Abstract
Mycobacterium abscessus and Kocuria species are rare causes of infections in humans. Endocarditis by these agents has been reported in only 11 cases. M. abscessus is a particularly resistant organism and treatment requires the association of antibiotics for a prolonged period of time. We report a case of native mitral valve bacterial endocarditis due to M. abscessus and Kocuria species in a 48-year-old man with a history of intravenous drug use. The case was complicated by a perforation of the posterior mitral valve leaflet, leading to surgical mitral valve replacement. Cultures from the blood and mitral valve disclosed M. abscessus and Kocuria species. The patient was treated for 6 months with clarithromycin, imipenem and amikacin, with resolution of symptoms. Repeated blood cultures were negative. Acid-fast staining should be done in subacute endocarditis in order to identify rapidly growing mycobacteria.
Background
Mycobacterium abscessus is a non-tuberculous, rapidly growing mycobacteria (RGM).1 This organism has been known to cause a wide variety of clinical diseases in humans, including skin and soft tissue infections, keratitis, catheter-related sepsis, osteomyelitis, septic arthritis and pulmonary infections.2–4 M. abscessus is also a rare aetiology of endocarditis. Only eight cases of M. abscessus endocarditis have been published in the literature. Kocuria species are Gram-positive bacteria rarely associated with human disease. There have been case reports of Kocuria-associated cholecystitis, bacteraemia and peritonitis.5–10 Only three cases of Kocuria endocarditis have been published. To the best of our knowledge, we present the first case of endocarditis due to M. abscessus and Kocuria species.
Case presentation
A 48-year-old Ukrainian man who had lived in the USA for 15 years, with a medical history significant for intravenous drug use, was admitted with a history of subjective fever, diffuse abdominal pain with blood per rectum and shortness of breath for 1 week. On interview, the patient endorsed night sweats and an unintentional 10-pound weight loss over the previous 2 months. He also mentioned worsening dyspnoea on exertion and dry cough. The patient had used intravenous drugs for 2 years, including crushed roxicet and cocaine. He admitted to last using drugs 2 days prior to admission and denied sharing syringes. On physical examination, the patient was febrile at 103°F; blood pressure 95/56 mm Hg; radial pulse 132 irregular beats per minute (bpm); 20 breaths/min; and oxygen saturation of 95% at room air. Auscultation was significant for a grade 3 pansystolic murmur at the apex radiating to the axilla. ECG disclosed atrial fibrillation with rapid ventricular response; laboratory analysis revealed a total white cell count of 15 000 cells/mL, with 89.2% of neutrophils; haemoglobin 11.1 mg/dL and haematocrit 35.4%. CT with contrast of the abdomen disclosed proximal coeliac artery thrombosis extending into the splenic artery. The superior mesenteric artery was intact. A transthoracic two-dimensional echocardiogram (2D echo) revealed mitral regurgitation with an anteriorly and medially regurgitant flow jet with regurgitant volumes of 70 mL, regurgitation fraction of 55% and a proximal isovelocity surface area radius of 1.1 cm. There was a large mitral leaflet vegetation of almost 2 cm (figure 1). The patient was started empirically on treatment for infectious endocarditis with intravenous vancomycin, ceftriaxone and gentamicin. Management consisted of β-blockers for atrial fibrillation rate control, intravenous fluids and vasopressors for sepsis and a heparin drip for thrombosis of the coeliac artery.
Figure 1.

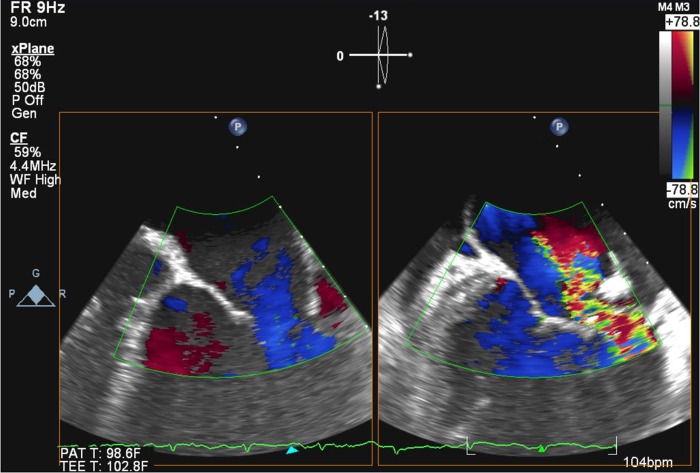
Large vegetation on mitral valve (1.9 cm).
