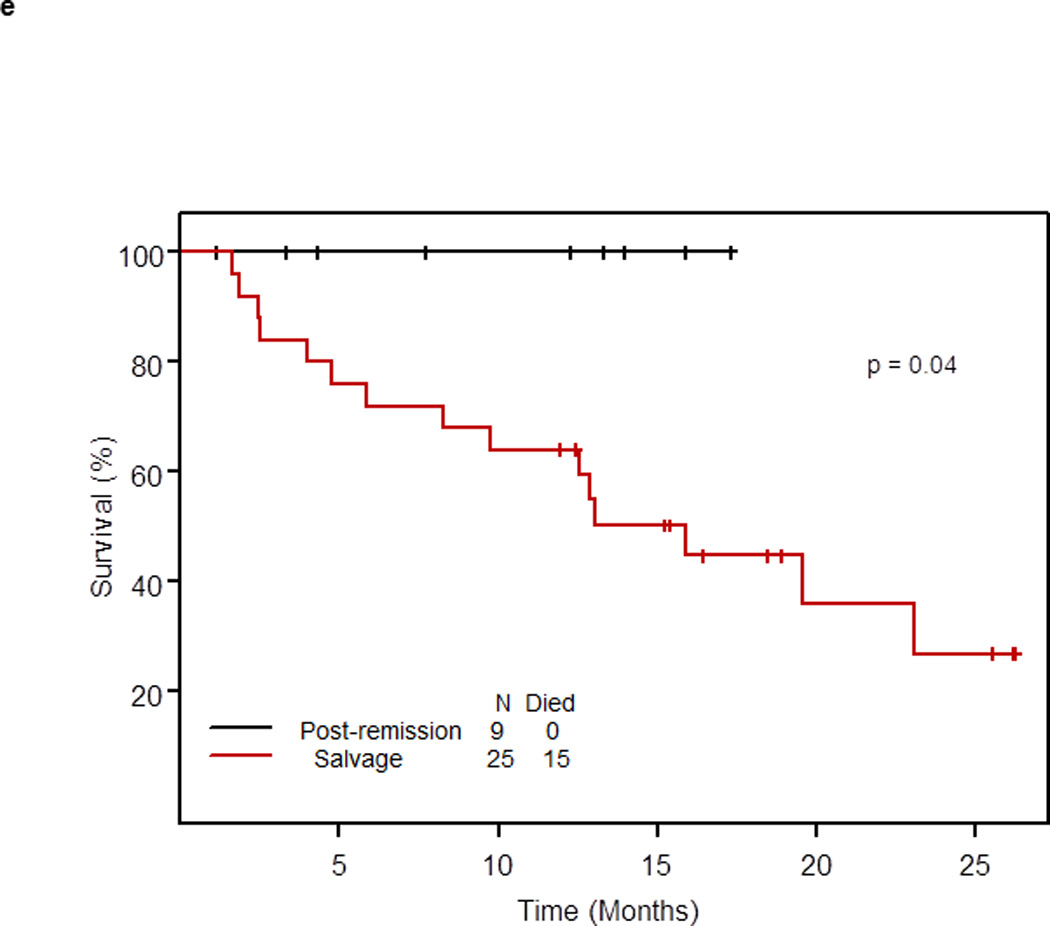
e. Survival of patients with RS or relapsed/refractory CLL who underwent SCT by status of disease
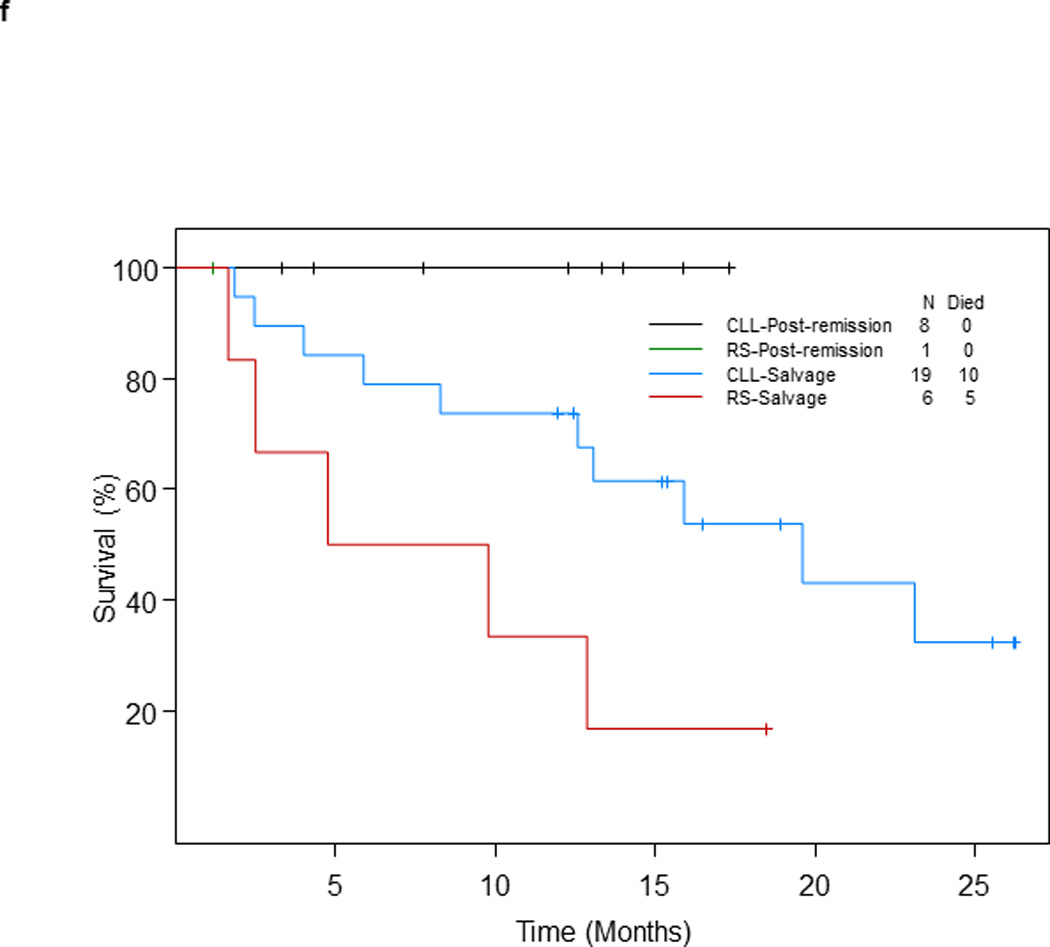
f. Survival of patients with RS or relapsed/refractory CLL who underwent SCT by status of disease at the time of SCT and diagnosis
In 102 patients (median follow-up of surviving patients, 26 months), the median survival was 12.6 months (95% CI: 7.5 – 20.6).
Failure-Free Survival
Eighty-two of 96 patients treated at the phase II recommended dose had treatment failure. The median FFS of 96 patients was 4.1 months (95% CI: 2.4 – 5.1, Figure 1a). The median FFS was 3.0 months (95% CI: 1.6 – 4.8) in patients with RS and 4.4 months (95% CI: 2.9 – 6.5) in patients with relapsed/refractory CLL (p= 0.12) (Figure 1d). At 1 year, 17% of patients with RS remained failure-free compared with 19% of patients with relapsed/refractory CLL. The median FFS of 102 patients was 4.1 months (95% CI: 2.4 – 5.1).
Stem Cell Transplantation after OFAR2
Thirty-four of 96 patients (35.4%) treated at the phase II recommended dose underwent allogeneic SCT after OFAR2 (post-remission therapy, n=9; and salvage therapy, n=25). With a median follow-up of 15.9 months from the date of SCT, none of the 9 patients who underwent SCT as post-remission therapy died; their median survival was not reached (> 25 months). With a median follow-up of 18.9 months, 15 of 25 patients who underwent SCT as salvage therapy have died; their median survival was 15.9 months (95% CI: 9.8 – 25+) (p = 0.04; Figure 1e); survival by diagnosis is shown in Figure 1f.
Comparison of OFAR2 with other regimens is shown in Supplemental File.
