Abstract
Emerging adults (18–25 years old) are often poorly retained in substance use disorder treatment. Office-based buprenorphine often enhances treatment retention among people with opioid dependence. In this study, we examined the records of a collaborative care buprenorphine treatment program to compare the treatment retention rates of emerging adults versus older adults. Subjects were 294 adults, 71 (24%) aged 18–25, followed in treatment with buprenorphine, nurse care management, and an intensive outpatient program followed by weekly psychosocial treatment. Compared to older adults, emerging adults remained in treatment at a significantly lower rate at 3 months (56% versus 78%) and 12 months (17% versus 45%), and were significantly more likely to test positive for illicit opioids, relapse, or drop out of treatment. Further research into factors associated with buprenorphine treatment retention among emerging adults is needed to improve treatment and long-term outcomes in this group.
Keywords: Buprenorphine, Opioid dependence, Emerging adult, Young adult, Retention, Toxicology, Outcome, Development, Treatment
1. Introduction
1.1. Opioid dependence among 18–25 year olds
Due to the availability of prescription pain medications, the prevalence of opioid dependence in the United States has increased dramatically over the past 15 years (Okie, 2010). Between 2004 and 2012, more than 16 million people in the United States initiated nonmedical use of prescription opioids (SAMHSA, 2013a) and between 12.2 and 13.5% of high school seniors misused prescription opioids each year (Johnston, O'Malley, Bachman, & Schulenberg, 2013). From 1998 to 2012, past-month prevalence of heroin use rose from 130,000 to 335,000 people (SAMHSA, 2013a). During this time, the frequency of overdose deaths associated with opioid analgesics rose dramatically (Warner, Chen, Makuc, Anderson, & Minino, 2011).
Particularly troublesome has been illicit opioid use among emerging adults (18–25 year olds) (Arnett, 2000). Rates of chronic prescription opioid misuse have been higher among 18–25 year olds than among any other age group (7.4 per 1000 of 18–25 year olds compared with 5.0 and 4.0 per 1000 among 26–34 and 35–49 year olds, respectively) (Jones, 2012). From 1998 to 2008, admissions for treatment of prescription opioid dependence recorded in the Treatment Episode Data Set increased by 350% among 18–25 year olds, a disproportionately greater rise than among all other age groups (SAMHSA, 2010). Since 2009, prescription opioid misuse has decreased from peak levels among 18–25 year olds; however, since heroin use has increased in parallel (Cicero, Ellis, & Surratt, 2012), levels of opioid dependence in this age group still remain high (SAMHSA, 2012).
1.2. Buprenorphine and treatment retention
One positive development slowing down the epidemic has been the introduction of office-based buprenorphine maintenance treatment for opioid dependence, which is now commonly available for adults in most urban areas, with more than 14,000 prescribers and 1800 programs nationwide (SAMHSA, 2013b). Maintenance treatment with buprenorphine has been demonstrated to be more effective for opioid dependence than detoxification or placebo (Fudala et al., 2003; Johnson, Jaffe, & Fudala, 1992; Weiss et al., 2011). Treatment retention among all adults in office-based buprenorphine treatment is generally between 40 and 50% at 12 months (Alford et al., 2011; Fiellin et al., 2008). Notably, in multiple studies, older age has been associated with improved outcomes in treatment for opioid dependence both with buprenorphine (Dreifuss et al., 2013; Marsch et al., 2005; Ohlin, Hesse, Fridell, & Tatting, 2011; Soeffing, Martin, Fingerhood, Jasinski, & Rastegar, 2009) and methadone (McHugh et al., 2013). The association between age and improved retention in buprenorphine treatment is important because retention in opioid agonist treatment predicts drug abstinence (Zhang, Friedmann, & Gerstein, 2003), and dropout is associated with a 7-fold increase in risk of overdose (Clausen, Anchersen, & Waal, 2008). Importantly, all of these studies of retention in opioid maintenance treatment consider age as a continuous variable in the analyses, and do not directly examine the influence of emerging adulthood, a specific developmental period of the lifespan referring to 18–25 year olds (Arnett, 2000, 2005).
1.3. Emerging adulthood
The concept of emerging adulthood as a developmental period differentiates 18–25 year olds from adolescents (< 18) and young adults (26–40) based on common demographic and psychological characteristics that are unique to 18–25 year olds in industrial societies (Arnett, 2000). Research on emerging adulthood has spread rapidly during the past decade through the fields of psychology and the social sciences. For instance, a database search for individual citations from 2000 through 2012 based on the terms “emerging adult” or “emerging adulthood” revealed 329 citations in PubMed, 882 in PsycINFO, and 1580 in Google Scholar. Most of this research has been conducted among non-addicted samples; however, the National Survey on Drug Use and Health demonstrated that 21% of 18–25 year olds reported illicit drug use in 2012, compared to 7% among people 26 and older (SAMHSA, 2013a).
1.4. Age-specific outcomes in addiction treatment
Several clinical outcome studies have begun to support the hypothesis that emerging adults are more difficult to engage in substance use disorder treatment when compared to older adults or adolescents. For instance, among intravenous drug users, one study has shown that emerging adults enrolled less frequently than older adults in continuing care programs after detoxification, and were less likely to enroll in opioid agonist therapy (Shin, Lundgren, & Chassler, 2007). In studies of the community reinforcement approach for youths with alcohol abuse, emerging adults had more days of alcohol use compared to adolescents, and were less likely to be abstinent (Smith, Godley, Godley, & Dennis, 2011). The difference in willingness to stop using substances was seen by the authors as partially due to lower levels of interpersonal motivation for abstinence among emerging adults, who were no longer having daily interactions with school and families of origin (Smith, Cleeland, & Dennis, 2010). Thus, accumulating evidence suggests that compared to older adults and adolescents, it may be more challenging to engage and retain emerging adults in treatment.
Since no study has examined treatment outcomes specifically for emerging adults in office-based buprenorphine treatment, it is crucial both to assess whether this age group benefits from office-based buprenorphine treatment and to understand which patient attributes may affect treatment for emerging adults. If emerging adults do not benefit as much as older adults do from office-based buprenorphine treatment, then novel age-specific interventions or alternative treatment approaches may be needed for this age group.
1.5. Developmental models
The developmental period model suggests that specific features of emerging adulthood (e.g., moving out of the parental home, absence of regular steady work, lack of marriage and/or cohabitation relationships, rapidly changing social networks, minimal responsibilities to family and others, less sense of need to adhere to adult norms) may contribute to patterns of increased substance use (Arnett, 2005; Stone, Becker, Huber, & Catalano, 2012). Additionally, other features of emerging adulthood, such as instability in environment and scheduling, frequent moves and transitions, self- and peer-directed identity exploration may contribute to poor engagement and retention in substance use disorder treatment (e.g., Arnett, 2011; Sheidow, McCart, Zajac, & Davis, 2012).
Neurodevelopmental models posit that during adolescence up until age 25 the developing brain interacts differently than an adult brain would with reinforcing substances, associative cues, and tasks requiring cognitive control, contributing to increased levels of substance use, difficulty remaining abstinent, and ultimately resulting in an inability to remain in treatment (Kellam, 2013). This model is supported by several findings: substance use during critical periods, e.g., cannabis use during adolescence, may differentially affect the developing brain, resulting in increased psychiatric co-morbidity (Malone, Hill, & Rubino, 2010); critical processes of cortical pruning and myelination needed for the development of executive function are underway during this time period (Giedd et al., 1999; Gogtay et al., 2004; Lebel & Beaulieu, 2011; Marsh, Gerber, & Peterson, 2008) and can be disrupted by substance abuse (Jacobus et al., 2009; Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007); aspects of normal cognitive development can be derailed (Hanson, Medina, Padula, Tapert, & Brown, 2011); and finally, these disruptions lead to an ongoing situation in which reward-based brain activation remains favored over inhibitory control processing needed to abstain from drug use and adhere to treatment program rules (Casey & Jones, 2010), both of which are often associated with remaining in treatment (Warden et al., 2012).
