SUMMARY
Haem (iron protoporphyrin IX) is both an essential growth factor and virulence regulator of the periodontal pathogens Porphyromonas gingivalis and Prevotella intermedia, which acquire it through the proteolytic degradation of haemoglobin and other haem-carrying plasma proteins. The haem-binding lipoprotein HmuY haemophore and the gingipain proteases of P. gingivalis form a unique synthrophic system responsible for capture of haem from haemoglobin and methaemalbumin. In this system, methaemoglobin is formed from oxyhaemoglobin by the activities of gingipain proteases and serves as a facile substrate from which HmuY can capture haem. This study examined the possibility of cooperation between HmuY and the cysteine protease interpain A (InpA) of P. intermedia in the haem acquisition process. Using UV-visible spectroscopy and polyacrylamide gel electrophoresis, HmuY was demonstrated to be resistant to proteolysis and thus capable to cooperate with InpA to extract heam from haemoglobin which was proteolytically converted to methaemoglobin by the protease. Spectroscopic pH titrations showed that both the iron(II) and iron(III) protoporphyrin IX-HmuY complexes were stable over the pH range 4 to 10, demonstrating that the haemophore could function over a range of pH which may be encountered in the dental plaque biofilm. This is the first demonstration of a bacterial haemophore working in conjunction with a protease from another bacterial species to acquire haem from haemoglobin and may represent mutualism between P. gingivalis and P. intermedia co-inhabiting the periodontal pocket.
Keywords: haemophore, interpain A, protease, haem, Porphyromonas, Prevotella
INTRODUCTION
Black-pigmenting anaerobic bacteria, including Porphyromonas gingivalis and Prevotella intermedia, are among the major pathogens associated with severe periodontitis (Holt and Ebersole, 2005), an inflammatory disease which damages the periodontal tissues supporting the teeth. Both P. gingivalis and P. intermedia have an absolute requirement for haem1, which they can store as an extracellular pigment, and which is composed of iron(III) protoporphyrin IX (Fe(III)PPIX) derived mainly from the proteolytic breakdown of haemoglobin, and from haem-carrying plasma proteins albumin and haemopexin. The haem pigment of P. gingivalis is composed of Fe(III)PPIX in the μ-oxo bishaem or dimeric form, [Fe(III)PPIX]2O (Smalley et al., 1998), whereas that from both P. intermedia and Prevotella nigrescens is in the monomeric form, haematin, Fe(III)PPIX.OH (Smalley et al., 2003). Development of the haem-containing black pigments by these organisms serves an important defensive role as the intrinsic catalase activity of ferrihaems destroys hydrogen peroxide (Smalley et al., 2000). Furthermore, formation of the μ-oxo bishaem-containing pigment by P. gingivalis is a chemical process which consumes oxygen and would thus promote anaerobiosis (Smalley et al., 1998, 2002). Neither P. gingivalis nor P. intermedia possess the genes encoding the biosynthesis of iron protoporphyrin IX, and are thus reliant on exogenous sources of haem such as haemoglobin.
The paradigm for haem acquisition from iron(II) oxyhaemoglobin by both P. gingivalis and P. intermedia involves a crucial initial haemoglobin oxidation stage which concomitantly reduces the affinity of the globin for the iron(III) protoporphyrin IX and renders the protein susceptible to further proteolytic degradation (Smalley et al., 2007, 2008; Byrne et al., 2009). In the case of P. gingivalis, the oxidized or methaemoglobin form of haemoglobin is produced proteolytically by the action of the arginine-specific cysteine protease gingipain, Rgp, and which is subsequently degraded by the lysine-specific gingipain, Kgp (Smalley et al., 2007; 2008). For P. intermedia, the proteolytic oxidation step is mediated by interpain A (InpA) (Byrne et al., 2009), a major extracellular cysteine protease (Mallorquí-Fernández et al., 2008), which is capable of disarming the host defences by degrading complement (Potempa et al., 2009; Potempa and Potempa, 2012). InpA can promote formation of both hydroxy- and aquomethaemoglobin under alkaline and acid conditions, respectively, which are also susceptible to degradation and haem release by InpA (Byrne et al., 2009). Whilst the agents responsible for haem deposition at the cell surface of P. gingivalis and P. intermedia have not been elucidated, binding of haem for purposes of internalisation by P. gingivalis is mediated by cell-surface haem-binding proteins such as haemin-binding protein 35 (Shoji et al., 2010) and the haemophore-like protein, HusA (Gao et al., 2010). To date, the most well characterised haem uptake system of P. gingivalis comprises the iron(III)- and iron(II)-haem-binding protein haemophore HmuY and its cognate receptor HmuR (Olczak et al., 2005; Wojtowicz et al., 2009b). Previously, we demonstrated that Rgp-mediated oxidation of iron(II) oxyhaemoglobin, facilitates the capture of the iron(III) protoporphyrin IX moiety by HmuY (Smalley et al., 2011).
Early work has shown that P. gingivalis, a member of the “red complex” of plaque organisms, requires the more prevalent “orange complex” members, including P. intermedia, for colonization (Socransky et al., 1998). Although other studies conflict with this view (Kolenbrander et al., 1985, 2006), more recent papers strongly show that such interactions exist. For example, co-aggregation between P. gingivalis and P. intermedia is shown to be mediated by adhesins encoded by the genes for gingipain (Kamaguchi et al., 2003). It has been widely accepted that co-aggregation promotes the generation of complex nutrient webs connecting species with distinct metabolic capabilities and increasing the pathogenicity of tightly interacting species. In this respect, a recent study has shown that bacterial loads of P. intermedia and P. gingivalis in plaque are strongly associated one with the other, and this co-colonisation is significantly linked to increased probing depth of diseased periodontitis sites (Nadkarni et al., 2012). These associations may be attributable to acquisition of haem, the essential growth factor for these two bacterial species. Given that haemoglobin oxidation is crucial to proteolytic haem release, and also the propensity for iron(III) protophyrin IX-containing haemoglobin to relinquish their haem to HmuY (Smalley et al., 2011), together with the demonstration that InpA can mediate oxidation of haemoglobin (Byrne et al., 2009), it is possible that HmuY, and by extension P. gingivalis, could benefit from the proteolytic activity of co-colonising or co-aggregating P. intermedia cells in acquiring haem. Here we support this statement by demonstrating that methaemoglobin formed by the activity of InpA is a facile substrate for transfer of iron(III) protoporphyrin IX to HmuY.
MATERIALS AND METHODS
Purification of HmuY
P. gingivalis apoHmuY (NCBI accession number CAM 31898) lacking the first 25 residues was expressed using a pHmuY11 plasmid and Escherichia coli ER2566 cells (New England Biolabs) and purified from a soluble fraction of the E. coli lysate as previously described (Olczak et al., 2008; Wojtowicz et al., 2009a).