Discussion
The therapeutic management of patients with Richter’s syndrome and relapsed/refractory CLL is challenging. We designed OFAR2 on the basis of our favorable experience with OFAR1, with the goal of improving clinical outcomes and decreasing toxicity. In patients with RS treated at the phase II recommended dose, the response rate was 38.7% (CR 6.5%), the median survival was 6.6 months, and the median FFS was 3 months. In patients with relapsed/refractory CLL treated at the phase II recommended dose, the response rate was 50.8% (CR, 4.6%), the median survival was 20.6 months, and the median FFS was 3 months. In patients with 17p deletion, the response rate was 31.0% and the median survival was 12.6 months. In patients with 11q deletion, the response rate was 54.5% and the median survival was 14.6 months. Keeping in mind that patients were heavily pretreated (median of 3 [RS] and 4 [relapsed/refractory CLL] prior therapies) and had high-risk biologic features, these results were encouraging. However, severe myelosuppression was also noted with OFAR2.
An intriguing finding was the survival advantage noted in patients treated with OFAR2 who underwent allogeneic SCT as post-remission therapy (Figure 2b). With a median follow-up of 15.9 months, no patient who underwent SCT as post-remission therapy died. These results demonstrate that OFAR2 is an effective cytoreductive regimen prior to referral for allogeneic SCT and confirm our prior observation that patients with RS or relapsed/refractory CLL who respond to antileukemic therapy and undergo SCT as post-remission therapy have long survival.
The major toxicities noted with OFAR2 were hematologic. Although the phase II OFAR2 regimen included lower doses of cytarabine compared to OFAR1 (500 mg/m2 for 3 days vs. 1 g/dL for 2 days, respectively) although doses of oxaliplatin were higher (30 mg/m2 for 4 days vs. 25 mg/m2 for 4 days), hematologic toxicity was significant. Administration of the phase II regimen was more feasible in patients with relapsed/refractory CLL than in patients with RS (median cycles: 3 vs. 2).
Although the number of patients in each study was relatively small, in RS, OFAR1(19) tended to be associated with superior outcomes compared to OFAR2. A possible explanation is that more intensive chemotherapy (higher doses of cytarabine) is needed to control a highly proliferative disease such as RS. This observation supports the need for individualized therapy in patients with this disease.
In conclusion, OFAR2 had activity in patients with relapsed/refractory CLL and RS, including patients with high-risk genomic aberrations such as 17p or 11q deletion. Cytopenias were the most common hematologic toxicities. SCT as post-remission therapy in patients treated with OFAR2 was associated with prolonged survival, as all patients who underwent SCT as post-remission therapy remain alive.
Supplementary Material
Acknowledgements
Donna Neuberg, Dana Farber, Biostatistics Director for limited statistical analysis.
Susan Lerner, Annette Jalayer and Susan C. Smith assisted with data collection (SL, SCS) and study coordination (AJ).
Christine Eberle for assistance with manuscript submission.
Financial Support: Research Funding (Sanofi to UT MD Anderson Cancer Center, ASCO Career Development Award to Dr. Tsimberidou), Grant Funding to UT MD Anderson Cancer Center, and Chronic Lymphocytic Leukemia Research Consortium to participating institutions.