1.6. Drug dependence inexperience model and treatment outcomes
Emerging adults generally have fewer years of experience with opioid dependence compared to older adults who often have had more years of life available for fostering an opioid habit. Some researchers have hypothesized that lack of motivation related to inexperience with opioid dependence may be a major reason for poor outcomes in buprenorphine treatment (Subramaniam et al., 2011). The health beliefs model predicts that people engage in health services when the perceived severity of illness and benefit of treatment are both high (Rosenstock, 1966). Derived from the health beliefs model, the opioid dependence inexperience model proposes that emerging adults may have less experience with negative consequences of addiction due to a shorter duration of substance use (e.g., less than 2 years of continuous opioid use), resulting in decreased perception of addiction severity (Finney & Moos, 1995), lack of motivation to refrain from substance use (McKellar, Kelly, Harris, & Moos, 2006), and less clear perceptions of the benefits of treatment. Based on clinical experience, we thought less than 2 years of continuous opioid use was a reasonable duration to operationalize the construct of opioid dependence inexperience, allowing us to examine this hypothesis. In addition, total years of opioid use and having started opioid use during adolescence, both of which are associated with opioid dependence experience, may also influence group differences in treatment retention (Soyka, Zingg, Koller, & Kuefner, 2008).
1.7. Predictors of buprenorphine treatment outcomes
To be able to examine whether emerging adulthood independently predicts attrition from treatment, it is essential to address characteristics associated with buprenorphine treatment outcomes, patterns of concurrent substance use, and indicators of drug dependence severity. Male gender (Marsch et al., 2005), recent cocaine use (Sullivan et al., 2010), cocaine dependence (Marsch et al., 2005), longer histories of opioid use (Soyka et al., 2008), more severe psychiatric problems and poorer psychosocial functioning (Pani, Maremmani, Pirastu, Tagliamonte, & Gessa, 2000) all have been associated with poorer buprenorphine treatment outcomes. Patterns of concurrent substance abuse (e.g. cannabis use) may vary by age group (SAMHSA, 2013a) and may influence treatment outcomes. Several well-known indicators of drug dependence severity have been shown to influence addiction treatment outcomes, but have mixed outcomes in buprenorphine treatment studies. Lifetime history of heroin use predicted poorer retention and more positive opioid toxicology screens among adults (Darke et al., 2005; Simpson, Joe, & Rowan-Szal, 1997), but heroin use history resulted in increased opioid abstinence in one study of buprenorphine treatment (Soeffing et al., 2009). Injection drug use history predicted lesser opioid use among adolescents prescribed buprenorphine (Subramaniam et al., 2011), but increased frequency of injection use at baseline has been associated with poor retention and less abstinence among heroin users (Darke et al., 2005; Simpson et al., 1997). Multiple medicated detoxifications have been shown to be associated with quicker relapse after substance use disorder treatment (Wright et al., 2007), though no data exist for buprenorphine treatment.
Opioid dependence inexperience together with concurrent substance misuse and well-known predictors of poor treatment outcomes could each wholly or partially account for age-related differences in buprenorphine treatment retention. For this reason, any examination of how emerging adult age status may influence buprenorphine treatment outcomes will need to account for these potential associations.
1.8. Study aims
We therefore compared outcomes between emerging adults and older adults during outpatient buprenorphine treatment for opioid dependence, to examine whether emerging adult age status was associated with higher levels of relapse and lower levels of retention. We also aimed to evaluate whether differences could be accounted for solely by the contribution of opioid dependence inexperience, concurrent substance abuse, or other well-known predictors of poor treatment outcomes.
2. Methods
2.1. Sample selection
This retrospective chart review was conducted in sequential admissions to an office-based opioid treatment (OBOT)(Gunderson & Fiellin, 2008) collaborative care buprenorphine maintenance program (Alford et al., 2011; Schuman-Olivier et al., 2010, 2013). The Cambridge Health Alliance human subjects review board approved the protocol and performed annual review of its progress. Subjects were exempted from providing informed consent. We conducted the chart review using standardized intake forms, quarterly interviews conducted by nurse care managers, and the electronic medical record.
2.1.1. Subjects
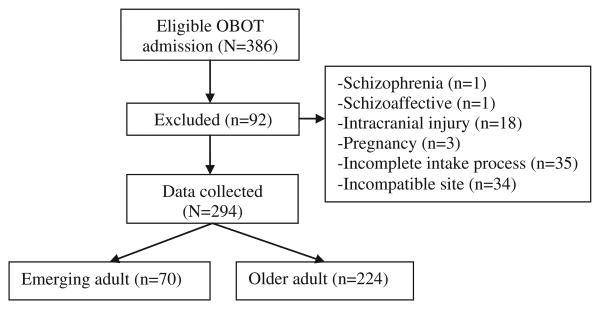
All newly eligible patients (N = 386) screened from November 2007 to June 2010 were included in this sequential admission study. Patients completed an intake process, which included comprehensive urine toxicology testing, a structured comprehensive substance abuse assessment interview by OBOT nurse care managers, a psychosocial assessment by a social worker, and a medical examination. Patients were prescribed buprenorphine/naloxone and assigned to an intensive outpatient program (IOP) for at least 2 weeks. Patients who completed an initial intake assessment and received a buprenorphine prescription were included in the chart review. People with psychosis, intracranial injury, or pregnancy were excluded (n = 23). Patients from a fifth site that deviated from treatment protocol (i.e., only 10% of newly eligible patients attended IOP), and those with an incomplete intake process (n = 35) were also excluded from the analysis, resulting in 294 complete patient records analyzed (Fig. 1). In this chart review, data for the final sample (N = 294) were recorded until either the date of OBOT discharge or 12 months after intake into OBOT treatment, whichever came first.
Fig. 1.
Consort diagram.
2.2. Procedure
2.2.1. Treatment
Two nurse care managers collaborated with multiple buprenorphine prescribers, coordinating urine toxicology screening, monitoring treatment adherence, overseeing medication management, and facilitating communication with addiction counselors. Prescribers were affiliated with an academic community healthcare system located in four Boston Metro-North cities, sharing an electronic medical record (EMR). Nurse care managers conducted a brief screening assessment by telephone or in-person to determine treatment program admission eligibility.
OBOT consisted of buprenorphine maintenance treatment prescribed by program-affiliated physicians from various medical specialties, including internal medicine, family medicine, and psychiatry. Within this program, clinicians encouraged brief inpatient detoxification before starting buprenorphine maintenance for patients with substantial co-morbidity (e.g., physiological dependence to other substances or significant medical problems); however, detoxification was not required when opioid dependence was the only substance use disorder present. Standard treatment consisted of buprenorphine initiation during a half-day in-office induction by a physician with nurse care manager collaboration. All buprenorphine prescriptions referred to throughout this study were for a sublingual buprenorphine/naloxone co-formulation tablet.
Patients were also required to attend consistent weekly psychosocial treatment sessions, either in a group or one-on-one format. Patients typically participated in an intensive outpatient program during the first 2 weeks of buprenorphine treatment and in response to substance use lapses. Buprenorphine prescriptions were first provided on a weekly basis. After clinical stabilization on weekly prescriptions, i.e., IOP completion, consistent abstinence from alcohol and other drugs, and regular attendance in weekly psychosocial treatment, then the prescription duration was increased to 2 weeks then monthly. If patients continued illicit opioid use, then in many cases, a trial of an increased buprenorphine dose was conducted. The program also required patients to step down after completing the IOP to a weekly relapse prevention group, unless psychiatric needs (e.g. hypomania and agitation disrupting group) precluded participation. The program provided individual therapy and psychopharmacology based on psychiatric need.
2.2.2. Nurse care manager assessments
Nurse care managers conducted structured comprehensive assessment interviews at intake using the OBOT-B comprehensive assessment form (Section 2.3.3.), lasting approximately 2 hours. They once again conducted a comprehensive assessment interview lasting 30 minutes every 3 months thereafter. At each 3-month interview, treatment retention was recorded based on a patient's history of adherence to buprenorphine treatment, urine toxicology screening, program rules, and psychosocial treatment recommendations. If the patient was discharged from the program, the disenrollment date and reasons for discharge were recorded, e.g., dropout, administrative non-compliance, or transfer to another program; however, if there was evidence for illicit opioid use just prior to discharge, then ‘relapse’ was the recorded reason.
