Abstract
Systemic juvenile idiopathic arthritis (sJIA) has long been recognized as unique among childhood arthritides, because of its distinctive clinical and epidemiological features, including an association with macrophage activation syndrome. Here, we summarize research into sJIA pathogenesis. The triggers of disease are unknown, although infections are suspects. Once initiated, sJIA seems to be driven by innate proinflammatory cytokines. Endogenous Toll-like receptor ligands, including S100 proteins, probably synergize with cytokines to perpetuate inflammation. These and other findings support the hypothesis that sJIA is an autoinflammatory condition. Indeed, IL-1 is implicated as a pivotal cytokine, but the source of excess IL-1 activity remains obscure and the role of IL-1 in chronic arthritis is less clear. Another hypothesis is that a form of hemophagocytic lymphohistiocytosis underlies sJIA, with varying degrees of its expression across the spectrum of disease. Alternatively, sJIA with MAS might be a genetically distinct subtype. Yet another hypothesis proposes that inadequate downregulation of immune activation is central to sJIA, supporting evidence for which includes ‘alternative activation’ of monocyte and macrophages and possible deficiencies in IL-10 and T regulatory cells. Some altered immune phenotypes persist during clinically inactive disease, which suggests that this stage might represent compensated inflammation. Despite much progress being made, many questions remain, providing fertile ground for future research.
Introduction
The clinical picture
Systemic juvenile idiopathic arthritis (sJIA) is a unique type of childhood chronic arthritis that is currently classified as a subtype of juvenile idiopathic arthritis (JIA).1 Unlike the other subtypes of JIA, sJIA shows no sex bias or peak age at onset during childhood. Extra-articular features, including daily spiking fevers, fleeting salmon-colored macular rash, lymphadenopathy, hepatosplenomegaly and polyserositis, can overshadow joint inflammation at disease onset. Erythrocyte sedimentation rate, C-reactive protein, and counts of neutrophils and platelets are typically quite elevated, reflecting systemic inflammation. The clinical course at later stages is highly variable. In one study, approximately 40% of patients with sJIA (followed on average for close to 5 years) had a monocyclic course, <10% had a polycyclic course and >50% had a persistent course (Box 1).2 The proportion of patients with monocyclic disease might be underestimated due to lack of ascertainment. In many patients with polycyclic or persistent sJIA, the systemic features subside during the initial months or years of disease, whereas arthritis is progressive. The joint disease is notable for early destructive changes, ankylosis of the cervical spine, wrists and mid-foot, and reduced responsiveness to treatments that are effective in polyarticular JIA. Responses to other treatments, including among patients. Polyarticular arthritis at 6 months after disease onset, particularly with hip involvement and highly increased platelet counts, is predictive of joint damage by 2 years.3,4 With persistent disease, significant growth impairment - beyond the extent attributable to steroid therapy - is typical. In North America and Europe, sJIA accounts for roughly 10% of cases of JIA; in India and Japan, where oligoarticular disease is less common, sJIA represents about 30% and 50% of reported JIA cases, respectively.5,6 Importantly, sJIA accounts for a disproportionate share of JIA-related mortality; this excess mortality is attributable primarily to complications of systemic inflammation (such as macrophage activation syndrome [MAS] and amyloidosis) and of immunosuppressive therapies.
Association with macrophage activation syndrome
The association of sJIA with MAS is a striking feature of the disease. MAS is a potentially fatal condition that is characterized by persistent fever, cytopenias, liver abnormalities, coagulopathy and central nervous system dysfunction. Well-differentiated macrophages with hemophagocytic activity are the pathologic hallmark of MAS.7 Presumed precipitating factors in children with sJIA include various infections and medications.7 Approximately 10% of patients with sJIA are diagnosed with MAS, some of whom suffer repetitive episodes.8,9 However, in as many as 50% of patients with active sJIA, MAS is evident in bone marrow aspirates.10,11 MAS is an acquired form of hemophagocytic lymphohistiocytosis (HLH), a group of histiocytic disorders that also includes inherited disorders.7
Pathophysiology of sJIA
Data from recent genetic and immunologic studies and from clinical trials of biologic therapies have provided substantial new insights into sJIA pathophysiology, but investigations have yet to reveal a primary underlying etiologic pathway for this disease. Indeed, the clinical heterogeneity of sJIA is paralleled by evidence for the involvement of various immune pathways and genetic influences. However, the studies to date have not associated immune variations with specific differences in disease course, response to medication or clinical outcomes, except for immune features associated with MAS. In this article, we review the current literature on sJIA pathogenesis, highlighting alternative mechanistic hypotheses, emerging themes and unanswered questions.
sJIA as a pathogen-triggered disease
The increased incidence of sJIA compared with that of adult-onset Still disease (the adult equivalent of sJIA) has implications for the etiology of sJIA. One scenario is that infectious agents that are typically encountered in childhood initiate sJIA; no single environmental trigger has been identified, although this lack of an obvious candidate could point to multiple common agents being capable of initiating sJIA. Consistent with the possibility of infectious triggers, onset of sJIA seemed to be seasonal in some studies.12–14 Alternatively, onset of disease during childhood could be associated with the presence of stronger or increased numbers of disease susceptibility alleles.
sJIA as an autoinflammatory disease
Autoimmune versus autoinflammatory diseases
Advances in elucidating the basic mechanisms of innate immunity have led to a new paradigm for understanding immune-mediated diseases, which is based on the relative contributions of abnormalities in the adaptive and innate immune responses. At one end of the spectrum are classic autoimmune diseases, such as rheumatoid arthritis or type 1 diabetes, in which adaptive immune responses are central. These diseases are associated with autoreactive antigen-specific T lymphocytes and high titers of autoantibodies, and typically show strong associations with MHC class II alleles. By contrast, abnormalities in pathways of innate immunity lead to a distinct group of pathologic conditions known as autoinflammatory diseases, in which monocytes and neutrophils, rather than lymphocytes, are the predominant effector cells.15 Initially, the autoinflammatory label was applied to a group of diseases with single-gene defects. These diseases have common clinical features including recurrent fevers and multisystem inflammation that usually affects the joints, skin, gastrointestinal tract and eyes, and are complicated by amyloidosis in the long term. A prototypical example, neonatal-onset multisystem inflammatory disease (NOMID), is caused by mutations in the NLRP3 gene, which encodes cryopyrin (also known as NACHT, LRR, and PYD domains-containing protein 3). Recently, the class of autoinflammatory diseases has been widened to encompass multigenic diseases in which innate inflammation is prominent, such as Crohn disease, type II diabetes and sJIA.15 Many clinical features of sJIA, including fever, arthritis and increased risk of amyloidosis, fit this classification. Notably, however, the occurrence of MAS is very rare in the monogenic autoinflammatory diseases.16
Genetic investigations
In commom with other autoinflammatory conditions, but unlike other subtypes of JIA, strong associations with MHC class II alleles have not been found for sJIA, although in some populations there might be an association with HLA-DR4 in patients with erosive arthritis.17 The inherited risk factors for sJIA most consistently described are polymorphisms in the promoter elements and genes encoding tumor necrosis factor (TNF),18 IL-6,19,20 IL-10,21,22 macrophage migration inhibitory factor (MIF)23,24 and the IL-1 family (specifically, IL1A, IL1RN, IL1R2 and possibly IL1F10).25 In addition, an association has been reported between susceptibility to sJIA with single nucleotide polymorphisms (SNPs) within SLC26A2, mutations in which cause diastrophic dysplasia.26 Another study found that 2 of 9 patients with sJIA had loss-of-function variants in P2X7, which encodes an ATP receptor that regulates IL-1 processing and secretion.27 Altogether, these findings support the model of sJIA as a multigenic disease, with each allele identified thus far providing a small contribution to inherited susceptibility.
