Abstract
This study first showed the behavioural benefits of novel combination therapy of l-dopa with acupuncture on Parkinson’s disease, and its underlying mechanisms within basal ganglia. The previous study reported that acupuncture may improve the motor function of a Parkinson’s disease (PD) mouse model by increasing the dopamine efflux and turnover ratio of dopamine. Hence, we hypothesised that combining l-dopa with acupuncture would have a behavioural benefit for those with PD. We performed unilateral injections of 6-OHDA into the striatum of C57Bl/6 mice to model hemi-Parkinsonian attributes. To test motor function and dyskinetic anomalies, we examined cylinder behaviour and abnormal involuntary movement (AIM), respectively. We found that (1) a 50% reduced dose of l-dopa (7.5 mg/kg) combined with acupuncture showed an improvement in motor function that was comparable to mice given the standard dose of l-dopa treatment (15 mg/kg) only, and that (2) the combination treatment (l-dopa +acupuncture) was significantly superior in reducing AIM scores when equivalent doses of l-dopa were used. The combination treatment also significantly reduces the abnormal increase of GABA contents in the substantia nigra compared to the standard l-dopa treatment. Furthermore, abnormal expression of FosB, the immediate early gene of l-dopa induced dyskinesia (LID), was mitigated in the striatum by the combination treatment. All of these results indicate that acupuncture enhances the benefits of l-dopa on motor function with reduced dose of l-dopa and alleviating LID by normalising neurochemical imbalance within the basal ganglia.
Keywords: Levodopa-induced dyskinesia, Acupuncture, Parkinson’s disease, l-dopa combination treatment, GABA, FosB
1. Introduction
Parkinson’s disease (PD) is characterised by the neurodegeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc), which leads to progressive dopamine depletion in the striatum. This characteristic of PD disrupts the balance of the basal ganglia circuit and subsequently disrupts motor functions, leading to rigidity, tremor, and akinesia. One to five per cent of the older population (over 50 years of age) suffers from this second most common neurodegenerative disease (Thomas and Beal, 2007). Unfortunately, there is not yet a perfectly effective clinical treatment for PD patients; despite promising laboratory data, dopamine agonist drugs engender tolerance and adverse effects (Antonini et al., 2009). Additionally, surgeries such as deep brain stimulation and cell transplantation are costly and invasive (Fahn, 2003). A vast number of studies have been directed at making these therapies more effective and less risky.
Because it results in a dramatic improvement in motor function in the early stages of the disease, administration of the dopamine precursor l-dopa is currently one of the gold standards for PD treatment. Notwithstanding its widespread use, l-dopa produces adverse effects, including hallucination, insomnia, nausea, and dyskinesia (Fahn et al., 2004). Among these, l-dopa-induced dyskinesia (LID) is the most severe and occurs in PD patients who have received a chronic administration of l-dopa (Calabresi et al., 2010). It has been reported that more than 50% of PD patients who receive chronic l-dopa suffer from this side effect within 5 years (Rascol et al., 2000). Research continues in an effort to resolve l-dopa-related pathologies; however, much regarding these pathologies cannot yet be studied. The dose of l-dopa is still the most significant variable in the development of dyskinesia (Nyholm et al., 2010; Sharma et al., 2008), and lowering the dose of l-dopa is the best strategy for avoiding l-dopa-induced adverse effects (Cedarbaum et al., 1991; Poewe et al., 1986; Weintraub et al., 2008). However, lowering the dose of l-dopa alone is not ideal because a too-low dose, though safer, is less effective at alleviating symptoms and can even lead to extraneous disability (Kurlan, 2005). Thus, a novel treatment method that allows effective l-dopa treatment with a low dosage is highly important.
Acupuncture is one of the most common complementary therapies in East Asia, Europe and the US. Approximately 40% of PD patients in the UK use one or more complementary therapies, including acupuncture (Ferry et al., 2002). A recent clinical study also demonstrated the effectiveness of acupuncture point stimulation using bee venom or needles in treating PD patients (Cho et al., 2012). In our previous research, acupuncture improved the motor function of a PD mouse model by increasing the dopamine efflux and turnover ratio of dopamine (Kim et al., 2011a). The results showed that acupuncture enhanced dopamine transmission, leading to normalisation of the basal ganglia system.
There is growing evidence that the problems with l-dopa treatment, including adverse effects, originate from abnormal synaptic transmission (Picconi et al., 2008) and a dysregulated basal ganglia system (Bagetta et al., 2011; Kumar et al., 2009). In line with this idea, we hypothesise that combining l-dopa therapy with acupuncture could mitigate the limitations of treatment with l-dopa alone. In this study, we investigated whether this combined treatment improves motor function in 6-OHDA-induced PD mice and simultaneously alleviates LID. Furthermore, we also investigated the underlying mechanisms at the level of GABA and glutamate in the substantia nigra and FosB expression in the striatum.
2. Results
2.1. Screening appropriate condition of l-dopa with/without acupuncture for finding most effective combination therapy
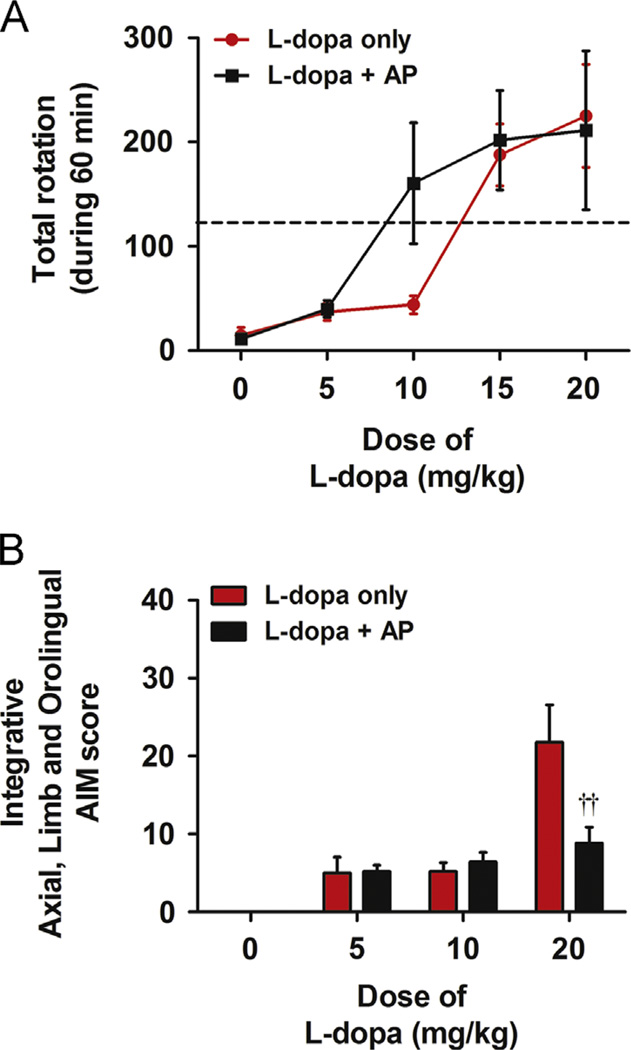
We analysed results from the rotation test to find the effective dose of l-dopa for combination treatment with acupuncture. We performed the rotational behaviour test in a clear cylinder. Five doses of l-dopa (0, 5, 10, 15, and 20 mg/kg) with or without acupuncture treatment were administered. With the combined treatment, the dose-response curve shifted to the left compared to those treated only with l-dopa (Fig. 1A). From the curve, the dose of l-dopa needed to produce 50% of the maximum turning behaviour was found to be approximately 15 mg/kg. This dose was set as the standard. Additionally, the dosage of l-dopa that was required to produce the same turning behaviour when combined with acupuncture was 7.5 mg/kg, half of the standard l-dopa dose (Fig. 1A). Therefore, we chose two doses (7.5 and 15 mg/kg) of l-dopa to test the effectiveness of l-dopa and acupuncture combinational therapy. Moreover, in the medium dose l-dopa (10 mg/kg), acupuncture did not show LID induction. Furthermore, acupuncture showed alleviation of LID when it combined with high dose of l-dopa (20 mg/kg). Therefore, we can conclude that this motor function improvement by acupuncture is not consequent of abnormal involuntary movement (Fig. 1B).
