Abstract
The need for antiretroviral therapy coupled with treatment of chronic co-morbidities places HIV-infected patients at risk for polypharmacy. However, few studies have described overall pill burden among HIV-infected patients. HIV-infected outpatients of the UNC Infectious Diseases Clinic were enrolled in this cross-sectional study. Subjects were contacted prior to a scheduled appointment and asked to bring all their medications to the visit. Daily total pill burden and medication type were recorded. 151 subjects were recruited: 76% male, 58% African American, 97% receiving antiretrovirals (ARVs). Median age was 48 (IRQ: 42–54) years. The median number of medications per subject was 8 (IQR: 6–11), and the median individual daily pill burden was 8 pills (IQR: 5–15): 3 pills (range: 2–5) for ARVs and 6 (range: 3–12.5) pills for non-ARVs. Duration of ART (per 2 years increase) and more than 3 co-morbidities was significantly associated with high pill burden (over 10 pills per day) with adjusted OR of 2.09 (95% CI, 1.14–3.84) and 8.04 (95% CI, 2.30–28.15), respectively. As patients with HIV age, strategies to reduce pill burden and number of medications will become increasingly critical to maintaining adherence, preventing medication errors, and serious drug–drug interactions.
Introduction
Antiretroviral therapy (ART) has led to substantial increases in life expectancy and quality of life for HIV-infected persons,1–4 and reduces transmission of the virus.5 As a result, treatment guidelines now recommend ART for all HIV-infected individuals.6 While ART has become more convenient, HIV infection still requires lifelong treatment. As HIV-infected individuals experience life expectancies that approach those without HIV, co-morbid conditions, including those associated with aging, become increasingly prevalent.7–13 Consequently, patients with HIV are likely to be prescribed a number of different medications both for HIV-related and -unrelated indications. Such polypharmacy risks drug interactions and overlapping toxicities, can be costly, and as medication complexity increases, may affect treatment adherence and virologic suppression.14–16
Estimates of medication burden among persons living with HIV infection vary. Studies conducted between 1988 and 2010 examining cohorts in Switzerland and Canada, countries with centralized healthcare systems, found a relatively high medication burden among HIV-infected persons, compared to those without HIV, especially among older patients.10,17 However, less is known about the medication burden and daily pill count for HIV-infected persons in the US, where there is a greater diversity in patient populations and less uniform access to HIV care and medication.
In this study, we quantified daily medication use, including pill and medication number and pharmacologic categories of medications taken, and examined factors associated with medication burden. The study population included HIV-infected patients receiving care at an outpatient clinic in the southern US, a region with a high prevalence and incidence not only of HIV but also co-morbidities such as diabetes mellitus, obesity, and cardiovascular disease.18–21
Methods
Participants
We recruited participants from the University of North Carolina at Chapel Hill (UNC) Center for AIDS Research (CFAR) Clinical Cohort (UCHCC) study, a prospective clinic-based cohort of HIV-infected patients.22 The vast majority (>90%) of UNC Infectious Diseases Clinic HIV-infected patients have consented to participate in the UCHCC. Cohort patients who were 18 years and older, English speaking, and who previously agreed to be contacted by phone regarding study opportunities were identified from weekly clinic appointment lists. Patients were not required to be on any medications to participate. Eligible patients were contacted by telephone within a week prior to their appointment to ask if they would be willing to participate in the study. Those who were interested were instructed to bring all their current medications, including over-the-counter (OTC) drugs and dietary supplements, to their upcoming clinical appointment. At the appointment, written informed consent for study participation was obtained. This project was approved by the Biomedical Institutional Review Board at the University of North Carolina at Chapel Hill.