Genes responsible for monogenic autoinflammatory diseases have been evaluated in sJIA. An initial study found no notable associations of sJIA with NLRP3, NOD2, MEFV or PSTPIP1, mutations in each of which cause such diseases.28 In a 2009 report, a subgroup (15%) of Turkish patients with severe sJIA harbored MEFV mutations.29 MEFV, the causal gene in familial Mediterranean fever, encodes pyrin - a protein that affects the IL-1β-activation pathway but whose precise function is unknown. Many patients with inherited autoinflammatory syndromes do not have defined genetic lesions, so additional overlap between risk factors for these diseases and for sJIA could yet be revealed. A genome-wide association study of sJIA is currently underway.
Innate immune activity in sJIA
Consistent with its classification as an autoinflammatory disorder, sJIA is characterized by prominent innate immune activity and a limited role for adaptive immunity. In active disease, circulating innate immune cells - monocytes, neutrophils and immature (CD34+ CD33+) myelomonocytic precursors - are expanded in number.30–32 Microarray studies of peripheral blood mononuclear cells (PBMCs) from patients with sJIA show increased expression of genes associated with the activation of the monocyte/macrophage lineage.30,33–36 Expression profiles also demonstrate upregulation of innate immune receptors (for example, CARD12 [NLR family CARD domain-containing protein 4] and Toll-like receptor [TLR] 5) and signaling pathways (such as TLR–IL-1, IL-6 and PPAR-γ signaling), as well as downregulation of gene networks involving processes related to natural killer (NK) cells, T cells and MHC.30,33–37 In one study, gene expression profiles from patients with sJIA overlapped to a greater extent with NOMID-associated transcripts than with those associated with systemic lupus erythematosus, polyarticular JIA or Kawasaki disease.34
Proinflammatory mediators
Many features of sJIA seem to be explained by the known effects of innate proinflammatory cytokines, IL-1 and IL-6 in particular but also macrophage colony-stimulating factor (M-CSF), TNF and IL-18 (Figure 1). The implicated cytokines are principally, though not exclusively, produced by monocytes, macrophages and neutrophils. Translational studies of patient samples also support the contributions of these mediators to disease.
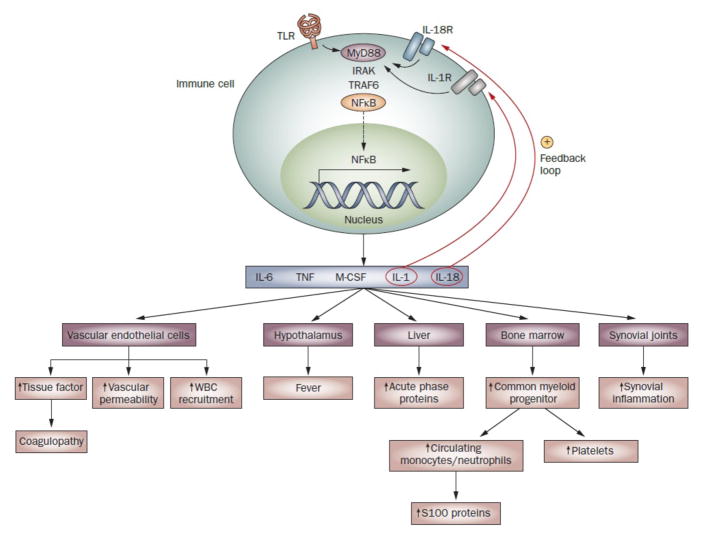
Figure 1.
Perpetuation of innate immune responses in sJIA. Innate immune pathways activated in sJIA are normally triggered by the recognition of pathogen-associated molecular patterns by TLRs expressed on innate immune cells, but can also be triggered by endogenous ligands in inflammatory conditions. These intracellular pathways lead to activation of the transcription factor NFκB, which is translocated into the nucleus where it upregulates expression of genes encoding several proinflammatory cytokines. These cytokines initiate the inflammatory cascade through their effects on the hypothalamus, bone marrow, liver and vascular endothelial cells. Activated vascular endothelial cells act as a procoagulant surface and are likely to contribute to the coagulopathy of sJIA.107 Antibodies against endothelial cells reportedly occur in sJIA,108 although the relevance of this latter finding is unclear. The cytokines also drive joint inflammation, by stimulating osteoclast-mediated bone resorption, osteoblast apoptosis, inhibition of chondrocyte proteoglycan synthesis and synoviocyte secretion of enzymes that degrade matrix and cartilage. Since signaling through IL-1R and IL-18R shares the downstream portion of the TLR4 signaling pathway, IL-1 and IL-18 provide positive feedback loops that further contribute to perpetuation of the inflammatory responses in sJIA. Abbreviations: IL-1R, IL-1 receptor; IL-18R, IL-18 receptor; M-CSF, macrophage colony-stimulating factor; NFκB, nuclear factor κB; sJIA, systemic juvenile idiopathic arthritis; TLR, Toll-like receptor; TNF, tumor necrosis factor; WBC, white blood cell.
IL–1 and S100 proteins
IL-1, a protein with pleiotropic effects, upregulates its own transcription as well as that of IL-6, among other cytokines. In addition to driving systemic inflammation, IL-1 stimulates the destruction of cartilage and bone; for example, by inducing follistatin-related protein 1, an inflammatory product of joint matrix cells and a possible biomarker of sJIA disease activity.38 Interestingly, gene expression studies of sJIA PBMCs have not usually shown increased expression of IL1B,30,33–36 but serum from patients with active sJIA can induce the transcription of various IL-1 related genes, including IL1B, IL1RN, IL1R1 and IL1R2, in PBMCs from healthy individuals.33 In addition, PBMCs from patients with untreated, new-onset sJIA demonstrated a possible IL-1-driven signature.30 A central role for IL-1 also emerged in pathway analysis of plasma proteins that are elevated at the time of sJIA flare.39
The most compelling evidence for the involvement of IL-1 in sJIA is the successful treatment of sJIA with inhibitors of IL-1 such as anakinra, a soluble IL-1 receptor antagonist (IL-1Ra) that is similar to naturally occurring IL-1Ra. Serum levels of endogenous IL-1RA are elevated in active sJIA, though not to the degree observed in polyarticular JIA.40–42 Augmenting IL-1 inhibition above these levels is rapidly therapeutic in a substantial proportion of patients with sJIA.33,43 Concurrent with a clinical response to anti-IL-1 therapy, many disease-associated changes in gene expression in PBMCs revert to normal.33,36,37
In initial studies of sJIA treatment with anakinra, about half of the patients treated were responders, whereas the other half had a transient response, followed by the return of arthritis and elevated levels of acute-phase proteins, although fever and rash did not typically recur.37,27 The incidence of poor response to anakinra in some patients suggested the possibility of biological subgroups, a hypothesis supported by the finding that this group had higher serum levels of granulocyte colony-stimulating factor and lower serum levels of IL-9 than those who responded to anakinra therapy.27 However, evidence from a recent retrospective study of 46 patients suggests that, if given as a first-line drug, anakinra leads to the resolution of both systemic symptoms and arthritis and protects against the development of persistent arthritis in approximately 90% of patients, and that the non-responders might have been underdosed.44 These data, which require confirmation, argue that IL-1 drives the initiating abnormalities in most cases of sJIA. The seemingly reduced success of IL-1 inhibition in patients with refractory arthritis implies that IL-1-independent mechanisms arise later in the disease course, if disease is uncontrolled.