Fig. 1.
Dose-responsed motor function curve and abnormal involuntary movement (AIM) scoring relating l-dopa dosage with or without acupuncture. (A) Different doses of l-dopa (0, 5, 10, 15, and 20 mg/kg) were administered to the mice with and without acupuncture treatment. The dashed line indicates half the behavioural effect elicited by maximal dose of l-dopa (20 mg/kg). The curve of the group treated with l-dopa and acupuncture combination was shifted to the left compared to the group treated only with l-dopa. The dose that had the same behavioural effect of l-dopa was lower in the curve of the group receiving the combination treatment. Data are presented as the mean±SEM. (B) Ten days after the l-dopa injection, AIM scoring tests were given to all mice. There was a significant difference between mice treated only with high dose of l-dopa (20 mg/kg) and those treated with l-dopa+AP combination. ††p<0.01, compared to group of l-dopa (20 mg/kg) only, analysed by a repeated-measures two-way ANOVA and Bonferroni’s post hoc test.
2.2. Synergistic effect of combined treatment on Parkinson’s disease mouse model
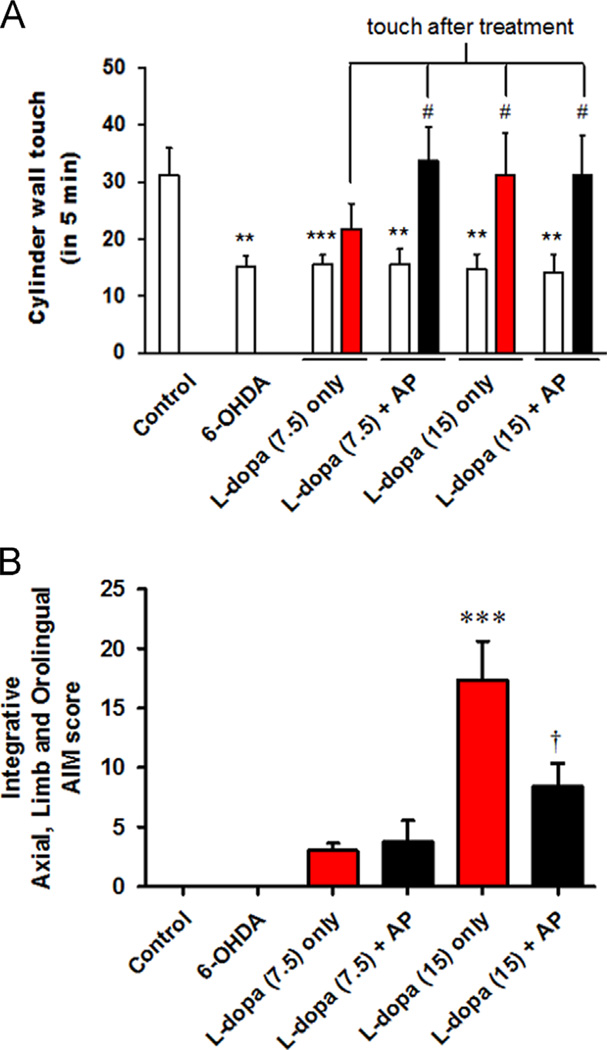
To find the beneficial effect of this combined treatment on PD, five main experimental treatment groups were created within 6-OHDA-induced PD mice: no-treatment, 7.5 mg/kg of l-dopa with or without acupuncture and 15 mg/kg of l-dopa with or without acupuncture. At the conclusion of the experiment, the mice were placed in a cylinder before and 2 h after l-dopa administration with or without acupuncture treatment. The 6-OHDA-depleted mice showed a significant decrease in cylinder wall touches compared to the control group (31.2±4.8 vs. 15.2±1.8, p<0.01 compared to Control). Groups treated only with a high dose of l-dopa (15 mg/kg) showed an increase in cylinder wall touches (31.2±7.4 vs. 14.8±2.6, p<0.05 compared to before) to a normal level, while those receiving the low dose of l-dopa (7.5 mg/kg) did not (21.8±4.6 vs. 15.6±1.7, p>0.05 compared to before). With acupuncture treatment, the low dose of l-dopa (7.5 mg/kg) was able to restore behaviour to normal levels (33.8±5.9 vs. 15.6±2.7, p<0.05 compared to before) (Fig. 2A). For testing adverse effects, we also assigned AIM scores after l-dopa injection. We found that the high dose (15 mg/kg) of l-dopa induced more dyskinesia (17.4±3.2 vs. 0.0±0.0, p<0.001, compared to the control and 6-OHDA groups) than the low dose (7.5 mg/kg) of l-dopa (3.0±0.6 vs. 0.0±0.0, p>0.05, compared to the control and 6-OHDA groups). Moreover, acupuncture combined with the high dose of l-dopa induced less dyskinesia than in those treated only with l-dopa (8.4±1.9 vs. 17.4±3.2, p<0.05 compared to only l-dopa treatment (15 mg/kg)) (Fig. 2B). A sub-analysis of AIM scores showed that all subcategories of the AIM scores also have this tendency (data not shown).
Fig. 2.
Cylinder test results showing the effects of l-dopa and acupuncture treatment on number of wall touches and abnormal involuntary movement (AIM) analysis. (A) All mice were given the cylinder test to extract behavioural quantification before l-dopa injection and 1 h after l-dopa injection with or without acupuncture treatment. Bar graph shows the mean values of the total number of forelimb contacts made with the cylinder wall. Blank bars represent the total counts of cylinder wall touches before the treatment. Filled bars represent the total cylinder wall touch counts at 1 h after the l-dopa injection with or without acupuncture treatment. **p<0.01 and ***p<0.001, compared to the value of control group, analysed by a one-way ANOVA, followed by Bonferroni’s post hoc test. #p<0.05, compared to previous value (before) of each group, analysed by a repeated-measures ANOVA, followed by Bonferroni’s post hoc test. (B) Integrative AIM score after l-dopa injection (7.5 and 15 mg/kg) with or without acupuncture treatment. There was a significant difference between the group treated only with l-dopa and l-dopa+AP when the dose of l-dopa was high (15 mg/kg). ***p<0.01 compared to the value of control and 6-OHDA group. †p<0.05 compared to the value of l-dopa (15 mg/kg) only group, analysed by a one-way ANOVA, followed by Bonferroni’spost hoc test. Data are presented as the mean±SEM.