Apostolia M. Tsimberidou, MD, PhD – Research Funding (Sanofi to UT MD Anderson Cancer Center, and ASCO Career Development Award), Consultancy outside the submitted work (Baxter HealthCare, Caris Life Sciences and Cephalon)
William G. Wierda, MD, PhD – Research Funding (Sanofi to UT MD Anderson Cancer Center), Consultancy (Outside the submitted work - Genentech, Celgene and Merck), Grants pending (Outside the submitted work – GlaxoSmithKline and Abbott), Lectures/Speakers Bureaus (Genentech and Celgene)
Susan O’Brien, MD – Research Support (Genentech)
Jeffry A. Jones, MD, MPH - Consultant/Advisory Relationship (GlaxoSmithKline and Millenium/Takeda), Research Funding (Abbott Labs)
Michael J. Keating, MD– Consultancy (Outside the submitted work – Celgene, Roche), Lectures/Speakers Bureaus (Xcenda)
Footnotes
Conflict of Interest:
Sijin Wen, PhD – No Conflict
William Plunkett – No Conflict
Thomas J. Kipps, MD, PhD – No Conflicts
Xavier Badoux, MD – No Conflict
HagopKantarjian, MD – No Conflict
Results of this study were presented at several conferences, including ASH 2010, Orlando, Florida as an oral presentation and at the 11th International Conference on Malignant Lymphoma International Lymphoma Conference - Lugano, Switzerland 2011
References
- 1.Richter MN. Generalized Reticular Cell Sarcoma of Lymph Nodes Associated with Lymphatic Leukemia. Am J Pathol. 1928 Jul;4(4):285–292. [PMC free article] [PubMed] [Google Scholar]
- 2.Tsimberidou AM, Keating MJ. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer. 2005 Jan 15;103(2):216–228. doi: 10.1002/cncr.20773. [DOI] [PubMed] [Google Scholar]
- 3.Tsimberidou AM, O'Brien S, Khouri I, Giles FJ, Kantarjian HM, Champlin R, et al. Clinical outcomes and prognostic factors in patients with Richter's syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006 May 20;24(15):2343–2351. doi: 10.1200/JCO.2005.05.0187. [DOI] [PubMed] [Google Scholar]
- 4.Velasquez WS, Cabanillas F, Salvador P, McLaughlin P, Fridrik M, Tucker S, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP) Blood. 1988 Jan;71(1):117–122. [PubMed] [Google Scholar]
- 5.Press OW, Livingston R, Mortimer J, Collins C, Appelbaum F. Treatment of relapsed non-Hodgkin's lymphomas with dexamethasone, high-dose cytarabine, and cisplatin before marrow transplantation. J Clin Oncol. 1991 Mar;9(3):423–431. doi: 10.1200/JCO.1991.9.3.423. [DOI] [PubMed] [Google Scholar]
- 6.Martelli M, Vignetti M, Zinzani PL, Gherlinzoni F, Meloni G, Fiacchini M, et al. High-dose chemotherapy followed by autologous bone marrow transplantation versus dexamethasone, cisplatin, and cytarabine in aggressive non-Hodgkin's lymphoma with partial response to front-line chemotherapy: a prospective randomized italian multicenter study. J Clin Oncol. 1996 Feb;14(2):534–542. doi: 10.1200/JCO.1996.14.2.534. [DOI] [PubMed] [Google Scholar]
- 7.Philip T, Chauvin F, Armitage J, Bron D, Hagenbeek A, Biron P, et al. Parma international protocol: pilot study of DHAP followed by involved-field radiotherapy and BEAC with autologous bone marrow transplantation. Blood. 1991 Apr 1;77(7):1587–1592. [PubMed] [Google Scholar]
- 8.Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, et al. ESHAP--an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994 Jun;12(6):1169–1176. doi: 10.1200/JCO.1994.12.6.1169. [DOI] [PubMed] [Google Scholar]
- 9.Watts MJ, Ings SJ, Leverett D, MacMillan A, Devereux S, Goldstone AH, et al. ESHAP and G-CSF is a superior blood stem cell mobilizing regimen compared to cyclophosphamide 1.5 g m(−2) and G-CSF for pre-treated lymphoma patients: a matched pairs analysis of 78 patients. Br J Cancer. 2000 Jan;82(2):278–282. doi: 10.1054/bjoc.1999.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozturk MA, Barista I, Altundag MK, Turker A, Yalcin S, Celik I, et al. Modified ESHAP as salvage chemotherapy for recurrent or refractory non-Hodgkin's lymphoma: results of a single-center study of 32 patients. Modified etoposide, methylprednisolone, cytarabine and cisplatin. Chemotherapy. 2002 Dec;48(5):252–258. doi: 10.1159/000066768. [DOI] [PubMed] [Google Scholar]