Data collection
Pill burden
At the clinic visit, the medications brought in by the patient were recorded and categorized. Patients who did not bring their medications were not enrolled unless they could produce a detailed medication list or were taking less than five medications and could readily recall each medication name and dose (91% of participants brought in their medications). Medication records were reviewed by at least two pharmacists or physicians to categorize medications and determine daily pill burden. Total medications included oral, inhaled, injectable, and topical medicines that patients were currently using. Pill burden was considered the number of pills taken on a daily basis and only applied to oral medications (including oral medications taken through gastric feeding tube). Each medication in non-OTC combination forms was recorded individually as a separate medication, while the pill burden was counted as one pill for one combination pills regardless of the number of medications in this combination (e.g., 1 tablet of fixed dose formulation of efavirenz, tenofovir, and emtricitabine was counted as three medications and 1 pill for pill burden). Oral medications taken as needed (i.e., PRN) were included in the pill burden if patients stated that they took the medication on a daily basis with the lowest daily pill number counted. In addition, patients were required to answer five multiple-choice questions regarding their perception of the pill burden (for questions and responses, see Supplementary Table S1; supplementary material is available online at www.liebertpub.com/apc).
Patient characteristics
Demographic information, insurance status at UNC HIV care initiation, and HIV clinical history (nadir CD4+ cell counts, duration of antiretroviral treatment, last CD4+ cell count, and last HIV RNA levels) were obtained from the UCHCC. Additionally, patients completed a brief questionnaire regarding demographic characteristics, including questions about race/ethnicity, current medication insurance status, and recent hospitalization. A physician investigator reviewed the clinical charts of all patients to identify and categorize co-morbid conditions. Co-morbid conditions occurring within the prior year that were recorded included: malignancy, diabetes mellitus, organ transplantation, hypercholesterolemia/hypertriglyceridemia, hypertension, chronic kidney disease, cardiovascular disease, chronic pain, psychiatric disorders (i.e., depression, anxiety, bipolar), osteoporosis, and hepatitis C virus (HCV) co-infection.
Statistical methods
Patient demographic and clinical characteristics were described using basic descriptive statistics. To identify patient characteristics associated with having a high total pill burden, we calculated unadjusted odds ratios (OR) and reported 95% confidence intervals (CI) as measures of precision. High pill burden was defined as the median cut-off of pills per day that study patients indicated too many to take based on the questionnaire. We also fit multivariable logistic regression to identify factors associated with high pill burden. These models were fit by including all factors associated with high pill burden in unadjusted analyses (p<0.2), and removing characteristics in a stepwise manner until only factors independently predictive of high pill burden with a p value<0.05 remained. We also employed a linear regression model for the same factors in the multivariable logistic regression model using pill burden as a continuous variable. We used SAS version 9.2 (SAS Institute, Cary, NC) for all analyses.
Results
Patient characteristics
A total of 605 HIV-infected patients scheduled for routine clinic appointments were screened between February and July 2012, and 474 met the inclusion criteria and were contacted by phone prior to a routinely scheduled clinic visit (Fig. 1). Of the 131 not meeting the inclusion criteria, 108 had indicated refusal to be contacted by phone when enrolled in the cohort, and 23 were non-English speakers. Of the 474 eligible patients, 226 were unable to be reached by phone and 19 would either not be attending their clinic appointment or could not bring in their medication. An additional 22 declined participation, and 56 agreed to participate but did not complete the study (i.e., missed scheduled clinic appointment).
FIG. 1.
Study recruitment.
Table 1 lists the demographic and clinical characteristics of the 151 study participants who completed the interview, as well as those of the patients who were screened but not enrolled. For both those screened but not enrolled and those interviewed, most were middle-aged, male, and African American. There were significantly more Hispanic patients in the screened but not enrolled group than in interviewed group (7% vs. 2%, p=0.01) as patients who spoke only Spanish were excluded. The insurance coverage at UNC HIV care initiation was comparable between the interviewed patients and the screened but not enrolled group. The majority of interviewed patients were supported by public healthcare plans (54%), followed by private insurance (22%); 21% of patients had low (ADAP or hospital charity program only) or no coverage for healthcare at the time of interview. Almost all the enrolled patients were receiving ART, and 87% had suppressed HIV RNA levels. In comparison, screened versus enrolled patients were less likely to be currently receiving ART (89% vs. 97%, p<0.01) and be virologically suppressed (78% vs. 87%, p=0.01). Among the interviewed patients, co-morbid conditions were common (Table 2). The median number of co-morbidities patients had was 1 [interquartile range (IQR): 1, 3], and the most commonly recorded conditions were hypertension (42%), psychiatric disorders (34%), hypercholesterolemia/hypertriglyceridemia (32%), and HCV co-infection (20%).