2.2.3. Urine toxicology screening
Nurse care managers conducted urine toxicology screening during the intake interview. Urine toxicology screens were then collected twice weekly during the first month. Once abstinence was attained, weekly urine toxicology screening was conducted for the first 6 months of treatment. If patients were clinically stable, then the program required at least monthly urine screening for the remainder of the year. If patient returned to drug use, frequency of urine toxicology screening would increase.
2.3. Materials and measures
2.3.1. Electronic medical record
The EMR recorded detailed descriptions from physician OBOT intake notes as well as urine toxicology results. We obtained these data for each patient until 12 months after intake into OBOT, or until the patient was discharged.
2.3.2. Urine toxicology tests
At intake, nurse care managers collected a standard urine toxicology panel from all patients, assessing for use of amphetamine, benzodiazepines, alcohol, cannabis, opioids, and cocaine. Urine toxicology results were recorded in the EMR throughout treatment; however, for this chart review, we extracted data only on illicit use of opioids and cocaine from urine screens during ongoing treatment. During the chart review, we did not extract data on other substances for several reasons: urine testing for alcohol using ethyl glucuronide was not consistent during treatment; cannabis metabolites are eliminated at variable rates, challenging our method for assigning positive screens to a specific treatment month; several patients had prescriptions for amphetamine (∼3%) and/or benzodiazepines (∼19%), offering potential confounders for the analysis. Urine toxicology used enzyme-mediated immunoassay techniques (Beckman Synchron, Beckman Coulter, Fullerton, CA), but rapid chromatographic immunoassays were used for oxycodone (Bio-Rad, Hercules, CA).
2.3.3. OBOT-Buprenorphine (OBOT-B) comprehensive assessment form
Using the OBOT-B intake assessment form widely used by all 19 OBOT-B programs funded by the state's department of public health, the nurse care managers conducted a standardized intake interview, obtaining a detailed history of substance use and demographic characteristics, including gender, race, employment, disability income, and psychiatric medication. The chart review collected the data from this interview record that were relevant to this analysis, including the following: (a) self-report of any use of alcohol, cannabis, cocaine, or other drugs in the past 30 days (recent amphetamine or benzodiazepine use was excluded from analysis because some people had prescriptions as described above); (b) drug dependence severity (number of previous detoxifications, lifetime use of heroin, any heroin use in past 30 days, and a history of injection drug use); (c) opioid dependence inexperience (age of initiation of opioid use, total years of opioid use, and short duration of continuous opioid use, i.e., less than or equal to 2 years).
2.4. Data analysis
We examined sample characteristics comparing emerging adults and older adults, using t-tests and chi-square analyses. To compare emerging adults and older adults on treatment retention, we conducted two logistic regression models with emerging adult age status as the independent variable and either 3- or 12-month retention as the dependent variable. We also used chi-square and logistic regression to examine whether there were age-group differences in the reasons recorded for disenrollment from treatment. Statistical significance was marked by p < 0.05 and 95 % confidence intervals were calculated when applicable.
We used a multivariable modeling approach to conduct a secondary analysis, in order to control for relevant covariates when comparing treatment retention outcomes differences between emerging adults and older adults. We performed separate multivariable analyses for both 3- and 12-month retention using logistic regression models conducted with a step-wise, user-driven approach. First, we conducted individual logistic regression models to examine the association of 20 potential predictors of treatment retention at 3 and 12 months. These predictors included demographic characteristics, substance use co-morbidity, well-known indicators of drug dependence severity, and opioid dependence inexperience model variables (e.g., short duration of opioid dependence [< 2 years], total years of opioid use). Next, we added each predictor that approached significance (p < 0.10) in individual models, into a final model in a step-wise approach, clustering with other related eligible variables. Multicollinearity was assessed by examination of tolerance (cutoff < 0.1) and variance inflation factors (cutoff > 10.0); variables did not exceed acceptable limits.
We conducted independent logistic regression models for each month with the presence of positive urine toxicology for illicit opioids or cocaine as the dependent variables. Because treatment dropout prevented future participation in toxicology screening, we limited our analysis of drug use outcomes to months in which levels of participation in toxicology screening were not significantly different between groups.
Post-hoc analyses compared the levels of buprenorphine prescribed to both groups at various study time points (i.e., week 2, day 30, day 90, and last dose). We conducted individual logistic regression to determine if the buprenorphine dose at any time point predicted treatment retention or opioid use. Significant predictors for individual modeling were included in multivariable modeling along with only emerging adult age status for treatment retention, opioid use, and disenrollment reasons. Finally, we added significant predictors to the full multivariable models of baseline variables previously described for treatment retention at 3- and 12-months, in order to examine if buprenorphine dose level influenced the relationship between emerging adult status and treatment outcomes.
3. Results
3.1. Sample characteristics
Subjects (N = 294) were 41.5% female and 92.2% Caucasian (Table 1). The median duration of opioid dependence was 6.0 years among emerging adults compared with 15.8 years among older adults. Emerging adults were more likely to have started opioid use before age 18 and to have used opioids for less than 2 years, and were less likely to have used psychiatric medications or receive disability income in the past year. Emerging adults were more likely to report recent cannabis use and have cannabis positive urine toxicology at intake. See Table 1.
Table 1.
Sample characteristics.
| Total | Emerging adults (18–25) | Older adults (26+) | p | ||
|---|---|---|---|---|---|
|
|
|
|
|||
| N = 294 | n = 70 | n = 224 | |||
| Demographics | |||||
| Age, mean (SD) | 35.6 (8.1) | 23.1 (1.7) | 39.5 (9.2) | < .01 | * |
| Gender, female % | 41.5 | 35.7 | 43.3 | 0.26 | |
| Race, Caucasian % | 92.2 | 97.1 | 90.6 | 0.08 | † |
| No. of days worked in past 30 days, mean (SD) | 3.9 (7.4) | 4.3 (7.2) | 3.7 (7.5) | 0.41 | |
| Currently receiving disability income, % | 22.5 | 2.9 | 28.6 | < 0.01 | * |
| Past-year history of psychiatric medication, % | 67.4 | 53.6 | 71.6 | < 0.01 | * |
| Recent substance use patterns | |||||
| Self-reported use in past 30 days | |||||
| Alcohol | 13.6 | 12.9 | 13.8 | 0.83 | |
| Cannabis | 12.2 | 21.4 | 9.4 | < 0.01 | * |
| Cocaine | 10.5 | 8.6 | 11.2 | 0.54 | |
| No other substance use | 39.8 | 34.3 | 41.5 | 0.28 | |
| Urine toxicology screening at intake | |||||
| Alcohol | 5.8 | 5.8 | 5.7 | 0.97 | |
| Cannabis | 27.9 | 41.4 | 23.7 | < 0.01 | * |
| Cocaine | 17.0 | 15.7 | 17.4 | 0.74 | |
| Amphetamine | 6.1 | 4.3 | 6.7 | 0.46 | |
| Benzodiazepine | 29.3 | 25.7 | 30.4 | 0.46 | |
| Opioid dependence severity | |||||
| No. of previous detoxification visits, mean (SD) | 1.9 (2.0) | 2.5 (1.9) | 3.0 (2.0) | 0.12 | |
| Lifetime heroin use, % | 77.9 | 81.4 | 76.8 | 0.41 | |
| Heroin use in the past 30 days, % | 18.0 | 22.9 | 16.5 | 0.23 | |
| History of injection drug use, % | 56.8 | 64.3 | 54.5 | 0.15 | |
| Opioid dependence experience | |||||
| Started during adolescence (< 18 years), % | 31.0 | 57.1 | 22.8 | < 0.01 | * |
| Total years of opioid use, mean (SD) | 13.5 (8.4) | 6.0 (2.3) | 15.8 (9.6) | < 0.01 | * |
| Short duration of opioid use (< 2 years), % | 2.4 | 7.1 | 0.9 | < 0.01 | * |
p < 0.05.
p < 0.10.