2.3. Postsynaptic neuronal FosB activation analyses
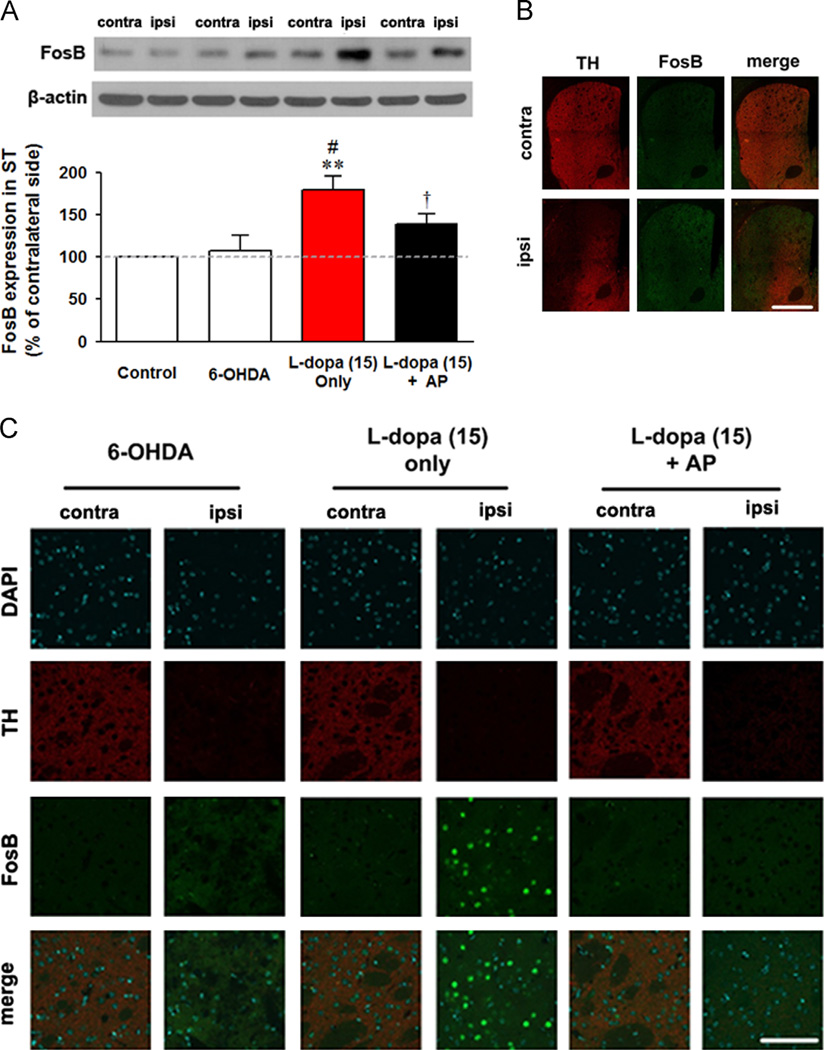
Next, we investigated the abnormal protein activation of FosB via western blot and immunofluorescence analysis. Abnormalities in FosB expression are associated with LID. Blot analyses showed that a high dose of l-dopa induces an abnormal increase of FosB (171.2±19.2 vs. 100.0±1.1, p<0.01 compared to the control; vs. 107.7±17.6, p<0.05 compared to the 6-OHDA group), while the combined therapy partially mitigated this increase (138.8±12.1, p<0.05 compared to the group treated only with l-dopa) (Fig. 3A). Additionally, these results were shown in histological data (Fig. 3B and C). This supports the results that combined therapy alleviates LID.
Fig. 3.
Effect of Levodopa-induced dyskinesia (LID) and acupuncture treatment on abnormal FosB activation in the striatum. (A) Western blotting analysis for markers of FosB in striatum. “contra” refers to contralateral (un-lesioned) side and “ipsi” refers to ipsilateral (lesioned) side striatum of each group. (B and C) Immunofluorescence image for the mice striatum. The mice striatum were double stained for tyrosine hydroxylase (TH) and FosB and then DAPI stained for nuclei at low magnification (B) and high magnification (C). (B) Low magnification image of the striatum of l-dopa (15 mg/kg) only group (scale bars indicate 400 µm). (C) High magnification image of the striatum of 6-OHDA, l-dopa (15 mg/kg) only, and l-dopa (15 mg/kg)+AP group (scale bars indicate 100 µm). FosB activations showed not only un-colocalisation with TH but also overactivation where dopamine fibres were depleted, which means that this protein activation was not affected by presynaptic TH protein and postsynaptic neuronal activation. **p<0.05 compared to the value of control group, #p<0.05 compared to the value of 6-OHDA group, and †p<0.05 compared to the value of l-dopa (15 mg/kg) only group, analysed by a one-way ANOVA, followed by Bonferroni’s post hoc test. Data are presented as the mean±SEM.
2.4. Neurotransmitters GABA and glutamate analyses
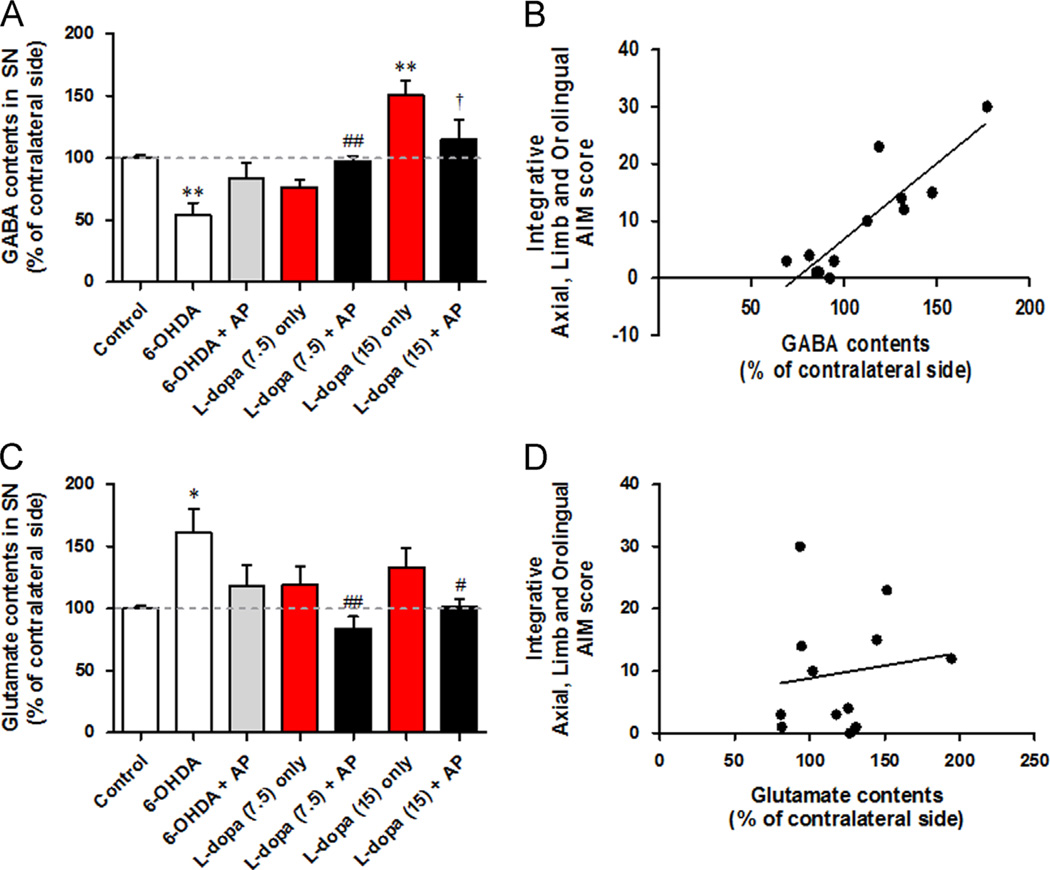
Following the behavioural experiments, we performed biochemical analyses of GABA and glutamate. Tissue samples of the substantia nigra pars reticulate (SNr) were analysed 20 min after l-dopa injection. Striatal GABA contents are presented as the ratio of the lesioned side to the intact side. HPLC analysis showed that 6-OHDA-depleted PD mice had significantly decreased GABA levels (99.8±2.2% vs. 54.2±9.7% of contralateral GABA, p<0.01 compared to the control group). The low dose (7.5 mg/kg) of l-dopa administration showed particularly up-regulated GABA contents (to 76.6±5.7%, p<0.05 compared to 6-OHDA). However, after a high dose (15 mg/kg) of l-dopa administration, GABA contents enormously increased to 150.4±11.9% (p<0.001 compared to 6-OHDA, p<0.001 compared to the control group). These high levels of GABA also mean that the basal ganglia system is in disequilibrium. The combination of acupuncture with l-dopa normalised the GABA levels, either as a decrease from 6-OHDA or as an increase from the high dosage of l-dopa to normal control levels (Fig. 4A). Acupuncture combination therapy using a low dose of l-dopa increased GABA levels to 97.1±4.6%, which is higher compared to those treated only with l-dopa (76.6±5.7%). The combination therapy using a high dose of l-dopa significantly decreased GABA levels to 115.3±16.0% compared to those treated only with l-dopa (150.4±11.9%, p<0.05).
Fig. 4.
Effect of Levodopa-induced dyskinesia (LID) and acupuncture treatment on GABA and glutamate contents in the substantia nigra. (A and C) The bar graph shows the per cent GABA (A) and glutamate (C) content of the ipsilateral (lesioned) side to the contralateral (un-lesioned) side of mice substantia nigra. A grey dashed line means the value of control group. (B and D) Regression test between GABA/glutamate contents and AIM score. (B) Increased GABA contents showed significant correlation with increasing AIM score. (D) There was no significant correlation between glutamate contents and AIM score. *p<0.05, **p<0.01 and ***p<0.001, compared to the value of control group, #p<0.05, ##p<0.01 and ###p<0.001, compared to the value of 6-OHDA group, †p<0.05, compared to the value of l-dopa (15 mg/kg) only group, analysed by a one-way ANOVA, followed by Bonferroni’s post hoc test. Data are presented as the percentages±SEM of the contralateral side values.
