Abstract
Background
While the incidence of inflammatory bowel disease (IBD) among African-Americans (AAs) is increasing, there is limited understanding of phenotypic differences and outcomes by race.
Aim
To describe disease characteristics of AA patients compared to Caucasian (Ca) patients in a tertiary care population.
Methods
We performed a cross-sectional review of the IBD registry at the University of Chicago from January 2008 to January 2013. Data regarding race, phenotype, disease onset, disease duration, medical therapy, and surgical treatment were abstracted from the database, then compared via Pearson’s chi-square analysis, Kruskal–Wallis analysis, and logistic regression with a significance level of p < 0.05.
Results
A total of 1,235 patients with Crohn’s disease (CD) and 541 patients with ulcerative colitis (UC) included 108 AA CD patients and 28 AA UC patients. AA CD patients had an increased rate of IBD-related arthralgias (36.5 vs. 23.9 %, p < 0.01) and surgery (p < 0.01), less ileal involvement (57.8 vs. 71.0 %, p < 0.01), and no differences for other extraintestinal manifestations or disease locations compared to Ca CD patients. AA UC patients were older at diagnosis, had an increased rate of arthralgias (28.6 vs. 14.6 %, p = 0.047) and ankylosing spondylitis/sacroiliitis (7.1 vs. 1.6 %, p = 0.035), with no differences for disease extent or rate of IBD-related surgeries compared to Ca UC patients. There were no differences in medication usage by race for CD and UC patients.
Conclusion
We identified significant differences in disease characteristics and extraintestinal manifestations between AA and Ca IBD patients in a large tertiary care population. These results have implications for future genotype-phenotype studies.
Keywords: Ulcerative colitis, Crohn’s disease, Epidemiology, Race, African-American
Introduction
The majority of data pertaining to the epidemiology, disease characteristics, and treatment of inflammatory bowel disease (IBD) is in Caucasian (Ca) patients. Recent studies indicate that the incidence of IBD in non-Ca minority groups, including African-Americans (AAs), is increasing [1–5]. Little is known, however, about disease-specific characteristics, medication usage, and outcomes in AA with IBD.
Several previous studies have examined phenotypic and outcome differences by race in IBD with conflicting results [5–15]. The majority of these evaluations, however, were limited by small sample sizes [5–7, 10, 11, 13]. The largest comprehensive multi-center study comparing 127 AA patients to 830 Ca patients found that AA with Crohn’s disease (CD) were more likely to have upper gastrointestinal involvement, perianal disease, uveitis, and sacroiliitis [8]. This report and several additional studies have also concluded that AA with CD are less likely to have ileal involvement [7, 8, 10, 11]. While there is less data regarding differences in disease extent by race in patients with ulcerative colitis (UC), Moore et al. [12] recently reported increased distal colitis and less pancolitis in AA patients with UC. In contrast, two recent comparably sized studies found no significant differences in disease location by race for either UC or CD [1, 13]. In agreement, a systematic review by Mahid et al. [9] found that the overall evidence suggests that AA patients with IBD have similar symptoms, extraintestinal manifestations, disease location, and behavior to Ca patients.
Similarly, several reports have differed in reported surgical rates for AA compared to Ca patients with IBD. Four retrospective studies have demonstrated no difference in overall rates of surgery by race [10, 12, 13, 16]. However, a nationwide analysis of hospital discharges over a 6-year period concluded that AA with CD were less likely to undergo surgery [17]. In contrast, a multi-center retrospective analysis showed more surgeries resulting in an ostomy in AA patients with CD [14].
Over the past decade, new medication classes and strategies have been integrated into the standard therapy for IBD. During this time, investigations have varied in their reports of utilization of medical therapy by race. AA IBD patients have been found in several analyses to receive less corticosteroids, azathioprine, 5-aminosalicylates (5-ASA), and infliximab [12, 13, 15]. However, these results have not been consistently reproduced in other study populations as two retrospective studies demonstrated no differences in medication utilization by race [7, 14].
Given the previous conflicting reports on differences in phenotype, outcomes, and medication utilization between AA and Ca patients with IBD, we sought to evaluate disease characteristics in a large prospectively collected cohort of patients in an urban tertiary care IBD center.
