Abstract
The cannabinoid type-1 receptor (CB1R) is one of the most abundant members of the G protein-coupled receptor family in the central nervous system. Once activated by their cognate ligands, endocannabinoids, CB1Rs generally limit the timing of neurotransmitter release at many cortical synapses. Prior studies have indicated the involvement of CB1R in neurodegeneration and in various neuronal insults, with an emphasis on their neuroprotective role. In the present study we used a novel selective CB1R radioligand to investigate regional variations in CB1R ligand binding as a factor of progressive Braak tau pathology in the frontal cortex of Alzheimer's disease (AD) patients. The frontal cortex was chosen for this study due to the high density of CB1Rs and their well-characterized involvement in the progression of AD. Post-mortem prefrontal cortex samples from AD patients from Braak stages I to VI and controls were subjected to CB1R autoradiography with [125I]SD-7015 as radioligand. Regional concentration of [125I]SD-7015, corresponding to, and thereby representing, regional CB1R densities, were expressed in fM/g_tissue. The results show that CB1R density inversely correlates with Braak tau pathology with the following tendency: controls <AD Braak stage V–VI <AD Braak stage III–IV <AD Braak stage I–II. Differences were significant between control and AD Braak stage I–II groups, as well as between controls and the AD group comprising all Braak stages. These findings indicate an up-regulation of the tissue binding of the selective CB1R radioligand [125I]SD7015 in human brains, allowing the detection of fine modalities of receptor expression and radioligand binding during the progression of AD.
Keywords: Alzheimer's disease, Braak classification, Endocannabinoid system, Molecular imaging, Biomarker, Human brain autoradiography, CB1R, [125I]SD7015
1. Introduction
The endocannabinoid (EC) system is generally viewed as a neuromodulatory system that interacts with, and regulates the functions of several neurotransmitter systems, including the dopaminergic (DA), cholinergic, serotoninergic, adrenergic, opiate, glutamatergic and GABAergic systems (Freund et al., 2003; Sperlágh et al., 2009). By way of retrograde signaling, endocannabinoids provide essential feedback to limit neurotransmitter release from presynaptic terminals (Bacci et al., 2004; Marinelli et al., 2009). CB1R are coupled primarily to Gi/o proteins and, under specific conditions, to Gs proteins. By coupling to Gi/o proteins, CB1Rs regulate the activity of many plasma membrane proteins and signal transduction pathways, including ion channels, enzymes producing cyclic nucleotide second messengers, and effector kinases (Micale et al. 2007; Westlake et al., 1994). An increasing body of evidence indicates the potent anti-inflammatory and neuroprotective effects of cannabinoids through CB1R-dependent and -independent mechanisms under neurode-generative conditions (Cannich et al.,2004; Eljaschewitsch et al., 2006; Micale et al., 2007; Ramirez et al., 2005).
The abundance of CB1Rs and their interactions with the most important neuronal networks could explain the effect of the EC system on learning, cognition and memory performance (Lee et al., 2010; Terranova et al., 1996; Walther et al., 2006), functions which become affected in Alzheimer's disease (AD). The progression of AD takes decades. In the preclinical phase, histopathological findings are already present, but there are no clinical manifestations. Braak staging of AD reveals that pathological changes follow a complex stereotyped neuroanatomical pattern (Braak and Braak 1991; Nelson et al., 2009). The progressive appearance of neurofibrillary tangles (NFTs) and neuropil threads (NTs) begins in the transentorhinal region followed by the entorhinal cortex in Braak stage II. In this stage the mildest if any cognitive impairment is detectable. In Braak stages III and IV, neurofibrillary degeneration invades the hippocampal formation but remains restricted to limbic regions. This associates clinically with impaired cognitive functioning and subtle changes in personality, representing incipient AD. Braak stage V coincides with the clinical diagnosis of Alzheimer-type dementia (Newell et al., 1999). End stages of AD (V and VI) show severe cognitive impairment, and are neuropathologically characterized by NFTs and NTs indiscriminately present in virtually all subdivisions of the cerebral cortex (Nelson et al., 2009).
The alteration of CB1R during the progression of AD is, according to the scanty recent literature data, a contentious issue. There is increasing evidence emphasizing the involvement of the endocannabinoid system in chronic neurodegenerative diseases, including AD (Di Marzo et al., 2000; Micale et al., 2007; Ramirez et al., 2005). The up-regulation of CB1R under specific neuropathologies, including neurodegeneration, has recently been demonstrated (Lastres-Becker et al., 2001a,b). Recent investigations have also documented the molecular reorganization of EC signaling in AD with enhanced signaling around regions with high plaque load Mulder et al., 2011). Other observations indicate no change or a reduction in the density of CB1Rs in neurodegenerative diseases, including AD and HD (Micale et al., 2007; Silverdale et al., 2001). There are further studies that could not demonstrate alterations of CB1Rs in AD brains in brain regions other than the hippocampus and the basal ganglia (Lee et al., 2010; Westlake et al., 1994). These controversial findings and interpretations may arise partly from the lack of appropriate radioligands that bind to CB1R with high affinity and selectivity.