3.2. Primary treatment outcomes
3.2.1. Treatment retention
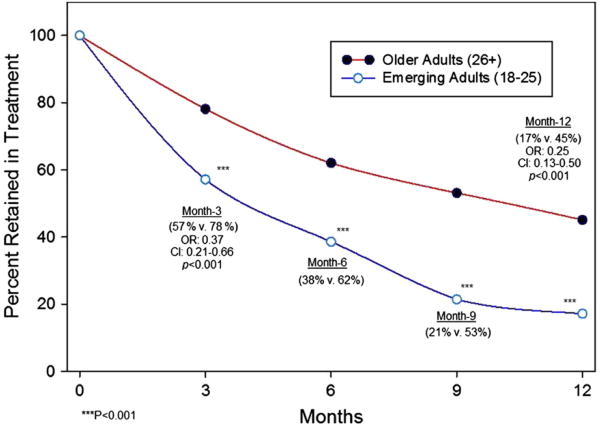
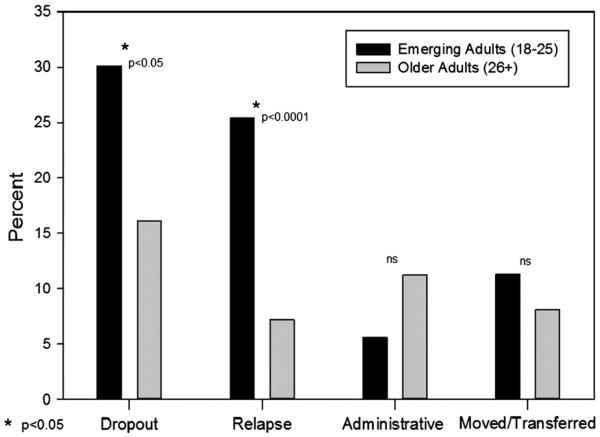
Logistic regression analysis showed a lower retention rate among emerging adults as compared to older adults at 3 months (57% versus 78%, p < 0.001; OR: 0.37 [0.21–0.66]) and 12 months (17% versus45%, p < 0.001; OR: 0.25 [0.13–0.50]) (Fig. 2). In examining reasons for lack of retention in treatment at 12 months, emerging adults were more likely than older adults to have left the program due to either known relapse (25.5% versus 7.1%, p < 0.0001; OR: 4.50 [2.15–9.42]) or drop out (without known relapse) (30.0% versus. 16.5%, p < 0.05; OR: 2.17 [1.16–4.03]); however, no difference emerged in disenrollment rates due to administrative non-compliance (5.7% versus 11.6%, p = 0.18; OR: 0.48 [0.16–1.44]), relocating or having their care transferred (11.4% versus 8.0%, p = 0.38; OR: 1.48 [0.61–3.56]) (Fig. 3).
Fig. 2.
Retention over time during 12 months of collaborative care buprenorphine treatment.
Fig. 3.
Emerging adults versus older adults compared on reasons for program disenrollment by 12 months after admission.
3.2.2. Individual logistic regression modeling for identifying covariates
We initially used individual logistic regression models to examine the association of 20 potential predictors with treatment retention at 3 and 12 months (Table 2). Results indicated that several well-known indicators of poor treatment outcomes, including cocaine-positive toxicology at intake, history of injection drug use, and lifetime heroin use were all predictors of poor 3-month treatment retention in individual logistic regression models. The primary opioid dependence inexperience model variable, short duration of continuous opioid use, which was a binary variable with only 7 seven subjects reporting less than 2 years of opioid use, demonstrated a moderate-to-large effect size and approached significance as a predictor of 3-month retention in individual logistic regression modeling (p = 0.087, Cohen's d = −0.72) (Chinn, 2000).
Table 2.
Individual logistic regression models for buprenorphine treatment retention at 3- and 12-months (N = 294).
| Predictor | Month-3 retention | Month-12 retention | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | p | OR | 95% CI | P | |
| Emerging adult (compared to older adult) | 0.37 | [0.21–0.66] | < 0.001* | 0.25 | [0.13–0.50] | < 0.001* |
| Demographics | ||||||
| Female (compared to male) | 1.22 | [0.72–2.07] | 0.458 | 1.20 | [0.75–1.93] | 0.450 |
| Caucasian | 0.74 | [0.27–2.06] | 0.565 | 0.45 | [0.19–1.06] | 0.069† |
| No. of days worked in past 30 days | 1.02 | [0.98–1.05] | 0.394 | 1.01 | [0.98–1.04] | 0.576 |
| Currently receiving disability income | 1.48 | [0.77–2.86] | 0.241 | 1.71 | [0.98–2.97] | 0.058† |
| Past-year history of psychiatric medication | 1.61 | [0.94–2.76] | 0.082† | 1.09 | [0.65–1.80] | 0.750 |
| Recent substance use patterns | ||||||
| Self-reported use in past 30 days | ||||||
| Alcohol | 2.29 | [0.92–5.68] | 0.075† | 0.96 | [0.48–1.90] | 0.896 |
| Cannabis | 0.70 | [0.33–1.48] | 0.352 | 0.42 | [0.18–0.95] | 0.037* |
| Cocaine | 0.89 | [0.39–2.02] | 0.774 | 0.52 | [0.23–1.21] | 0.132 |
| No other substance use | 1.11 | [0.65–1.89] | 0.699 | 2.18 | [1.35–3.53] | 0.002* |
| Urine toxicology screening at intake | ||||||
| Alcohol | 1.21 | [0.38–3.82] | 0.749 | 1.13 | [0.42–3.06] | 0.811 |
| Cannabis | 0.61 | [0.35–1.06] | 0.082† | 0.38 | [0.21–0.68] | 0.001* |
| Cocaine | 0.39 | [0.21–0.73] | 0.003* | 0.51 | [0.26–1.00] | 0.050† |
| Amphetamine | 1.31 | [0.42–4.09] | 0.647 | 2.10 | [0.80–5.49] | 0.130 |
| Benzodiazepine | 0.79 | [0.45–1.38] | 0.404 | 0.87 | [0.48–1.36] | 0.421 |
| Opioid dependence severity | ||||||
| No. of previous detoxification visits | 1.02 | [0.89–1.16] | 0.786 | 1.09 | [0.97–1.23] | 0.154 |
| Lifetime heroin use | 0.48 | [0.24–0.98] | 0.043* | 0.44 | [0.25–0.77] | 0.004* |
| Heroin use in the past 30 days | 0.73 | [0.39–1.40] | 0.346 | 0.41 | [0.20–0.81] | 0.011* |
| History of injection drug use | 0.44 | [0.25–0.76] | 0.004* | 0.49 | [0.30–0.79] | 0.003* |
| Opioid dependence experience | ||||||
| Started during adolescence (< 18 years) | 0.65 | [0.38–1.11] | 0.116 | 0.53 | [0.31–0.91] | 0.021* |
| Total years of opioid use | 1.03 | [1.00–1.06] | 0.087† | 1.03 | [1.01–1.06] | 0.009* |
| Short duration of opioid use (< 2 years) | 0.27 | [0.06–1.21] | 0.087† | 0.26 | [0.03–2.19] | 0.215 |
Note: Each row represents an individual logistic regression model.
p < 0.05.
p < 0.10.
In addition to the substance use disorder variables that were predictive of 3-month retention, several other variables were also predictive of 12-month retention. For instance, cannabis or heroin use in the 30 days prior to intake, cannabis-positive toxicology at intake, starting opioid abuse during adolescence, and total duration of opioid use were predictors of lower rates of 12-month treatment retention in individual logistic regression models, while no alcohol or illicit drug use in 30 days prior to intake and a history of starting opioid use during adolescence were both associated with greater 12-month treatment retention. Nine covariates of 3-month retention and twelve covariates for 12-month retention with p < 0.1 in individual logistic modeling were included in their respective multivariable models.
3.2.3. Multivariable regression modeling for treatment retention
Group differences in treatment retention related to emerging adult age status remained significant at 3 months (p < 0.05, OR: 0.44 [0.22–0.89]) and 12 months (p < 0.05, OR: 0.43 [0.19–0.98]) in multivariable modeling even after adjusting for significant covariates related to demographic characteristics, substance use co-morbidity, drug dependence severity, and opioid dependence inexperience. In the 3-month multivariable model, only cocaine-positive toxicology at intake was also independently predictive of retention (Table 3). In the 12-month multivariable model, only emerging adult age status was predictive of treatment retention. However, when all of the covariates were included in the multivariable model, the association between emerging adult age status and 12-month retention was diminished from an odds ratio of 0.25 to 0.43. This reduction in the multivariable model's variance explained by emerging adulthood was mostly due to the addition of the combination of two opioid dependence inexperience variables (i.e., total duration of opioid use and started during adolescence) which shifted the odds ratio by 28% (Table 4). When these two variables were added to a model with emerging adult age status, both individually and in combination, only emerging adult age status remained significant.