Interpain A purification
InpA was expressed recombinantly in E. coli and purified by affinity chromatography on Fast Flow Ni-NTA (Ni2+-nitrilo-triacetate)–Sepharose (Qiagen) followed by anion- exchange chromatography (MonoQ, GE Healthcare) as described previously (Mallorquí-Fernández et al., 2008). Before use, InpA was pre-activated by incubation for 15 min in 2 mM dithiothreitol (DTT) in 0.1 M NaCl, 0.1 M Tris-HCl, pH 7.5. For use in haemoglobin degradation assays, the above buffer was replaced with that without DTT by ultrafiltration using 10 kDa cut-off Microcons (Amicon).
Polyacrylamide gel electrophoresis (PAGE and SDS-PAGE)
SDS-PAGE was carried out as previously described (Charalabous et al., 2007). Gels were firstly stained for protein-bound haem with tetramethylbenzidine-H2O2 (TMB-H2O2) and counterstained for protein with Coomassie Brilliant Blue R-250 (CBB) (Byrne et al., 2009). Samples were solubilised in application buffer at 37°C for 1 h without DTT. For native PAGE, urea and SDS were omitted from the separating gel, and the sample solubilisation was also performed in application buffer without SDS, urea and DTT as previously described (Smalley et al., 2011).
Haemoglobin preparations
Oxyhaemoglobin, prepared from fresh horse erythrocytes as previously described (Smalley et al., 2008), was stored in 0.14 M NaCl, 0.1 M Tris-HCl, pH 7.5 at −80°C until required. Methaemoglobin was prepared proteolytically by treatment of oxyhaemoglobin (16 μM with respect to haemoglobin subunit) with InpA (2 μM) for 7 h at 37°C in 0.14 M NaCl, 0.1 M Tris-HCl, pH 7.5 (Byrne et al., 2009). This haemoglobin preparation comprised 44 % methaemoglobin as calculated from A577nm and A630nm values (Smalley et al., 2007).
UV-visible spectroscopy
UV-visible spectra were recorded using an Ultrospec 2000 spectrophotometer (Biochrom Ltd) using 1 cm pathlength cuvettes.
HmuY-haem complex formation
The HmuY-ferrihaem complex was prepared by reacting equimolar amounts of iron(III) protoporphyrin IX with HmuY at 0.14M NaCl, 0.1M Tris-HCl, pH 7.5, at 37°C, the reaction being monitored by UV-visible spectroscopy which showed a spectrum with a 411nm Soret and lower intensity Q bands at 527 and 558 nm as previously reported for the six-coordinate, bis-histidine ligated, low-spin complex (Smalley et al., 2011; Wojtowicz et al., 2009b).
Spectrophotometric pH titrations of haem with HmuY
For UV-visible absorption analysis, 10 μl of the iron(III) protporphyrin IX-HmuY complex (1:1 molar protein:haem ratio; 20 [.proportional]M HmuY) were diluted into 1 ml of either 50 mM citrate buffer adjusted to pH 2.4, 2.7, 2.9, 3.1, 3.4, 50 mM acetate buffer pH 3.3, 5.2, 50 mM MES buffer pH 6.1, 50 mM phosphate buffer pH 7.1, 50 mM Tris/HCl buffer pH 7.9, 9.4, or 20 mM N-cyclohexyl-3-aminopropanesulfonic acid (CAPS) buffer pH 9.2 and 12.0; all buffers also containing 0.14 M NaCl. HmuY samples were equilibrated in the above buffers at 4°C for 16 h, after which the pH was checked. Samples of the iron(II) protoporphyrin IX-HmuY complex were made in the above series of buffers after addition of an excess of sodium dithionite (Na2S2O4) as reported previously (Wojtowicz et al., 2009b). Non-linear curve fitting was carried out using OriginPro 7.5 software.
RESULTS
HmuY resistance to InpA proteolysis
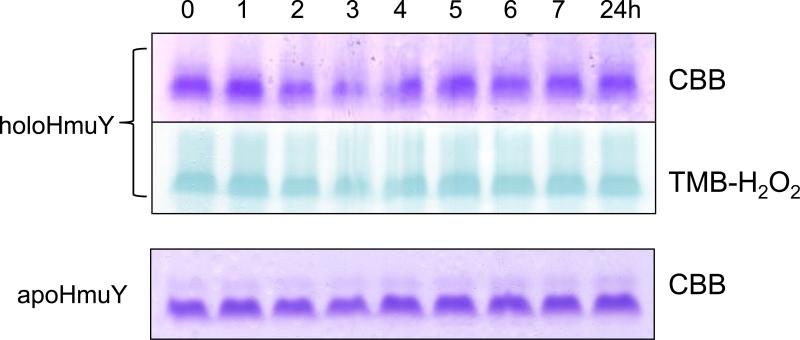
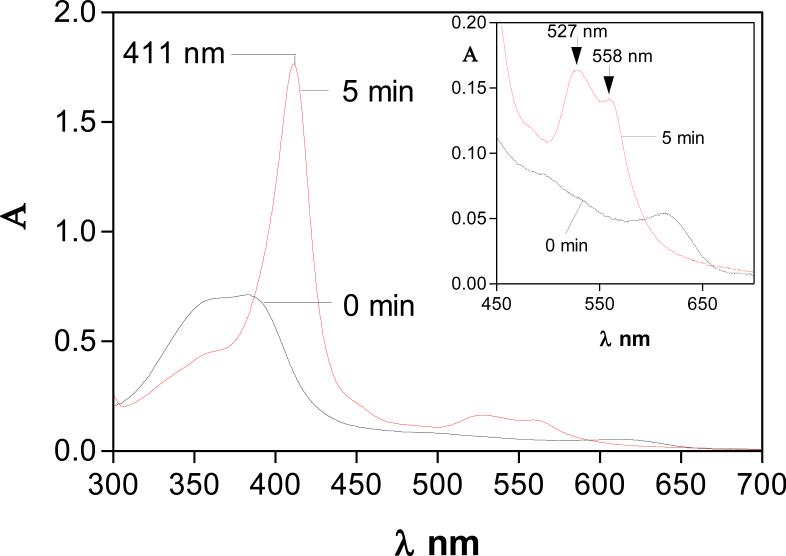
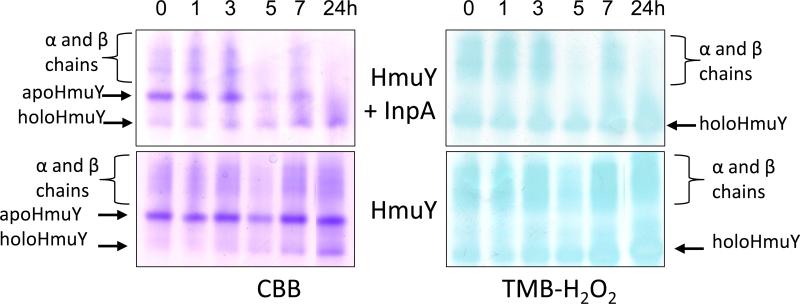
We observed that both the holo- and apo-forms of HmuY were completely resistant to InpA activity (Fig 1, panels A and B), and in the case of haem-liganded form, there was no evidence of haem loss as revealed by TMB-H2O2 staining (Fig 1A). However, in keeping with previous observations (Wójtowicz et al., 2009a), we found that denaturation by heating at 100°C for 10 min rendered both apo- and holoHmuY susceptible to InpA (Fig 1, panel C). Additionally, pre-treatment of apoHmuY for 24 h with InpA at 37°C did not affect subsequent haem pickup as demonstrated by rapid formation of a spectrum typical of the iron(III) protoporphyrin IXHmuY complex (Fig 2).