The patient presented with partial clinical improvement for the following 2 days. Atrial fibrillation persisted, but rate controlled at 90 bpm and blood cultures drawn initially revealed Enterococcus faecalis. Antibiotics were subsequently switched to ampicillin and gentamicin after 3 days on the prior regimen. The transoesophageal echocardiogram disclosed a small patent foramen ovale and 1.9 cm vegetation (figure 2) with associated perforation in the posterior mitral valve leaflet (figure 3) leading to severe mitral regurgitation (figure 4). As the patient presented with an altered mental status and febrile state; a CT of the brain was obtained and it did not show any signs of acute ischaemia, thrombus or enhancing lesions suggestive of septic emboli. He improved later. Repeated-blood cultures after 6 days on ampicillin and gentamicin were started disclosed no growing organism. Therefore, surgical mitral valve replacement was done using a bioprosthetic valve rather than a mechanical valve in order to avoid anticoagulation therapy in a patient with questionable medication compliance. Postsurgical blood cultures and mitral valve tissue cultures revealed Kocuria species and M. abscessus. Culture results were based on colony morphology, pigmentation pattern, growth rate and results of biochemical testing. Ampicillin and gentamicin were then discontinued and the patient was started on amikacin, imipenem and clarithromycin. Since the patient was bacteraemic at the time of valve replacement, the replaced valve was considered to be infected and another surgery was indicated. Unfortunately, the patient had a poor nutritional status and became haemodynamically unstable, requiring intravenous pressors. He was deemed a poor surgical candidate. At this point, blood cultures revealed susceptibility only to amikacin and clarithromycin, so imipenem was discontinued. Another set of blood cultures after antibiotics adjustment still revealed M. abscessus. 2D echo was repeated and neither vegetation nor mitral regurgitation was observed on the prosthetic valve (figure 5). The patient was restarted on imipenem with amikacin and clarithromycin. Later, two blood cultures disclosed no growing organism. The patient did not hold medical insurance and therefore stayed in hospital for continued antibiotic and heparin administration for the following 6 months as an alternative treatment for a possibly infected prosthetic valve. Other investigative tests during his hospitalisation disclosed a negative HIV test and positive hepatitis C, genotyope 3a with 72 000 copies. He was never started on treatment for that. A repeated CT of the abdomen with contrast disclosed only stable areas of splenic infarct from previous arterial thrombosis. The final conclusion about the splenic artery thrombosis was due to septic emboli. After discharge, there was no follow-up as the patient never presented to outpatient clinic. He was admitted again 4 months after discharge, presenting with respiratory failure and septic shock. He expired on the next day due to multiorgan failure. Owing to the rapid deterioration of his condition, only a few investigative studies were conducted initially that included blood cultures. They disclosed M. abscessus as well.
Figure 2.

Perforation of the mitral valve posterior leaflet, resulting in severe mitral regurgitation.
Figure 3.
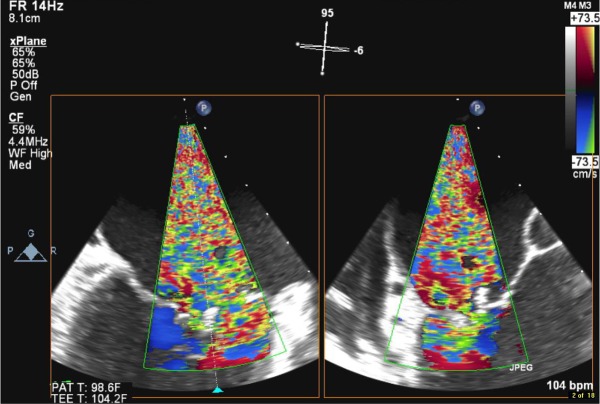
Three-dimensional transoesophageal echocardiogram discloses large vegetation on the mitral valve.