Despite the substantial effort invested in developing CB1R SPECT and PET radioligands to allow in vivo imaging of this receptor system, an “ideal” CB1R ligand has not yet been found (Horti and Van Laere, 2008). Compared to synthetic cannabinoid ligands (e.g. SR141716A, WIN55212-2), endogenous CB1R ligands have only moderate affinity, are highly lipophilic, subject to metabolism, and not selective whereas phytocannabinoids exhibit high lipophilic characters. Pyrazole derivatives are high affinity antagonists at CB1Rs, however, with still relatively high lipophilicity, low brain up-take or rapid clearance. Aminoalkylindoles lack a replaceable iodine or fluoride group whose presence is essential for PET or SPECT. Iodinated derivatives were also described but they presented either relatively low affinity or low brain uptake (Gifford et al., 2002). The four, recently tested, novel PET radioligands, 18F-MK-9470 (Burns et al., 2007), [11C]JHU75528 (also known as [11C]OMAR) (Horti et al., 2006; Wong et al., 2010) and 11C-MePPEP (Donohue et al., 2008; Yasuno et al., 2008; Terry et al., 2009, 2010a,b), have various advantages and disadvantages for routine use. However, the selective high affinity CB1R radioligand 125I SD-7015 (Donohue et al., 2009) appears as a superior tool for molecular imaging allowing in vitro and in vivo imaging studies of the CB1R and its changes in the human brain during physiological settings or under disease conditions. In particular we set out to measure changes in CB1R densities during the progression of AD in brain samples obtained from patients with different Braak stages as well as from control subjects.
In order to investigate the aforementioned conundrum regarding the alterations of CB1R densities in AD, we set out to measure the changes of CB1R densities during the progression of the disease as validated by Braak staging with [125I]SD-7015 in autoradiography experiments using brain samples obtained from AD patients with different Braak stages as well as from control subjects.
2. Material and methods
2.1. Radioligand and chemicals
The detailed synthesis of [125I]SD7015 was described earlier (Donohue et al., 2009). The specific radioactivity of the radioligand was 2175 Ci/mmol. Tris–HCl, bovine serum albumin (BSA), pargy-line hydrochloride (selective MAO-B inhibitor), GBX Developer and Fixer Twin Pack were all purchased from Sigma–Aldrich (Budapest, Hungary). The selective CB1R antagonist rimonabant (SR141716A) was purchased from Cayman Chemicals (Michigan, USA). All other chemicals were obtained from commercial sources and were analytical grade.
2.2. Brain tissue and processing
The study was approved by the Local Ethical Committee of the University of Debrecen (protocol number: DEOEC RKEB/IKEB M2547a-2006). Cortical specimens were dissected in the Human Brain Tissue Bank, Budapest. The study material consisted of eleven prefrontal cortex specimens (Brodmann area 10) from individuals with AD-related neurofibrillary degeneration (3 samples Braak stage I–II; 4 samples Braak stage III–IV; 4 samples Braak stage V– VI) and five control samples from the identical cortical area of subjects with no documented history of neurological or psychiatric disorders. Neuropathological data excluded any significant pathological finding in controls whereas AD brains met the neuropatho-logical criteria for different Braak stages of AD-related neurofibrillary degeneration (Braak and Braak, 1991). Mean age (±SD) of controls and individuals with AD-related changes was 45.5 ± 15.4 (control), 77.8 ± 11.3 (Braak I–II), 74.8 ± 9.2 (Braak III– IV), 87 ± 5.4 (Braak V–VI), and the post-mortem delays were 5.8 ± 2.8, 4.5 ± 2.7, 4.0 ± 2.1, 5.3 ± 1.0 (hours), respectively.
Frozen brain samples were sectioned at 20 lm thickness on a cryostat microtome (Leica, CM 1850) at −20 °C. Sections were thaw mounted onto glass slides, air-dried and stored at −20 °C until processing.