Figure 1.
Non-reducing SDS-PAGE showing apoHmuY and holoHmuY are resistant to InpA degradation. HoloHmuY and apoHmuY (both at 16 μM) were incubated with 2 μM InpA in 0.1 M NaCl, 0.1 M Tris-HCl, pH 7.5, at 37°C, for the times indicated and subjected to non-reducing SDS-PAGE. Panels A and B, native holo- and apoHmuY plus InpA. Panels C and D, holo- and apoHmuY denatured by heating at 100°C for 10 min. The presence of haem complexed with HmuY is confirmed by haem peroxidase staining using tetramethylbenzidine-H2O2 (TMB-H2O2) before counterstaining with Coomassie Brilliant Blue R-250 (CBB). 10 μg of HmuY were loaded per track.
Figure 2.
UV-visible spectroscopic demonstration of the formation of the HmuY-iron(III) protoporphyrin IX complex by HmuY pre-treated with InpA. HmuY (16 μM) was pre-incubated at 37°C for 24 h with InpA (2 μM), before exposure to an equimolar amount of iron(III) protoporphyrin IX. Experimental conditions were as in Fig 1. Inset graph shows ordinate expansion of the Q band region.
HmuY-haem complex formation during incubation of oxyhaemoglobin pre-treated with InpA
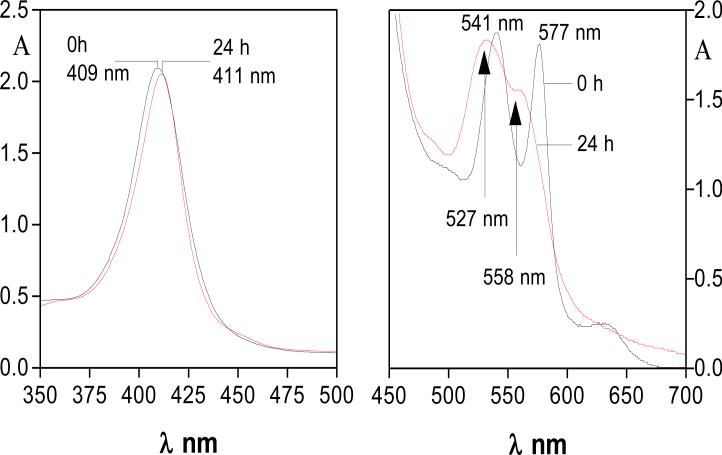
To experimentally verify whether haem extraction by HmuY is aided by InpA (through mediating oxyhaemoglobin oxidation), oxyhaemoglobin was proteolytically oxidised by 7 h pre-incubation with InpA (2 μM) to yield a starting substrate comprising 44 % methaemoglobin. This was incubated with HmuY (16 μM), which resulted in the rapid formation of an iron(III) protoporphyrin IX-HmuY spectrum (411 nm Soret λmax and 527 and 558 nm Q bands; Fig 3). This spectrum remained stable over 24 h. Importantly, iron(III) protoporphyrin IX-HmuY formation from oxyhaemoglobin was greatly reduced in the absence of InpA (data not shown; starting methaemoglobin concentration in the preparation = 15 %), which is likely a direct result of the low rate of auto-oxidation observed at this pH (Tsuruga et al., 1998).
Figure 3.
HmuY-iron(III) protoporphyrin IX formation from methaemoglobin induced by the action of InpA on oxyhaemoglobin. Black line, methaemoglobin at time zero; red line, 5 mins after addition of HmuY. Methaemoglobin was formed by incubation of oxyhaemoglobin (16 μM; with respect to subunit) with InpA (2 μM) at 37°C, in 0.14 M NaCl, 0.1 M Tris-HCl, pH 7.5.
However, to confirm that haem was in complex with HmuY and not still present as haemoglobin, the incubation mixture was treated with 10 mM Na2S2O4 to concomitantly remove dioxygen and to reduce the haem iron. This yielded a spectrum with a 423 nm Soret2, plus visible bands at 526 and 556 nm (data not presented), indicative of the iron(II) protoporphyrin IX-HmuY complex (Smalley et al., 2011; Wojtowicz et al., 2009). This unequivocally demonstrated that haem had become complexed to HmuY during incubation with the InpA-treated oxyhaemoglobin.
It is noteworthy that the InpA-induced methaemoglobin substrate showed little haem loss relative to the auto-oxidized control as judged by the Soret band intensity (data not shown) and agrees with the previous observation of limited proteolysis at pH 7.5 during which time InpA-mediated oxidation is taking place (Byrne et al., 2009). SDS-PAGE and densitometry confirmed that incubation of InpA with oxyhaemoglobin at pH 7.5 resulted in immeasurably low levels of haem release after 7 h (data not shown). Therefore it is unlikely that binding by HmuY of the low levels of any haem liberated proteolytically as a result of InpA activity could account for the strong intensity of the iron(III) protoporphyrin IX-HmuY spectrum produced upon addition of HmuY to the InpA-oxyhaemoglobin incubation. From this it was confirmed that holoHmuY formation was, in the main, due to the direct acquisition of haem from methaemoglobin induced by InpA activity.