Figure 4.
Severe mitral regurgitation flow by transoesophageal echocardiogram.
Figure 5.
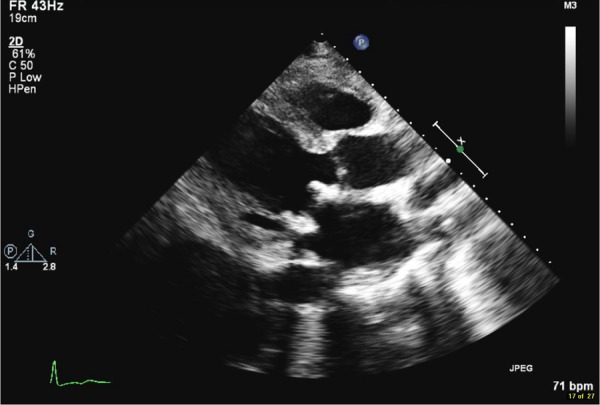
Bioprosthetic mitral valve in place; no mitral regurgitation or vegetations.
Investigations
ECG: atrial fibrillation with rapid ventricular response; CT with contrast of the abdomen: proximal coeliac artery thrombosis extending into the splenic artery. The superior mesenteric artery was intact.
Transthoracic 2D echo: mitral regurgitation with a large mitral leaflet vegetation of almost 2 cm.
Blood cultures: initially E. faecalis, then Kocuria species and M. abscessus.
Transoesophageal echocardiogram: small patent foramen ovale and 2 cm vegetation with associated perforation in the posterior mitral valve leaflet leading to severe mitral regurgitation.
Differential diagnosis
Bacterial endocarditis
Connective tissue disorder
Tuberculosis
Congestive heart failure
Treatment
Surgical mitral valve replacement
Clarithromycin, imipenem and amikacin
Outcome and follow-up
The patient was discharged after 6 months of antibiotics. He achieved resolution of symptoms and his blood cultures were repeatedly negative. He was lost to follow-up but was readmitted 4 months later with sepsis and expired on the day after his admission.
Discussion
We presented a case of a concomitant bacteraemia and valve infection by Kocuria and non-tuberculous mycobacteria. On the basis of the course of the presentation, we believe that after manipulating the mitral valve for repair the bacteria might have gotten dislodged and spread to the blood. More interestingly, after he was successfully treated for 6 months, the same type of bacteraemia recurred 4 months later. This could be due to reinfection by intravenous drug use or the infection was not successfully treated.
Kocuria species are Gram-positive bacteria members of the Micrococcus family. The main agents are K. kristinae, K. rosea, K. varians and K. palustris. Infection with Kocuria in humans is extremely rare. A previous literature search from 1995 to 2010 identified only 15 cases.6 Immunocompromised individuals are at greater risk. Only three cases of Kocuria endocarditis have been reported (table 1). Common risk factors were not identified in previously published cases.
Table 1.