2.3. CB1R autoradiography with [125I-]SD-7015
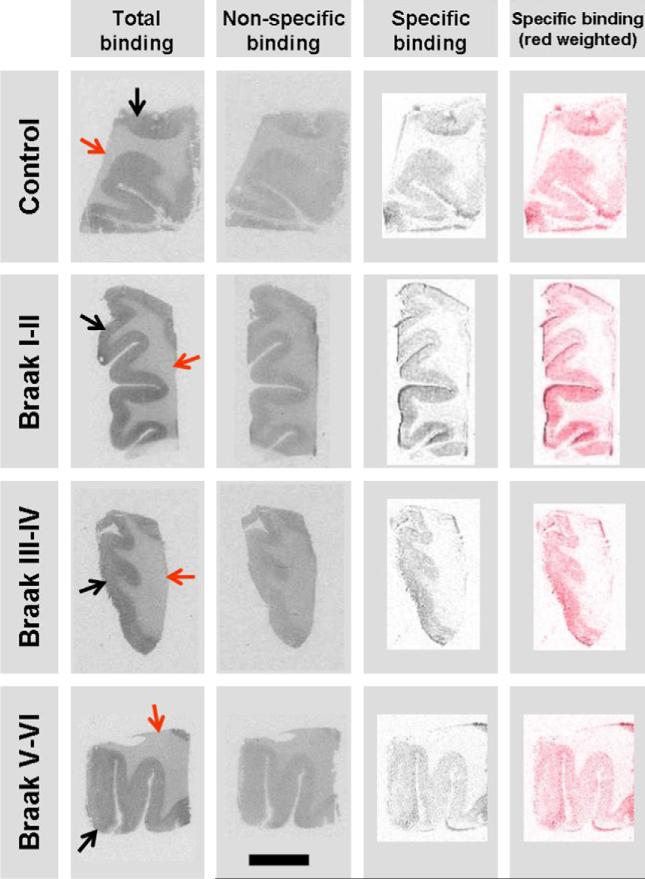
Consecutive tissue sections of prefrontal cortex were used in quadruplicates (11 brains) or triplicates (5 brains) in “one-point experiments”, using one radioligand concentration below saturation level. Tissues were incubated for 60 min with [125I]SD-7015 (40 pM) at a radioactivity concentration of 0.174 mCi/ml in a Tris buffer (50 mM, pH 7.4) containing NaCl (120 mM), KCl (5 mM), CaCl2 (2 mM), MgCl2 (1 mM), ascorbic acid (0.1% w/v), 10 μM pargyline and BSA (0.1%). Non-specific binding was determined in the presence of 10 μM rimonabant. The sections were then washed in the same buffer three times for thirty minutes each and briefly dipped in ice-cold distilled water. The sections were dried on a warm plate and subsequently exposed to γ-radiation sensitive film (Kodak Biomax MS, Sigma–Aldrich, Budapest, Hungary) for 24 h. The autoradiograms were digitized using a high-resolution scanner (Epson Perfection V750 Pro). Adobe Photoshop CS2 software was used for image analysis and processing. Specific binding images (Fig. 1) were obtained using the RGB mode of Adobe Photoshop CS2 by overlaying of non-specific binding and total binding images of the same brain sample. Optical densities of the overlaid images were subtracted using the ‘difference’ application of the program. The gray specific binding images were transformed to red weighted ones using the Photo filter application in order to make our pictures more expressive.
Fig. 1.
Characteristic CB1R autoradiographs of prefrontal cortex samples obtained with [125I]-SD-7015 as radioligand. The scale bar is 10 mm. In the fourth column the specific binding images (shown in the third column as grey-scale images) are red weighted. Red arrow = white matter, black arrow = cortex.
Results of multiple measurements, expressed as mean pixel values of optical densities in a region of interest, were averaged for each subject. Then, these values were used to calculate the difference between total binding and blocked (non-specific) binding, which consequently represents the mean specific binding of the radioligand.
For quantification, 14C-calibration scales (American Radiolabelled Chemicals Inc, St Louis, MO, USA) were used as described by Baskin and Wimpy (1989). Briefly, the radioactivity concentrations of the 14C plastic standards, supplied by the manufacturer, were transformed in tissue equivalent concentrations of 125I expressed as disintegrations per minute per mm2 (DPM/mm2). The transformation was based on the following quadratic equation:
where x values represent 14C radioactivity in μCi/g plastic. Mean pixel values of optical densities were converted into DPM/mm2 applying the obtained 125I DPM/mm2 values of the scale. Taking into consideration the standard specific radioactivity value of the radio-ligand (2200 Ci/mM), the 1 DPM = 451 fCi conversion factor and the slide thickness (20 μM), DPM/mm2 values were converted into fM/g_tissue concentration of the radioligand bound to the receptors, i.e. the radioligand's specific binding value.
Statistical analysis was performed with Student's t-test (two tailed, unequal variance). A p < 0.05 value was considered as significant.
3. Results
Fig. 1 provides an overview of characteristic autoradiographs showing total binding, blocked or non-specific binding, and specific binding obtained in a control brain and three AD brains with various Braak grades.