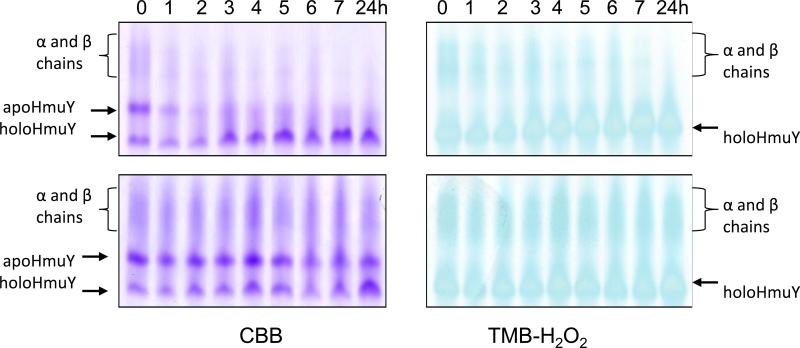
Previously we reported that under native PAGE, apoHmuY migrates as a single band with an Rf greater than that of the diffusely-stained α and β haemoglobin chains (Smalley et al., 2011). In addition, the mobility of the haem-complexed HmuY band is increased relative to apoHmuY, which is attributed to the increased negative charge of the iron(III) protoporphyrin IX-HmuY complex imparted by the ionised carboxylate groups of the ligated haem (Smalley et al., 2011). In the current study during native PAGE of HmuY incubated with InpA-pre-treated oxyhaemoglobin, the CBB and TMB-H2O2 staining of the slightly faster migrating HmuY-haem band increased in intensity while that of the apoHmuY displayed a reciprocal reduction (Fig 4, upper panels, arrowed) indicating the progressive formation of the iron(III) protoporphyrin IX-haemophore complex. This was accompanied by reduced TMB-H2O2 staining of the haemoglobin α and β chains indicating loss of haem (Smalley et al., 2008). HoloHmuY formation occurred at a greater rate in the presence of InpA than without it, which corroborated the UV-visible spectroscopic data. Formation of some haem-stained holoHmuY was also observed for oxyhaemoglobin incubated in the absence of InpA (Fig 4, lower panels, arrowed), which is attributable to formation of methaemoglobin via natural auto-oxidation during incubation.
Figure 4.
Native PAGE showing the effect of HmuY on oxyhaemoglobin pre-treated with or without with InpA. Oxyhaemoglobin (16 μM, with respect to subunit) was pre-incubated at 37°C with (upper panels) or without InpA (2 μM) (lower panels) at pH 7.5 for 7 h in 0.1 M NaCl, 0.1 M Tris-HCl, and then exposed to HmuY (16 μM) and sampled at the times shown. Gels were stained for the presence of haem with TMB-H2O2 and counterstained with CBB.
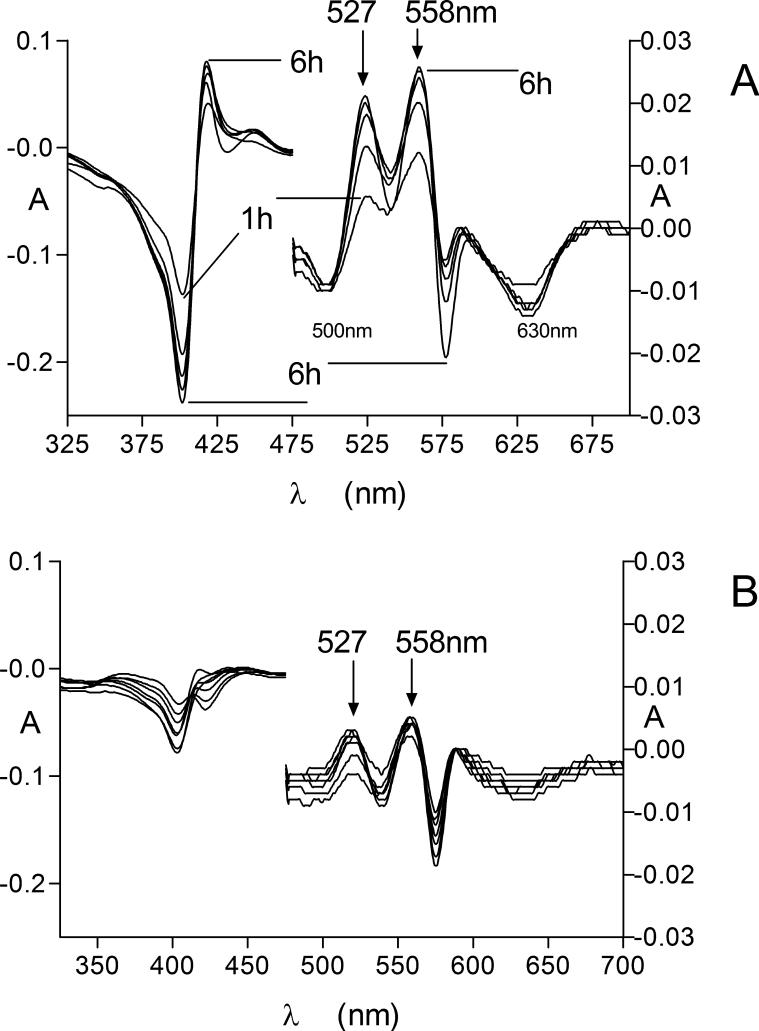
The facile transfer of iron(III) protoporphyrin IX from haemoglobin to HmuY was further corroborated by assessment of the relative amount of iron(III) protoporphyrin IX-HmuY formed with time during incubation of HmuY with oxyhaemoglobin pre-incubated with or without InpA for 7 h. Difference spectra were derived by subtracting the control time zero spectrum of the InpA-pre-treated oxyhaemoglobin plus HmuY from the spectra at each subsequent time interval (Fig 5 A). Negative absorbances (troughs) relating to loss of methaemoglobin at 500 nm and at 630 nm, and absorbance increases at 527 nm and 558 nm, attributable to the formation of the iron(III) protoporphyrin IX-HmuY complex, were observed. Note that the relative absorbance intensities were altered to those seen in the spectrum of the HmuY-iron(III) protoporphyrin IX as in Fig 2, which arises from subtraction of the control spectra from those of the tests (Smalley et al., 2011). In contrast there was little change in the difference spectra in both the Soret and Q band regions for the control spectra (oxyhaemoglobin not pre-treated with InpA and containing only auto-oxidised haemoglobin) over the same time period above (Fig 5 B). These data clearly demonstrated a greater degree of HmuY-iron(III) protoporphyrin IX complex formation from oxyhaemoglobin pre-treated with InpA, in turn a result of the greater rate of methaemoglobin formation mediated by the action of InpA.
Figure 5.
Difference spectra showing the generation of the HmuY-iron(III) protoporphyrin IX complex Q bands during incubation of HmuY with InpA-pre-treated oxyhaemoglobin. InpA-pretreated oxyhaemoglobin (A); auto-oxidised haemoglobin. Experimental conditions were as described in Figure 4. See text for details.
HmuY-haem complex forms during co-incubation of oxyhaemoglobin with both InpA and HmuY
Using native PAGE we monitored the effect of incubating equimolar amounts of HmuY and oxyhaemoglobin (16 μM with respect to the monomer) in the presence of InpA (2 μM). We observed a time-dependent increase in the amount of the faster migrating holoHmuY (CBB-stained panel, Fig 6), which also became progressively stained with TMB-H2O2, indicating transfer of haem to HmuY (Fig 6). Both the TMB-H2O2 and CBB staining of the slower migrating haemoglobin α and β bands demonstrated a reciprocal decrease in intensity, which is typical during the formation of haem-free globin chains (Smalley et al., 2008). A smaller amount of the faster migrating holoHmuY was also generated (notably after 24 h) when oxyhaemoglobin was incubated only with HmuY (Fig 6), and is attributable to haem pickup by HmuY from auto-oxidised methaemoglobin.