In the glutamate analysis, 6-OHDA-depleted mice showed a significant increase in glutamate levels in the substantia nigra (99.1±1.7% vs. 160.8±19.4% of contralateral glutamate, p<0.05 compared to the control group). Both l-dopa treatments normalised this increase of glutamate (Fig. 4C); their levels showed no significant difference (7.5 mg/kg vs. 15 mg/kg, 118.7±15.7% vs. 133.4±17.2%). In the combination therapy, more significant decreases were found with both the low dose (160.8±19.4% vs. 84.0±9.6%, p<0.01 compared to 6-OHDA) and the high dose of l-dopa (160.8±19.4% vs. 101.5±6.2%, p<0.05 compared to 6-OHDA). Additionally, GABA levels were significantly correlated with the AIM score (r=0.8970, p<0.0001) (Fig. 4B), while glutamate levels were not (r=0.1452, p=0.6526) (Fig. 4D).
3. Discussion
l-dopa shows great potential in the treatment of PD patients; however, it has serious adverse effects. Thus, researchers have sought to find a novel therapy for reducing l-dopa’s adverse effects while enhancing its benefits. In this study, we found acupuncture and l-dopa in combination can enhance the benefits of l-dopa and alleviate the adverse effects.
In past works studying l-dopa-induced dyskinesia (LID), both MPTP (Ding et al., 2011) and 6-OHDA (Santini et al., 2007) mouse models are used. However, because the main target of LID is mostly related with abnormal plasticity of dopaminergic system (Santini et al., 2008), 6-OHDA induced hemi-PD rodent model was more frequently used. Thus, we decided in this first study to concentrate on looking changes of dopamine plasticity using 6-OHDA rodent model. However, further studies are also needed to study combined treatment in another LID mouse model, induced by MPTP.
To determine the most effective conditions for combination therapy, the best method of acupuncture and optimal dose of l-dopa were discovered. When combined with l-dopa, acupuncture treatment at GB34 had the best effect on alleviation of dyskinesia model compared to another acupoint candidate and a non-acupoint. The results in PD mice also suggested the acupoint GB34 is the most effective for improving motor function. Next, we sought to determine whether the acupuncture and l-dopa combination therapy lowered the effective dose of l-dopa and improved motor function. In the results, the combination therapy shifted the dose-dependent behavioural response curve to the left. As anticipated, mice who received combined acupuncture and l-dopa therapy required half the dose of l-dopa to increase turning responses.
An interesting finding was that there are similar rotation between the combined treatment and l-dopa alone under high dose condition. We have a few speculations as to why the two groups—combined and l-dopa at high dose (20 mg/kg) and l-dopa alone (20 mg/kg)—yielded similar rotation behaviours. First, the behaviours could reflect pharmacokinetics, whereby the dose-dependent activity is lost beyond the maximum (saturation) point. Popoli et al. demonstrates this loss of dose-dependency in behavioural responses of dopamine D1/D2 agonist-treated mice. Once the optimal dose was identified, further increases in dosage past that point showed not only an unpairing of the dose-dependent relationship, but also a decrease in performance (Popoli et al., 2000). Second, the physical limitations of mice pose problems for accurately reflecting the internal state through motor behaviour. Szcypka et al. showed that administering l-dopa to dopamine-deficient mice induced hyperactivity, but only up to a certain threshold as defined by his motor limitations (Szczypka et al., 1999). Another experiment compared the tail flick latency between morphine-treated dopamine-deficient mice, to that of controls. Morphine creates hypersensitivity in the dopamine-deficient group. Upon reaching the peak locomotor response, the mice could no longer display dose-dependent hypersensitivity, and matched the motor performance of the controls (Hnasko et al., 2005). Similarly, we believe our behavioural results between the two 20mg/kg groups demonstrate this reaching of maximum threshold.
With these preconditions (acupuncture at point GB34 and the half-dose of l-dopa) for combination therapy, we investigated the effect of the combined treatment on PD mice model. In cylinder tests designed to check motor functions, the 6-OHDA-induced PD mice showed significantly decreased forelimb contact with the cylinder wall relative to normal control mice. Mice that received the combination treatment showed motor function improvement that matched the normal control mice and the mice receiving purely the double dose of l-dopa. In other words, the combination therapy reduced the effective dose of l-dopa necessary to improve motor function without inducing the severe LID that a double dose of l-dopa would have otherwise produced. Additionally, we found that AIM shown in the standard l-dopa group was significantly mitigated by acupuncture. Overall, we can conclude that acupuncture and l-dopa in combination reduce the effective dose of l-dopa, alleviating adverse effects and enhancing its benefits.
The basal ganglia system is known to be involved in numerous neurological systems, including motor function, habit learning, reinforcement, and addiction; the primary role of the basal ganglia is to relay cortical signals and, accordingly, to modify brain function (Crittenden and Graybiel, 2011). The signalling cascade starts from autonomous paced dopamine transmission release from the SNc to the striatum. The dopamine secreted into the striatum transmits its signal to the SNr via two GABAergic pathways, the “direct pathway” and the “indirect pathway”.The “direct pathway” involves dopamine activation of receptor D1 in the striatum. When this receptor is activated by dopamine, the striatal neurons directly transmit GABA signal to the SNr. We found that 6-OHDA decreases GABA contents in SNr, and this decrease in GABA levels was soon restored by l-dopa treatment, indicating that dopamine replacement replenishes the 6-OHDA-induced abnormality in the basal ganglia. Interestingly, the combination therapy increased GABA contents to normal levels more effectively than treatment with solely l-dopa or acupuncture (Fig. 4A). With support from a previous study (Kim et al., 2011a), we suggest that acupuncture-induced modulation of dopamine transmission works synergistically with l-dopa-induced replacement of dopamine. High doses of l-dopa led to abnormal increases of GABA contents in SNr, and combination therapy reduced this increase. Because high levels of GABA in SNr are correlated with abnormal dyskinesia (Bido et al., 2011) and this study showed correlations between increased GABA contents and the AIM dyskinesia score (Fig. 4B), we could conclude that the combination treatment alleviates the adverse effects of l-dopa by modulating GABA in the SNr. In the “indirect pathway”, dopamine interacts with receptor D2 and triggers GABA transmission to the subthalamic nucleus, suppressing the excitatory glutamatergic activity to the SNr. HPLC analyses showed that l-dopa significantly normalises the 6-OHDA induced increases in the glutamate contents of the SNr. In the indirect pathway, dopamine depletion induced by 6-OHDA ultimately leads to an increase in glutamate input to SNr, acting in contrary to the decrease of GABA input to SNr by the direct pathway. Thus, the replacement of dopamine by l-dopa mitigated the above conditions. Combination therapy also showed synergistic effects in regulating glutamate contents (Fig. 4C), which means that dopamine replacement by l-dopa and dopamine enhancement by acupuncture both operate via the indirect pathway. Unlike GABA, a high dose of l-dopa therapy did not induce an abnormal decrease of glutamate contents. Furthermore, we found no correlation between AIM scores and glutamate contents, suggesting that l-dopa-induced abnormal changes are more closely related to the direct pathway than the indirect pathway.
Next, we examined protein activation changes brought about by LID. It was previously determined that high doses of l-dopa in PD patients induce abnormal protein changes in postsynaptic neurons (Feyder et al., 2011). The immediate early gene FosB is an important final protein responsible for abnormal plasticity in LID (Andersson et al., 1999); striatal overexpression of FosB reproduces involuntary movements and abnormal responsive activity to dopamine (Cao et al., 2010). High doses of l-dopa increased FosB expression compared to the 6-OHDA group.
All of these mechanistic analyses support behavioural benefits by the combined treatment to alleviate LID by normalising FosB activation and neurotransmissions within basal ganglia system (Fig. 5).