Methods
This study was approved by the Institutional Review Board at the University of Chicago under the study protocol 12-0209. We performed a cross-sectional review of the IBD registry at the University of Chicago Medicine. The IBD registry is a prospectively collected database that captures outpatients seen in the IBD clinics at the University of Chicago. Study participants consisted of patients with diagnosed IBD, either CD or UC, who were being evaluated in the outpatient IBD clinics.
Data were collected from study participants by a structured questionnaire filled out by the patient, and a standardized physician documentation template that was completed by the treating physicians. Both sources of information were integrated into a comprehensive database for the period between January 2008 and January 2013. Data regarding diagnosis, year of diagnosis, date of birth, self-identified race, family history, disease location, extent, and extraintestinal manifestations were collected during the initial clinic visit during the study period. Patients were classified as CD or UC by physician documentation. Patients with a diagnosis of indeterminate colitis were excluded from the analysis. Age of diagnosis was determined by subtracting the year of birth from the year of diagnosis. Similarly, duration of disease was determined by subtracting year of diagnosis from the year of the most recent clinic visit. Family history was provided by the patient for first degree relatives. Disease location or extent was determined from physician documentation. For the purposes of this study, ileal involvement in CD was defined as evidence of ileal disease at any point in the disease course. Extraintestinal manifestations were determined from physician documentation and included arthralgias related to underlying IBD activity, ankylosing spondylitis/sacroiliitis, erythema nodosum, pyoderma gangrenosum, oral aphthous ulcers, ocular inflammation, osteoporosis, primary sclerosing cholangitis, or other liver disease. Data regarding current medications and surgical history were collected during the initial clinic visit and updated during any subsequent follow-up visits to the clinic. Surgeries were limited to those for complications or treatment of either CD or UC. For CD, this included intestinal resections, stoma creation, stricturoplasty, incision and drainage, abdominal abscess drainage, perianal fistulotomy, seton placement, stoma revision, advancement flap, or an ileal pouch-anal anastomosis (IPAA). For UC, this included colectomy, IPAA formation, or permanent ileostomy. Information on disease phenotype was abstracted from the clinical documentation and patient-reported history.
The resulting data were subsequently abstracted from the database and divided into groups based on their self-identified race. Patients who self-identified as Ca or AA were included in the analysis. Patients who had no race data available, identified as Asian, Native Hawaiian/Pacific Islander, American Indian/Alaskan Native, or self-identified as mixed race, were not included in the analysis. Mixed race was not included because the ambiguity of the category would limit the conclusions that could be drawn from the population. Other groups aside from Ca and AA were not included in the analysis because of their limited numbers.
Statistical analyses were performed using STATA 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX, USA: StataCorp LP). The mean age of diagnosis was compared between groups using the Student’s t test. The mean duration of disease for AA and Cas with both UC and CD was compared using the Kruskal–Wallis equivalence-of-populations rank test. Disease characteristics variables between Cas and AAs were compared via Pearson’s chi-square analysis. Odds ratios for likelihood of surgery were calculated using logistic regression to adjust for location or extent of disease for each group. A predetermined significance level of p < 0.05 was used for all analyses.
Results
Population Characteristics
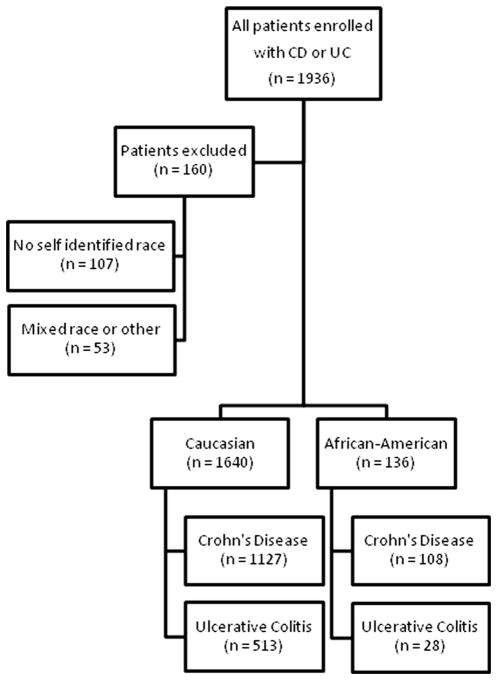
1,776 AA and Ca patients were identified in the database that had completed at least one data questionnaire. There were 1,235 patients with CD and 541 patients with UC (Fig. 1). Among patients with CD, 1,127 (91 %) Ca and 108 (9 %) AA patients were included in the analysis. Additionally, 513 (95 %) Ca and 28 (5 %) AA patients with UC were included in the analysis. There were 107 patients excluded due to no self-identified race information and 53 patients were excluded due to reporting mixed race or other races than Ca or AA (Fig. 1). Women represented a higher percentage of AA CD patients (77/108, 71.3 vs. 610/1,127, 54.1 %, p < 0.01) compared to Ca patients. AA patients with UC were diagnosed at an older mean age than Ca patients with UC (39.0 vs. 31.3 years, p = 0.01). AA patients with UC also had a shorter mean duration of disease than Ca patients with UC (8.7 vs. 11.5 years, p = 0.026). There were no significant differences for mean age of diagnosis or mean duration of disease between AA or Ca patients with CD (Table 1). Among all patients with IBD, Ca and AA patients reported similar prevalence of having a parent, sibling, or child affected by IBD (Table 1).