Figure 6.
Native PAGE showing the effect of co-incubating oxyhaemoglobin in the presence of both InpA and HmuY. Oxyhaemoglobin (16 μM) was incubated together with InpA (2 μM) plus HmuY (16 μM) at 37°C in 0.1 M NaCl, 0.1 M Tris-HCl, pH 7.5, for the times indicated and subjected to native PAGE before staining for the presence of haem with TMB-H2O2 and then counterstained for protein with CBB.
HmuY binds haem over a range of pH
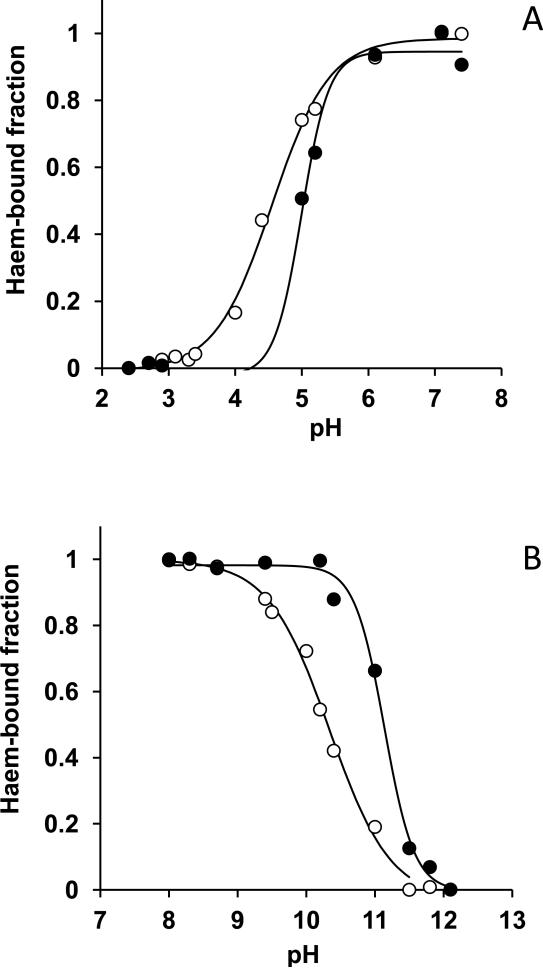
We examined the haem binding ability of HmuY under different pH conditions which may be encountered in the oral cavity. UV-visible absorbtion spectral changes were measured over a range of pH between 2 and 12. This was done by spectroscopically titrating HmuY with a molar equivalent of haem and plotting the change in absorbance at 411nm and 425nm for the iron(III)- and iron(II) protoprphyrin IX-HmuY complexes, respectively. From this, the fraction of total haem bound to the haemophore was calculated (Fig 7). This revealed that both the iron(II) and iron(III) protoporphyrin IX-HmuY complexes were stable within the pH range 4 to 10, whereas significant decreases in Soret band intensities at 411nm (for the iron(III)-form) and at 425 nm (for the iron(II)-form) were observed for pH values lower than ~4 and above ~10, indicating haem release (Fig 7). Nevertheless, these data showed that HmuY could remain functionally active over a range of pH which may be encountered in dental plaque.
Figure 7.
Spectrophotometric titrations of HmuY with iron(II) and iron(III) protoporphyrin IX over a range of pH. Spectra of the acidic (upper panel) and basic (lower panel) species of HmuY-haem at a 1:1 molar ratio were analyzed under oxidized (empty circles) and reduced (filled circles) conditions. Soret band absorbance changes were monitored at 411 nm (oxidized) and 425 nm (reduced) and from these the haem-bound fractions of the HmuY complexes were calculated. Solid lines indicate the sigmoidal fits.
DISCUSSION
P. gingivalis exists predominantly in complex biofilm communities within the periodontal pocket, co-aggregating with other species such as Actinomyces viscosus, Streptococcus mutans, Streptococcus gordonii, and Fusobacterium nucleatum (Nonaka et al. 2001; Li et al., 1991; Kinder and Holt, 1989). It is also known that P. gingivalis gingipains mediate co-aggregation with P. intermedia (Kamaguchi et al., 2003). Such interactions are considered to provide growth substrates and reduce oxygen tension allowing anaerobic growth. In such an environment, P. intermedia plays an important role in the maturation of plaque by forming a complex with P. nigrescens and F. nucleatum which bridges early colonizing Gram-positive bacteria with Gram-negative species of the mature biofilm (Socransky et al., 1998), and thus contributes indirectly to periodontal disease progression.
Others have hypothesised that a degree of mutualism exists between P. gingivalis and P. intermedia. For example, binding of IgG by P. intermedia cell-surface Fc receptor may protect P. gingivalis from IgG (Labbé and Grenier, 1995), whilst fermentation of glutamic and aspartic acids by P. intermedia and F. nucleatum results in ammonia generation and thus a more neutral pH which could benefit acid-sensitive species including P. gingivalis (Takahashi, 2003). Cooperative inhibition of complement activation, and hence the defensive sequelae associated with the inflammatory response through the synergistic degradation of complement factor C3b by InpA of P. intermedia and Kgp of P. gingivalis, has also been described (Potempa and Potempa, 2012). It is also known that the stronger haemolytic activity of P. intermedia may advantage the growth of P. gingivalis by augmenting the levels of free haemoglobin (Okamoto et al., 1999). In this study, we have demonstrated that, in addition to deployment of its own gingipain proteases to engender methaemoglobin formation (Smalley et al., 2011) to facilitate HmuY haemophore haem capture, P. gingivalis may also benefit from the P. intermedia haem acquisition system, i.e. the proteolytic activity of InpA.
For HmuY to be instrumental in co-operative haem acquisition, an important pre-requisite would be resistance to proteases, which could otherwise jeopardise haem binding ability. In this respect, Wójtowicz et al. (2009a) have shown that HmuY is completely resistant to the activities of trypsin, Kgp, and R-gingipains HRgpA and RgpB, making it a very durable agent to work in concert with proteases for haem extraction. It is noteworthy that higher levels of total serum IgG and IgG1 specific for P. gingivalis HmuY have been measured in chronic periodontitis patients compared to non-periodontitis controls (Trindade et al., 2012), demonstrating that HmuY is expressed in vivo. In addition, Potempa et al. (2009) found that the majority of P. intermedia strains freshly isolated from chronic and aggressive periodontitis sites carry and express the InpA gene. Since P. gingivalis and P. intermedia inhabiting the same environmental niche may concurrently deploy InpA and HmuY, it was relevant to investigate their concomitant effects on oxyhaemoglobin. Importantly, we found that both iron(III) protoporphyrin IX-complexed and apoHmuY were completely resistant to proteolysis by InpA and that apoHmuY pre-treated with InpA remained capable of forming the HmuY-iron(III) protoporphyrin IX complex. This indicated that HmuY could remain functionally active in a protease-rich environment, a feature which would be important in establishing a co-operative relationship between the two proteins and hence between the two microorganisms for haem capture.