Table 3.
Logistic regression odds ratios for retention in buprenorphine treatment at 3 months (n = 294).
| Predictor | Model 0 | Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Emerging Adult (compared 10 older adult) | 0.37 | [0.21–0.66]* | 0.39 | [0.22–0.70]* | 0.36 | [0.20–0.66]* | 0.41 | [0.22–0.75]* | 0.44 | [0.22–0.89]* |
| Demographics | ||||||||||
| Past-year history of psychiatric medication | 1.41 | [0.81–2.45] | 1.36 | [0.77–2.39] | 1.44 | [0.81–2.56] | 1.44 | [0.81–2.56] | ||
| Recent substance use patterns | ||||||||||
| Self-reported use in past 30 days | ||||||||||
| Alcohol | 2.32 | [0.91–5.92]† | 2.46 | [0.95–6.34]† | 2.36 | [0.91–6.10]† | ||||
| Urine toxicology screening at intake | ||||||||||
| Cannabis | 0.75 | [0.42–1.35] | 0.73 | [0.40–1.32] | 0.70 | [0.39–1.29] | ||||
| Cocaine | 0.38 | [0.20–0.74] * | 0.42 | [0.22–0.81]* | 0.42 | [0.21–0.81]* | ||||
| Opioid dependence severity | ||||||||||
| Lifetime heroin use | 0.81 | [0.33–2.00] | 0.83 | [0.33–2.07] | ||||||
| History of injection drug use | 0.52 | [0.25–1.04]† | 0.42 | [0.25–1.03]† | ||||||
| Opioid dependence experience | ||||||||||
| Total years of opioid use | 1.00 | [0.96–1.03] | ||||||||
| Short duration of opioid use (< 2 years) | 0.38 | [0.07–1.99] | ||||||||
p < 0.05.
p < 0.10.
Table 4.
Logistic regression odds ratios for retention in buprenorphine treatment at 12 months (N = 294).
| Predictor | Model 0 | Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Emerging adult (compared 10 older adult) | 0.25 | [0.13–0.50]* | 0.27 | [0.14–0.55]* | 0.30 | [0.15–0.61]* | 0.32 | [0.16–0.66]* | 0.43 | [0.19–0.98]* |
| Demographics | ||||||||||
| Caucasian | 0.52 | [0.22–1.26] | 0.49 | [0.19–1.22] | 0.46 | [0.18–1.19] | 0.47 | [0.18–1.19] | ||
| Currently receiving disability income | 1.29 | [0.73–2.30] | 1.09 | [0.60–2.00] | 1.16 | [0.62–2.14] | 1.06 | [0.57–2.00] | ||
| Recent substance use patterns | ||||||||||
| Self-reported use in past 30 days | ||||||||||
| Cannabis | 0.88 | [0.35–2.24] | 0.74 | [0.29–1.93] | 0.77 | [0.30–2.00] | ||||
| No other substance use | 1.91 | [1.12–3.26]* | 1.63 | [0.90–2.93] | 1.63 | [0.90–2.96] | ||||
| Urine toxicology screening at intake | ||||||||||
| Cannabis | 0.53 | [0.28–0.99]* | 0.53 | [0.28–1.01]† | 0.56 | [0.29–1.07]† | ||||
| Cocaine | 0.58 | [0.28–1.19] | 0.65 | [0.31–1.34] | 0.65 | [0.32–1.36] | ||||
| Opioid dependence severity | ||||||||||
| Lifetime heroin use | 0.59 | [0.28–1.26] | 0.54 | [0.25–1.15] | ||||||
| Heroin use in the past 30 days | 0.65 | [0.29–1.47] | 0.63 | [0.28–1.44] | ||||||
| History of injection drug use | 0.71 | [0.38–1.33] | 0.75 | [0.40–1.40] | ||||||
| Opioid dependence experience | ||||||||||
| Started during adolescence (< 18 years) | 0.78 | [0.40–1.52] | ||||||||
| Total years of opioid use | 0.98 | [0.94–1.01] | ||||||||
p < 0.05.
p < 0.10.
3.2.4. Urine toxicology
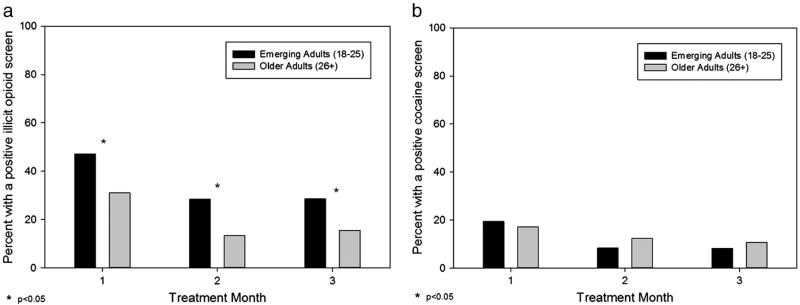
During the first 3 months of treatment, emerging adults were more likely than older adults to test positive for illicit use of opioids (p < 0.0001; OR: 2.20 [1.53–3.15]). Specifically, during month 1, 47.9% of emerging adults versus 31.0% of older adults tested positive for illicit use of opioids, with similar group differences during month 2 (28.3% versus 13.3%) and month 3 (28.6% versus 15.4%) (Fig. 4a). In contrast, no group differences were found for cocaine toxicology during the first 3 months: month 1 (19.7% versus 17.2%), month 2 (8.3% versus 12.3%), and month 3 (8.2% versus 10.8%) (Fig. 4b). After 3 months of treatment, emerging adults were less likely to participate in urine toxicology testing (i.e., participation rates in toxicology screening became significantly different between month 3 and month 4 [month 3: 66% versus 75%, p = 0.13; month 4: 57% versus 73%, p = 0.014]), which is consistent with the between-group differences in retention seen after 3 months of treatment.
Fig. 4.
Emerging adults versus older adults compared on presence of a positive toxicology for illicit use of (a) opioids and (b) cocaine during each treatment month.
3.3. Post-hoc analysis
Daily buprenorphine dose was generally higher among older adults versus emerging adults throughout the study: week 2 [15.3 (6.8) versus 12.8 (5.8), F = 8.2, p < 0.01], day 30 [16.3 (6.3) versus 13.6 (5.6), F = 9.9, p < 0.01], day 90 [18.0 (6.2) versus 15.7 (6.3), F = 5.1, p < 0.05], last dose [16.9 (7.2) versus 14.2 (6.1), F = 8.0, p < 0.01]. Day 30 dose was predictive of treatment retention at 12 months (OR: 0.96 [0.92–0.99], p < 0.05), and approached significance for 3 month retention (OR: 0.96 [0.92–1.00], p = 0.08). Buprenorphine dosing levels at other time points did not predict retention, opioid use, or disenrollment reason; therefore, day 30 dose was the only additional variable included in multivariable modeling. When day 30 dose was included in multivariable models along with only emerging adult age status, the main effect of emerging adult age status remained an independent predictor of treatment retention (p < 0.01), opioid use (p < 0.01), and disenrollment reasons [relapse (p < 0.001), dropout (p < 0.05)]. Neither the main effect of day 30 dose nor the interaction effect of day 30 dose and emerging adult age status were significant when added to all of these models with emerging adult age status. A recent meta-analysis reported that higher prescribed buprenorphine dosage was associated with increased retention and less illicit opioid use among adults of all ages (Fareed, Vayalapalli, Casarella, & Drexler, 2012). In contrast, higher day 30 dose levels among emerging adults in this study were paradoxically associated with increased rates of positive toxicology for illicit opioids in the second month of treatment (p < 0.05). This further demonstrates that even though emerging adults had a lower mean buprenorphine dose than older adults at multiple time points, buprenorphine dosing did not independently influence group differences in retention and opioid use in this study when controlling for emerging adult age status.