The basis of the observed excess IL-1 activity in sJIA is not known. The initial rapid response and continued dependence on IL-1 inhibition in many patients with sJIA are reminiscent of responses seen in NLRP3-associated autoinflammatory syndromes. In vitro, however, NLRP3-mutant cells show impaired redox homeostasis associated with rapid secretion of IL-1,45 whereas sJIA monocytes do not.27 Monocytes from both disease groups are resistant to enhancement by ATP of lipopolysaccharide-stimulated IL-1β secretion in vitro, but probably for different reasons (Figure 2).
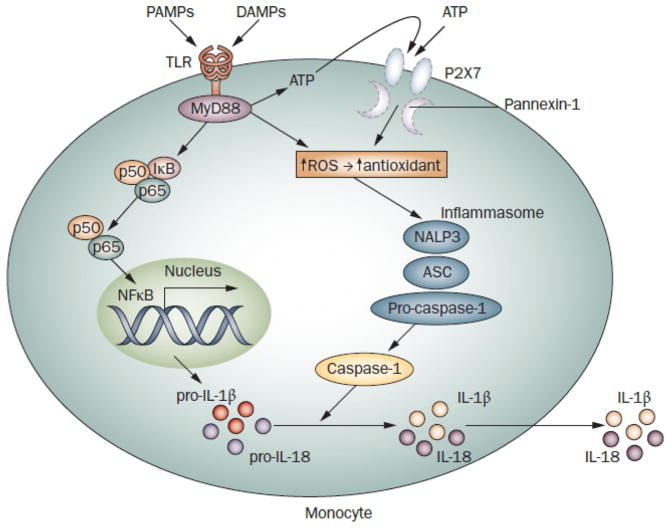
Figure 2.
Secretion of IL-1β by monocytes in inflammatory diseases. Ligation of pattern recognition receptors, such as TLRs, induces expression of inactive pro-IL-1β; its cleavage by caspase-1 leads to mature IL-1β and secretion.109 Activation of caspase-1 requires assembly of the inflammasome, a multimolecular complex that includes NALP3 (cryopyrin) and the adaptor protein ASC.110 Exogenous ATP triggers the receptor P2X7 and its associated pore, pannexin-1, inducing a strong redox response (generation of ROS followed by upregulation of antioxidant systems) and facilitating assembly/activation of the inflammasome. TLR signaling also stimulates ROS generation and release of endogenous ATP, resulting in autocrine stimulation. NLRP3 mutant monocytes (from patients with cryopyrin-associated periodic syndromes) have altered basal and TLR-stimulated redox states, causing increased and abnormally fast secretion of LPS-stimulated IL-1β. IL-1 secretion is not increased further by ATP, seemingly due to depletion of this pathway after LPS alone.27,45,111 sJIA monocytes do not share this overall phenotype,45 but are resistant to ATP-enhanced IL-1 secretion.37 Anakinra treatment results in increased P2X7 transcripts in sJIA blood cells,37 suggesting that excess IL-1 activity leads to suppression of P2X7. IL-18 processing and secretion is similar to that of IL-1; however, pro-IL-18 is constitutively produced by monocytes. Abbreviations: ASC, apoptosis-associated speck-like protein containing a CARD; DAMP, damage-associated molecular pattern molecule; IL, interleukin; LPS, lipopolysaccharide; PAMP, pathogen-associated molecular pattern molecule; P2X7, P2X purinoceptor 7; ROS, reactive oxygen species; sJIA, systemic juvenile idiopathic arthritis; TLR, Toll-like receptor.
The occurrence of a clinical response to IL-1 inhibition, despite the absence of excess IL-1 secretion by sJIA blood monocytes in vitro, suggests that increased IL-1 activity in vivo might involve other cell types, privileged sites, or different triggers or pathways, and some evidence supports these possibilities. IL-1 levels in synovial fluid are higher in active sJIA than in active polyarticular JIA, whereas levels in circulating plasma are lower in sJIA.46 Pathways other than the caspase-1 - inflammasome pathway that is shown in Figure 2 can contribute to IL-1β secretion in inflammatory diseases (including models of arthritis).47,48 For example, proteinase-3 in neutrophils,48 secreted serine proteases of mast cells and neutrophils which, in turn, further increases production of S100 proteins (Figure 3). In vitro, the disruption of this loop reduces the production of IL-1β by PBMCs.50
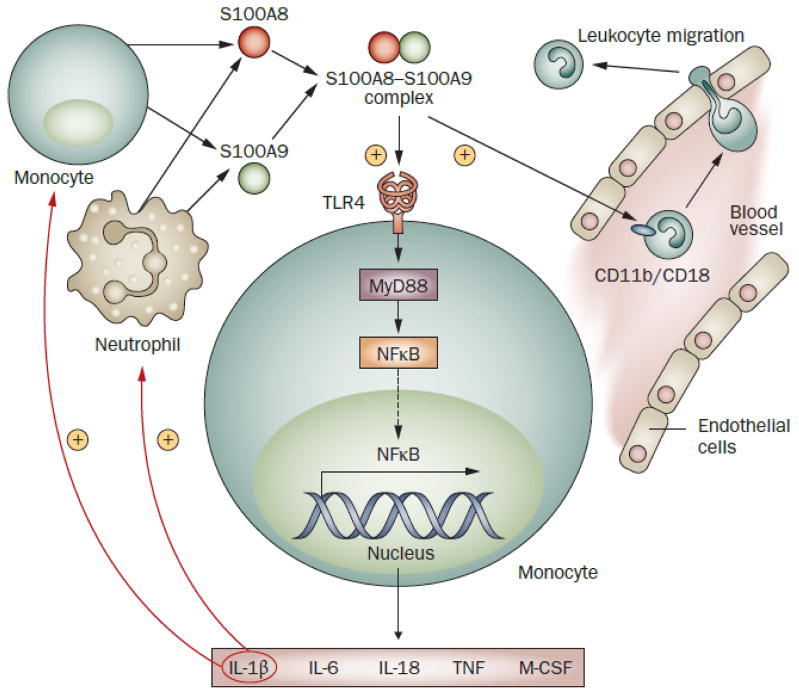
Figure 3.
A possible positive feedback cycle involving IL-1β and S100 proteins contributes to perpetuation of chronic inflammation in sJIA. Various pro-inflammatory stimuli, including IL-1β, lead to activation of monocytes and neutrophils. The activation of these cells is accompanied by increased secretion of S100 proteins, including S100-A8, S100-A9 and S100-A12. The proteins S100-A8 and S100-A9, which are markedly elevated in sJIA, form a complex called calprotectin that can serve as an endogenous agonist of TLR4 and trigger TLR signaling pathways, leading to activation of the transcription factor NFκB. Translocation of activated NFκB into the nucleus upregulates expression of IL-1β. In turn, increased IL-1β leads to further secretion of S100 proteins. In addition, S100-A8–S100-A9 complexes activate endothelial cells and also bind the integrin receptor CD11b/CD18 on phagocytes and stimulate their transendothelial migration into tissues.112,113 Abbreviations: IL, interleukin; M-CSF, macrophage colony-stimulating factor; NFκB, nuclear factor κB; TNF, tumor necrosis factor.