Table 1.
Demographic and Clinical Characteristics of Study Patients
| N (%) or median (IQR) | ||
|---|---|---|
| Characteristics | Interviewed patients (n=151) | Screened and not interviewed patients (n=454) |
| Age (years) | 48 (42–54) | 47 (39–55) |
| Sex, male | 114 (76%) | 359 (79%) |
| Race | ||
| African American | 88 (58%) | 238 (53%) |
| Caucasian | 49 (32%) | 155 (34%) |
| Multiracial/others/unknown | 6 (4%) | 17 (2%) |
| Native American | 4 (3%) | 8 (2%) |
| Latino | 3 (2%) | 34 (7%) |
| Asian | 1 (1%) | 1 (0%) |
| Insurance status at UNC HIV care initiation | ||
| Private | 43 (28%) | 125 (28%) |
| Public | 39 (26%) | 128 (28%) |
| None | 69 (46%) | 200 (44%) |
| Current insurance status | ||
| Private (stand-alone or combined with public healthcare plans) | 33 (22%) | NA |
| Public (Medicare and/or Medicaid, with or without ADAP or charity programs) | 81 (54%) | NA |
| Low or no coverage (ADAP, hospital charity program or no coverage) | 31 (21%) | NA |
| Other plans | 4 (3%) | NA |
| Unknown | 2 (1%) | NA |
| Prior ART use | 149 (99%) | 449 (99%) |
| Current ART use | 146 (97%) | 406 (90%) |
| Duration of ART, years | 10 (4–16) | 11 (5–16) |
| Nadir CD4 cell count, cells/μL | 126 (27–280) | 174 (39–312) |
| Most recent CD4 cell count, cells/μL | 575 (385–779) | 567 (355–768) |
| Most recent HIV RNA level <50 copies/mL | 131 (87%) | 352 (78%) |
ADAP, AIDS Drug Assistance Programs; ART, antiretroviral therapy; IQR, interquartile range; NA, not available.
Table 2.
Selected Co-Morbidities in Study Participants
| Selected co-morbidities | N (%) |
|---|---|
| Hypertension | 63 (42%) |
| Psychiatric disorders (depression, anxiety, bipolar) | 51 (34%) |
| hypercholesterolemia/hypertriglyceridemia | 49 (32%) |
| Hepatitis C | 30 (20%) |
| Chronic pain | 27 (18%) |
| Chronic kidney disease | 20 (13%) |
| Diabetes mellitus | 16 (11%) |
| Coronary heart disease | 9 (6%) |
| Malignancy | 5 (3%) |
Medication burden
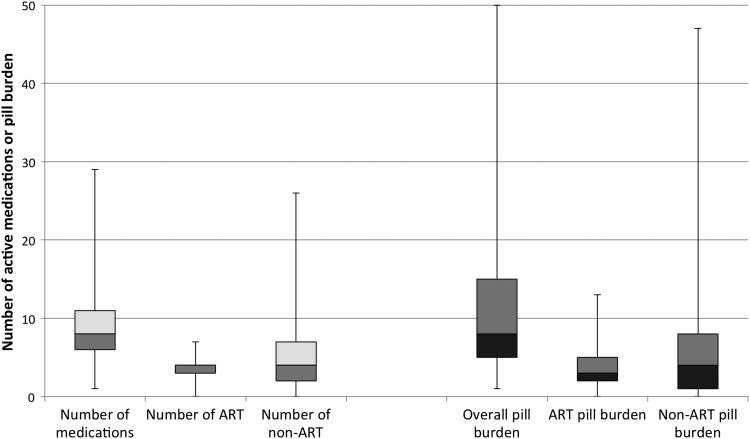
Overall, the 151 participants were taking a total of 1394 medications, of which 847 (61%) were non-ART. The median number of medications per patient was 8 (IQR: 6–11) and the median individual daily pill burden was 8 pills (IQR: 5–15): a median 3 pills (IQR: 2–5) were ART and 4 pills (IQR: 1–8) were non-ART (Fig. 2). Patients over 50 years of age had a median daily pill burden of 10 (IQR: 6.5–16), of which 3 pills (IQR: 2–5) were ART and 6 (IQR: 3–12.5) were non-ART.