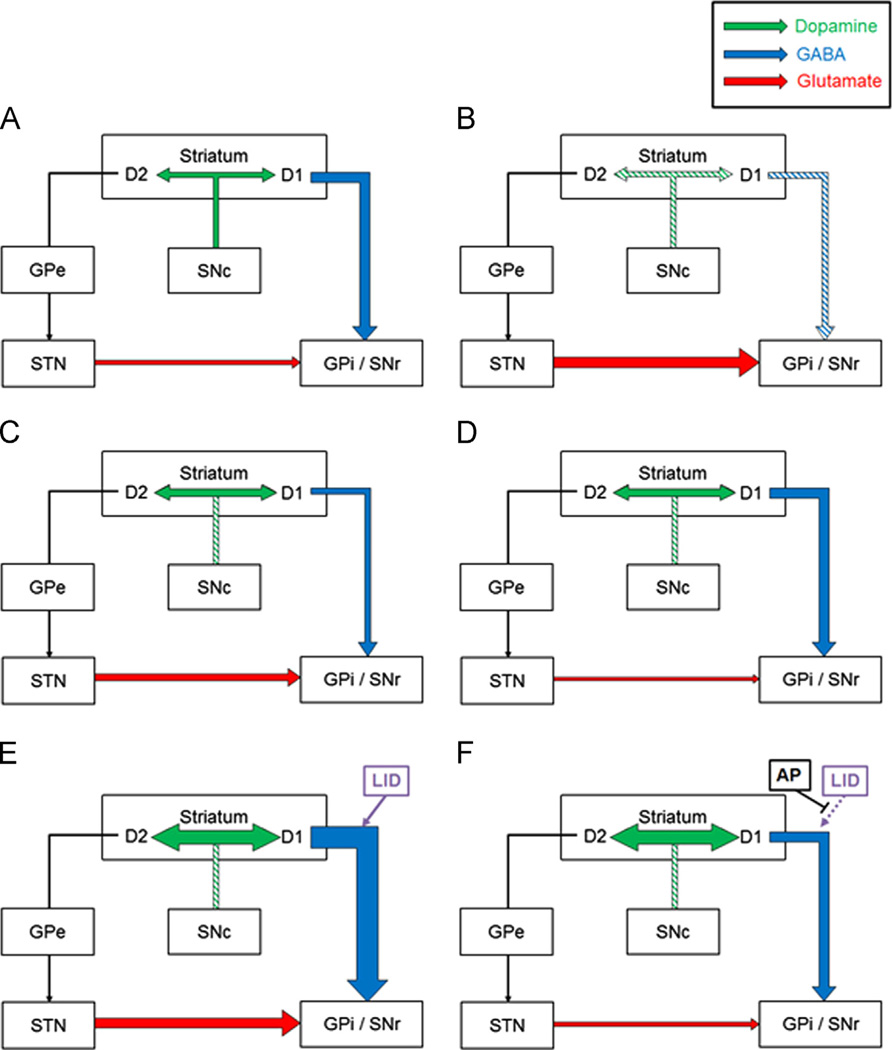
Fig. 5.
Schematic hypothetical mechanism illustration of this experiment. (A) Healthy basal ganglia in normal control group. The dopaminergic pathway describes the projection of dopaminergic neurons from the substantia nigra pars compacta (SNc) to the striatum. Those nigrostriatal dopaminergic neurons reach the striatal output neurons through either the direct or indirect GABAergic pathways. The excitatory dopamine D1 receptors activate the direct GABAergic pathway; striatal neurons project to the substantia nigra pars reticulata (SNr) directly. The indirect pathway describes the course that inhibitory dopamine D2 receptors activate; the D2 activated striatal neurons project to the external globus pallidus (GPe). Upon receiving GABAergic input, the GPe proceeds to inhibit the subthalamic nucleus (STN), which, in turn, inhibits excitatory glutamatergic pathway that projects to the internal globus pallidus (GPi) and SNr. Signals from the direct and indirect pathways are integrated and sent to the thalamus and spinal cord to control movement (illustration not shown). (B) In the mice of the 6-OHDA group, decreased dopamine content affects downstream GABA/glutamatergic input to the SNr. (C) Treating Parkinsonian mice with l-dopa replaces dopamine and normalises basal ganglia system by changing the GABA/glutamatergic projection to the SNr. (D) Because acupuncture treatment enhances dopamine availability and l-dopa replaces dopamine contents, the combined therapy of l-dopa with acupuncture shows a synergistic effect in normalising the basal ganglia system. (E) In the mice that received 6-OHDA+ l-dopa (high) only, overdose of l-dopa not only replaces dopamine at an excessive concentration but also overactivates the D1 receptor to cause hyperactivity of the direct GABAergic projection to SNr. This increased activity of the direct pathway is representative of the pathophysiology of Levodopa-induced dyskinesia (LID). (F) With acupuncture treatment, we found a significant normalisation of abnormal GABA/glutamate inputs to the SNr and inhibition of the pathophysiology of LID.
Nevertheless, there are limitations to this study; the translation from a biochemical assay to neuron interaction rests heavily on speculation and assumptions. For example, GABA has recently been found to be released from not only GABAergic neurons but also from glial neurons, which means that GABA contents also have other roles in interactions between glia and neurons (Lee et al., 2010). Different studies have reported that l-dopa increases the glutamate in the striatum or SNr of Parkinsonian rat models (Marti et al., 2012; Mela et al., 2012). These discrepancies may stem from the multifaceted roles of glutamate and GABA in the brain as a whole as neurotransmitters and from different methods of biochemical assay. Electrophysiological studies of specific brain regions and close analyses of other neurotransmitters may provide more concrete evidence to rule out some of the speculations. Further studies are needed to investigate the detailed mechanism underlying this effect of acupuncture.
This study demonstrated that the effect of l-dopa treatment is significantly enhanced when it is combined with acupuncture. The administration of low-dose l-dopa in conjunction with acupuncture potentiates motor function improvement without the onset of adverse effects such as LID. This shows much promise as a potential novel therapeutic method for the management of PD. Because in actuality, the only choice of treatment for PD patients is a dopamine replacement drug in clinics. However, drug treatment has limitations in alleviating the whole symptoms of PD patients. Moreover, adverse effects and tolerance of drug produce another problem to patients. Based on this study, the additional benefits of acupuncture to l-dopa not only complement the limitations of l-dopa but also show the possibility of acupuncture to give beneficial additions to conventional medicine in clinics so that PD patients could choose more options to treat their condition. Though further research is needed to fully comprehend the mechanisms of the combination therapy, this study offers preclinical scientific evidence for an integrated approach to PD management.
In summary, we found that acupuncture and l-dopa combination therapy reduces the effective dose of l-dopa and alleviates LID, an adverse effect of l-dopa. Acupuncture modulates the basal ganglia system to enhance the benefits of l-dopa while mitigating its adverse effects. This is the first demonstration of the beneficial effects of a complementary integrative medicinal method for l-dopa, and it sheds light on the promise of such integrative approaches. These novel findings provide preclinical evidence that acupuncture and l-dopa combination therapy would be beneficial to control PD’s motor dysfunction and medication-related dyskinesia.
4. Experimental procedures
4.1. Design
This study is composed of preliminary experiment and main experiment. In the preliminary experiment, to find the most effective level of l-dopa to use in the combination therapy, contralateral rotations were counted in the l-dopa-induced rotational test as previously described (Malmlof et al., 2010) and abnormal involuntary movement (AIM) were scoring. Five doses of l-dopa were used (0, 5, 10, 15, and 20 mg/kg) to construct a dose-dependent curve of motor function. Because dopamine agonists, including l-dopa, reacted to the sensitised side of the brain in the hemi-Parkinsonian mice model, we tested contralateral turning behaviour and its relationship with the gradual increase in the dose of l-dopa. The dose-dependent curve and subsequently the effective quantity of l-dopa were derived from the total number of contralateral rotations during the 60-min interval after l-dopa injection. The optimal dose of l-dopa to be used in combination therapy was found by identifying the dose at which the combination therapy results matched those of a standard l-dopa therapy. After the injection with or without acupuncture treatment 10 days more, AIM were scored for test the side effect in addition to motor function improvement by same treatment. In the main experiment, normal control and 6-OHDA-induced PD mice were used. PD mice were divided into five groups: no treatment and two by two (2 × 2) designed combination treatment, consisting of two doses of l-dopa (7.5 mg/kg and 15 mg/kg) with or without acupuncture. The anti-Parkinsonian effect was examined by the cylinder test, and dyskinetic behaviour was evaluated by scoring the animals for AIMs at the end of experiment.