Fig. 1.
Study enrollment by inclusion and exclusion criteria
Table 1.
Family history and extraintestinal manifestations
| AA–CD | Ca–CD | p | AA–UC | Ca–UC | p | |
|---|---|---|---|---|---|---|
| General characteristics | ||||||
| Female gender | 77/108 (71.3 %) | 610/1,127 (54.1 %) | < 0.01 | 17/28 (60.7 %) | 258/513 (50.3 %) | 0.28 |
| Mean age of diagnosis (SD) | 28.2 (13.27) | 27.1 (13.09) | 0.41 | 39.0 (15.94) | 31.3 (14.13) | 0.01 |
| Mean duration of disease (SD) | 15.0 (10.76) | 15.6 (11.81) | 0.81 | 8.7 (10.90) | 11.5 (9.69) | 0.026 |
| Family history | ||||||
| Affected parent | 3/105 (2.9 %) | 70/1,103 (6.4 %) | 0.15 | 1/27 (3.7 %) | 36/501 (7.2 %) | 0.49 |
| Affected child | 1/108 (0.9 %) | 57/1,127 (5.1 %) | 0.05 | 0/28 (0.0 %) | 15/513 (2.9 %) | 0.36 |
| Any family member | 3/105 (2.9 %) | 89/1,103 (8.1 %) | 0.05 | 2/27 (7.4 %) | 43/501 (8.6 %) | 0.83 |
| Extraintestinal manifestations | ||||||
| Joint symptoms | 39/107 (36.5 %) | 265/1,111 (23.9 %) | < 0.01 | 8/28 (28.6 %) | 73/499 (14.6 %) | 0.047 |
| Ankylosing spondylitis/sacroiliitis | 3/107 (2.8 %) | 32/1,115 (2.9 %) | 0.97 | 2/28 (7.1 %) | 8/503 (1.6 %) | 0.035 |
| Erythema nodosum | 6/107 (5.6 %) | 41/1,117 (3.7 %) | 0.32 | 0/28 (0.0 %) | 4/502 (0.8 %) | 0.64 |
| Pyoderma | 3/107 (2.8 %) | 17/1,116 (1.5 %) | 0.32 | 1/28 (3.6 %) | 4/503 (0.8 %) | 0.14 |
| Oral aphthous ulcers | 4/108 (3.7 %) | 57/1,117 (5.1 %) | 0.52 | 2/28 (7.1 %) | 20/504 (4.0 %) | 0.41 |
| Ocular inflammation | 4/108 (3.7 %) | 42/1,118 (3.8 %) | 0.98 | 2/28 (7.1 %) | 11/503 (2.2 %) | 0.10 |
| Osteoporosis | 6/106 (5.7 %) | 104/1,111 (9.4 %) | 0.20 | 1/28 (3.6 %) | 24/499 (4.8 %) | 0.76 |
| Primary sclerosing cholangitis | 2/107 (1.9 %) | 6/1,117 (0.5 %) | 0.10 | 0/28 (0.0 %) | 6/501 (1.2 %) | 0.56 |
| Other liver disease | 0/107 (0.0 %) | 14/1,116 (1.3 %) | 0.24 | 0/27 (0.0 %) | 6/501 (1.2 %) | 0.57 |
Mean duration of disease and mean age is presented with standard deviations for this population. Other results are presented as the number of patients with the condition as a proportion of the total patients for whom data were available with the corresponding percentage
AA–CD African-American patients with Crohn’s disease, AA–UC African-American patients with ulcerative colitis, Ca–CD Caucasian patients with Crohn’s disease, Ca–UC Caucasian patients with ulcerative colitis
Compared to Ca patients, there were significantly more AA patients with arthralgias associated with disease activity for both CD (39/107, 36.5 vs. 265/1,111, 23.9 %, p < 0.01) and UC (8/28, 28.6 vs. 73/499, 14.6 %, p = 0.047). Additionally, AA patients with UC had a significantly higher prevalence of ankylosing spondylitis and sacroiliitis than Ca patients with UC (2/28, 7.1 vs. 8/503, 1.6 %, p = 0.035). There were no significant differences in ankylosing spondylitis and sacroiliitis, however, among CD patients. Furthermore, there were no significant differences between the two groups in prevalence of erythema nodosum, pyoderma gangrenosum, oral aphthous ulcers, ocular inflammation, osteoporosis, liver disease, or primary sclerosing cholangitis (Table 1).