4. Discussion
This naturalistic investigation of differences in office-based buprenorphine treatment outcomes, comparing emerging adults (18–25 year olds) and older adults (26+), had several key findings. First, emerging adults had substantially lower levels of treatment retention at 3 and 12 months, in line with previous research, which suggested that retaining younger patients in treatment is more challenging than older adults (Dreifuss et al., 2013; Marsch et al., 2005; Soeffing et al., 2009). Second, emerging adults were more likely than older adults to continue illicit use of opioids during the first 3 months, suggesting that ongoing opioid use after treatment entry may be associated with poor treatment retention. Similarly, in this study, emerging adults were more likely than older adults to be disenrolled from buprenorphine treatment because of either known relapse during treatment or dropout; the latter is strongly associated with later relapse (Zhang et al., 2003), especially among drug users under 30 years old (Grella, Hser, Joshi, & Anglin, 1999). These data support the assertion that low rates of buprenorphine treatment retention among emerging adults are associated with relapse or dropout as opposed to a result of administrative non-compliance, transition to a new program, or relocation to another region. Third, while several demographic characteristics, opioid dependence inexperience variables, prescribed dosage of buprenorphine, and indicators of drug dependence severity predicted treatment retention in individual regression models, our multivariable modeling demonstrated that these variables cannot fully account for the differences found between emerging adults and older adults on treatment retention, suggesting a need for further testing of developmental explanatory models in future studies.
While previous studies have identified directional associations (Dreifuss et al., 2013; Marsch et al., 2005) and trends (Soeffing et al., 2009) between age and retention in buprenorphine treatment, this study is the first to report a difference between emerging adults and older adults on treatment retention rates in outpatient-based opioid treatment at 12 months. Similarly, this study is the first to report that these retention differences are specifically associated with opioid relapse and/or drop out from treatment. The National Drug Abuse Treatment Clinical Trials Network (CTN) multi-site trial of 154 opiate users aged 15–21 demonstrated a 3-month retention rate of 71.6% in buprenorphine maintenance (Woody et al., 2008). In this CTN study, several baseline characteristics (e.g., hallucinogen use, heroin use) and early treatment factors (e.g., positive opioid toxicology screens early in treatment, buprenorphine adherence less than 5 days a week) predicted attrition from buprenorphine treatment (Warden et al., 2012). Additionally, baseline internalizing mental health symptoms predicted less opioid use (Subramaniam et al., 2011). Because emerging adults with opioid dependence treated with buprenorphine in the United States generally receive the prescription from outpatient physicians or adult outpatient opioid treatment programs in the community and are not usually treated within time-limited, structured treatment settings with daily supervised medication administration, such as was used in this trial, it remained unknown whether these findings in 15–21 year olds could generalize to treatment of emerging adults in the community. Therefore, the new findings reported here with emerging adults in office-based opioid treatment with buprenorphine over a 12-month period generalize more easily to current practice and more accurately represent current treatment with this specific emerging adult age group.
The duration of continuous opioid use is significantly different between emerging adults and older adults with about a 9-year mean difference between groups (Table 1). Duration of opioid use, measured by years of use as a continuous variable, was a predictor of retention, with fewer years of continuous opioid use being associated with fewer weeks in treatment. Similarly, those who started opioid use during adolescence had fewer weeks in treatment. However, when duration of opioid use or adolescent start was included in multivariable modeling with emerging adult status, only emerging adult age status remained an independent predictor of retention. Therefore, duration of opioid use and adolescent start were not mediators of the negative effect of emerging adult age status on retention; rather, they were both dependent on the emerging adult effect.
In order to further evaluate the opioid inexperience model, derived from the health beliefs model, this study also evaluated the role of opioid inexperience by using a dichotomous predictor, namely continuous opioid use for less than 2 years. The data demonstrated that opioid inexperience was not the underlying reason for the substantially negative effect of emerging adult age status on treatment retention in this study. Yet, this study could not definitively rule out a role for the opioid inexperience model among the small sample of adults with short duration of opioid abuse. The substantial effect size of the dichotomous short duration of opioid use variable suggests that continuous opioid abuse for less than 2 years may cause a negative effect on treatment retention, even if it is does not mediate the negative effect of emerging adult age status on treatment outcomes in this study. Further research with a larger sample of patients with short duration of continuous opioid dependence is needed to evaluate whether short duration of opioid use is independently associated with poor treatment retention.
While variables related to substance use disorder co-morbidity, prescribed dosage of buprenorphine, drug dependence severity, and opioid inexperience did account for some of the variability in retention patterns, they could not account for the significant effect of emerging adult age status on retention rates; several untested factors may contribute to the strength of this effect. For instance, emerging adults with opioid dependence have been found to have high rates of conduct disorder (Subramaniam, Ives, Stitzer, & Dennis, 2010), which has been shown to contribute to difficulties remaining in buprenorphine treatment (Ohlin et al., 2011), and may be especially high among transition-age youth growing out of juvenile legal services and social services (Heflinger & Hoffman, 2008). Given the common reliance of drug dependence treatment models on concomitant involvement in self-help programs, low levels of sustained involvement in twelve-step programs during emerging adulthood (Kelly, Brown, Abrantes, Kahler, & Myers, 2008) may eliminate a recovery synergy from which older adults often benefit. From a neurodevelopmental perspective, delayed onset of executive function capabilities related to continuous use of neurotoxic substances during brain development (Hanson et al., 2011; Jacobus et al., 2009) may influence the ability to comply with opioid treatment program rules. Finally, this study suggests that in addition to several well-known indicators of drug dependence severity and a potential negative effect of short duration of opioid dependence on treatment retention, some unidentified factors associated with the developmental period of emerging adulthood (e.g., demographic, psychological) may influence buprenorphine treatment retention (Arnett, 2005). Although we were unable to assess these additional factors in this study, we speculate that transitions in social networks (family, romantic relationships, employment/school, peer group, housemates and cohabitants), perceptions about recovery that conflict with developmental goals of individuation, as well as an optimistic bias about the possibilities for change matched with a strong drive for autonomy and independence from external assistance may each be differentially affecting emerging adults' decisions to abstain and remain in treatment.
Future studies should test novel methods for increasing retention in buprenorphine treatment. As previously mentioned, buprenorphine adherence has been associated with increased retention among opioid dependent youth (Warden et al., 2012). For this reason, using remote technology to promote buprenorphine adherence, such as secure electronic pill dispensers (Uosukainen, Pentikainen, & Tacke, 2013), offers promise. Development of specific behavioral therapy manuals targeting emerging adult developmental tasks may be helpful. Smartphone apps for relapse prevention (McTavish, Chih, Shah, & Gustafson, 2012) may be integrated into treatment in order to improve medication adherence and increase recovery support. Emerging adults are significantly more likely than any other age group to utilize mobile data applications beyond voice technology (A. Smith, 2010), and have highest prevalence of smartphone health app use (Fox, 2012). Smartphone ownership in young adults is high (79% of 18–24 year olds own smartphones versus 56% of all adults) (Smith, 2013), continues to increase (Pew, 2013), and is relatively impervious to socioeconomic differences: smartphone ownership prevalence ranges from 77 to 90% based on income in adults aged 18–29. Thus, smartphone ownership and use characteristics make emerging adults the ideal candidates for smartphone-enhanced opioid treatment delivery systems.
Several alternative treatment approaches may enhance long term outcomes among emerging adults or offer benefits in this difficult to retain age group. Involving families (Klostermann & O'Farrell, 2013) or peers (Smith, Cleeland, Middleton, & Godley, 2013) may offer a way to increase interpersonal consequences of substance use and provide additional recovery support. While contingency management with financial incentives can increase retention rates in opioid dependence (Petry & Carroll, 2013), community programs need to surmount the widespread hurdle of perceived infeasibility (Hartzler & Rabun, 2013). Since 30 days of no alcohol or illicit drug use prior to initiating OBOT treatment was associated with increased retention in this study, initiating treatment in a residential setting followed by strong continuity into outpatient treatment may improve outcomes, while also enhancing momentum and recovery skills often absent among emerging adults (Kelly, Urbanoski, Hoeppner, & Slaymaker, 2012). Other models such as chronic disease care management (Saitz, Larson, Labelle, Richardson, & Samet, 2008) or assertive community treatment (Mueser, Bond, Drake, & Resnick, 1998) may lower the level of expectations for participation and abstinence necessary to remain in treatment, allowing for therapeutic relationships to develop over time.