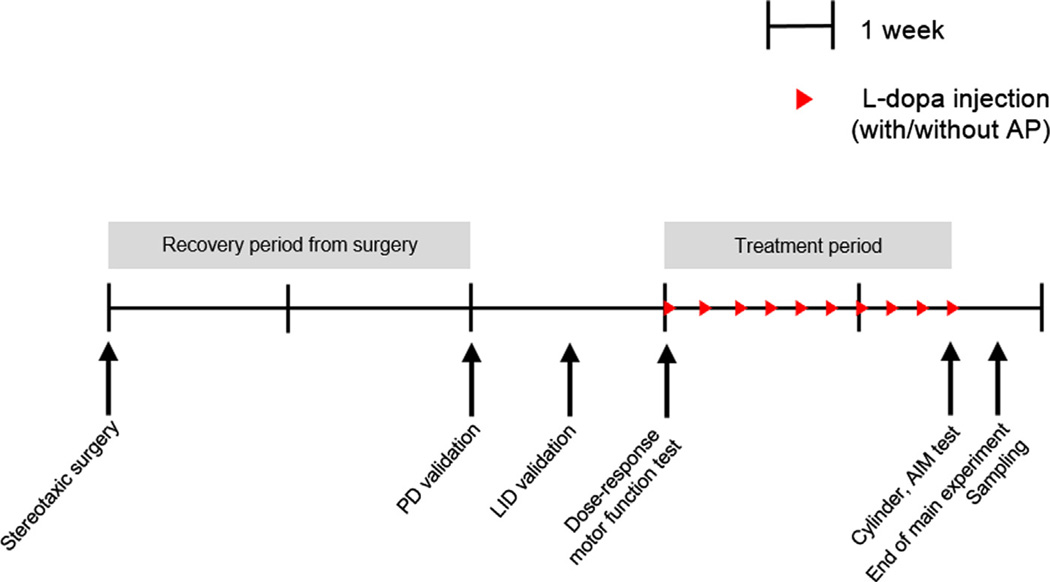
Experimental schedule is illustrated in Fig. 6. From day 0 of the experiment, all mice received stereotaxic injections of saline or 6-OHDA with two weeks of recovery time from surgery. Because not all mice show the same severity of dyskinesia (Santini et al., 2007), we tested the mice’s dopamine-depleted and LID-induction states. Through this validation test, we screened and excluded any un-induced or moderately induced mice from this study. Finally PD-and LID-validated mice were used for the whole experiment. After this strict alignment of the LID state across all groups, the mice in the main study were divided into 6 groups as indicated in the experimental design. After an additional week, mice received the drug injection, acupuncture treatment, or both, according to their schedule. To test dose-responsed motor function resulting from treatment with l-dopa with or without acupuncture, the mice were given a rotational test on the first treatment day. Following continued l-dopa therapy, the motor benefits and adverse effects from chronic treatment were evaluated with two behavioural tests on day 30: the cylinder test and AIM test. At the end of the experiment, substantia nigra and striatum tissue samples were collected for post-experimental analysis (Fig. 6).
Fig. 6.
Schematic flow-chart of experimental design. Capped line represents 1 week time progression from left to right. Red arrow denotes the daily l-dopa injection with or without acupuncture treatment. On day 0, all mice were given saline or 6-hydroxydopamine (6-OHDA) injections as determined by their group assignments (dopamine depleted or control). Two weeks later, an apomorphine test and an acute l-dopa induction test were performed to validate Parkinson’s disease (PD) and Levodopa-induced dyskinesia (LID), respectively. Through these validation tests, PD or LID un-induced mice were exclusively screened. Three weeks after surgery, all mice were further subdivided into experimental groups for the main experiment. If necessary, behavioural tests were performed on day 22 to examine the dose-response motor function. On day 30, final behavioural tests were performed with cylinder test and abnormal involuntary movement scoring. All experiments were finished on day 31.
4.2. Animals
Nine-week-old male C57BL/6 mice (Central Lab. Animal Inc., Seoul, Republic of Korea), weighing 24–26 g each, were used. All experiments were approved by the Kyung Hee University Animal Care Committee for animal welfare [KHUASP(SE)-09-046] and maintained in strict accordance with Guidelines of the NIH and Korean Academy of Medical Sciences. Stereotaxic surgery was performed to create unilateral 6-OHDA lesions on mice’s striata.
4.3. Stereotaxic surgery for unilateral lesion
For stereotaxic surgery, mice were anesthetised with a mixture of tiletamine+zolazepam (30 mg/kg; Zoletil 50, Virbac, France) and xylazine (10 mg/kg; Rompun, Bayer Korea, Republic of Korea) in physiological saline and mounted in a stereotactic frame with a mouse adaptor (Stoelting Co., USA). A 6-OHDA-HCl mixture (3.0 mg/ml; Sigma Aldrich, USA) was dissolved in 0.02% ascorbic acid-based physiological saline. Mice received saline or 6-OHDA injections (2 × 2 µl) into the right striatum unilaterally at the following coordinates according to the mouse brain atlas (BJ and GP, 2008): +1.0 mm of AP, −2.1 mm of ML, −3.2 mm of DV; and +0.3 mm of AP, −2.3 mm of ML, −3.2 mm of DV. The flow rate of each injection was 0.5 µl/min.
4.4. Validation of the dopaminergic lesions
Following surgery, the mice were given two weeks for recovery. Five minutes after a subcutaneous injection with 0.6 mg/kg of apomorphine (Sigma–Aldrich, USA), screening of rotational activity during 10 min was performed for validation of the dopaminergic lesions. The number of turns was then recorded during 10 min period after the administration of apomorphine. The cut-off number of turns was set to at least five contralateral turns (turn to un-lesioned side) per minute, which correlates to approximately 70% striatal dopamine denervation according to the western blotting results of preliminary experiments (data not shown). Thus, all animals selected for the following studies showed severe motor impairment and dopaminergic depletion.