Disease Location or Extent
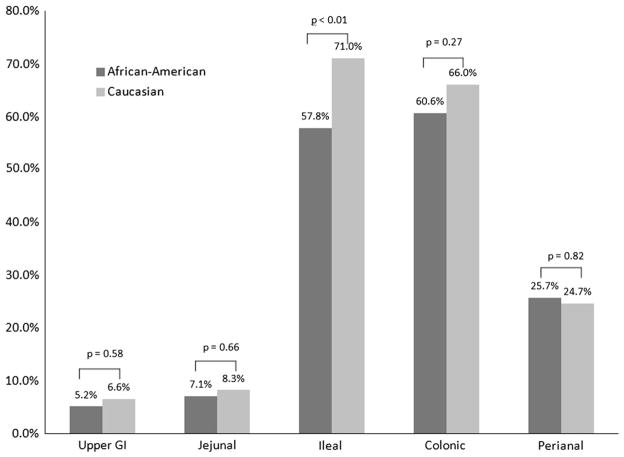
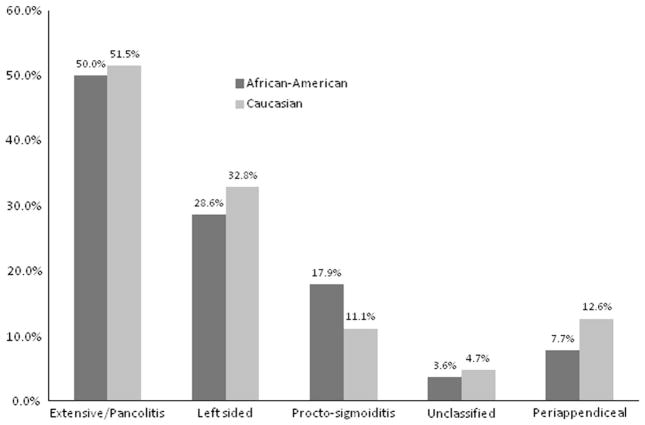
AA patients with CD demonstrated significantly less ileal involvement than Ca patients (59/102, 57.8 vs. 778/1,096, 71.0 %, p < 0.01) (Fig. 2). There were no significant differences between AA and Ca with CD in the percentage of patients with upper gastrointestinal, jejunal, colonic, or perianal involvement. Among patients with UC, there were no significant differences in disease extent identified between the two groups nor was there a difference in the rate of periappendiceal involvement (Fig. 3).
Fig. 2.
Crohn’s disease location. Percent of patients with CD involvement in AA patients with CD and Caucasian patients with CD. p values for CD location represent the results of Pearson’s chi-square tests for each variable. Light gray Caucasian, dark gray African-American
Fig. 3.
Ulcerative colitis disease extent. Percent of patients with UC extent in AA patients with UC and Caucasian patients with UC. UC disease extent was tested as an overall comparison with a single Pearson’s chi-square test (p = 0.73). Periappendiceal involvement was compared by a separate Pearson’s chi-square test (p = 0.46). Light gray Caucasian, dark gray African-American
Surgery History
AA patients with CD were found to have had significantly more Crohn’s related surgeries (Table 2), which remained present when adjusted for disease location with an odds ratio of 1.9 for AA patients (95 % CI 1.26–2.86, p = 0.002). For UC patients, there were no differences in surgical rates between racial groups prior to or after adjusting for disease extent (Table 2).