IL–6
In addition to IL-1, IL-6 also contributes to sJIA pathogenesis. Serum levels of IL-6 rise and fall in parallel with sJIA fever spikes, and increased IL-6 production is associated with thrombocytosis, microcytic anemia, growth retardation, and osteopenia.52–54 In some, but not all, reports, IL-6 is spontaneously released in vitro from circulating immune cells in sJIA,55,56 although this phenotype is not unique to sJIA.57 Importantly, neutralizing IL-6 with tocilizumab in sJIA led to clinical improvement in preliminary studies.58,59 Positive response to this therapy is associated with the upregulation of cartilage oligometric matrix protein, a marker of growth cartilage, supporting a role for IL-6 in growth impairment in sJIA.60 Another consequence of elevated IL-6 in active sJIA is modulation of levels of proteases and their regulators (for example, MMP-9 and its inhibitor TIMP-1).61–64 Presumably as a consequence of these changes, novel fragments of various proteins can be detected in plasma39 and urine63 during flare; these peptides might provide a novel source of sJIA biomarkers.
sJIA as a defect of Immunoregulation
Anti-inflammatory responses are also stimulated in active sJIA, presumably in an attempt to quell inflammation. In untreated early disease, PBMCs show increases in transcripts for negative regulators of innate immune responses, such as IL1RN (encoding IL-1Ra) and SOCS3 (encoding suppressor of cytokine signaling 3), which is induced by IL-1 and IL-6 and inhibits their signaling pathways.30,34 Additional anti-inflammatory pathways are detected during sJIA, as discussed below. It seems that none are sufficient to resolve the inflammation in sJIA. The exception might be disease resolution in monocyclic sJIA but this possibility has not been investigated.
IL–10
Expression of the immunoregulatory cytokine IL-10 is detectable during active disease. IL-10 mediates a broad range of anti-inflammatory action with effects on both innate and adaptive immune responses. The IL10 allele associated with sJIA is a ‘low expressor’,21,22 and average levels of IL-10 in plasma and synovial fluid during active disease, although elevated compared with inactive disease, are lower than levels in active poly articular JIA.46,65 These results have suggested that the anti-inflammatory IL-10 response is deficient in sJIA. One downstream effect of IL-10 is induction of the enzyme heme oxygenase 1 (HO-1), which mediates anti-inflammatory effects through its products (discussed below). Serum levels of HO-1 in sJIA correlate with erythrocyte sedimentation rate.66
Alternatively activated monocyte/macrophages
The monocyte/macrophage lineage is strikingly plastic in response to environmental cues and undergoes different forms of polarized activation. ‘Classically activated’ M1 macrophages are highly proinflammatory, whereas ‘alternatively activated’ M2 macrophages fine-tune or resolve inflammatory responses, perform scavenger functions and promote tissue remodeling and repair.67 M1 and M2 macrophages also participate in type 1 T helper (TH1) and TH2 responses, respectively. Interferon (IFN)-γ is the key cytokine driving the M1 pathway, whereas the addition of IL-4, IL-3, IL-10 or steroids to monocytes in culture promotes their differentiation into M2 macrophages.67 Subtypes of M2 macrophages have been described, in association with different polarizing conditions (Table 1). sJIA monocytes express transcripts related to the M2 pathway(s), for example, the genes MS4A4A, GPR84, CCL2, ARG1, CD163 and IL10.30,34 The M2 phenotype in sJIA monocytes is not the result of steroid treatment, because this phenotype is observed in untreated patients.30 sJIA monocytes are also M2-like in their resistance to apoptotic stimuli.68,69 In addition, the absence of IFN-induced gene expression signatures in PBMCs from patients with active sJIA is consistent with a more-M2-like phenotype.30,34,35,70 Interestingly, anakinra treatment might reverse this aspect of the phenotype; in a recent study, elevated type I IFN-regulated transcripts were observed in PBMCs of the majority of anakinra-treated subjects, regardless of their clinical response.37 This finding implies that IL-1 might contribute (directly or indirectly) to inhibition of IFN signaling, for example through inducing SOCS-3. As M2 polarization of monocyte/macrophages is a ‘deactivating’ program, it seems plausible that it is induced to suppress the inflammatory state.
Table 1.
| Characteristic | M1 | M2a* | M2b* | M2c* |
|---|---|---|---|---|
| In vitro induction | IFN-γ plus ipopolysaccharide | IL-4, IL-13 | Immune complex plus lipopolysaccharide or IL-1β | IL-10, TGF-β, glucocorticoids |
| Surface expression | MHC class II, CD40, CD80, IL-2Rα (CD25), IL-7Rα(CD127) | MHC class II, MMR (CD206), CD209 (DC-SIGN), CD20 antigen-like 1 (MS4A4A), CD20 antigen-like 3 (MS4A6A), SRAP, CD302, Dectin 1 | No unique markers defined | CD163, IL-21R, TLR1, TLR8 |
|
| ||||
| Cytokines | IL-1β, IL-6, IL-12, IL-15, IL-23, TNF | IL-1Ra, IL-10, TGF-β, IGF1, Fibronectin 1, β-ig-H3, PDGFC, Coagulation factor XIIIa | IL-10 | IL-10 |
|
| ||||
| CC-chemokine ligands | CCL8, CCL15, CCL19, CCL20 | CCL2, CCL13, CCL14, CCL17, CCL18, CCL22, CCL23, CCL24, CCL26 | CCL1, CCL20 | CCL18 |
| CXC-chemokine ligands | CXCL9, CXCL10, CXCL11, CXCL13 | GRO-α (CXCL1), CXCL2, CXCL3 | ||
| Transcription factor | IRF5 | STAT6, IRF4 | IRF4 | IRF4 |
Subtypes of alternatively activated M2 cells have been defined,69,115 Abbreviations: β-ig-H3, TGFβ-inducible protein ig-H3; CCL CC-chemokine ligand; CXCL, CXC-chemokine ligand; DCL1, dicer-like 1; DC-SIGN, dendritic cell-specific ICAM-3-grabbing non-integrin 1; GROα, growth-regulated protein α; IGF, insulin-like growth factor; IFN, interferon; IL-1Ra, irrterleukin-1 receptor antagonist; lL-2Rα; interleukin-2 receptor subunit α; IL-7Rα, interleukin-7 receptor subunit α; IL-21R, interleukin-21 receptor; IRF, interferon regulatory factor; MMR, macrophage mannose receptor; PDGFC, platelet-derived growth factor C; SRAR steroid receptor RNA activator protein; STAT6, signal transducer and activator of transcription 6; TGF, transforming growth factor; TLR, Toll-like receptor; TNF, tumor necrosis factor.