4.5. Acupuncture treatment
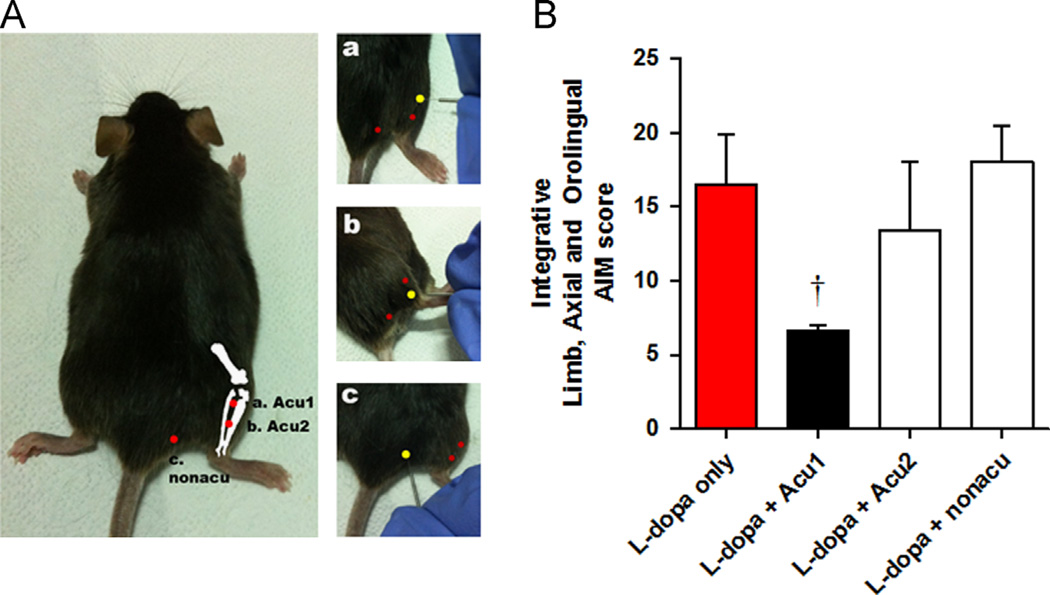
The mice were lightly immobilised to minimise stress. Acupuncture needles (DongBang Acupuncture Inc., Republic of Korea) were bilaterally inserted to a depth of 3 mm at each acupuncture point, turned at a rate of two spins per second for 15 s, and then immediately removed. The control group was also immobilised for the same amount of time in order to experience restraint equivalent to the treatment groups. To determine which acupuncture point is the most effective, LID-validated mice were divided into four groups; all groups received the standard l-dopa treatment, and three groups also received one of three different acupuncture methods: “Acu1” (GB34), “Acu2” (GB36) and “non-acupoint” (control point) (Fig. 7A). Acupuncture point GB34 has been used to treat movement disorders in traditional East Asian medicine, and recent neuroimaging studies also discuss its effects on motor function (Chae et al., 2009; Huang et al., 2010; Na et al., 2009). Moreover, it also showed effective function on Parkinson’s disease mouse model in our previous research (Jeon et al., 2008; Kim et al., 2010, 2011a,b). The point is located at the intersection of the anterior and posterior margin of the fibular head. This location corresponds anatomically to the acupuncture point used in humans. GB36, another specific acupuncture point, is the midpoint of the line connecting the prominence of the lateral malleolus and the GB34 point. We used GB36 as another target point because of its physical proximity to GB34. In addition to the two acupoints GB36 and GB34, we also used the “nonacu” to control the non-specific effects of acupuncture stimulation. This point was set at a point that was approximately 3 mm to the lateral side of the tail on the gluteus muscle. After grouping, chronic l-dopa administration with 20 mg/kg dosage with or without acupuncture was performed for 10 days to induce LID. At the end of the experiment, observers who were blind to the group assignment measured AIM scores for all mice. As a result showed, we found that Acu1 was most effective on alleviation of LID, so that we choose Acu1 to treating point in the whole experiment (Fig. 7B).
Fig. 7.
Acupuncture treatment method. (A) Location of acupuncture treating point. This figure shows the anatomical location of where acupuncture was performed on the mice. The yellow point indicates the treated point and depicts its relative anatomical location to other points (red point). (a) “Acu1” point is located anterior to the fibular head. (b) “Acu2” point is located in the middle of the mouse hindlimb between the tibia and fibula. (c) “nonacu” point is located 3 mm to the lateral side of the tail on the gluteus muscle. (B) Abnormal involuntary movement (AIM) scoring for selection of acupuncture point which is the most effective to LID. Ten days after l-dopa injection, AIM scoring tests were given to all mice. There was a significant difference between mice treated only with l-dopa and those treated with l-dopa+Acu1 combination. †p<0.05, compared to group of l-dopa only, analysed by a repeated-measures one-way ANOVA and Bonferroni’s post hoc test.
4.6. Cylinder test
The dopamine-depleted PD mice showed decreased movement behaviour, shown in fewer forelimb contacts with the supporting wall; this behaviour can be restored by l-dopa treatment. The mice were placed in a plastic cylinder (12 cm in diameter and 20 cm tall) without habituation and recorded with a video camera for five minutes. After the recording, observers who were blind to the experiments counted the number of forelimb wall contacts.
4.7. Abnormal involuntary movement (AIM) score
To quantify LID, each mouse was placed in a separate cage for a blinded observer to assess abnormal behaviours. The observations were made individually every 20 min from 20 to 140 min after the injection of l-dopa or vehicle (Cenci et al., 1998). Movements were considered LID-induced if their displayed behaviour satisfied the following criteria: (1) movement developed after injection of l-dopa; (2) movement inclined to contralateral side of the lesion; and (3) repetitive, purposeless, clearly unnatural movements. AIM scores were then classified into subtypes: (1) axial AIM, twisted posture of the body and neck toward contralateral side; (2) limb AIM, repetitive rhythmical spasm or dystonic posture of the forelimb; and (3) orolingual AIM, abnormal chewing movements and occasional tongue flourish. For scoring these subtypes, dyskinesia was scored on a four-point scale reflective of frequency (0, absent; 1, occasional; 2, frequent; 3, continuous but interrupted by external stimuli; 4, continuous, severe, not interrupted by external stimuli). After scoring, we integrated three subtypes of AIM scores (axial, limb and orolingual AIM) for group comparison.
4.8. High performance liquid chromatography (HPLC)
The SNr were dissected from the mice brain on 30 min after injection, according to mouse brain atlas (BJ and GP, 2008). They were immediately frozen by liquid nitrogen and stored at −80 °C until used. Then, they were homogenised with methanol and prepared for analysis. GABA and glutamate content in SNr were determined by an HPLC system (Waters Co., USA) with a fluorescence detector (FLD; Waters Co., USA) coupled to an automated pump (Waters Co., USA). The system was equipped with a 5 µm particle size (C18, 150 mm × 4.6 mm) analytical column (Atlantis, Waters Co., Ireland). Analytic software (Waters Co., USA) was used to analyse the chromatographic data. The mobile phase consisted of 50% 0.05 M sodium acetate, 1% tetrahydrofuran and 49% methanol (v/v) adjusted to pH 4.0 by acetate (Sigma, USA). All materials soluted in the mobile phase were filtered through 0.45 µm Durapore membrane filters (Millipore, USA). SNr soluted in 90% methanol were eluted isocratically over a 15 min runtime at a flow rate of 1 ml/min. The fluorescent detector was set at an excitation wavelength of 337 nm and an emission wavelength of 454 nm. GABA and glutamate were characterised by their peak retention times as determined by standard solutions (Sigma, USA). The integrated data were measured by calculating the total area under the sample peaks compared to calibrate standard samples through the integrator system.
4.9. Western blotting
Thirty minutes after the last l-dopa injection, the mice striata were quickly dissected and lysed in 2.5 M NaCl, 1 M tris–HCl, 0.5 M sodium diphosphate, 1 M NaF, 0.5 M EDTA, 0.5 M Na3VO4, and 10% Triton X-100. Samples (30 µg) were resolved on 10% SDS PAGE gels and electrotransferred to PVDF membranes. The membranes were shaken in tris-buffered saline containing 0.1% tween-20. To block non-specific signals, the membranes were shaken in 5% skim milk for 1 h at room temperature. We looked for the expression of the following primary antibodies: FosB (Cell Signalling, USA), tyrosine hydroxylase (Santa Cruz, USA), and β-actin (Sigma Aldrich, USA). After the membranes were shaken in primary antibody solution overnight, they were shaken for 1 h at room temperature in an HRP-conjugated secondary antibody (Pierce Biotechnology, USA) and visualised with a chemiluminescence kit (West Pico; Pierce Biotechnology, USA). The band intensities of the detected proteins were measured by computer software.
4.10. Immunofluorescence
The animals were sacrificed and perfused transcardially with 4% paraformaldehyde in 0.2 M phosphate buffer. The brains were removed, post-fixed, and cryo-protected. The brain tissues were then prepared in free-floating sections (40 µm thickness) that encompassed the entire striatum (level between AP +0.38~+0.98 mm from bregma in accordance with the atlas of the mouse brain (BJ and GP, 2008)). After incubation with 3% H2O2 in 0.05 M phosphate-buffered saline, the sections were blocked with 1% BSA and normal goat serum. Then, the sections were incubated overnight at room temperature in primary antibodies. Mouse tyrosine hydroxylase (Santa Cruz, USA) and rabbit FosB (Cell Signalling, USA) were used in this study as primary antibodies. The tissue sections were incubated with anti-rabbit Alexa-488 and anti-mouse Alexa-594 immunoglobulin G (Molecular probes, USA) for 1 h at room temperature for double staining. Fluorescence was examined using a confocal laser scanning microscope (Fluoview FV10i; Olympus, Japan). All procedures for analyses were performed in a blind manner to minimise the possibility of observer bias.