Table 2.
Number of surgeries by race and diagnosis
| CD
|
UC
|
||||||
|---|---|---|---|---|---|---|---|
| Total surgeries | AA (n = 108) | Ca (n = 1,127) | p | Surgery | AA (n = 28) | Ca (n = 513) | p |
| 0 | 44 (40.7 %) | 597 (53.0 %) | No | 27 (96.4 %) | 494 (96.3 %) | ||
| 1 | 31 (28.7 %) | 320 (28.4 %) | Yes | 1 (3.6 %) | 19 (3.7 %) | ||
| 2 | 24 (22.2 %) | 178 (15.8 %) | |||||
| 3+ | 9 (8.3 %) | 32 (2.8 %) | < 0.01 | 0.97 | |||
Number of surgeries for each group and overall p values
AA African-American patients, Ca Caucasian patients, UC ulcerative colitis, CD Crohn’s disease
Medication Use
Medications included in the analysis were corticosteroids, 5-ASA, 6-mercaptopurine/azathioprine, methotrexate, calcineurin inhibitors, antibiotics, anti-tumor necrosis factor antibodies, and natalizumab. There were no significant differences among all IBD patients between AA and Ca for medication use reported in clinic. Furthermore, Ca and AA patients with both CD and UC had similar use of these medications (Table 3).
Table 3.
Percentage of patients prescribed or continued on medication during clinic visit
| CD medications | AA (n = 108) | Ca (n = 1,127) | p | UC medications | AA (n = 28) | Ca (n = 513) | p |
|---|---|---|---|---|---|---|---|
| Corticosteroids | 26 (24.1 %) | 279 (24.8 %) | 0.88 | Corticosteroids | 9 (32.1 %) | 146 (28.5 %) | 0.68 |
| Aminosalicylates | 26 (24.1 %) | 238 (21.1 %) | 0.47 | Aminosalicylates | 17 (60.7 %) | 310 (60.4 %) | 0.98 |
| 6MP/AZA | 44 (40.7 %) | 459 (40.7 %) | 0.99 | 6MP/AZA | 7 (25.0 %) | 166 (32.4 %) | 0.42 |
| Methotrexate | 6 (5.6 %) | 97 (8.6 %) | 0.27 | Methotrexate | 0 (0.0 %) | 7 (1.4 %) | 0.53 |
| Calcineurin inhibitor | 1 (0.9 %) | 5 (0.4 %) | 0.49 | Calcineurin inhibitor | 0 (0.0 %) | 4 (0.8 %) | 0.64 |
| Antibiotics | 18 (16.7 %) | 161 (14.3 %) | 0.50 | Antibiotics | 0 (0.0 %) | 37 (7.2 %) | 0.14 |
| Anti-TNF | 38 (35.2 %) | 481 (42.7 %) | 0.13 | Anti-TNF | 5 (17.9 %) | 99 (19.3 %) | 0.85 |
| Natalizumab | 1 (0.9 %) | 21 (1.9 %) | 0.48 |
AA African-American patients, Ca Caucasian patients, UC ulcerative colitis, CD Crohn’s disease, 6MP 6-mercaptopurine, AZA azathioprine, Anti-TNF Anti-tumor necrosis factor antibodies
Discussion
In our analysis of one of the largest single-center cohorts of AA IBD patients described to date, we identified several phenotypic differences between AAs and Cas with IBD. AAs with UC were significantly older when IBD was diagnosed and had shorter duration of disease than Cas with UC. AA CD patients were also less likely than Ca CD patients to have ileal involvement. Across both AA CD and AA UC, we found higher reported arthralgias associated with disease activity, as well as more ankylosing spondylitis/sacroiliitis in AA UC. In addition to phenotypic differences between AAs and Cas with IBD, AA patients with CD were more likely to have had surgery in this population. Although not directly measured by this study, this finding may reflect a more aggressive disease course.