For assimilation of haem by P. gingivalis from any oxyhaemoglobin released into a periodontal pocket as a result of bleeding, the haemoglobin must firstly be converted to the methaemoglobin form before haem sequestration by the HmuY haemophore (Smalley et al., 2011). However, at the slightly alkaline pH of these environments (Eggert et al., 1991; Bickel and Cimasoni et al., 1985) the natural auto-oxidation rates of both α and β chains of oxyhaemoglobin are lowest (Tsuruga et al., 1998), which would limit HmuY haem extraction. This problem is overcome through the proteolytically mediated oxidation of oxyhaemoglobin by Rgp which facilitates both subsequent attack and haem release mediated by Kgp (Smalley et al., 2008) and iron(III) protoporphyrin IX extraction by HmuY (Smalley et al., 2011). Oxyhaemoglobin oxidation mediated by InpA is minimal at pH 7.5 (i.e., at a pH typical of the diseased periodontal pocket and inflamed gingival crevice) compared to that at lower pH (Byrne et al., 2009), at which the protease is optimally active against protein substrates (J. Potempa, unpublished findings). Nevertheless, pre-treatment of oxyhaemoglobin with InpA at pH 7.5 rendered it susceptible to haem extraction by the P. gingivalis haemophore and formation of a stable HmuY-iron(III) protoporphyrin IX complex. These data showed that InpA would be effective at slightly alkaline pH in “priming” oxyhaemoglobin for haem extraction by HmuY.
P. gingivalis may encounter fluctuations in environmental conditions since it exists in the oral fluids, colonizes oral surfaces, and invades host cells. One of the factors which may change is pH. Despite individual variations, plaque pH is the range 5.6-6.5, whereas the pH of resting saliva is about 6.7 (Kleinberg, 1970; Moreno and Margolis, 1988). The pH of the gingival crevice has been shown to be alkaline (Kleinberg and Hall, 1969) and development of periodontitis is associated with a rise in pH above 8.0 (Bickel et al., 1985; Eggert et al., 1991), resulting in conditions favouring Gram-negative bacteria, including P. gingivalis (McDermid et al., 1988; Bickel and Cimasoni, 1985). P. intermedia also displays a saccharolytic metabolism generating an acidic terminal growth pH in both liquid (Takahashi & Yamada, 2000) and on solid media (Smalley et al., 2003). In contrast, P. gingivalis grows best in neutral to alkaline environments which suit its proteolytic activity (McDermid et al., 1988). Therefore, HmuY binding of both iron(II) and iron(III) protoporphyrin IX over the pH range 2.5 to 12 was determined. We observed that both the iron(II) and iron(III) protoporphyrin IX-HmuY complexes were stable over the pH range 4 to 10, demonstrating that the haemophore could actively bind haem under extremes of pH which might be encountered in the plaque biofilm. In light of these findings, we conclude that P. gingivalis may also be able to gain haem via the HmuY haemophore at pHs outside its growth optimum, and especially at acid pH where InpA is more efficient in oxidising haemoglobin (Byrne et al., 2009) compared to R-gingipain which has a neutral to alkaline pH optimum (Potempa et al., 1998). It is also noteworthy that P. gingivalis can elevate the pH of its environment through base production when in a medium which is initially poised in the acid range (Takahashi et al., 1997), and as a consequence may be capable of surviving local depressions in pH caused by acid generating species such as P. intermedia.
At the molecular level, oxyhaemoglobin oxidation to the met-state results in relaxation of the affinity of globin for iron(III) protoporphyrin IX compared to that of iron(II) species (Hargrove et al., 1996). In the circulation, iron(II)haemoglobin species released from damaged and senescent erythrocytes gradually transform into methaemoglobin, from which serum albumin and/or haemopexin having association constants for iron(III) protoporphyrin IX of ≈ 108 and 2×1014 M−1, respectively (Adams and Berman 1980; Hrkal et al. 1974), can extract the iron porphyrin. This enables both haem transport to the spleen and liver for reprocessing and limits its bioavailabilty to any invading bacteria. However, it would appear that both P. intermedia and P. gingivalis also take advantage of the above chemistry through evolution of a proteolytic mechanism to circumvent host haem sequestration by promoting methaemoglobin formation. Here we have shown that InpA of P. intermedia, through proteolytically oxidising haemoglobin, facilitates iron(III) protoporphyrin IX extraction by the HmuY haemophore of P. gingivalis. Of significance is the fact that both apoHmuY and its haem-ligated form are completely resistant to proteolysis by InpA, indicating that HmuY could function alongside the protease for haem acquisition. This co-operative action is not without precedent as synergistic acquisition of iron from haemoglobin has been shown during abscess formation for Bacteroides fragilis and E. coli which produces a haemoglobin protease (Otto et al., 2002). However, to our knowledge, this is the first documented demonstration of a bacterial haemophore acting synthrophically with a specific “haemoglobinase” from a different bacterial species. These findings support a potential mutualistic relationship between P. intermedia and P. gingivalis for haem acquisition. It is noteworthy that a HmuY-like outer-membrane lipoprotein has been reported in P. intermedia strain 17 (Lewis et al., 2006), which is dramatically up-regulated in iron-deplete conditions (Yu et al., 2007). It is likely that such a molecule may function in a similar fashion to HmuY by extracting haem from InpA proteolytically “primed” oxyhaemoglobin. Taken together, the apparent similarity of the haem uptake mechanisms suggests the possibility of convergent evolution of the haem acquisition systems of P. gingivalis and P. intermedia. In a complex polymicrobial environment such a system would satisfy the nutritional needs of each species. As a consequence it would be expected that the efficiency of haem acquisition in co-aggregates of P. gingivalis and P. intermedia would be much higher, and would encourage a higher degree of inter species cooperativity.