FIG. 2.
Box plots of daily total medications, ART medications, non-ART medications, total pill burden, ART pill burden, non-ART pill burden per patient with median values, maximum values, minimum values, and interquartile ranges.
Among all patients receiving ART (n=146), 80% were treated with a NRTI plus an anchor agent: in 40% a protease inhibitor (PI), in 30% a NNRTI, and in 10% an integrase inhibitor (InSTI). An additional 8% were on a NRTI plus both an InSTI and PI, 2% were on a NRTI plus both a PI and a NNRTI, and 3% were on a PI plus an InSTI only (Table 3). Eighty participants (53%) were taking once-daily ART regimens and 31 (21%) participants were on a single tablet regimen.
Table 3.
Antiretroviral Regimens
| Regimens | Number of patients taking the regimen (N=146) |
|---|---|
| NRTI+one anchor agent | 117 (80%) |
| NRTI+PI | 58 (40%) |
| NRTI+NNRTI | 44 (30%) |
| 3 drug FDC | 31 (21%) |
| NRTI+InSTI | 15 (10%) |
| NRTI+NNRTI+PI | 3 (2%) |
| NRTI+InSTI+PI | 12 (8%) |
| NRTI+NNRTI+InSTI | 2 (1%) |
| InSTI+PI | 4 (3%) |
| NRTI+NNRTI+PI+InSTI | 3 (2%) |
| Others | 5 (3%) |
FDC, fixed dose combination; InSTI, intergrase inhibitor; NNRTI, non-nucleotide reverse transcriptase inhibitor; NRTI, nucleotide/nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
For the 141 patients (93% of total interviewed patients) receiving non-ART medications, these drugs were analgesics in 52%, antihypertensives in 43%, vitamin/minerals in 40%, antidepressants/antipsychotics/anxiolytics in 34%, and lipid-lowering agents in 34% (Table 4). Twenty percent of the non-ART medications were OTC, and 55% of patients were taking OTC medications.
Table 4.
Non-Antiretroviral (ART) Medication Categories Among 141 Patients Receiving Non-ART Medications
| Class of medications | Number of patients taking medications (%) |
|---|---|
| Analgesics | 74 (52%) |
| Antihypertensives | 61 (43%) |
| Vitamins/minerals | 56 (40%) |
| Antidepressants/antipsychotics/ anxiolytics | 48 (34%) |
| Lipid lowering agents | 46 (33%) |
| Acid secretion | 25 (18%) |
| Insomnia | 25 (18%) |
| Antivirals (non-HIV) | 21 (15%) |
| Other antibiotic | 17 (12%) |
| OI prophylaxis | 15 (11%) |
| Antidiarrheal/constipation | 15 (11%) |
| Antifungals | 14 (10%) |
| Non-vitamin/non-mineral supplement | 10 (7%) |
| Hormone replacement | 9 (6%) |
| Insulin | 8 (6%) |
| Oral hyperglycemic agents | 8 (6%) |
| Nausea/vomiting | 7 (5%) |
| Anticoagulants | 3 (2%) |
| Smoking cessation | 3 (2%) |
Factors associated with high daily pill burden
Overall, 64 (42%) patients were considered to have a high pill burden (taking ≥10 pills per day—a threshold based on patient survey response as described below). Factors identified as being associated with high pill burden are listed in Table 5. Gender, race, HCV co-infection, and last viral load ≥50 copies/mL were not significantly associated with high pill burden. Age (per 10 years increase), nadir CD4 (per 100 cells/μL decrease), duration of ART (per 2 years increase), and number of co-morbidities were associated with high pill burden with p values<0.2. When these were included in the multivariable regression model, duration of ART (per 2 years increase) and number of co-morbidities >2 remained significantly associated with high pill burden with an adjusted OR of 2.09 (95% CI: 1.14–3.84, p=0.02) and 8.04 (95% CI: 2.30–28.15, p<0.01), respectively. The adjusted odds ratio for age (per 10 year increase) was 1.52 (95% CI: 1.00–2.31, p=0.05).