In common with alternatively activated circulating monocytes, hemophagocytic bone-marrow macrophages, observed in occult10 or overt MAS, express CD163. CD163 can bind hemoglobin–haptoglobin (Hb–HP) complexes, initiating pathways that facilitate adaptation to the oxidative stress that is caused by free heme and iron.71 Uptake of Hb–HP complexes by CD163+ macrophages induces intracellular HO-1 activity. HO-1 degrades the heme subunit of Hb into biliverdin, which is subsequently converted to the anti-inflammatory components bilirubin, carbon monoxide and free iron. The free iron is either sequestered with ferritin within the cell or transported to red blood cell precursors in the bone marrow. Thus, the CD163+ macrophages seem to have a protective, anti-inflammatory role.10,11,72,73
TREG cell–TH17 cell balance
Another anti-inflammatory mechanism that might be deficient in sJIA is the action of T regulatory (TREG) cells. Reduced frequency of circulating TREG cells has been noted in active sJIA.74 In the setting of autologous stem cell transplantation, clinical improvement correlates with normalized TREG cell frequency in blood.74 Restoration of TREG cells by this method implies that TREG cell deficiency is not a primary defect in sJIA. In inflamed joints of patients with various subtypes of JIA, including sJIA, TREG cells are found in inverse proportion to the highly inflammatory CD4+ subset of TH17 cells, 75,76 which are strongly implicated in destructive arthritis.77,78 TH17 cells develop from naïve T cells or TREG cells, and IL-1β is a critical cytokine in TH17 differentiation, synergizing with IL-6 and IL-23. The balance of TREG cells and TH17 cells is implicated as a determinant of joint-related outcomes,75 and might have a role in the chronic arthritis stage of sJIA.80,81
sJIA as an HLH variant
The association of sJIA with MAS, which shares features with acquired HLH in other disease settings, has led to the hypothesis that sJIA represents an HLH variant. Evidence for occult MAS in a substantial proportion of patients with sJIA supports this possibility.10
HLH genes and sJIA
As one test of this hypothesis, genes responsible for familial HLH have been studied in sJIA. Initial SNP analysis limited to four genes, encoding two cytoxic effectors, perforin (PRF1) and granzyme B (GZMB), and two regulators of secretory cytotoxic granule function (RAB27A and UNC13D, which is also known as Munc13-4), did not reveal any associations with sJIA.82 However, more extensive SNP typing, albeit in small numbers of patients, has yielded provocative results. For example, Munc13-4 sequences with HLH-associated mutations were found in 2 of 18 patients with sJIA and MAS, and a novel Munc13-4 SNP haplotype was present in more than half of the remaining sJIA patients with MAS versus ~10% of controls or of those with sJIA but not MAS.83 In another study, missense mutations in PRF1, many of which were associated with perforin dysfunction, were present in 20% of patients with sJIA and were more frequent in sJIA with MAS than without MAS.84 Likewise, polymorphisms in IRF5, which encodes a transcription factor in the proinflammatory TLR signaling pathway, were associated with susceptibility to sJIA with MAS but not without MAS.85 Thus, emerging evidence suggests sJIA with MAS might represent a genetically distinct subtype of sJIA that is an HLH variant.
HLH–related immune features in sJIA with MAS
Interferon γ production
HLH is thought to stem from excessive activation and expansion of (mostly CD8+) T cells, which produce IFN-γ and stimulate macrophages to produce proinflammatory cytokines. Serum levels of IFN-γ and neopterin, a product of IFN-driven macrophages, are higher in sJIA with MAS than without MAS.86,87 Liver tissue from patients with MAS demonstrates massive infiltration by IFN-γ-producing CD8+ T cells and hemophagocytic macrophages that produce TNF and IL-6.88 Whether IL-1 has a role in HLH is not clear. MAS can develop in children being treated with anakinra as a first-line drug;44 conversely, anakinra has been effective in the treatment of MAS.89
Impaired cytotoxic function
In inherited HLH, the underlying gene defects impair cytotoxic functions,90 indicating a link between this deficiency and the uncontrolled proliferation of macrophage and activation of T cells. In one possible mechanism for this link, defective killing of infected cells results in persistent antigenic stimulation of T cells, leading them to produce high levels of macrophage-activating cytokines, such as IFN-γ. In another mechanism, abnormal cytotoxic cells fail to remove activated macrophages and IFN-γ-producing cells during the contraction phase of the immune response, perpetuating inflammation.91
Consistent with a mechanistic similarity between the genetic HLH diseases and sJIA, deficient cytolytic activity in circulating NK cells distinguishes sJIA from other subtypes of JIA and is prominent in sJIA with MAS.92–94 A recent study indicates that NK cell dysfunction in sJIA is associated with defective phosphorylation and signaling by the β chain of the IL-18 receptor.95 Interestingly, a member of the IL-1 family, IL-1F7b, could contribute to this phenotype, because the complex formed by its attachment to IL-18 binding protein (IL-18BP) binds to the IL-18 receptor β chain and blocks IL-18 signal transduction.96 Another source of cytolytic dysfunction in sJIA could be polymorphisms in relevant genes, as described above. Along these lines, in a study of new-onset, untreated sJIA, patients in a ‘very high serum ferritin’ subgroup differed from those in a ‘normal ferritin’ group in having higher expression levels of RAB27A and lower expression levels of SH2D1A, genes that are critical for activation of the cytolytic pathway.30 The ‘high ferritin’ subgroup was also at higher apparent risk for MAS.
Enhanced red blood cell turnover
‘High ferritin’ patients are also distinguished by a gene expression signature, originating from CD71+ erythroid precursors.32 This erythropoiesis signature and expansion of erythroid precursors are found in HLH, probably as a result of increased blood cell turnover. In new-onset sJIA, this signature might reflect subclinical hemophagocytosis.32
Excess IL–18 activity
Uniquely high serum levels of biologically active IL-18 are observed in active sJIA, in comparison with other inflammatory diseases.65,86 A contribution of this cytokine to MAS is suggested by elevated circulating levels of IL-18 during MAS, and by the role of IL-18 in the induction of IFN-γ production by CD8+ T cells and NK cells.30,95 IL-18 bioactivity is regulated by its soluble decoy receptor, IL-18BP, and a severe imbalance between levels of IL-18 and IL-18BP has been hypothesized to drive MAS and other forms of acquired HLH.97 Data also suggest that the IL-18 receptor binds an unknown ligand that delivers an anti-inflammatory signal, and competition from high levels of IL-18 will suppress this response.98 However, in the absence of IL-12, which is not expressed at very high levels in sJIA, IL-18 can divert TH1 cells to cells that produce IL-13 and IL-4, raising the alternative possibility that IL-18 participates in an immunoregulatory (negative) feedback loop.99 The cellular source of serum IL-18 in sJIA has not been extensively studied, but is suggested by one report to be bone marrow macrophages.100 Intriguing, but not well understood, is the strong correlation of IL-18 levels with serum ferritin in sJIA.101 Ferritin, which is critical in iron homeostasis, is also an acute-phase protein with an antioxidant and cytoprotective role during inflammatory states. IL-18 induces production and secretion of H-ferritin by macrophages,102 whereas serum ferritin is typically L-ferritin. However, serum ferritin in malignant histiocytosis consists mainly of H-ferritin.103
Insights from a new animal model of MAS
The most recent animal model of MAS, derived in normal mice by chronic TLR9 stimulation, provides several new insights.104 The mice developed features characteristic of MAS: splenomegaly, pancytopenia, hyperferritinemia, fibrin microthrombi, elevated serum levels of IFN-γ and hepatic inflammation. A small population of CD8+ T cells was activated, but activation of NK cells was more robust. Strikingly, hemophagocytic cells were absent in the bone marrow, arguing against a role for these cells in disease initiation. However, after blockade of the receptor for IL-10, hemophagocytic cells appeared, indicating a critical protective function for IL-10. Interestingly, experiments in this model also suggested a contribution of an as-yet unidentified IFN-γ-producing myeloid cell.
Inactive sJIA as compensated inflammation
Several observations suggest that some immunologic alterations remain during periods of sJIA quiescence. Levels of canonical monocyte subset surface markers, CD14 and CD16, remain elevated during inactive disease, although only a few patients off medication were assayed.31 Levels of the acute-phase protein serum amyloid A are also elevated in samples obtained from patients with clinically inactive sJIA (off medication).105 Notably, serum amyloid A is able to induce expansion of TREG cells, which may contribute to controlling inflammation.105 Elevated levels of certain cytokines, notably MIF65 and IL-18,65 during inactive disease (with NSAID treatment only or with no medication) suggest some continued immune activity despite clinical quiescence. As these studies did not use a validated definition of inactive sJIA or remission, the results require confirmation in future studies. Nonetheless, taken together, the results raise the possibility that quiescence (off medication) in sJIA represents a state of compensated inflammation.