ACKNOWLEDGEMENTS
We acknowledge the financial support (to DPB) from the School of Dental Science, University of Liverpool, and from the British Council under the British-Polish Young Scientist Programme (project reference number: WAR/342/146). The research in the laboratories of JP is supported by grants from: the National Institutes of Health (Grant DE 09761, USA), National Science Centre (2011/01/B/NZ6/00268, Kraków, Poland), the European Community (FP7-HEALTH-2010-261460 “Gums & Joints” and FP7-PEOPLE- 2011-ITN-290246 “RAPID”), MNiSW (2136/7. PR UE/2011/2, Warszawa, Poland), and the Foundation for Polish Science (TEAM project DPS/424-329/10) (to J.P.). The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of structural funds from the European Union (POIG.02.01.00-12-064/08). We also thank Halina Wojtowicz for curve fitting and for calculation of the HmuY haem-bound fractions from the pH titration data.
Footnotes
As a common convention haem will be used to refer to iron protoporphyrin IX irrespective of iron oxidation state. Where necessary, the iron oxidation state will be denoted as either iron(III) or iron(II).
The presence of a 429 nm Soret and single 555 nm Q band would have signified the presence only of haem in the form of deoxyhaemoglobin.
REFERENCES
- Adams PA, Berman MC. Kinetics and mechanism of the interaction between human serum albumin and monomeric haemin. Biochem J. 1980;191:95–102. doi: 10.1042/bj1910095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel M, Cimasoni G. The pH of human crevicular fluid measured by a new microanalytical technique. J Periodont Res. 1985;20:35–40. doi: 10.1111/j.1600-0765.1985.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Bickel M, Munoz JL, Giovannini P. Acid-base properties of human gingival crevicular fluid. J Dent Res. 1985;64:1218–1220. doi: 10.1177/00220345850640100801. [DOI] [PubMed] [Google Scholar]
- Byrne DP, Wawrzonek K, Jaworska A, Birss AJ, Potempa J, Smalley J,W. Role of the cysteine protease interpain A of Prevotella intermedia in breakdown and release of haem from haemoglobin. Biochem J. 2009;425:257–264. doi: 10.1042/BJ20090343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalabous P, Risk JM, Jenkins R, Birss AJ, Hart CA, Smalley JW. Characterization of a bifunctional catalase-peroxidase of Burkholderia cenocepacia. FEMS Immunol Med Microbiol. 2007;50:37–44. doi: 10.1111/j.1574-695X.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- Eggert FM, Drewell L, Bigelow JA, Speck JE, Goldner M. The pH of gingival crevices and periodontal pockets in children, teenagers and adults. Arch Oral Biol. 1991;36:233–238. doi: 10.1016/0003-9969(91)90091-8. [DOI] [PubMed] [Google Scholar]
- Gao J-L, Nguyen K-A, Hunter N. Characterisation of a hemophore-like protein from Porphyromonas gingivalis. J Biol Chem. 2010;285:40028–40038. doi: 10.1074/jbc.M110.163535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove MS, Wilkinson AJ, Olson JS. Structural factors governing hemin dissociation from metmyoglobin. Biochemistry. 1996;35:11300–11309. doi: 10.1021/bi960372d. [DOI] [PubMed] [Google Scholar]
- Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the red complex, a prototype polybacterial pathogenic consortium in periodontitis. Periodont 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- Hrkal Z, Vodrážka Z, Kalousek I. Transfer of haem from ferrihemoglobin and ferrihemoglobin isolated chains to hemopexin. Eur J Biochem. 1974;43:73–78. doi: 10.1111/j.1432-1033.1974.tb03386.x. [DOI] [PubMed] [Google Scholar]
- Kamaguchi A, Ohiyama T, Sakai E, Nakamura R, Watanabe T, Baba H, Nakayama K. Adhesins encoded by the gingipain genes of Porphyromonas gingivalis are responsible for the co-aggregation with Prevotella intermedia. Microbiology. 2003;149:1257–1264. doi: 10.1099/mic.0.25997-0. [DOI] [PubMed] [Google Scholar]
- Kinder SA, Holt SC. Characterisation of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect Immun. 1989;57:3425–3433. doi: 10.1128/iai.57.11.3425-3433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberg I, Hall G. pH and depth of gingival crevices in different areas of the mouths of fasting humans. J Periodontal Res. 1969;4:109–117. doi: 10.1111/j.1600-0765.1969.tb01955.x. [DOI] [PubMed] [Google Scholar]
- Kleinberg I. Formation and accumulation of acid on the tooth surface. J Dent Res. 1970;49:1300–1317. doi: 10.1177/00220345700490062301. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, Andersen RN, Holdeman LV. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985;48:741–746. doi: 10.1128/iai.48.3.741-746.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander PE, Palmer RJ, Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Labbé S, Grenier D. Characterisation of the human immunoglobulin G Fc-binding activity of Prevotella intermedia. Infect Immun. 1995;63:2785–2789. doi: 10.1128/iai.63.7.2785-2789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JP, Plata K, Yu F, Rosato A, Anaya C. Transcriptional organization, regulation and role of the Porphyromonas gingivalis W83 hmu haemin-uptake locus. Microbiol. 2006;152:3367–3382. doi: 10.1099/mic.0.29011-0. [DOI] [PubMed] [Google Scholar]
- Li J, Ellen RP, Hoover CI, Felton JR. Association of proteases of Porphyromonas (Bacteroides) gingivalis with its adhesion to Actinomyces viscosus. J Dent Res. 1991;70:82–86. doi: 10.1177/00220345910700021501. [DOI] [PubMed] [Google Scholar]
- Mallorquí-Fernández N, Manandhar SP, Mallorquí-Fernández G, Usón I, Wawrzonek K, Kantyka T, Solá M, Thøgersen IB, Enghild JJ, Potempa J, Gomis-Rüth FX. A new autocatalytic activation mechanism for cysteine proteases revealed by Prevotella intermedia interpain A. J Biol Chem. 2008;283:2871–2882. doi: 10.1074/jbc.M708481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermid AS, McKee AS, Marsh PD. Effect of environmental pH on enzyme activity and growth of Bacteroides gingivalis W50. Infect Immun. 1988;56:1096–1100. doi: 10.1128/iai.56.5.1096-1100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno EC, Margolis HC. Composition of human plaque fluid. J Dent Res. 1988;67:1181–1189. doi: 10.1177/00220345880670090701. [DOI] [PubMed] [Google Scholar]
- Nadkarni MA, Browne GV, Chhour KL, Byun R, Nguyen KA, Chapple CC, Jacques NA, Hunter N. Pattern of distribution of Prevotella species/phylotypes associated with healthy gingiva and periodontal disease. Eur J Clin Microbiol Infect Dis. 2012;31:2989–2999. doi: 10.1007/s10096-012-1651-5. [DOI] [PubMed] [Google Scholar]