Table 5.
Patient Demographic and Clinical Characteristics Associated with High Pill Burden
| Characteristics | Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
|---|---|---|---|---|
| Age, per 10 year increase | 2.03 (1.41–2.94) | <0.001 | 1.52 (1.00–2.31) | 0.05 |
| Female | 1.62 (0.77–3.41) | 0.21 | ||
| Caucasians | 0.91 (0.46–1.82) | 0.79 | ||
| HCV co-infection | 1.24 (0.56–2.78) | 0.60 | ||
| Nadir CD4, per 100 cells/μL decrease | 1.01 (0.99–1.03) | 0.16 | 1.01 (0.99–1.03) | 0.26 |
| Last CD4, per 100 cells/μL decrease | 1.00 (0.99–1.01) | 0.33 | ||
| Duration of ART, per 2 years increase | 2.92 (1.70–5.03) | <0.001 | 2.09 (1.14–3.84) | 0.001 |
| Number of co-morbidities | ||||
| 0 (control) | 1 | NA | 1 | NA |
| 1–2 | 2.48 (0.92–6.72) | 0.07 | 2.30 (0.80–6.61) | 0.12 |
| ≥3 | 11.25 (3.57–35.50) | <0.001 | 8.04 (2.30–28.15) | 0.001 |
| Last viral load ≥50 copies/mL | 0.89 (0.34–2.33) | 0.82 | ||
Factors with p value<0.2 in univariate analysis were included in multivariable logistic regression. (Age, nadir CD4, duration of ART, number of co-morbidities).
ART, antiretroviral therapy; CI, confidence interval; OR, odds ratio.
The multivariable linear regression model using pill burden as a continuous variable revealed the same significant factors associated with pill burden as logistic regression model (duration of ART per 2 years increase, p=0.005, co-morbidity categories, p=0.001 and there was a trend with increasing age (per 10 year increase, p=0.07).
Perceptions of pill burden
Fourteen percent of patients reported that their overall pill burden was “too high” and 10% reported they were taking medications “too often.” Ten percent of patients on ART responded that their ART pill burden was “too high” and 7% reported they were taking ART medications “too often.” The median cut-off of pills per day that participants indicated was too many to take was 10 pills (IQR: 5–20) (Supplementary Table S1).
Discussion
In this cross-sectional study of patients living with HIV infection engaged in medical care, we found polypharmacy to be common. Half of the patients were taking 8 or more medications, resulting in a median daily burden of 8 pills. ART accounted for less than half of the medications taken, and treatment for co-morbid hypertension, dyslipidemia, mental health disorders, and pain accounted for the majority of prescribed medication burden. Over-the-counter agents and dietary supplements were being taken by 55% of the patients. However, the vast majority of patients did not perceive the total daily pill burden or medication dosing frequency to be too high and considered a daily burden of 10 or more pills per day to be excessive. Older age, duration of HIV infection, and number of co-morbid conditions were each associated with pill counts above this threshold.