The finding that AA patients with UC are diagnosed later in life is similar to findings by White et al. [18]. While their study concluded that AA children with UC were more likely to be diagnosed after the age of 12, ours found a mean age of diagnosis in AA patients with UC of nearly 8 years older than Ca patients with UC. Previous studies have similarly found no significant difference between AA and Ca patients with CD for age of diagnosis [5, 8, 10]. Our finding that AA with UC have a shorter duration of disease has not been reported and is likely a reflection of the later age at disease diagnosis in this cross-sectional database [5, 7]. Reasons leading to these findings are varied due to the complex interaction of genetics and environment in the natural history of UC. While it is possible that this reflects a difference in the phenotypic expression of genetic differences, there is likely a significant effect of disparities in healthcare access and other socioeconomic factors. Since socioeconomic factors were not measured by this study, the size of this effect is not known.
Our observation that AA patients with CD have a lower incidence of ileal disease is consistent with what has been reported in several smaller studies [7, 10, 11]. Differences in UC disease extent and upper gastrointestinal CD involvement have each been shown in a single study, but other studies, including ours, have not redemonstrated these differences [1, 7, 12, 13, 15]. In addition, we identified differences in IBD extraintestinal manifestations in AA patients compared to Ca patients in both our UC and CD populations, which is similar with what has been reported in previous studies [7, 8]. The predominant extraintestinal manifestation in our population was arthralgias related to disease activity, consistent with prior data [9]. In our analysis, IBD-related arthralgias were more common in AA IBD patients. One explanation is that this could represent a different phenotype of IBD. However, there is a possibility that the results are biased by a difference in the prevalence of osteoarthritis. In fact, the NHANES-III study found that knee osteoarthritis was more common in AA patients than in Ca patients in the general population [19].
In contrast to previous studies, we did not identify a disparity in 5-ASA, steroid, biologic, or immunomodulator therapy for either our CD or UC populations [7, 12, 13, 15]. In addition, our study population did not show differences in positive family history in either CD or UC, contrary to previous investigations [7, 10, 12]. We did, however, observe a significant difference in the rate of surgeries in our AA CD patient population, which was not previously identified by several other studies [5, 9, 10, 12–14]. This finding is not likely biased by the age of diagnosis or duration of disease as these did not differ among patients with CD. Our data set does not provide enough details about the type of surgery or the indication for surgery to be able to reliably compare our populations.
In addition to the growing literature examining difference in IBD disease characteristics by race in adults, there have been several studies in pediatrics which also demonstrate inconsistencies in reported disease-specific differences by race. Eidelwein et al. [20] found that AA children with IBD were less often diagnosed before the age of 6, more likely to have stricturing or penetrating CD, and more exposure to corticosteroids, early azathioprine use, and infliximab. Ogunbi et al. [5] similarly found that AA children with IBD were diagnosed later in life, but did not demonstrate differences in CD surgery or disease location. In 2008, White et al. [18] reported on a multicenter registry of pediatric IBD patients. They found AA children with IBD were more likely to be diagnosed after the age of 12, although had similar locations of disease, prevalence of extraintestinal manifestations, and complications at diagnosis.
Similar to adults, the pediatric literature examining racial differences in IBD demonstrates evidence of regional variability, with single centers tending to report differences between the groups, while broader studies show more similarities. This likely results from referral bias, variation in management between centers, and complex socioeconomic factors that affect the phenotype of these complex diseases.
Because of the complexity of IBD, there is significant effort being spent on identifying all possible cellular mechanisms of disease by genetic analysis. Our study supports the hypothesis that these genetic factors are likely variable between populations. To date, there have been 163 risk-associated genetic loci identified for IBD. This includes 30 loci associated with CD, 23 loci associated with UC, and 110 shared loci [21]. The loci are estimated to explain 7.5 % of the variance of UC and 13.6 % of the variance of CD [21]. Studies that have contributed to these conclusions, however, were either predominantly or entirely composed of samples from populations of European descent [22, 23]. As a result, no data regarding the variability between populations of different genetic backgrounds is available at present, which further highlights the need for including different populations in biomedical research.