Conclusions
It remains to be determined whether sJIA is primarily a disease of exuberant innate inflammation that outstrips normal anti-inflammatory controls, or whether the primary immune dysfunction is defective downregulation of initially normal inflammatory responses to (probably infectious) triggers. Indeed, although the clinical picture is one of excess inflammation, it is possible that the underlying abnormality causes some degree of immunodeficiency that results in secondary (chronic) inflammation, such as occurs in some HLH disorders and has been proposed for Crohn disease.106 For example, exuberant IL-1 activity could downregulate the efficacy of type I IFN pathways,37 attenuating the clearance of viral infections. In any of these scenarios, sJIA might result from an immune abnormality produced by different additive molecular etiologies in different patient subgroups.
A number of additional questions are raised by the available data. What are the initial triggers of sJIA? What are the mechanistic differences between sJIA patients with a monocyclic course and those with a more chronic course? What circulating factors stimulate expression of IL1 family genes in normal PBMCs, and how do these factors relate to the primary sJIA defect? What is the molecular basis of excess IL-1 activity in sJIA? What is the relationship between IL-1 and IL-6 production in sJIA? What is the role of neutrophils? Do the cells that drive inflammation operate from a privileged site? Are there two phases, with an IL-1-dependent process followed by one driven by other cells or mediators? Is there a critical role for TH17 cells in persistent sJIA? How is an IL-1-driven disease linked to a complication that is driven by reduced cytotoxic function? Is sJIA with MAS a disease variant that has a distinct genetic profile, or is it a severe (environmentally triggered) subtype of disease along a continuum in which all sJIA includes subclinical MAS? Which antigen-presenting cells have a proinflammatory role in MAS? The next years of sJIA research promise to be very exciting as answers to these questions emerge.
Supplementary Material
Acknowledgments
C. P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape, LLC-accredited continuing medical education activity associated with this article.
Footnotes
Author contributions
All authors contributed equally to researching data for the article, providing substantial contributions to discussions of content and writing the article.
E. D. Mellins reviewed and edited the manuscript before submission.
References
- 1.Petty RE, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 2.Singh-Grewal D, Schneider R, Bayer N, Feldman BM. Predictors of disease course and remission in systemic juvenile idiopathic arthritis: significance of early clinical and laboratory features. Arthritis Rheum. 2006;54:1595–1601. doi: 10.1002/art.21774. [DOI] [PubMed] [Google Scholar]
- 3.Sandborg C, et al. Candidate early predictors for progression to joint damage in systemic juvenile idiopathic arthritis. J Rheumatol. 2006;33:2322–2329. [PubMed] [Google Scholar]
- 4.Spiegel LR, et al. Early predictors of poor functional outcome in systemic-onset juvenile rheumatoid arthritis: a multicenter cohort study. Arthritis Rheum. 2000;43:2402–2409. doi: 10.1002/1529-0131(200011)43:11<2402::AID-ANR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Sawhney S, Magalhães C. Paediatric rheumatology—a global perspective. Best Pract Res Clin Rheumatol. 2006;20:201–221. doi: 10.1016/j.berh.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Fujikawa S, Okuni M. Clinical analysis of 570 cases with juvenile rheumatoid arthritis: results of a nationwide retrospective survey in Japan. Acta Paediatr Jpn. 1997;39:245–249. doi: 10.1111/j.1442-200x.1997.tb03593.x. [DOI] [PubMed] [Google Scholar]
- 7.Deane S, Selmi C, Teuber S, Gershwin ME. Macrophage activation syndrome in autoimmune disease. Int Arch Allergy Immunol. 2010;153:109–120. doi: 10.1159/000312628. [DOI] [PubMed] [Google Scholar]
- 8.Grom A. Natural killer cell dysfunction: A common pathway in systemic-onset juvenile rheumatoid arthritis, macrophage activation syndrome, and hemophagocytic lymphohistiocytosis? Arthritis Rheum. 2004;50:689–698. doi: 10.1002/art.20198. [DOI] [PubMed] [Google Scholar]
- 9.Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child. 2001;85:421–426. doi: 10.1136/adc.85.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34:1133–1138. [PubMed] [Google Scholar]
- 11.Bleesing J, et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor α-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:965–971. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 12.Lindsley CB. Seasonal variation in systemic onset juvenile rheumatoid arthritis. Arthritis Rheum. 1987;30:838–839. doi: 10.1002/art.1780300719. [DOI] [PubMed] [Google Scholar]
- 13.Oen K, Fast M, Postl B. Epidemiology of juvenile rheumatoid arthritis in Manitoba, Canada, 1975–1992: cycles in incidence. J Rheumatol. 1995;22:745–750. [PubMed] [Google Scholar]
- 14.Uziel Y, et al. Seasonal variation in systemic onset juvenile rheumatoid arthritis in Israel. J Rheumatol. 1999;26:1187–1189. [PubMed] [Google Scholar]
- 15.Masters S, Simon A, Aksentijevich I, Kastner D. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Ann Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rigante D, et al. First report of macrophage activation syndrome in hyperimmunoglobulinemia D with periodic fever syndrome. Arthritis Rheum. 2007;56:658– 661. doi: 10.1002/art.22409. [DOI] [PubMed] [Google Scholar]
- 17.Nepom BS, Glass DN. Juvenile rheumatoid arthritis and HLA: report of the Park City III workshop. J Rheumatol Suppl. 1992;33:70–74. [PubMed] [Google Scholar]
- 18.Date Y, et al. Identification of a genetic risk factor for systemic juvenile rheumatoid arthritis in the 5′-flanking region of the TNFα gene and HLA genes. Arthritis Rheum. 1999;42:2577–2582. doi: 10.1002/1529-0131(199912)42:12<2577::AID-ANR10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 19.Fishman D, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogilvie EM, et al. The -174G allele of the interleukin-6 gene confers susceptibility to systemic arthritis in children: a multicenter study using simplex and multiplex juvenile idiopathic arthritis families. Arthritis Rheum. 2003;48:3202–3206. doi: 10.1002/art.11300. [DOI] [PubMed] [Google Scholar]
- 21.Fife MS, et al. Novel IL10 gene family associations with systemic juvenile idiopathic arthritis. Arthritis Res Ther. 2006;8:R148. doi: 10.1186/ar2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moller J, et al. IL10 promoter polymorphisms are associated with systemic onset juvenile idiopathic arthritis (SoJIA) Clin Exp Rheumatol. 2010;28:912–918. [PubMed] [Google Scholar]
- 23.Donn RP, Shelley E, Ollier WE, Thomson W. A novel 5′-flanking region polymorphism of macrophage migration inhibitory factor is associated with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2001;44:1782–1785. doi: 10.1002/1529-0131(200108)44:8<1782::AID-ART314>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 24.De Benedetti F, et al. Functional and prognostic relevance of the –173 polymorphism of the macrophage migration inhibitory factor gene in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2003;48:1398–1407. doi: 10.1002/art.10882. [DOI] [PubMed] [Google Scholar]
- 25.Stock CJ, et al. Comprehensive association study of genetic variants in the IL-1 gene family in systemic juvenile idiopathic arthritis. Genes Immun. 2008;9:349–357. doi: 10.1038/gene.2008.24. [DOI] [PubMed] [Google Scholar]
- 26.Lamb R, Thomson W, Ogilvie E, Donn R. Positive association of SLC26A2 gene polymorphisms with susceptibility to systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:1286–1291. doi: 10.1002/art.22444. [DOI] [PubMed] [Google Scholar]
- 27.Gattorno M, et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:1505–1515. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- 28.Day TG, et al. Autoinflammatory genes and susceptibility to psoriatic juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:2142–2146. doi: 10.1002/art.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayaz NA, et al. MEFV mutations in systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford) 2009;48:23–25. doi: 10.1093/rheumatology/ken409. [DOI] [PubMed] [Google Scholar]