The total pill burden for HIV-infected patients is influenced by a number of epidemiologic and therapeutic factors. Foremost, the HIV-infected population is aging. It is projected that by 2015, more than half of all persons living with HIV in the US will be over 50 years of age.23 In addition, over 10% of new HIV infections are among those older than 50 years.2 As the proportion of persons living with HIV who are middle-aged or older rises, so too does the risk of co-morbid conditions requiring treatment; several studies suggest the risk of co- and multi-morbidity among HIV-infected patients is greater than that of age-matched controls without HIV.24–26 Treatment of HIV is also now recommended for all patients, regardless of CD4+ cell count, especially those aged 50 years or greater, leading to more widespread prescription of ART. On the other hand, HIV therapies are becoming simpler and co-formulation of new and existing antiretroviral agents, in particular, reduces pill number and dosing frequency.
Our results are remarkably concordant with a similar HIV clinical cohort study in Alberta, Canada, where median total daily pill burden in 2010 was found to be 6.7, with ART accounting for 51% of medications taken.17 As in our study, age, nadir CD4+ cell count, and duration of HIV infection influenced pill burden. Similarly, our findings mirror those from the Swiss HIV Cohort Study, which found an increasing medication burden among HIV-infected patients accompanying aging and the accumulation of co-morbid conditions.10 That study did not report a median daily pill burden and examined data from 2008 through 2010.
While not perceived to be onerous by patients, the extent of polypharmacy evident in this population has a number of implications. The management of older patients with high pill burdens may be challenging. A high percent of the patients we studied were receiving antihypertensives, psychotropic medications, and therapy for metabolic disorders. Some widely used medications in these categories may have serious drug–drug interactions (DDIs) with ART. For example, certain statin agents are contraindicated with ritonavir boosted PIs due to increased risk of rhabdomyolysis.27 In addition, although several studies28,29 indicate that older people have better adherence to ART compared to younger patients, the greater pill burden and treatment complexity attending polypharmacy is known to challenge adherence.30–34 Pharmacists specializing in HIV services have been shown to improve overall ART adherence and may be uniquely positioned to identify patient-specific barriers to adherence.35–39 Our findings suggest that the role of pharmacists can be important, not only for obtaining accurate ART and non-ART medication reconciliation, but also identifying medication discrepancies and preventing potential DDIs.
There are a number of strengths of our investigation including direct ascertainment of medications actually being taken via in person interviews during which patients were asked to bring their medication for review. Over 91% of patients brought their medications to the visit. All patients had medication and co-morbidity data available within a single electronic medical records system, facilitating data collection. Selection of the participants was also designed to be representative of the clinic population and accounted for clinic health care provider and day of the week seen in the clinic. The study was conducted in the US South, the region of the country with the most people living with HIV and where co-morbid conditions including diabetes and obesity are also most prevalent.19,20 Our study also assessed perceptions regarding medication burden among patients. The responses provided useful information about the subjective medication taking experience including a general acceptance of the number of medications and pills being taken. The respondents also provided a 10 pill per day threshold of excessive pill burden that can be useful in future assessments of medication use in HIV-infected patients.
Limitations to our investigation include the study of patients within one HIV clinic, located within an academic center. It could be that the medication and pill burden at other types of practices might be different. However, the concordance of our results with those from other countries supports the generalizability of our findings. In addition, a significant proportion of those screened for the study were not enrolled, largely as a consequence of not meeting the inclusion criteria, not being able to be reached by phone, or not attending their scheduled clinic appointment. However, the generalizability of our findings to the overall clinic population is supported by the similar characteristics of the interviewed patients and patients who were screened but not interviewed. This study was not designed to assess or quantify medication adherence, and while we aimed to ascertain the true pill burden, patients may have brought with them medications that they were not actually taking; therefore, in such cases our account of pill burden would be an overestimate.