4.1. Strengths and limitations
This study integrated clinical and laboratory information from a large collaborative care OBOT program that coordinated care for buprenorphine prescribers from 4 cities. Sample findings were similar to trends found in previous studies (Dreifuss et al., 2013; Marsch et al., 2005; Soeffing et al., 2009; Subramaniam et al., 2011; Warden et al., 2012), supporting the overall generalizability of study results. This study was a systematic, retrospective chart review, and thus variable selection was limited. We were not, for example, able to include more sensitive well-validated measures of substance use disorder severity or other relevant variables listed in the previous paragraph that may further explain the treatment retention differences between emerging and older adults. Because our focus was on retention rather than engagement, we excluded those who did not complete intake assessments or receive a buprenorphine prescription; however, this potentially could have contributed to some minor sampling bias.
4.2. Conclusions
Compared to older adults, emerging adults more often continued using illicit opioids during buprenorphine treatment and discontinued treatment at higher rates due to relapse or dropout. These effects on treatment retention are not accounted for by group differences in demographic characteristics, co-morbid substance abuse, drug dependence severity, dosage of prescribed buprenorphine, or opioid dependence inexperience. More research is needed to understand psychological, developmental, environmental, neurologic, and clinical factors affecting buprenorphine treatment for 18–25 year olds with opioid dependence.
Acknowledgments
We appreciate the contribution of Kevin Wall and Emeric Bojarski to data entry and database auditing. We thank Francyne Puopolo, RN, Lola Roland, RN, and David Mysels, MD for their consultation during the data entry process. We appreciate mentoring and editorial support from the MGH Center for Addiction Medicine, especially A. Eden Evins, MD, MPH and John Kelly, PhD.
This study was conducted without a direct funding source; however, support for authors was provided through various related grants: Harvard Medical School Department of Psychiatry Dupont-Warren Fellowship (ZSO), Tufts University Summer Undergraduate Research Fellowship (JB), K24DA022288 (RW), U10DA15831 (RW), K01DA027097 (BH), in addition to UL1RR025758 (Harvard Catalyst) for REDCAP database services.
References
- Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: Five-year experience. Archives of Internal Medicine. 2011;171(5):425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. American Psychologist. 2000;55(5):469–480. [PubMed] [Google Scholar]
- Arnett JJ. The developmental context of substance use in emerging adulthood. Journal of Drug Issues. 2005;35(2):235–254. [Google Scholar]
- Arnett JJ. Emerging adulthood(s): The cultural psychology of a new life stage. In: Jensen LA, editor. Bridging Cultural and Developmental Approaches to Psychology. Oxford University Press; 2011. pp. 255–275. [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Statistics in Medicine. 2000;19(22):3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. New England Journal of Medicine. 2012;367(2):187–189. doi: 10.1056/NEJMc1204141. [DOI] [PubMed] [Google Scholar]
- Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): A national prospective cross-registry study. Drug and Alcohol Dependence. 2008;94(1–3):151–157. doi: 10.1016/j.drugalcdep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J, Teesson M, Ali R, Cooke R, Ritter A, et al. Factors associated with 12 months continuous heroin abstinence: Findings from the Australian Treatment Outcome Study (ATOS) Journal of Substance Abuse Treatment. 2005;28(3):255–263. doi: 10.1016/j.jsat.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Dreifuss JA, Griffin ML, Frost K, Fitzmaurice GM, Potter JS, Fiellin DA, et al. Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: Results from a multisite study. Drug and Alcohol Dependence. 2013;131(1–2):112–118. doi: 10.1016/j.drugalcdep.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed A, Vayalapalli S, Casarella J, Drexler K. Effect of buprenorphine dose on treatment outcome. Journal of Addictive Diseases. 2012;31(1):8–18. doi: 10.1080/10550887.2011.642758. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Moore BA, Sullivan LE, Becker WC, Pantalon MV, Chawarski MC, et al. Long-term treatment with buprenorphine/naloxone in primary care: Results at 2-5 years. American Journal on Addictions. 2008;17(2):116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- Finney JW, Moos RH. Entering treatment for alcohol abuse: A stress and coping model. Addiction. 1995;90(9):1223–1240. doi: 10.1046/j.1360-0443.1995.90912237.x. [DOI] [PubMed] [Google Scholar]
- Fox S. Mobile Health 2012. Pew Internet & American Life Project; 2012. from http://pewinternet.org/∼/media//Files/Reports/2012/PIP_MobileHealth2012_FINAL.pdf. [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. New England Journal of Medicine. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella CE, Hser YI, Joshi V, Anglin MD. Patient histories, retention, and outcome models for younger and older adults in DATOS. Drug and Alcohol Dependence. 1999;57(2):151–166. doi: 10.1016/s0376-8716(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Gunderson EW, Fiellin DA. Office-based maintenance treatment of opioid dependence: How does it compare with traditional approaches? CNS Drugs. 2008;22(2):99–111. doi: 10.2165/00023210-200822020-00002. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of adolescent alcohol and drug use on neuropsychological functioning in young adulthood: 10-Year outcomes. Journal of Child & Adolescent Substance Abuse. 2011;20(2):135–154. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Rabun C. Community opioid treatment perspectives on contingency management: Perceived feasibility, effectiveness, and transportability of social and financial incentives. Journal of Substance Abuse Treatment. 2013;45(2):242–248. doi: 10.1016/j.jsat.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflinger CA, Hoffman C. Transition age youth in publicly funded systems: Identifying high-risk youth for policy planning and improved service delivery. Journal of Behavioral Health Services and Research. 2008;35(4):390–401. doi: 10.1007/s11414-006-9042-2. [DOI] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, et al. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31(6):349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267(20):2750–2755. [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975–2012: Volume I, secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- Jones CM. Frequency of prescription pain reliever nonmedical use: 2002–2003 and 2009–2010. Archives of Internal Medicine. 2012;172(16):1265–1267. doi: 10.1001/archinternmed.2012.2533. [DOI] [PubMed] [Google Scholar]
- Kellam H. The neuro-developmental pathway origins of addiction. Early Brain Development and Addiction for Undergraduate Medical Education. 2013 [podcast] Retrieved on 8/31/2013 from http://chec-cesc.afmc.ca/resource/the-neuro-developmental-pathway-origins-of-addiction-an-ebbda-introductory-podcast.