The specific binding values of [125I]SD7015 to CB1R's, expressed in fM/g-tissue, are displayed in Table 1. Values for individual AD groups were on or above than those obtained for the control group, but only the comparisons with the AD Braak I–II group revealed significantly increased CB1R binding relative to the control group (p < 0.05). However, when all AD brains were grouped together, the density value of the “indiscriminate AD group” was significantly higher than that of the control group (p < 0.05).
Table 1.
Specific binding values (average ± SEM) of [125I]SD7015 to CB1Rs in human prefrontal cortex samples (fM/g_tissue). There were significant differences between control and AD I–II values (p = 0.0459) and control and AD I–VI values (p = 0.0452), but no significant differences were present for other group comparisons.
| Sample group: | Control | AD Braak I–II | AD Braak III–IV | AD Braak V–VI | AD Braak I–VI | Control + AD Braak I–II | AD Braak III–VI | |
|---|---|---|---|---|---|---|---|---|
| Specific binding value of [125I]SD7015, (fM/g_tissue) | Average | 204.70 | 267.83 | 264.45 | 228.99 | 252.46 | 233.91 | 246.72 |
| SEM | 15.07 | 12.26 | 42.13 | 38.95 | 20.30 | 17.73 | 27.37 | |
| Number of | Brains | 5 | 3 | 4 | 4 | 11 | 8 | 8 |
| Sections | 15 | 12 | 12 | 16 | 40 | 27 | 28 |
In several studies on AD brain specimens, those belonging to Braak I II are often grouped and treated together with the control specimens. We also calculated the average density for the combined control plus AD Braak I II group for comparison with the AD III–VI group values. In this case, the density values were not significantly different.
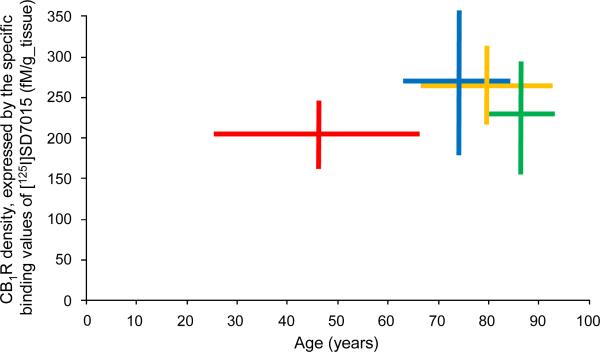
When the specific binding values (minimum–maximum range) for each age group (cf. Table 1) were plotted against the age range of each group (control, Braak I–II, Braak III–IV, Braak V–VI), the plot showed the up-regulation of the CB1Rs in Braak I–II as compared to their level in the control group. This elevated level of CB1Rs decreased in Braain III–IV, and further did so in Braan V–VI; however, even in Braain V–VI the CB1R density surpassed that in the control group (Fig. 2).
Fig. 2.
Age-ranges (horizontal lines) and CB1R density ranges, expressed by the specific binding values of [125I]SD7015, (vertical lines) for the four groups investigated in the present study (red: control, blue: Braak I–II, orange: Braak III– IV, green: Braak V–VI).
4. Discussion
The present study aimed to explore CB1R density changes in the prefrontal cortex of AD subjects, including all Braak stages, and in controls by using human brain receptor autoradiography with 125I-SD-7015, a novel high affinity CB1R agonist radioligand.
Data about CB1R alteration in AD are controversial. Westlake et al. (1994) found in samples from end-stage AD patients a reduced CB1R density in the entorhinal cortex, hippocampus, caudate nucleus, medial part of globus pallidus and substantia nigra pars reticulate, but without any alteration in some parts of the insular and temporal cortex. However, no association was found between reduced CB1R expression and characteristic neuropathological signs of AD. It was concluded that CB1R loss was probably a consequence of normal aging, resulting in extensive neuronal loss and the reduced density of synapses. Ramirez et al. (2005), using immunohistochemical techniques, demonstrated in frontal cortex samples markedly decreased CB1R protein expression in AD patients. Other studies found no alteration of CB1R density in AD brains including the entorhinal cortex (Benito et al., 2003), frontal cortex, anterior cingulated cortex, hippocampus, and caudate nucleus (Lee et al., 2010). In their study of the 17 AD patients 15 were neuropathologically classified to be in Braak stage V and VI, two in Braak stage III–IV, whereas brain specimens from Braak I or II stage were not investigated separately.