| First author | Age, gender | Valve affected | Organism | Risk factors | Culture source | Antibiotics | Disease duration, months | Antibiotic duration, weeks | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Al-Benwan11 | 54, male | Mitral, native | Mycobacterium abscessus | Hepatitis C, alcoholic cirrhosis, ESRD, haemodialysis | Blood, catheter-tip | Clarithromycin and tigecycline | 5.5 | 7 | Died |
| Altmann12 | 45, male | Aortic, prosthesis | M. abscessus | Previous endocarditis, rheumatic disease, recent aortic valve replacement | Blood, prosthesis | Lincomycin+cloxacillin | 4.5 | 8 | Died |
| Liebeskind13 | 35, male | Mitral, native | M. abscessus | NA | Blood, CSF, BM | Clarithromycin+imipenem | 7 | 10 | Died |
| Tsai14 | 29, male | Tricuspid, native | M. abscessus | IV drug use, previous endocarditis | Blood, sputum | Clarithromycin+amikacin+ciprofloxacin | 9 | 14 | Alive |
| Viscidi15 | 55, male | Mitral, prosthesis | M. abscessus | Rheumatic heart disease | Blood, urine, BM, sputum, wound | Amikacin, erythromycin, ethionamide | 5 | 4 | Died |
| Wallace3 | 53, male | Prosthesis | M. abscessus | NA | Blood | NA | NA | NA | Died |
| Wallace3 | 50, male | Prosthesis | M. abscessus | NA | Blood | NA | NA | NA | Died |
| Williamson16 | 29, female | Mitral, native | M. abscessus | ESRD, haemodialysis, renal transplant, anaemia, corticoid immunosuppression | Blood | Imipenem, clarithromycin, moxifloxacin | 1.5 | 6.5 | Died |
| Citro17 | 74, male | Mitral, native | Kocuria kristinae | Diabetes, hypertension, foot ulcer | Blood | Ampicillin–sulbactam+gentamicin | 1 | 3 | Died |
| Lai7 | 89, female | NA | K. kristinae | Ischaemic bowel disease status post surgery, TPN | Blood | Vancomycin+teicoplanin | NA | NA | Alive |
| Srinivasa18 | 35, male | Mitral, native | K. rosea | Rheumatic mitral regurgitation | Blood | Gentamicin+ceftriaxone | 2 | 4 | Alive |
| Present case | 48, male | Mitral, native | M. abscessus and Kocuria species | IV drug use | Blood, mitral valve | Amikacin+clarithromycin+imipenem | 8 | 24 | Alive |
BM, bone marrow; CSF, cerebrospinal fluid; ESRD, end-stage renal disease; IV, intravenous; NA, not applicable or not available; TPN, total parenteral nutrition.
Unlike other non-tuberculous mycobacteria, RGM grow within 7 days in a wide variety of culture media.1 The majority of infections due to RGM are caused by M. abscessus, M. fortuitum and M. chelonae.19 20 M. abscessus is a ubiquitous bacteria; it can be identified worldwide in dust, soil and water.20–22 Many risk factors have been associated with M. abscessus infections, such as haemodialysis, surgical procedures, immunosuppression, prosthetic devices and cystic fibrosis.3 16 23–25
Infective endocarditis due to M. abscessus is a rare condition. After a literature review, to the best of our knowledge, only eight cases have been reported (table 1). The mitral valve was affected in five cases and there was an equivalent distribution among native and prosthetic valves. The mortality of M. abscessus-related endocarditis is very high. We report the second case where the patient was successfully treated and discharged from the hospital. The main associated risk factors identified in a literature review were intravenous drug use, end-stage renal disease on dialysis and immunosuppression. In the current case, the patient endorsed intravenous drug use over the past 2 years.
Antibiotic resistance is an important issue in patients with M. abscessus infections. This organism is resistant to many antibiotics and there have been reports of variable susceptibility to chemotherapeutic agents.19 26–29 Therefore, infections due to rapidly growing mycobacteria should be treated with at least two antibiotics.30 The most commonly used antibiotics include amikacin, cefoxitin, clarithromycin, imipenem, linezolid and tigecycline.11 20 31 No current guidelines include a recommended treatment for Kocuria-related endocarditis.17 In our case, the patient was successfully treated with a prolonged triple regimen of amikacin, clarithromycin and imipenem.
Initial blood cultures from our patient disclosed E. faecalis. Treatment with gentamicin might have treated the Enterococcus infections, explaining why further cultures did not grow this organism. Another possibility is misidentification of M abscessus. In Switzerland, 50 samples of rapidly growing mycobacteria were sent to study participant centres for bacteriological investigation. Only 13 (26%) determined the correct diagnosis. The most common misdiagnosis was Nocardia.32 Another study has reported misidentification of rapidly growing bacteria as Corynebacterium.33 Given that E. faecalis and M. abscessus are Gram-positive bacilli, this misidentification is conceivable.