- Kelly JF, Brown SA, Abrantes A, Kahler CW, Myers M. Social recovery model: An 8-year investigation of adolescent 12-step group involvement following inpatient treatment. Alcoholism, Clinical and Experimental Research. 2008;32(8):1468–1478. doi: 10.1111/j.1530-0277.2008.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Urbanoski KA, Hoeppner BB, Slaymaker V. “Ready, willing, and (not) able” to change: Young adults' response to residential treatment. Drug and Alcohol Dependence. 2012;121(3):224–230. doi: 10.1016/j.drugalcdep.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann K, O'Farrell TJ. Treating substance abuse: Partner and family approaches. Social Work in Public Health. 2013;28(3–4):234–247. doi: 10.1080/19371918.2013.759014. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: Epidemiology and neurodevelopmental models. British Journal of Pharmacology. 2010;160(3):511–522. doi: 10.1111/j.1476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Stephens MA, Mudric T, Strain EC, Bigelow GE, Johnson RE. Predictors of outcome in LAAM, buprenorphine, and methadone treatment for opioid dependence. Experimental and Clinical Psychopharmacology. 2005;13(4):293–302. doi: 10.1037/1064-1297.13.4.293. [DOI] [PubMed] [Google Scholar]
- Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(11):1233–1251. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Murray HW, Hearon BA, Pratt EM, Pollack MH, Safren SA, et al. Predictors of dropout from psychosocial treatment in opioid-dependent outpatients. American Journal on Addictions. 2013;22(1):18–22. doi: 10.1111/j.1521-0391.2013.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar J, Kelly J, Harris A, Moos R. Pretreatment and during treatment risk factors for dropout among patients with substance use disorders. Addictive Behaviors. 2006;31(3):450–460. doi: 10.1016/j.addbeh.2005.05.024. [DOI] [PubMed] [Google Scholar]
- McTavish FM, Chih MY, Shah D, Gustafson DH. How patients recovering from alcoholism use a smartphone intervention. Journal of Dual Diagnosis. 2012;8(4):294–304. doi: 10.1080/15504263.2012.723312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007;29(1):141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Bond GR, Drake RE, Resnick SG. Models of community care for severe mental illness: A review of research on case management. Schizophrenia Bulletin. 1998;24(1):37–74. doi: 10.1093/oxfordjournals.schbul.a033314. [DOI] [PubMed] [Google Scholar]
- Ohlin L, Hesse M, Fridell M, Tatting P. Poly-substance use and antisocial personality traits at admission predict cumulative retention in a buprenorphine programme with mandatory work and high compliance profile. BMC Psychiatry. 2011;11:81. doi: 10.1186/1471-244X-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okie S. A flood of opioids, a rising tide of deaths. New England Journal of Medicine. 2010;363(21):1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- Pani PP, Maremmani I, Pirastu R, Tagliamonte A, Gessa GL. Buprenorphine: A controlled clinical trial in the treatment of opioid dependence. Drug and Alcohol Dependence. 2000;60(1):39–50. doi: 10.1016/s0376-8716(99)00140-4. [DOI] [PubMed] [Google Scholar]
- Petry NM, Carroll KM. Contingency management is efficacious in opioid-dependent outpatients not maintained on agonist pharmacotherapy. Psychology of Addictive Behaviors. 2013;27(4):1036–1043. doi: 10.1037/a0032175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew. Cell phone and smartphone ownership over time. Pew Internet & American Life Project; 2013. from http://www.pewinternet.org/Trend-Data-%28Adults%29/Device-Ownership.aspx. [Google Scholar]
- Rosenstock IM. Why people use health services. The Milbank Memorial Fund Quarterly. 1966;44(3 Suppl):94–127. [PubMed] [Google Scholar]
- Saitz R, Larson MJ, Labelle C, Richardson J, Samet JH. The case for chronic disease management for addiction. Journal of Addiction Medicine. 2008;2(2):55–65. doi: 10.1097/ADM.0b013e318166af74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samhsa. The TEDS Report: Characteristics of substance abuse treatment admissions reporting primary abuse of prescription pain relievers: 1998 and 2008. Rockville, MD: Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2010. [Google Scholar]
- Samhsa. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings NSDUH Series H-44, HHS Publication No (SMA) 12-4713. Rockville, MD: Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2012. [Google Scholar]
- Samhsa. Substance Abuse and Mental Health Services Administration. Rockville, MD: 2013a. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- Samhsa. Buprenorphine physician and program treatment locator. 2013b Retrieved 5/19/2013, from http://buprenorphine.samhsa.gov/bwns_locator/dr_search.htm.
- Schuman-Olivier Z, Albanese M, Nelson SE, Roland L, Puopolo F, Klinker L, et al. Self-treatment: Illicit buprenorphine use by opioid-dependent treatment seekers. Journal of Substance Abuse Treatment. 2010;39(1):41–50. doi: 10.1016/j.jsat.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Schuman-Olivier Z, Hoeppner BB, Weiss RD, Borodovsky J, Shaffer HJ, Albanese MJ. Benzodiazepine use during buprenorphine treatment for opioid dependence: Clinical and safety outcomes. Drug and Alcohol Dependence. 2013;132(3):580–586. doi: 10.1016/j.drugalcdep.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheidow AJ, McCart M, Zajac K, Davis M. Prevalence and impact of substance use among emerging adults with serious mental health conditions. Psychiatric Rehabilitation Journal. 2012;35(3):235–243. doi: 10.2975/35.3.2012.235.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, Lundgren L, Chassler D. Examining drug treatment entry patterns among young injection drug users. American Journal of Drug and Alcohol Abuse. 2007;33(2):217–225. doi: 10.1080/00952990601174774. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Rowan-Szal GA. Drug abuse treatment retention and process effects on follow-up outcomes. Drug and Alcohol Dependence. 1997;47(3):227–235. doi: 10.1016/s0376-8716(97)00099-9. [DOI] [PubMed] [Google Scholar]
- Smith A. Mobile Access 2010. Pew Internet & American Life Project; 2010. from http://pewinternet.org/∼/media//Files/Reports/2010/PIP_Mobile_Access_2010.pdf. [Google Scholar]
- Smith A. Smartphone ownership—2013 update. Pew Internet & American Life Project; 2013. from http://pewinternet.org/∼/media//Files/Reports/2013/PIP_Smartphone_adoption_2013.pdf. [Google Scholar]
- Smith DC, Cleeland L, Dennis ML. Reasons for quitting among emerging adults and adolescents in substance-use-disorder treatment. Journal of Studies on Alcohol and Drugs. 2010;71(3):400–409. doi: 10.15288/jsad.2010.71.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Cleeland L, Middleton A, Godley MD. Willingness and appropriateness of peers participating in emerging adults' substance misuse treatment. Journal of Substance Abuse Treatment. 2013;45(1):148–154. doi: 10.1016/j.jsat.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Godley SH, Godley MD, Dennis ML. Adolescent Community Reinforcement Approach outcomes differ among emerging adults and adolescents. Journal of Substance Abuse Treatment. 2011;41(4):422–430. doi: 10.1016/j.jsat.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeffing JM, Martin LD, Fingerhood MI, Jasinski DR, Rastegar DA. Buprenorphine maintenance treatment in a primary care setting: Outcomes at 1 year. Journal of Substance Abuse Treatment. 2009;37(4):426–430. doi: 10.1016/j.jsat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: Results from a randomized study. International Journal of Neuropsychopharmacology. 2008;11(5):641–653. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- Stone AL, Becker LG, Huber AM, Catalano RF. Review of risk and protective factors of substance use and problem use in emerging adulthood. Addictive Behaviors. 2012;37(7):747–775. doi: 10.1016/j.addbeh.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Subramaniam GA, Ives ML, Stitzer ML, Dennis ML. The added risk of opioid problem use among treatment-seeking youth with marijuana and/or alcohol problem use. Addiction. 2010;105(4):686–698. doi: 10.1111/j.1360-0443.2009.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam GA, Warden D, Minhajuddin A, Fishman MJ, Stitzer ML, Adinoff B, et al. Predictors of abstinence: National Institute of Drug Abuse multisite buprenorphine/naloxone treatment trial in opioid-dependent youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(11):1120–1128. doi: 10.1016/j.jaac.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Moore BA, O'Connor PG, Barry DT, Chawarski MC, Schottenfeld RS, et al. The association between cocaine use and treatment outcomes in patients receiving office-based buprenorphine/naloxone for the treatment of opioid dependence. American Journal on Addictions. 2010;19(1):53–58. doi: 10.1111/j.1521-0391.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uosukainen H, Pentikainen H, Tacke U. The effect of an electronic medicine dispenser on diversion of buprenorphine-naloxone-experience from a medium-sized Finnish city. Journal of Substance Abuse Treatment. 2013;45(1):143–147. doi: 10.1016/j.jsat.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Warden D, Subramaniam GA, Carmody T, Woody GE, Minhajuddin A, Poole SA, et al. Predictors of attrition with buprenorphine/naloxone treatment in opioid dependent youth. Addictive Behaviors. 2012;37(9):1046–1053. doi: 10.1016/j.addbeh.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug poisoning deaths in the United States, 1980–2008. NCHS Data Brief. 2011;81:1–8. [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, et al. Adjunctive counseling during brief and extended buprenorphine–naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, et al. Extended vs short-term buprenorphine–naloxone for treatment of opioid-addicted youth: A randomized trial. JAMA. 2008;300(17):2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TM, Myrick H, Malcolm R, Randall P, Boyle E, Henderson S, et al. Impact of lifetime alcohol quit attempts and medicated detoxifications on time to relapse during an index alcohol detoxification. Journal of Addiction Medicine. 2007;1(1):15–20. doi: 10.1097/ADM.0b013e318044ce4f. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Friedmann PD, Gerstein DR. Does retention matter? Treatment duration and improvement in drug use. Addiction. 2003;98(5):673–684. doi: 10.1046/j.1360-0443.2003.00354.x. [DOI] [PubMed] [Google Scholar]