In the present study we investigated AD brain specimens from all Braak stages and compared them with specimens obtained from control subjects. The samples with AD-related tau pathology were divided in three groups based on neuropathological classification, using the Braak criteria. The changes in receptor densities were explored by measuring the specific binding to CB1R's of [125I]SD7015, a novel CB1 radioligand. The specific binding values of the radioligand in the frontal cortex were found in the range between 200 and 270 fM/g_tissue, similar to the range found with other radioligands such as [3H]CP55,940 (Biegon and Kerman, 2001) as well as with [35S]GTPγS assay (Mato et al., 2003). Our results suggest an increased CB1R density in prefrontal cortex in all neuropathological stages of AD as compared to controls. The difference is significant between the control group and the AD Braak I–II group, as well as the control group and the combined AD Braak I–VI group. No significance was found between controls and AD Braak stage III– IV, AD Braak stage V–VI sample groups. It is accepted that CBs through CB1Rs are capable of functional modulation of neurotrans-mission and inflammatory processes (Cannich et al.,2004; Eljaschewitsch et al., 2006; Micale et al., 2007; Ramirez et al., 2005). Our results indicate a possible up-regulation of the CB1R system in the early stages of AD, followed by a diminution of increased receptor density during the progress of the disease. The decrease of CB1R density could be due to intensified neuroinflamation and degeneration of neurons containing primarily the CB type-1 receptors. In this case our results already reflects the diminished CB1R population in later stages (Braak V–VI) of AD, showing, however, that CB1R receptor density levels do not decrease below control levels neither at the end-stage of AD.
CB1R densities found in our AD Braak I–II and III–IV sample groups cannot be directly compared to former studies either because other researchers had investigated regions that were affected by the pathological process others than the frontal cortex (Benito et al., 2003) or because brain samples from deceased individuals showing the earliest stages of AD-related neurofibrillary degeneration (i.e. Braak I–II) were not examined or separated from controls (Lee et al., 2010; Ramirez et al., 2005; Westlake et al., 1994). In this regard it is worth pointing out that the Braak I–II phase is regarded by several studies in the literature as still being part of the population of clinically asymptomatic control subjects. In Braak I–II neurofibrillary tangle deposits are restricted to the entorhinal cortex and the hippocampus and it is regarded as a pre-clinical, non-dementia phase of AD. In line with this approach, we have also grouped the brain specimens so that the control and the AD Braak I–II groups formed one group and the AD IV–VI groups. In this case there was no significant difference between the two groups.
Endocannabinoids take part in neuroprotection by preventing Aβ-induced neurotoxicity by indirect or direct modulation of immune responses through neuronal CB1Rs and/or microglial CB2Rs, respectively (Micale et al., 2007; Porter and Felder 2001; Ramirez et al., 2005). In addition, administration of CB1R antagonists improved memory in a model of AD probably through modulation of ACh release (Terranova et al., 1996). In late dementia the daily use of dronabinol (cannabinoid agonist) for two weeks significantly improved the Neuropsychiatric Inventory total score and the subscores for agitation and aberrant motor and nighttime behaviors (Walther et al., 2006). Jung et al. (2011, submitted) report about significantly lower endocannabinoid anandamide levels in postmortem mid-frontal and temporal cortex of subjects with AD. They identified positive correlations between cortical anandamide content and the patient's performance in cognitive measures of psychomotor speed and language. Furthermore, they found that anandamide and N-arachidonoyl-substituted phosphatidylethanolamine species (NArPE) levels in midfrontal cortex of the AD subjects are inversely correlated with levels of the neurotoxic amyloid peptide, amyloid β-protein (Aβ)42. These data suggest a protective role for the EC system during the course of AD.
In aged Sprague–Dawley rats entorhinal and temporal cortices showed increased CB1 protein expression whereas postrhinal cortex presented significant decrease; there were no changes in the dorsal and ventral hippocampus and the perirhinal cortex (Liu et al., 2003). Other authors reported age-related decreased expression of CB1 receptors in aged rat basal ganglia, layer I and VI of cerebral cortex, some cerebellar and hypothalamic structure and different alterations in distinct hippocampal areas (Berrendero et al., 1998; Romero et al., 1998). There were no alterations in other layers of cerebral cortex or other brain regions of rats. Mato and Pazos (2004) found a 8–10% decreases of CB receptor (CB1 and CB2) density per decade in human frontal cortex. In our study we found a slight age-related decreasing tendency of CB1R density in control group, however, these age-related changes did not reach significance. If we combined control and AD Braak I–II stage sample groups this tendency disappeared. In the light of these our results suggest that CB1R density does not decrease with age. Moreover, it shows a clear increasing tendency in initial stages of AD (Braak I–II). Our data draws our attention to the possibility that CB1R related changes could take place already in the early stages of AD. This supports the idea that AD Braak stage I–II samples should be studied separately from controls to investigate early alterations of CB system. This could reveal early reactions of the CB system to neurodegenerative processes, acting through the modulation of neurotransmission and neuroprotection.