The main indications for surgical treatment in endocarditis are mechanical complications and therapy failure. Possible mechanical complications that require surgical management include perforation, rupture, dehiscence, repeated embolic events from vegetations or a significant perivalvular abscess. In addition, persistent infection manifested by persistently positive blood cultures within 1 week of antibiotics also may require surgical treatment.34 35 Our patient suffered a posterior mitral valve leaflet perforation with consequent severe mitral regurgitation and therefore required mitral valve replacement.
In conclusion, M. abscessus may represent an important aetiology of bacterial endocarditis. In negative cultures or in atypical cases of subacute endocarditis, particularly if antibiotic resistance is an issue, acid-fast staining should be done in order to increase the diagnosis of this infection.
Learning points.
Mycobacterium abscessus may represent an important aetiology of bacterial endocarditis, given that the agent may be underdiagnosed or misidentified.
In atypical presentations, if cultures are negative or in antibiotic resistant cases, consider an acid-fast stain for rapidly growing mycobacteria.
Infections due to M. abscessus should be treated with a combination of antibiotics for a prolonged period of time (at least 2 weeks).
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Brown-Elliott BA, Wallace RJ., Jr Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev 2002;15:716–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia DC, Sandoval-Sus J, Razzaq K, et al. Vertebral osteomyelitis caused by Mycobacterium abscessus. BMJ Case Rep 2013;2013:pii:bcr2013009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace RJ, Jr, Swenson JM, Silcox VA, et al. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis 1983;5:657–79 [DOI] [PubMed] [Google Scholar]
- 4.Wang SX, Yang CJ, Chen YC, et al. Septic arthritis caused by Mycobacterium fortuitum and Mycobacterium abscessus in a prosthetic knee joint: case report and review of literature. Intern Med 2011;50:2227–32 [DOI] [PubMed] [Google Scholar]
- 5.Basaglia G, Carretto E, Barbarini D, et al. Catheter-related bacteremia due to Kocuria kristinae in a patient with ovarian cancer. J Clin Microbiol 2002;40:311–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn R, Bares S, David MZ. Central venous catheter-related bacteremia caused by Kocuria kristinae: case report and review of the literature. Ann Clin Microbiol Antimicrob 2011;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai CC, Wang JY, Lin SH, et al. Catheter-related bacteraemia and infective endocarditis caused by Kocuria species. Clin Microbiol Infect 2011;17:190–2 [DOI] [PubMed] [Google Scholar]
- 8.Ma ES, Wong CL, Lai KT, et al. Kocuria kristinae infection associated with acute cholecystitis. BMC Infect Dis 2005;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlini A, Mattei R, Lucarotti I, et al. Kocuria kristinae: an unusual cause of acute peritoneal dialysis-related infection. Perit Dial Int. 2011;31:105–7 [DOI] [PubMed] [Google Scholar]
- 10.Cheung CY, Cheng NH, Chau KF, et al. An unusual organism for CAPD-related peritonitis: Kocuria kristinae. Perit Dial Int 2011;31:107–8 [DOI] [PubMed] [Google Scholar]
- 11.Al-Benwan K, Ahmad S, Mokaddas E, et al. Diagnosis of endocarditis caused by Mycobacterium abscessus. Ann Saudi Med 2010;30:408–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altmann G, Horowitz A, Kaplinsky N, et al. Prosthetic valve endocarditis due to Mycobacterium chelonei. J Clin Microbiol 1975;1:531–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebeskind DS, Ostrzega N, Wasterlain CG, et al. Neurologic manifestations of disseminated infection with Mycobacterium abscessus. Neurology. 2001;56:810–13 [DOI] [PubMed] [Google Scholar]