4.11. Data analysis
All procedures, assessments, and analyses were performed blindly to minimise observer bias. SPSS 17.0 (SPSS Inc., Chicago, USA) was used for statistical procedures. All data are expressed as the mean±SEM. Among experimental groups, the comparison of the cylinder test, AIM score, biochemical and protein assay were analysed using a one-way ANOVA. Comparisons of difference after treatment among groups in the cylinder test was analysed using a repeated-measured ANOVA. In all of the analyses, statistically significant differences were considered at p<0.05.
Acknowledgments
We thank Dr. Seong-Uk Park (Department of Cardiology and Neurology of Korean Medicine, College of Korean Medicine, Kyung Hee University, Seoul, Republic of Korea) for the great contribution of advice in clinical aspect of the manuscript. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) [2005-0049404 and 2010-0008834] and a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. HI13C0540). Jongbae J. Park was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health (award K12DE022793).
REFERENCES
- Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol. Dis. 1999;6:461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- Antonini A, et al. A reassessment of risks and benefits of dopamine agonists in Parkinson’s disease. Lancet Neurol. 2009;8:929–937. doi: 10.1016/S1474-4422(09)70225-X. [DOI] [PubMed] [Google Scholar]
- Bagetta V, et al. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson’s disease. J. Neurosci. 2011;31:12513–12522. doi: 10.1523/JNEUROSCI.2236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bido S, Marti M, Morari M. Amantadine attenuates levodopa-induced dyskinesia in mice and rats preventing the accompanying rise in nigral GABA levels. J. Neurochem. 2011;118:1043–1055. doi: 10.1111/j.1471-4159.2011.07376.x. [DOI] [PubMed] [Google Scholar]
- BJ K, GP F. The Mouse Brain in Stereotaxic Coordinates. California: Academic; 2008. [Google Scholar]
- Calabresi P, et al. Levodopa-induced dyskinesias in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol. 2010;9:1106–1117. doi: 10.1016/S1474-4422(10)70218-0. [DOI] [PubMed] [Google Scholar]
- Cao X, et al. Striatal overexpression of DeltaFosB reproduces chronic levodopa-induced involuntary movements. J. Neurosci. 2010;30:7335–7343. doi: 10.1523/JNEUROSCI.0252-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum JM, Gandy SE, McDowell FH. "Early" initiation of levodopa treatment does not promote the development of motor response fluctuations, dyskinesias, or dementia in Parkinson’s disease. Neurology. 1991;41:622–629. doi: 10.1212/wnl.41.5.622. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. l-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur. J. Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- Chae Y, et al. Parsing brain activity associated with acupuncture treatment in Parkinson’s diseases. Mov. Disord. 2009;24:1794–1802. doi: 10.1002/mds.22673. [DOI] [PubMed] [Google Scholar]
- Cho SY, et al. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Parkinsonism Relat. Disord. 2012;18:948–952. doi: 10.1016/j.parkreldis.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front. Neuroanat. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, et al. Enhanced striatal cholinergic neuronal activity mediates l-DOPA-induced dyskinesia in parkinsonian mice. Proc. Natl. Acad. Sci. U.S.A. 2011;108:840–845. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann. N. Y. Acad. Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fahn S, et al. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Ferry P, Johnson M, Wallis P. Use of complementary therapies and non-prescribed medication in patients with Parkinson’s disease. Postgrad. Med. J. 2002;78:612–614. doi: 10.1136/pmj.78.924.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyder M, Bonito-Oliva A, Fisone G. l-DOPA-Induced dyskinesia and abnormal signaling in striatal medium spiny neurons: focus on dopamine D1 receptor-mediated transmission. Front. Behav. Neurosci. 2011;5:71. doi: 10.3389/fnbeh.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- Huang Y, et al. Complementary acupuncture in Parkinson’s disease: a spect study. Int. J. Neurosci. 2010;120:150–154. doi: 10.3109/00207450903316527. [DOI] [PubMed] [Google Scholar]
- Jeon S, et al. Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment in a Parkinson’s disease mouse model. Proteomics. 2008;8:4822–4832. doi: 10.1002/pmic.200700955. [DOI] [PubMed] [Google Scholar]
- Kim SN, et al. Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson’s disease. PLoS One. 2011a;6:e27566. doi: 10.1371/journal.pone.0027566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SN, et al. Phosphatidylinositol 3-kinase/Akt signaling pathway mediates acupuncture-induced dopaminergic neuron protection and motor function improvement in a mouse model of Parkinson’s disease. Int. J. Neurosci. 2011b;121:562–569. doi: 10.3109/00207454.2011.591515. [DOI] [PubMed] [Google Scholar]
- Kim ST, et al. The effect of electroaucpuncture for 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced proteomic changes in the mouse striatum. J. Physiol. Sci. 2010;60:27–34. doi: 10.1007/s12576-009-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, et al. Evaluation of D2 and D3 dopamine receptor selective compounds on l-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology. 2009;56:956–969. doi: 10.1016/j.neuropharm.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlan R. "Levodopa phobia": a new iatrogenic cause of disability in Parkinson disease. Neurology. 2005;64:923–924. doi: 10.1212/01.WNL.0000152880.77812.5B. [DOI] [PubMed] [Google Scholar]
- Lee S, et al. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- Malmlof T, et al. Deuterium substitutions in the l-DOPA molecule improve its anti-akinetic potency without increasing dyskinesias. Exp. Neurol. 2010;225:408–415. doi: 10.1016/j.expneurol.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Marti M, et al. Nociceptin/orphanin FQ receptor agonists attenuate l-DOPA-induced dyskinesias. J. Neurosci. 2012;32:16106–16119. doi: 10.1523/JNEUROSCI.6408-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela F, et al. In vivo evidence for a differential contribution of striatal and nigral D1 and D2 receptors to l-DOPA induced dyskinesia and the accompanying surge of nigral amino acid levels. Neurobiol. Dis. 2012;45:573–582. doi: 10.1016/j.nbd.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Na BJ, et al. An fMRI study of neuronal specificity of an acupoint: electroacupuncture stimulation of Yanglingquan (GB34) and its sham point. Neurosci. Lett. 2009;464:1–5. doi: 10.1016/j.neulet.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Nyholm D, et al. Large differences in levodopa dose requirement in Parkinson’s disease: men use higher doses than women. Eur. J. Neurol. 2010;17:260–266. doi: 10.1111/j.1468-1331.2009.02866.x. [DOI] [PubMed] [Google Scholar]
- Picconi B, et al. l-DOPA dosage is critically involved in dyskinesia via loss of synaptic depotentiation. Neurobiol. Dis. 2008;29:327–335. doi: 10.1016/j.nbd.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Poewe WH, Lees AJ, Stern GM. Low-dose l-dopa therapy in Parkinson’s disease: a 6-year follow-up study. Neurology. 1986;36:1528–1530. doi: 10.1212/wnl.36.11.1528. [DOI] [PubMed] [Google Scholar]
- Popoli P, Reggio R, Pezzola A. Effects of SCH 58261, an adenosine A(2A) receptor antagonist, on quinpirole-induced turning in 6-hydroxydopamine-lesioned rats. Lack of tolerance after chronic caffeine intake. Neuropsychopharmacology. 2000;22:522–529. doi: 10.1016/S0893-133X(99)00144-X. [DOI] [PubMed] [Google Scholar]
- Rascol O, et al. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N. Engl. J. Med. 2000;342:1484–1491. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- Santini E, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in l-DOPA-induced dyskinesia. J. Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Valjent E, Fisone G. Parkinson’s disease: levodopa-induced dyskinesia and signal transduction. FEBS J. 2008;275:1392–1399. doi: 10.1111/j.1742-4658.2008.06296.x. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, et al. Feeding behavior in dopamine-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12138–12143. doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma JC, et al. Relationship between weight, levodopa and dyskinesia: the significance of levodopa dose per kilogram body weight. Eur. J. Neurol. 2008;15:493–496. doi: 10.1111/j.1468-1331.2008.02106.x. [DOI] [PubMed] [Google Scholar]
- Thomas B, Beal MF. Parkinson’s disease. Hum. Mol. Genet. 2007;16(Spec no. 2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Comella CL, Horn S. Parkinson’s disease—Part 2: treatment of motor symptoms. Am. J. Manage. Care. 2008;14:S49–S58. [PubMed] [Google Scholar]