Our present findings suggest an early reaction of the CB1R population in AD prefrontal cortex to the imminent neurodegenerative process. However, whether or not our results reflect compensatory or neuroprotective mechanisms or both is still unknown. For this reason the herewith demonstrated up-regulation of CB1Rs warrants for detailed studies of the CB1Rs’ possible role in AD. Furthermore, our results also confirm that this receptor population could serve as target for treatment in AD. However, a better understanding of the relationship between CB system and AD request further investigations. For instance, a wider selection of brain regions, including all regions routinely analysed for neuropathological staging according to Braak & Braak would be a valuable aspect for future studies.
Apart from observations in AD, in animal models of Parkinson's disease as well as that of Huntington's disease reactive changes affecting CB1Rs were reported as early events (Lastres-Becker et al., 2001a,b; Romero et al., 2002). Therefore an appropriate CB1R ligand which is able to determine in vivo the degree of CB1R occupancy, to evaluate CB1Rs up- or down-regulation or to follow the loss of particular neuronal cell types in neurodegenerative diseases might be used as molecular marker during normal ageing or for diagnosis of pathological processes.
A radioligand for imaging brain CB1 receptors would ideally possess adequately high affinity and moderate lipophilicity to promote rapid development of a high ratio of receptor-specific binding to non-specific binding in brain in vivo. Additionally, the proportion of radioligands available to cross the blood–brain barrier is determined by its plasma-free fraction (Donohue et al., 2009). The aforementioned criteria represents the deficiencies apply to most radiotracers developed to date for the CB1 receptor. Recently, the modification of rimonabant substituents led to the development of two promising radioligands: [11C]JHU75528 (also called [11C[OMAR) and [11C[JHU75575 (Donohue et al., 2008; Fan et al., 2006; Horti et al., 2006). These ligands showed different kinetics in the white matter than in cortical regions and/or partial volume effects may not be completely eliminated even for high-resolution research tomograph (Wong et al., 2010). In addition [11C]JHU75528 had low to moderate uptake in baboon brain and were displaced by non-radioactive ligands. (Horti et al., 2006). An other, recent developed CB1R ligand was [11C]MePPEP which presented higher peak brain uptake in monkey and in human brain compared to [11C]JHU75528. However, the [11C]MePPEP plasma-free fraction was quite low and generated radiometabolite(s) that entered monkey brain (Yasuno et al., 2008), furthermore it has relatively high lipophilicity (cLog D7.4: 5.42) (Donohue et al., 2009). The cLog D7.4 value of SD7015 is 4.14 and appears more favorable for a radioligand than that of [11C]MePPEP (Donohue et al., 2009). [11C[MePPEP had also relatively poor retest and intersubject variabilities, probably due to short radioactive half-life of 11C (20.4 min). An 18F isotope labeled analogue of [11C]MePPEP showed greater accuracy in quantification of receptor density as distribution volume, in comparison with the 11C analogue (Terry et al., 2010b). A PET radioligand using a radionuclide with a longer half-life (e.g. 18F, 109.7 min) would provide for extended measurements from arterial plasma and hopefully allow more accurate quantification of CB1 receptors in the brain with compartmental modeling (Terry et al., 2010b). SD7015 presented acceptable CB1 receptor affinity (Ki 3.40 ± 0.43 vs. only 1.38 ± 0.17 for rimonabant) and lipophilicity for development as a SPECT radioligand, furthermore, its physiochemical and pharmacological properties compare well with other successful PET radioligands (Donohue et al., 2009). SD7015 provides the advantage of developing a CB1R specific ligand labeled with 123I, 131I or 125I isotopes characterized by different physical decays (half-life: 13 h, 8 days and 59 days, respectively) and making possible to test this ligand by in vitro autoradiography followed by in vivo brain imaging.
As the novel selective CB1R agonist radioligand [125I[SD-7015 showed specific binding properties in every sample groups, we conclude that it may represent a promising radioligand for studying disease related CB1R alterations in the central nervous system.
Acknowledgements
The study was supported by the Hungarian National Science Found (OTKA) (K 68864 and 62893). VWP and SRD were supported by the Intramural Research Program of NIH (NIMH). The study has partly been performed within the frame of a master research agreement between Karolinska Institutet, Mediso Medical Imaging Systems and CROmed Translational.