In summary, patients engaged in HIV care in a southern specialty clinic were confirmed to be taking a median of 8 pills per day, with most of their medication burden comprised of non-ART agents. Older age, co-morbidity, and longer duration of HIV infection were associated with high pill burden. As patients with HIV age, strategies to reduce pill burden and number of medications, and avoidance of polypharmacy will become increasingly critical to maintaining adherence, preventing adverse events such as medication errors, and serious drug–drug interactions.
Supplementary Material
Acknowledgments
This study was supported by University of North Carolina Center for Infectious Diseases and Center For AIDS Research (CFAR) (AI50410).
Author Disclosure Statement
Conflict of interest and source of funding: this study was supported by University of North Carolina Center for Infectious Diseases and University of North Carolina Center for AIDS Research (AI50410).
References
- 1.Global report: UNAIDS Report on the Global AIDS Epidemic 2012. Joint United Nations Programme on HIV/AIDS (UNAIDS), 2012 [Google Scholar]
- 2.HIV surveillance–United States, 1981–2008. MMWR Morb Mortal Wkly Rep 2011;60:689–693 [PubMed] [Google Scholar]
- 3.Antiretroviral Therapy Cohort Collaborations. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet 2008;372:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodger AJ, Lodwick R, Schechter M, et al. . Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 2013;27:973–979 [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. . Prevention of HIV-1 infection with early antiretroviral therapy. New Engl J Med 2011;365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson MA, Aberg JA, Hoy JF, et al. . Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA 2012;308:387–0402 [DOI] [PubMed] [Google Scholar]
- 7.Wohl D, Scherzer R, Heymsfield S, et al. . The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr 2008;48:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallant JE, Parish MA, Keruly JC, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis 2005;40:1194–1198 [DOI] [PubMed] [Google Scholar]
- 9.Wester CW, Koethe JR, Shepherd BE, et al. . Non-AIDS-defining events among HIV-1-infected adults receiving combination antiretroviral therapy in resource-replete versus resource-limited urban setting. AIDS 2011;25:1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasse B, Ledergerber B, Furrer H, et al. . Morbidity and aging in HIV-infected persons: The Swiss HIV cohort study. Clin Infect Dis 2011;53:1130–1139 [DOI] [PubMed] [Google Scholar]
- 11.Yanik EL, Napravnik S, Cole SR, et al. . Incidence and timing of cancer in HIV-infected individuals following initiation of combination antiretroviral therapy. Clin Infect Dis 2013;57:756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freiberg MS, Chang CC, Kuller LH, et al. . HIV infection and the risk of acute myocardial infarction. JAMA Int Med 2013;173:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: A cohort and nested case-control study using Quebec's public health insurance database. J Acquir Immune Defic Syndr 2011;57:245–253 [DOI] [PubMed] [Google Scholar]
- 14.Onen NF, Overton ET, Seyfried W, et al. . Aging and HIV infection: A comparison between older HIV-infected persons and the general population. HIV Clin Trials 2010;11:100–109 [DOI] [PubMed] [Google Scholar]
- 15.Nachega JB, Hsu AJ, Uthman OA, Spinewine A, Pham PA. Antiretroviral therapy adherence and drug–drug interactions in the aging HIV population. AIDS 2012;26:S39–S53 [DOI] [PubMed] [Google Scholar]
- 16.Nachega JB, Parienti JJ, Uthman OA, et al. . Lower pill burden and once-daily dosing antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis 2014;58:1297–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krentz HB, Cosman I, Lee K, Ming JM, Gill MJ. Pill burden in HIV infection: 20 years of experience. Antiviral Ther 2012;17:833–840 [DOI] [PubMed] [Google Scholar]
- 18.HIV Surveillance Report, 2011. Centers Dis Control Prevent 2013
- 19.Centers for Disease Control and Prevention. Diabetes Report Card 2012. Atlanta, GA: US Department of Health and Human Services, 2012 [Google Scholar]