Despite being able to demonstrate several clinically relevant disease-specific differences between AA and Ca patients with IBD, this study does have several limitations. First, the use of self-identified race in biomedical research is controversial. Race is a complex social construct that includes both ancestry and cultural factors, which can obscure the connection between race and genetics. Due to this inaccuracy, the field of genetic research is searching for suitable alternative categories to describe a population’s genetic background [24–26]. Future studies of population differences in IBD should consider using ancestry in place of self-identified race. This has the advantage of reducing dependence on historically complex definitions of racial groups based on culture and instead provides information about geographically defined heritage [25]. The second limitation is that our database did not collect socioeconomic variables. Further information regarding the socioeconomic makeup of our patient population would have helped control for significant differences in environmental exposure and would have helped clarify the genetic and environmental components that contribute to the observed differences. Future studies should consider socioeconomic data, including patient zip code, income, family size, and education level as vital to a comprehensive patient database. Additionally, our single-center location limited our ability to recruit a patient population that accurately reflects the general population and restricted our ability to recruit a larger sample size of AA UC patients. Indeed, the small sample size of AA UC patients limited the power of the analysis.
The primary strength of our study is that it is one of the largest single-center populations of AA patients with IBD reported. Additionally, data were collected prospectively on a comprehensive set of variables with significant clinical importance. Because of these strengths, we were able to demonstrate differences in the rates of surgery for patients with CD that were not previously reported. Our study did not differentiate between non-penetrating, non-stricturing CD and fistulizing CD, or stricturing CD. However, a higher rate of IBD-related surgery suggests a higher rate of the indications for surgery, such as fistulizing disease or stricturing disease. This finding lends support to the hypothesis that AA with CD has a more aggressive disease course.
In conclusion, we demonstrate differences in disease characteristics between AA and Ca patients with IBD in our tertiary care hospital. With less classical ileal disease and more prominent joint symptoms associated with disease activity, special attention must be paid toward this atypical disease profile among AA patients. Our study further underscores the need for inclusion of racial and ethnic minorities into the study of IBD and has implications for further genotype-phenotype studies.
Supplementary Material
Acknowledgments
Funded in part by the following Grants: P30DK42086 (Digestive Diseases Research Core Center) and K08DK090152 (J.P.).
Abbreviations
- IBD
Inflammatory bowel disease
- Ca
Caucasian
- AA
African-American
- CD
Crohn’s disease
- UC
Ulcerative colitis
- 5-ASA
5-Aminosalicylate
- IPAA
Ileal pouch-anal anastomosis
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10620-014-3160-0) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Contributor Information
M. Anthony Sofia, Email: masofia@uchicago.edu, Department of Medicine, University of Chicago, Chicago, IL, USA. 5841 S. Maryland Avenue, MC 7082, Chicago, IL 60637, USA.
David T. Rubin, Email: drubin@medicine.bsd.uchicago.edu, Department of Medicine, University of Chicago, Chicago, IL, USA. Inflammatory Bowel Disease Center, University of Chicago, Chicago, IL, USA. 5841 S. Maryland Avenue, MC 4076, Chicago, IL 60637, USA
Ningqi Hou, Email: nhou@health.bsd.uchicago.edu, Department of Health Studies, University of Chicago, Chicago, IL, USA. 5841 S. Maryland Avenue, MC 2007, Chicago, IL 60637, USA.
Joel Pekow, Email: jpekow@medicine.bsd.uchicago.edu, Department of Medicine, University of Chicago, Chicago, IL, USA. Inflammatory Bowel Disease Center, University of Chicago, Chicago, IL, USA. 900 East 57th St., MB #9, Chicago, IL 60637, USA.