References
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Wimpy H. Calibration of [14C] plastic standards for quantitative autoradiography of [125I] labeled ligands with Amersham Hyperfilm β-max. Neurosci. Lett. 1989;104:171–177. doi: 10.1016/0304-3940(89)90350-9. [DOI] [PubMed] [Google Scholar]
- Benito C, Núñez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J. Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Romero J, García-Gil L, Suarez I, De la Cruz P, Ramos JA, Fernández-Ruiz JJ. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim. Biophys. Acta. 1998;1407:205–214. doi: 10.1016/s0925-4439(98)00042-8. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS, Gibson R, Ryan C, Connolly B, Patel S, Krause S, Vanko A, Van Hecken A, Dupont P, De Lepeleire I, Rothenberg P, Stoch SA, Cote J, Hagmann WK, Jewell JP, Lin LS, Liu P, Goulet MT, Gottesdiener K, Wagner JA, de Hoon J, Mortelmans L, Fong TM, Hargreaves RJ. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc. Natl. Acad. Sci. USA. 2007;104:9800–9805. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem. 2004;11:625–632. doi: 10.1101/lm.77904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson's disease. FASEB J. 2000;14:1432–1438. doi: 10.1096/fj.14.10.1432. [DOI] [PubMed] [Google Scholar]
- Donohue SR, Krushinski JH, Pike VW, Chernet E, Phebus L, Chesterfield AK, Felder CC, Halldin C, Schaus JM. Synthesis, ex vivo evaluation, and radiolabeling of potent 1, 5-diphenylpyrrolidin-2-one cannabinoid subtype-1 receptor ligands as candidates for in vivo imaging. J. Med. Chem. 2008;51:5833–5842. doi: 10.1021/jm800416m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue SR, Varnäs K, Jia Z, Gulyás B, Pike VW, Halldin C. Synthesis and in vitro autoradiographic evaluation of a novel high-affinity radioiodinated ligand for imaging brain cannabinoid subtype-1 receptors. Bioorg. Med. Chem. Lett. 2009;19:6209–6212. doi: 10.1016/j.bmcl.2009.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Fan H, Ravert HT, Holt DP, Dannals RF, Horti AG. Synthesis of 1- (2, 4-dichlorophenyl)-4-cyano-5-(4-[11C]methoxyphenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide ([11C]JHU75528) and 1- (2-bromophenyl)-4-cyano-5-(4-[11C]methoxyphenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide ([11C] JHU75575) as potential radioligands for PET imaging of cerebral cannabinoid receptor. J. Label. Compd. Radiopharm. 2006;49:1021–1036. [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Makriyannis A, Volkow ND, Gatley SJ. In vivo imaging of the brain cannabinoid receptor. Chem. Phys. Lipids. 2002;121:65–72. doi: 10.1016/s0009-3084(02)00148-2. [DOI] [PubMed] [Google Scholar]
- Horti AG, Fan H, Kuwabara H, Hilton J, Ravert HT, Holt DP, Alexander M, Kumar A, Rahmim A, Scheffel U, Wong DF, Dannals RF. 11CJHU75528: a radiotracer for PET imaging of CB1 cannabinoid receptors. J. Nucl. Med. 2006;47:1689–1696. [PubMed] [Google Scholar]
- Horti AG, Van Laere K. Development of radioligands for in vivo imaging of type 1 caanabinoid receptors (CB1) in human brain. Curr. Pharm. Des. 2008;14:3363–3383. doi: 10.2174/138161208786549380. [DOI] [PubMed] [Google Scholar]
- Jung KM, Astarita G, Yasar S, Vasilevko V, Cribbs DH, Head E, Cotman CW, Piomelli D. An amyloid β(42)-dependent deficit in anandamide mobilization is associated with cognitive dysfunction in Alzheimer's disease. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.03.012. E-published ahead of print, PMID: 21546126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, Kerman IA. Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. Neuroimage. 2001;14:1463–1468. doi: 10.1006/nimg.2001.0939. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Fezza F, Cebeira M, Bisogno T, Ramos JA, Milone A, Fernández-Ruiz J, Di Marzo V. Changes in endocannabinoid transmission in the basal ganglia in a rat model of Huntington's disease. Neuroreport. 2001a;12:2125–2129. doi: 10.1097/00001756-200107200-00017. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Cebeira M, de Ceballos M, Zeng B-Y, Jenner P, Ramos JA, Fernandez-Ruiz JJ. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson's disease and MPTP-treated marmosets. Eur. J. Neurosci. 2001b;14:1827–1832. doi: 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Agacinski G, Williams JH, Wilcock GK, Esiri MM, Francis PT, Wong PT, Chen CP, Lai MK. Intact cannabinoid CB1 receptors in the Alzheimer's disease cortex. Neurochem. Int. 2010;57:985–989. doi: 10.1016/j.neuint.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Liu P, Bilkey DK, Darlington CL, Smith PF. Cannabinoid CB1 receptor protein expression in the rat hippocampus and entorhinal, perirhinal, postrhinal and temporal cortices: regional variations and age-related changes. Brain Res. 2003;979:235–239. doi: 10.1016/s0006-8993(03)02872-5. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Pacioni S, Cannich A, Marsicano G, Bacci A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat. Neurosci. 2009;12:1488–1490. doi: 10.1038/nn.2430. [DOI] [PubMed] [Google Scholar]
- Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci. 2003;17:1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- Mato S, Pazos A. Influence of age, postmortem delay and freezing storage period on cannabinoid receptor density and functionality in human brain. Neuropharmacology. 2004;46:716–726. doi: 10.1016/j.neuropharm.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Micale V, Mazzola C, Drago F. Endocannabinoids and neurodegenerative diseases. Pharmacol. Res. 2007;56:382–392. doi: 10.1016/j.phrs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Mulder J, Zilberter M, Pasquaré SJ, Alpár A, Schulte G, Ferreira SG, Köfalvi A, Martín-Moreno AM, Keimpema E, Tanila H, Watanabe M, Mackie K, Hortobágyi T, de Ceballos ML, Harkany T. Molecular reorganization of endocannabinoid signalling in Alzheimer's disease. Brain. 2011;134:1041–1060. doi: 10.1093/brain/awr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KL, Hyman BT, Growdon JH. Application of the National Institute on Aging (NIA)–Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J. Neuropathol. Exp. Neurol. 1999;58:1147–1155. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Porter AC, Felder CC. The endocannabinoid nervous system:unique opportunities for therapeutic intervention. Pharmacol. Therapeut. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. receptors in nonhuman primate brain. J. Neurosci. 25, 1904–1913.