- 20.Vital signs: State-specific obesity prevalence among adults—United States, 2009. MMWR Morb Mortal Wkly Rep 2010;59:951–955 [PubMed] [Google Scholar]
- 21.Prevalence of stroke–United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012;61:379–382 [PubMed] [Google Scholar]
- 22.Napravnik S, Eron JJ, Jr., McKaig RG, Heine AD, Menezes P, Quinlivan E. Factors associated with fewer visits for HIV primary care at a tertiary care center in the Southeastern U.S. AIDS Care 2006;18:S45–S50 [DOI] [PubMed] [Google Scholar]
- 23.Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA 2013;309:1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverberg MJ, Chao C, Leyden WA, et al. . HIV infection and the risk of cancers with and without a known infectious cause. AIDS 2009;23:2337–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Womack JA, Goulet JL, Gibert C, et al. . Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PloS One 2011;6:e17217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverberg MJ, Leyden WA, Xu L, et al. . Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr 2014;65:160–166 [DOI] [PubMed] [Google Scholar]
- 27.Gerber JG, Rosenkranz SL, Fichtenbaum CJ, et al. . Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: Results of AIDS Clinical Trials Group 5108 Study. J Acquir Immune Defic Syndr 2005;39:307–312 [DOI] [PubMed] [Google Scholar]
- 28.Branas F, Berenguer J, Sanchez-Conde M, et al. . The eldest of older adults living with HIV: Response and adherence to highly active antiretroviral therapy. Am J Med 2008;121:820–824 [DOI] [PubMed] [Google Scholar]
- 29.Nogueras M, Navarro G, Anton E, et al. . Epidemiological and clinical features, response to HAART, and survival in HIV-infected patients diagnosed at the age of 50 or more. BMC Infect Dis 2006;6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Libby AM, Fish DN, Hosokawa PW, et al. . Patient-level medication regimen complexity across populations with chronic disease. Clin Therap 2013;35:385–398e381. [DOI] [PubMed] [Google Scholar]
- 31.Dragomir A, Cote R, Roy L, et al. . Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Med Care 2010;48:418–425 [DOI] [PubMed] [Google Scholar]
- 32.Krousel-Wood M, Islam T, Muntner P, et al. . Association of depression with antihypertensive medication adherence in older adults: Cross-sectional and longitudinal findings from CoSMO. Ann Behav Med 2010;40:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May HT, Sheng X, Catinella AP, Horne BD, Carlquist JF, Joy E. Antilipidemic adherence post-coronary artery disease diagnosis among those with and without an ICD-9 diagnosis of depression. J Psychosom Res 2010;69:169–174 [DOI] [PubMed] [Google Scholar]
- 34.McLean DL, McAlister FA, Johnson JA, et al. . A randomized trial of the effect of community pharmacist and nurse care on improving blood pressure management in patients with diabetes mellitus: Study of cardiovascular risk intervention by pharmacists-hypertension (SCRIP-HTN). Arch Int Med 2008;168:2355–2361 [DOI] [PubMed] [Google Scholar]
- 35.Murphy P, Cocohoba J, Tang A, Pietrandoni G, Hou J, Guglielmo BJ. Impact of HIV-specialized pharmacies on adherence and persistence with antiretroviral therapy. AIDS Patient Care STDs 2012;26:526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kibicho J, Owczarzak J. A patient-centered pharmacy services model of HIV patient care in community pharmacy settings: A theoretical and empirical framework. AIDS Patient Care STDs 2012;26:20–28 [DOI] [PubMed] [Google Scholar]
- 37.Cocohoba JM, Murphy P, Pietrandoni G, Guglielmo BJ. Improved antiretroviral refill adherence in HIV-focused community pharmacies. J Am Pharm Assoc 2012;52:e67–e73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kibicho J, Owczarzak J. Pharmacists' strategies for promoting medication adherence among patients with HIV. J Am Pharm Assoc 2011;51:746–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cocohoba J, Comfort M, Kianfar H, Johnson MO. A qualitative study examining HIV antiretroviral adherence counseling and support in community pharmacies. J Manag Care Pharm 2013;19:454–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.