- 11.Kidani Y, Noji M, Tashiro T. Antitumor activity of platinum(II) complexes of 1,2-diamino-cyclohexane isomers. Gann. 1980 Oct;71(5):637–643. [PubMed] [Google Scholar]
- 12.Mathe G, Kidani Y, Segiguchi M, Eriguchi M, Fredj G, Peytavin G, et al. Oxalato-platinum or 1-OHP, a third-generation platinum complex: an experimental and clinical appraisal and preliminary comparison with cis-platinum and carboplatinum. Biomed Pharmacother. 1989;43(4):237–250. doi: 10.1016/0753-3322(89)90003-6. [DOI] [PubMed] [Google Scholar]
- 13.Chollet P, Bensmaine MA, Brienza S, Deloche C, Cure H, Caillet H, et al. Single agent activity of oxaliplatin in heavily pretreated advanced epithelial ovarian cancer. Ann Oncol. 1996 Dec;7(10):1065–1070. doi: 10.1093/oxfordjournals.annonc.a010500. [DOI] [PubMed] [Google Scholar]
- 14.Wang YF, Curtis JE, Lipton J, Minkin S, McCulloch EA. Cytosine arabinoside (ara-C) and cis-dichlorodiammineplatinum II (cisplatin) alone and in combination: effects on acute myeloblastic leukemia blast cells in culture and in vivo. Leukemia. 1991 Jun;5(6):522–527. [PubMed] [Google Scholar]
- 15.Yang LY, Li L, Keating MJ, Plunkett W. Arabinosyl-2-fluoroadenine augments cisplatin cytotoxicity and inhibits cisplatin-DNA cross-link repair. Mol Pharmacol. 1995 May;47(5):1072–1079. [PubMed] [Google Scholar]
- 16.Li L, Keating MJ, Plunkett W, Yang LY. Fludarabine-mediated repair inhibition of cisplatin-induced DNA lesions in human chronic myelogenous leukemia-blast crisis K562 cells: induction of synergistic cytotoxicity independent of reversal of apoptosis resistance. Mol Pharmacol. 1997 Nov;52(5):798–806. doi: 10.1124/mol.52.5.798. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Liu X, Glassman AB, Keating MJ, Stros M, Plunkett W, et al. Fludarabine triphosphate inhibits nucleotide excision repair of cisplatin-induced DNA adducts in vitro. Cancer Res. 1997 Apr 15;57(8):1487–1494. [PubMed] [Google Scholar]
- 18.Gandhi V, Nowak B, Keating MJ, Plunkett W. Modulation of arabinosylcytosine metabolism by arabinosyl-2-fluoroadenine in lymphocytes from patients with chronic lymphocytic leukemia: implications for combination therapy. Blood. 1989 Nov 1;74(6):2070–2075. [PubMed] [Google Scholar]
- 19.Tsimberidou AM, Wierda WG, Plunkett W, Kurzrock R, O'Brien S, Wen S, et al. Phase I-II study of oxaliplatin, fludarabine, cytarabine, and rituximab combination therapy in patients with Richter's syndrome or fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2008 Jan 10;26(2):196–203. doi: 10.1200/JCO.2007.11.8513. [DOI] [PubMed] [Google Scholar]
- 20.Moufarij MA, Sampath D, Keating MJ, Plunkett W. Fludarabine increases oxaliplatin cytotoxicity in normal and chronic lymphocytic leukemia lymphocytes by suppressing interstrand DNA crosslink removal. Blood. 2006 Dec 15;108(13):4187–4193. doi: 10.1182/blood-2006-05-023259. [Clinical Trial Comparative Study Research Support, N.I.H., Extramural]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999 Apr;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996 Jun 15;87(12):4990–4997. [PubMed] [Google Scholar]
- 23.Tsimberidou AM, Kantarjian HM, Cortes J, Thomas DA, Faderl S, Garcia-Manero G, et al. Fractionated cyclophosphamide, vincristine, liposomal daunorubicin, and dexamethasone plus rituximab and granulocyte-macrophage-colony stimulating factor (GM-CSF) alternating with methotrexate and cytarabine plus rituximab and GM-CSF in patients with Richter syndrome or fludarabine-refractory chronic lymphocytic leukemia. Cancer. 2003 Apr 1;97(7):1711–1720. doi: 10.1002/cncr.11238. [DOI] [PubMed] [Google Scholar]
- 24.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002 Jan 24;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 25.Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S, et al. Cyclophosphamide, fludarabine, alemtuzumab, and rituximab as salvage therapy for heavily pretreated patients with chronic lymphocytic leukemia. Blood. 2011 Aug 25;118(8):2085–2093. doi: 10.1182/blood-2011-03-341032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011 Mar 17;117(11):3016–3024. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