- 30.Fall N, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56:3793–3804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 31.Macaubas C, et al. Distribution of circulating cells in systemic juvenile idiopathic arthritis across disease activity states. Clin Immunol. 2010;134:206–216. doi: 10.1016/j.clim.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinze C, et al. Immature cell populations and an erythropoiesis gene-expression signature in systemic juvenile idiopathic arthritis: implications for pathogenesis. Arthritis Res Ther. 2010;12:R123. doi: 10.1186/ar3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogilvie EM, Khan A, Hubank M, Kellam P, Woo P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:1954–1965. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 35.Barnes M, et al. Subtype-specific peripheral blood gene expression profiles in recent-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:2102–2112. doi: 10.1002/art.24601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allantaz F, et al. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. J Exp Med. 2007;204:2131–2144. doi: 10.1084/jem.20070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quartier P, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Ann Rheum Dis. 2011;70:747–754. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson D, et al. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2010;62:2510–2516. doi: 10.1002/art.27485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling X, et al. Plasma profiles in active systemic juvenile idiopathic arthritis: biomarkers and biological implications. Proteomics. 2010;10:4415–4430. doi: 10.1002/pmic.201000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verbsky J, White A. Effective use of the recombinant interleukin 1 receptor antagonist anakinra in therapy resistant systemic onset juvenile rheumatoid arthritis. J Rheumatol. 2004;31:2071–2075. [PubMed] [Google Scholar]
- 41.Irigoyen PI, Olson J, Hom C, Ilowite NT. Treatment of systemic onset juvenile rheumatoid arthritis with anakinra. Arthritis Rheum. 2004;50:S437. [Google Scholar]
- 42.Henrickson M. Efficacy of anakinra in refractory systemic arthritis. Arthritis Rheum. 2004;50:S438. [Google Scholar]
- 43.Muzaffer MA, et al. Differences in the profiles of circulating levels of soluble tumor necrosis factor receptors and interleukin 1 receptor antagonist reflect the heterogeneity of the subgroups of juvenile rheumatoid arthritis. J Rheumatol. 2002;29:1071–1078. [PubMed] [Google Scholar]
- 44.Nigrovic P, et al. Anakinra as first-line disease modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 2010;63:545–555. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- 45.Tassi S, et al. Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1β secretion. Proc Natl Acad Sci USA. 2010;107:9789–9794. doi: 10.1073/pnas.1000779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Ham HJ, de Jager W, Bijlsma JWJ, Prakken BJ, de Boer RJ. Differential cytokine profiles in juvenile idiopathic arthritis subtypes revealed by cluster analysis. Rheumatology (Oxford) 2009;48:899–905. doi: 10.1093/rheumatology/kep125. [DOI] [PubMed] [Google Scholar]
- 47.Guma M, et al. Caspase 1-independent activation of interleukin-1β in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joosten LAB, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1 β. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metkar SS, et al. Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity. 2008;29:720–733. doi: 10.1016/j.immuni.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Frosch M, et al. The myeloid-related proteins 8 and 14 complex, a novel ligand of Toll-like receptor 4, and interleukin-1β form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:883–891. doi: 10.1002/art.24349. [DOI] [PubMed] [Google Scholar]
- 51.Wittkowski H, et al. S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 2008;58:3924–3931. doi: 10.1002/art.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Benedetti F, et al. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1991;34:1158–1163. doi: 10.1002/art.1780340912. [DOI] [PubMed] [Google Scholar]
- 53.de Benedetti F, et al. Effect of IL-6 on IGF binding protein-3: a study in IL-6 transgenic mice and in patients with systemic juvenile idiopathic arthritis. Endocrinology. 2001;142:4818–4826. doi: 10.1210/endo.142.11.8511. [DOI] [PubMed] [Google Scholar]
- 54.Cazzola M, et al. Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood. 1996;87:4824–4830. [PubMed] [Google Scholar]
- 55.Pignatti P, et al. Abnormal regulation of interleukin 6 in systemic juvenile idiopathic arthritis. J Rheumatol. 2001;28:1670–1676. [PubMed] [Google Scholar]
- 56.Muller K, Herner EB, Stagg A, Bendtzen K, Woo P. Inflammatory cytokines and cytokine antagonists in whole blood cultures of patients with systemic juvenile chronic arthritis. Rheumatology (Oxford) 1998;37:562–569. doi: 10.1093/rheumatology/37.5.562. [DOI] [PubMed] [Google Scholar]
- 57.Bradshaw EM, et al. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing TH17 cells. J Immunol. 2009;183:4432–4439. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yokota S, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 59.De Benedetti F, et al. Tocilizumab in patients with systemic juvenile idiopathic arthritis: efficacy data from the placebo-controlled 12-week part of the phase 3 TENDER trial. Arthritis Rheum. 2010;62:S596. [Google Scholar]
- 60.Nakajima S, et al. Improvement of reduced serum cartilage oligomeric matrix protein levels in systemic juvenile idiopathic arthritis patients treated with the anti-interleukin-6 receptor monoclonal antibody tocilizumab. Mod Rheumatol. 2009;19:42–46. doi: 10.1007/s10165-008-0115-3. [DOI] [PubMed] [Google Scholar]
- 61.Sarma PK, Misra R, Aggarwal A. Elevated serum receptor activator of NFκB ligand (RANKL), osteoprotegerin (OPG), matrix metalloproteinase (MMP)3, and ProMMP1 in patients with juvenile idiopathic arthritis. Clin Rheumatol. 2008;27:289–294. doi: 10.1007/s10067-007-0701-3. [DOI] [PubMed] [Google Scholar]
- 62.Silacci P, et al. Interleukin (IL)-6 and its soluble receptor induce TIMP-1 expression in synoviocytes and chondrocytes, and block IL-1-induced collagenolytic activity. J Biol Chem. 1998;273:13625–13629. doi: 10.1074/jbc.273.22.13625. [DOI] [PubMed] [Google Scholar]
- 63.Ling X, et al. Urine peptidomic and targeted plasma protein analyses in the diagnosis and monitoring of systemic juvenile idiopathic arthritis. Clin Proteomics. 2010;6:175–193. doi: 10.1007/s12014-010-9058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kossakowska AE, et al. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood. 1999;94:2080–2089. [PubMed] [Google Scholar]
- 65.de Jager W, et al. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. 2007;66:589–598. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi A, et al. The role of hemeoxygenase-1 in systemic-onset juvenile idiopathic arthritis. Mod Rheumatol. 2009;19:302–308. doi: 10.1007/s10165-009-0152-6. [DOI] [PubMed] [Google Scholar]
- 67.Martinez F, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 68.Roca H, et al. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Srivastava S, et al. Monocytes are resistant to apoptosis in systemic juvenile idiopathic arthritis. Clin Immunol. 2010;136:257–268. doi: 10.1016/j.clim.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Porta C, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor κB. Proc Natl Acad Sci USA. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kristiansen M, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 72.Avcin T, Tse SML, Schneider R, Ngan B, Silverman E. Macrophage activation syndrome as the presenting manifestation of rheumatic diseases in childhood. J Pediatrics. 2006;148:683–686. doi: 10.1016/j.jpeds.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 73.Schaer D, et al. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol. 2005;74:6–10. doi: 10.1111/j.1600-0609.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 74.de Kleer I, et al. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107:1696–1702. doi: 10.1182/blood-2005-07-2800. [DOI] [PubMed] [Google Scholar]