- 14.Tsai WC, Hsieh HC, Su HM, et al. Mycobacterium abscessus endocarditis: a case report and literature review. Kaohsiung J Med Sci 2008;24:481–6 [DOI] [PubMed] [Google Scholar]
- 15.Viscidi R, Geller A, Caplan W, et al. Prosthetic valve endocarditis caused by Mycobacterium chelonei: case report and literature review. Heart Lung 1982;11:555–9 [PubMed] [Google Scholar]
- 16.Williamson JC, Miano TA, Morgan MR, et al. Fatal Mycobacterium abscessus endocarditis misidentified as Corynebacterium spp. Scand J Infect Dis 2010;42:222–4 [DOI] [PubMed] [Google Scholar]
- 17.Citro R, Prota C, Greco L, et al. Kocuria kristinae endocarditis related to diabetic foot infection. J Med Microbiol 2013;62(Pt 6):932–4 [DOI] [PubMed] [Google Scholar]
- 18.Srinivasa KH, Agrawal N, Agarwal A, et al. Dancing vegetations: Kocuria rosea endocarditis. BMJ Case Rep 2013;2013: pii:bcr2013010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombo RE, Olivier KN. Diagnosis and treatment of infections caused by rapidly growing mycobacteria. Semin Respir Crit Care Med 2008;29:577–88 [DOI] [PubMed] [Google Scholar]
- 20.Medjahed H, Gaillard JL, Reyrat JM. Mycobacterium abscessus: a new player in the mycobacterial field. Trends Microbiol 2010;18:117–23 [DOI] [PubMed] [Google Scholar]
- 21.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416 [DOI] [PubMed] [Google Scholar]
- 22.Morimoto K, Manago E, Iioka H, et al. Rare complication after stripping operation: a case report of mycobacterium abscessus infection. Ann Vasc Dis 2010;3:232–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esther CR, Jr, Esserman DA, Gilligan P, et al. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 2010;9:117–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith DE, Girard WM, Wallace RJ., Jr Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis 1993;147:1271–8 [DOI] [PubMed] [Google Scholar]
- 25.Sermet-Gaudelus I, Le Bourgeois M, Pierre-Audigier C, et al. Mycobacterium abscessus and children with cystic fibrosis. Emerg Infect Dis 2003;9:1587–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nessar R, Cambau E, Reyrat JM, et al. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 2012;67:810–18 [DOI] [PubMed] [Google Scholar]
- 27.Sanguinetti M, Ardito F, Fiscarelli E, et al. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J Clin Microbiol 2001;39:816–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection 2004;32:257–70 [DOI] [PubMed] [Google Scholar]
- 29.Woods GL, Bergmann JS, Witebsky FG, et al. Multisite reproducibility of results obtained by the broth microdilution method for susceptibility testing of Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum. J Clin Microbiol 1999;37:1676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis 2006;42:1756–63 [DOI] [PubMed] [Google Scholar]
- 31.Petrini B. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS 2006;114:319–28 [DOI] [PubMed] [Google Scholar]
- 32.von Graevenitz A, Punter-Streit V. Failure to recognize rapidly growing mycobacteria in a proficiency testing sample without specific request—a wider diagnostic problem?. Eur J Epidemiol 1998;14:519–20 [DOI] [PubMed] [Google Scholar]
- 33.Garg P, Athmanathan S, Rao GN. Mycobacterium chelonei masquerading as Corynebacterium in a case of infectious keratitis: a diagnostic dilemma. Cornea 1998;17:230–2 [DOI] [PubMed] [Google Scholar]
- 34.Pierce D, Calkins BC, Thornton K. Infectious endocarditis: diagnosis and treatment. Am Fam Physician 2012;85:981–6 [PubMed] [Google Scholar]
- 35.Cabell CH, Abrutyn E, Fowler VG, Jr, et al. Use of surgery in patients with native valve infective endocarditis: results from the International Collaboration on Endocarditis Merged Database. Am Heart J 2005;150:1092–8 [DOI] [PubMed] [Google Scholar]