- Nonaka E, Kiyama-Kishikawa M, Hayakawa M. Identification of 40-kDa outer membrane as an aggregation factor of Porphyromonas gingivalis to Streptococcus gordonii. J Oral Sci. 2001;43:239–243. doi: 10.2334/josnusd.43.239. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Meada N, Kondo K, Leung KP. Hemolytic and hemagglutinating activities of Prevotella intermedia and Prevotella nigrescens. FEMS Microbiol Lett. 1999;178:299–304. doi: 10.1111/j.1574-6968.1999.tb08691.x. [DOI] [PubMed] [Google Scholar]
- Olczak T, Simpson W, Liu X, Genco CA. Iron and haem utilisation in Porphyromonas gingivalis. FEMS Microbiol Rev. 2005;29:119–144. doi: 10.1016/j.femsre.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Olczak T, Sroka A, Potempa J, Olczak M. Porphyromonas gingivalis HmuY and HmuR-further characterisation of a novel mechanism of haem utilisation. Arch Microbiol. 2008;183:197–210. doi: 10.1007/s00203-007-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto BR, van Dooren SJ, Dozois CM, Luirink J, Oudega B. Escherichia coli hemoglobin protease autotransporter contributes to synergistic abscess formation and haem-dependent growth of Bacteroides fragilis. Infect Immun. 2002;70:5–10. doi: 10.1128/IAI.70.1.5-10.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thøgersen IB, Enghild JJ, Travis J. Comparative properties of two cysteine proteinases (gingipain Rs), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21657. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- Potempa M, Potempa J, Wawrzonek K, Manandhar SP, Nguyen K, Popadiak K, Riesbeck K, Eick S, Blom AM. Interpain A, cysteine proteinase from Prevotella intermedia inhibits complement by degrading complement factor C3. PLoS Pathog. 2009;5(2):e1000316. doi: 10.1371/journal.ppat.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Potempa M. Protease-dependent mechanisms of complement evasion by bacterial pathogens. Biol Chem. 2012;393:873–888. doi: 10.1515/hsz-2012-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Shibata Y, Shiroza T, Yukitake H, Peng B, Chen Y, Sato K, Naito M, Abiko Y, Reynolds E, Nakayama K. Characterisation of hemin-binding protein 35 (HBP35) in Porphyromonas gingivalis: its cellular distribution, thioredoxin activity and role in haem utilisation. BMC Microbiol. 2010;10:152–163. doi: 10.1186/1471-2180-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley JW, Birss AJ, Silver J. The periodontal pathogen Porphyromonas gingivalis harnesses the chemistry of the μ-oxo bishaem of iron protoporphrin IX to protect against hydrogen peroxide. FEMS Microbiol Lett. 2000;183:159–164. doi: 10.1111/j.1574-6968.2000.tb08951.x. [DOI] [PubMed] [Google Scholar]
- Smalley JW, Silver J, Marsh PJ, Birss AJ. The periodontal pathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the mu-oxo dimeric form: an oxidative buffer and possible pathogenic mechanism. Biochem J. 1998;331:681–685. doi: 10.1042/bj3310681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley JW, Birss AJ, Withnall R, Silver J. Interactions of Porphyromonas gingivalis with oxyhaemoglobin and deoxyhaemoglobin. Biochem J. 2002;362:239–245. doi: 10.1042/0264-6021:3620239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley JW, Silver J, Birss AJ, Withnall R, Titler PJ. The haem pigment of the oral anaerobes Prevotella nigrescens and Prevotella intermedia is composed of iron (III) protoporphyrin IX in the monomeric form. Microbiol. 2003;149:1711–1718. doi: 10.1099/mic.0.26258-0. [DOI] [PubMed] [Google Scholar]
- Smalley JW, Birss AJ, Szmigielski B, Potempa J. Sequential action of R- and K-specific gingipains of Porphyromonas gingivalis in the generation of the haem-containing pigment from oxyhaemoglobin. Arch Biochem Biophys. 2007;465:44–49. doi: 10.1016/j.abb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Smalley JW, Birss AJ, Szmigielski B, Potempa J. Mechanism of methaemoglobin breakdown by the lysine-specific gingipain of the periodontal pathogen Porphyromonas gingivalis. Biol Chem. 2008;389:1235–1238. doi: 10.1515/BC.2008.XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley JW, Byrne DP, Birss AJ, Wojtowicz H, Sroka A, Potempa J, Olczak T. HmuY haemophore and gingipain proteases constitute a unique syntrophic system of haem acquisition by Porphyromonas gingivalis. PLoS ONE. 2011;6(2):e17182. doi: 10.1371/journal.pone.0017182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaques. J Clin Periodont. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Yamada T. Glucose metabolism by Prevotella intermedia and Prevotella nigrescens. Oral Microbiol Immunol. 2000;15:188–195. doi: 10.1034/j.1399-302x.2000.150307.x. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Saito K, Schachtele CF, Yamada T. Acid tolerance and acid-neutralizing activity of Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum. Oral Microbiol Immunol. 1997;12:323–328. doi: 10.1111/j.1399-302x.1997.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Takahashi N. Acid-neutralizing activity during amino acid fermentation by Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum. Oral Microbiol Immunol. 2003;18:109–113. doi: 10.1034/j.1399-302x.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- Trindade SC, Olczak T, Gomes-Filho IS, Moura-Costa LF, Cerqueira EM, Galdino-Neto M, Alves H, Carvalho-Filho PC, Xavier MT, Meyer R. Induction of interleukin (IL)-1β, IL-10, IL-8 and immunoglobulin G by Porphyromonas gingivalis HmuY in humans. J Periodont Res. 2012;47:27–32. doi: 10.1111/j.1600-0765.2011.01401.x. [DOI] [PubMed] [Google Scholar]
- Tsuruga M, Matsuoka A, Hachimori A, Sugawara Y, Shikama K. The molecular mechanisms of autoxidation for human oxyhemoglobin: tilting of the distal histidine causes non-equivalent oxidation of the β chain. J Biol Chem. 1998;273:8607–8615. doi: 10.1074/jbc.273.15.8607. [DOI] [PubMed] [Google Scholar]
- Wójtowicz H, Guevara T, Tallant C, Olczak M, Sroka A, Potempa J, Solà M, Olczak T, Gomis-Rüth FX. Unique structure and stability of HmuY, a novel haem-binding protein of Porphyromonas gingivalis. PLoS Pathog. 2009a;5:e1000419. doi: 10.1371/journal.ppat.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójtowicz H, Wojaczyński J, Olczak M, Króliczewski J, Latos-Grazyński L, Olczak T. Haem environment of Porphyromonas gingivalis HmuY haem-binding protein. Biochem Biophys Res Commun. 2009b;382:178–182. doi: 10.1016/j.bbrc.2009.03.148. [DOI] [PubMed] [Google Scholar]
- Yu F, Anaya C, Lewis JP. Outer membrane proteome of Prevotella intermedia 17: Identification of thioredoxin and iron-repressible hemin uptake loci. Proteomics. 2007;7:403–412. doi: 10.1002/pmic.200600441. [DOI] [PubMed] [Google Scholar]