References
- 1.Malaty HM, Hou JK, Thirumurthi S. Epidemiology of inflammatory bowel disease among an indigent multi-ethnic population in the United States. Clin Exp Gastroenterol. 2010;3:165–170. doi: 10.2147/CEG.S14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sewell JL, Yee HF, Jr, Inadomi JM. Hospitalizations are increasing among minority patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2010;16:204–207. doi: 10.1002/ibd.21008. [DOI] [PubMed] [Google Scholar]
- 3.Veluswamy H, Suryawala K, Sheth A, et al. African-American inflammatory bowel disease in a southern U.S. Health Center. BMC Gastroenterol Engl. 2010;10:104. doi: 10.1186/1471-230X-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 5.Ogunbi SO, Ransom JA, Sullivan K, Schoen BT, Gold BD. Inflammatory bowel disease in african-american children living in Georgia. J Pediatr. 1998;133:103–107. doi: 10.1016/s0022-3476(98)70187-8. [DOI] [PubMed] [Google Scholar]
- 6.Simsek H, Schuman BM. Inflammatory bowel disease in 64 black patients: analysis of course, complications, and surgery. J Clin Gastroenterol. 1989;11:294–298. doi: 10.1097/00004836-198906000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Basu D, Lopez I, Kulkarni A, Sellin JH. Impact of race and ethnicity on inflammatory bowel disease. Am J Gastroenterol. 2005;100:2254–2261. doi: 10.1111/j.1572-0241.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and Non-Hispanic Whites: characterization of a large North American cohort. Am J Gastroenterol. 2006;101:1012–1023. doi: 10.1111/j.1572-0241.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 9.Mahid SS, Mulhall AM, Gholson RD, Eichenberger MR, Galandiuk S. Inflammatory bowel disease and African Americans: a systematic review. Inflamm Bowel Dis. 2008;14:960–967. doi: 10.1002/ibd.20389. [DOI] [PubMed] [Google Scholar]
- 10.Deveaux PG, Kimberling J, Galandiuk S. Crohn’s disease: presentation and severity compared between black patients and white patients. Dis Colon Rectum. 2005;48:1404–1409. doi: 10.1007/s10350-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 11.Jackson JF, III, Dhere TT, Repaka A, Shaukat A, Sitaraman S. Crohn’s disease in an African-American population. Am J Med Sci. 2008;336:389–392. doi: 10.1097/MAJ.0b013e31816a5c06. [DOI] [PubMed] [Google Scholar]
- 12.Moore L, Gaffney K, Lopez R, Shen B. Comparison of the natural history of ulcerative colitis in African Americans and Non-Hispanic Caucasians: a historical cohort study. Inflamm Bowel Dis. 2012;18:743–749. doi: 10.1002/ibd.21796. [DOI] [PubMed] [Google Scholar]
- 13.Sewell JL, Inadomi JM, Yee HF., Jr Race and inflammatory bowel disease in an urban healthcare system. Dig Dis Sci. 2010;55:3479–3487. doi: 10.1007/s10620-010-1442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straus WL, Eisen GM, Sandler RS, Murray SC, Sessions JT. Crohn’s disease: does race matter? The mid-atlantic Crohn’s disease study group. Am J Gastroenterol. 2000;95:479–483. doi: 10.1111/j.1572-0241.2000.t01-1-01531.x. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen GC, LaVeist TA, Harris ML, Wang MH, Datta LW, Brant SR. Racial disparities in utilization of specialist care and medications in inflammatory bowel disease. Am J Gastroenterol. 2010;105:2202–2208. doi: 10.1038/ajg.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer ME, Machan JT, Kawatu D, et al. Factors that determine risk for surgery in pediatric patients with Crohn’s disease. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2010;8:789–794. doi: 10.1016/j.cgh.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen GC, Bayless TM, Powe NR, Laveist TA, Brant SR. Race and health insurance are predictors of hospitalized Crohn’s disease patients undergoing bowel resection. Inflamm Bowel Dis. 2007;13:1408–1416. doi: 10.1002/ibd.20200. [DOI] [PubMed] [Google Scholar]
- 18.White JM, O’Connor S, Winter HS, et al. Inflammatory bowel disease in African American children compared with other racial/ethnic groups in a multicenter registry. Clin Gastroenterol Hepatol. 2008;6:1361–1369. doi: 10.1016/j.cgh.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the third national health and nutrition examination survey 1991–94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 20.Eidelwein AP, Thompson R, Fiorino K, Abadom V, Oliva-Hemker M. Disease presentation and clinical course in black and white children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:555–560. doi: 10.1097/MPG.0b013e3180335bb3. [DOI] [PubMed] [Google Scholar]
- 21.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimura JH, Rajagopalan R. Different differences: the use of ‘genetic ancestry’ versus race in biomedical human genetic research. Soc Stud Sci. 2011;41:5–30. doi: 10.1177/0306312710379170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torres JB, Kittles RA. The relationship between “race” and genetics in biomedical research. Curr Hypertens Rep. 2007;9:196–201. doi: 10.1007/s11906-007-0035-1. [DOI] [PubMed] [Google Scholar]
- 26.Collier R. Race and genetics in the doctor’s office. Can Med Assoc J. 2012;184:752–753. doi: 10.1503/cmaj.109-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.