- 75.Nistala K, et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008;58:875–887. doi: 10.1002/art.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olivito B, et al. TH17 transcription factor RORC2 is inversely correlated with FOXP3 expression in the joints of children with juvenile idiopathic arthritis. J Rheumatol. 2009;36:2017–2024. doi: 10.3899/jrheum.090066. [DOI] [PubMed] [Google Scholar]
- 77.Agarwal S, Misra R, Aggarwal A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J Rheumatol. 2008;35:515–519. [PubMed] [Google Scholar]
- 78.Peck A, Mellins E. Breaking old paradigms: TH17 cells in autoimmune arthritis. Clin Immunol. 2009;132:295–304. doi: 10.1016/j.clim.2009.03.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manel N, Unutmaz D, Littman DR. The differentiation of human TH17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toh ML, et al. Role of interleukin 17 in arthritis chronicity through survival of synoviocytes via regulation of synoviolin expression. PLoS ONE. 2010;5:e13416. doi: 10.1371/journal.pone.0013416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koenders M, et al. Interleukin-1 drives pathogenic TH17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 2008;58:3461–3470. doi: 10.1002/art.23957. [DOI] [PubMed] [Google Scholar]
- 82.Donn R, et al. Genetic loci contributing to hemophagocytic lymphohistiocytosis do not confer susceptibility to systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:869–874. doi: 10.1002/art.23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang K, et al. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis is associated with MUNC13–4 polymorphisms. Arthritis Rheum. 2008;58:2892–2896. doi: 10.1002/art.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vastert S, et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford) 2010;49:441–449. doi: 10.1093/rheumatology/kep418. [DOI] [PubMed] [Google Scholar]
- 85.Yanagimachi M, et al. Association of IRF5 polymorphisms with susceptibility to macrophage activation syndrome in patients with juvenile idiopathic arthritis. J Rheumatol. 2011;38:769–774. doi: 10.3899/jrheum.100655. [DOI] [PubMed] [Google Scholar]
- 86.Shimizu M, et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology (Oxford) 2010;49:1645–1653. doi: 10.1093/rheumatology/keq133. [DOI] [PubMed] [Google Scholar]
- 87.Imagawa T. Differences between systemic onset juvenile idiopathic arthritis and macrophage activation syndrome from the standpoint of the proinflammatory cytokine profiles. Arthritis Rheum. 2004;50:S92. [Google Scholar]
- 88.Billiau AD, Roskams T, Van Damme-Lombaerts R, Matthys P, Wouters C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-γ-producing lymphocytes and IL-6- and TNF-α-producing macrophages. Blood. 2005;105:1648–1651. doi: 10.1182/blood-2004-08-2997. [DOI] [PubMed] [Google Scholar]
- 89.Bruck N, et al. Rapid and sustained remission of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome through treatment with anakinra and corticosteroids. J Clin Rheum. 2011;17:23–27. doi: 10.1097/RHU.0b013e318205092d. [DOI] [PubMed] [Google Scholar]
- 90.Filipovich A. Hemophagocytic lymphohistiocytosis and other hemophagocytic disorders. Immunol Allergy Clin North Am. 2008;28:293–313. viii. doi: 10.1016/j.iac.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 91.Voskoboinik I, Smyth M, Trapani J. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 92.Wulffraat NM, Rijkers GT, Elst E, Brooimans R, Kuis W. Reduced perforin expression in systemic juvenile idiopathic arthritis is restored by autologous stem-cell transplantation. Rheumatology (Oxford) 2003;42:375–379. doi: 10.1093/rheumatology/keg074. [DOI] [PubMed] [Google Scholar]
- 93.Grom AA, et al. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Pediatrics. 2003;142:292–296. doi: 10.1067/mpd.2003.110. [DOI] [PubMed] [Google Scholar]
- 94.Villanueva J, et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther. 2005;7:R30–R37. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Jager W, et al. Defective phosphorylation of interleukin-18 receptor β causes impaired natural killer cell function in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:2782–2793. doi: 10.1002/art.24750. [DOI] [PubMed] [Google Scholar]
- 96.Bufler P, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci USA. 2002;99:13723–13728. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mazodier K, et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106:3483–3489. doi: 10.1182/blood-2005-05-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nold-Petry CA, et al. Increased cytokine production in interleukin-18 receptor α-deficient cells is associated with dysregulation of suppressors of cytokine signaling. J Biol Chem. 2009;284:25900–25911. doi: 10.1074/jbc.M109.004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sugimoto T, et al. Interleukin 18 acts on memory T helper cells type 1 to induce airway inflammation and hyperresponsiveness in a naive host mouse. J Exp Med. 2004;199:535–545. doi: 10.1084/jem.20031368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maeno N, et al. Increased interleukin-18 expression in bone marrow of a patient with systemic juvenile idiopathic arthritis and unrecognized macrophage-activation syndrome. Arthritis Rheum. 2004;50:1935–1938. doi: 10.1002/art.20268. [DOI] [PubMed] [Google Scholar]
- 101.Coffey AJ, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 102.Recalcati S, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol. 2010;40:824–835. doi: 10.1002/eji.200939889. [DOI] [PubMed] [Google Scholar]
- 103.Lukina EA, Levina AA, Mokeeva RA, YuN T. The diagnostic significance of serum ferritin indices in patients with malignant and reactive histiocytosis. Br J Haematol. 1993;83:326–329. doi: 10.1111/j.1365-2141.1993.tb08289.x. [DOI] [PubMed] [Google Scholar]
- 104.Behrens EM, et al. Repeated Toll-like receptor 9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. doi: 10.1172/JCI43157. in press. [DOI] [PMC free article] [PubMed]
- 105.Nguyen KD, et al. Serum amyloid A overrides TREG anergy via monocyte-dependent and TREG-intrinsic, SOCS3-associated pathways. Blood. 2011;117:3793–3798. doi: 10.1182/blood-2010-11-318832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith AM, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009;206:1883–1897. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scott JP, Gerber P, Maryjowski MC, Pachman LM. Evidence for intravascular coagulation in systemic onset, but not polyarticular, juvenile rheumatoid arthritis. Arthritis Rheum. 1985;28:256–261. doi: 10.1002/art.1780280304. [DOI] [PubMed] [Google Scholar]
- 108.Bloom B, Toyoda M, Petrosian A, Jordan S. Anti-endothelial cell antibodies are prevalent in juvenile idiopathic arthritis: implications for clinical disease course and pathogenesis. Rheumatol Int. 2007;27:655–660. doi: 10.1007/s00296-006-0276-3. [DOI] [PubMed] [Google Scholar]
- 109.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 110.Church L, Cook G, McDermott M. Primer: inflammasomes and interleukin 1β in inflammatory disorders. Nat Clin Pract Rheumatol. 2008;4:34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 111.Gattorno M, et al. Pattern of interleukin-1β secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 2007;56:3138–3148. doi: 10.1002/art.22842. [DOI] [PubMed] [Google Scholar]
- 112.Srikrishna G, et al. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J Immunol. 2001;166:4678–4688. doi: 10.4049/jimmunol.166.7.4678. [DOI] [PubMed] [Google Scholar]
- 113.Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the β2 integrin Mac-1 on neutrophils. J Immunol. 1998;160:1427–1435. [PubMed] [Google Scholar]
- 114.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Ann Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 115.El Chartouni C, Schwarzfischer L, Rehli M. Interleukin-4 induced interferon regulatory factor (Irf) 4 participates in the regulation of alternative macrophage priming. Immunobiology. 2010;215:821–825. doi: 10.1016/j.imbio.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 116.Krausgruber T, et al. IRF5 promotes inflammatory macrophage polarization and TH1–TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.