- Romero J, Berrendero F, Garcia-Gil L, de la Cruz P, Ramos JA, Fernández-Ruiz JJ. Loss of cannabinoid receptor binding and messenger RNA levels and cannabinoid agonist-stimulated [35S]guanylyl-5’O-(thio)-triphosphate binding in the basal ganglia of aged rats. Neuroscience. 1998;84:1075–1083. doi: 10.1016/s0306-4522(97)00552-6. [DOI] [PubMed] [Google Scholar]
- Romero J, Lastres-Becker I, de Miguel R, Berrendero F, Ramos JA, Fernández-Ruiz J. The endogenous cannabinoid system and the basal ganglia. Biochemical, pharmacological, and therapeutic aspects. Pharmacol. Ther. 2002;95:137–152. doi: 10.1016/s0163-7258(02)00253-x. [DOI] [PubMed] [Google Scholar]
- Silverdale MA, McGuire S, McInnes A, Crossman AR, Brotchie JM. Striatal cannabinoid CB1 receptor mRNA expression is decreased in the reserpine-treated rat model of Parkinson's disease. Exp. Neurol. 2001;169:400–406. doi: 10.1006/exnr.2001.7649. [DOI] [PubMed] [Google Scholar]
- Sperlágh B, Windisch K, Andó RD, Sylvester Vizi E. Neurochemical evidence that stimulation of CB1 cannabinoid receptors on GABAergic nerve terminals activates the dopaminergic reward system by increasing dopamine release in the rat nucleus accumbens. Neurochem. Int. 2009;54:452–457. doi: 10.1016/j.neuint.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Terranova J-P, Storme J-J, Lafor N, Perio A, Rinaldi-Carmona M, Le Fur G, Soubrie P. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR141716A. Psychopharmacology. 1996;126:165–172. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- Terry GE, Hirvonen J, Liow JS, Seneca N, Tauscher JT, Schaus JM, Phebus L, Felder CC, Morse CL, Pike VW, Halldin C, Innis RB. Biodistribution and dosimetry in humans of two inverse agonists to image cannabinoid CB1 receptors using positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging. 2010a;37:1499–1506. doi: 10.1007/s00259-010-1411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry GE, Hirvonen J, Liow JS, Zoghbi SS, Gladding R, Tauscher JT, Schaus JM, Phebus L, Felder CC, Morse CL, Donohue SR, Pike VW, Halldin C, Innis RB. Imaging and quantitation of cannabinoid CB1 receptors in human and monkey brains using (18)F-labeled inverse agonist radioligands. J. Nucl. Med. 2010b;51:112–120. doi: 10.2967/jnumed.109.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry GE, Liow JS, Zoghbi SS, Hirvonen J, Farris AG, Lerner A, Tauscher JT, Schaus JM, Phebus L, Felder CC, Morse CL, Hong JS, Pike VW, Halldin C, Innis RB. Quantitation of cannabinoid CB1 receptors in healthy human brain using positron emission tomography and an inverse agonist radioligand. NeuroImage. 2009;48:362–370. doi: 10.1016/j.neuroimage.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Mahlberg R, Eichmann U, Kunz D. D-9-Tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology. 2006;185:524–528. doi: 10.1007/s00213-006-0343-1. [DOI] [PubMed] [Google Scholar]
- Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiographic and in situ hybridization histochemistry study of normal aged and Alzheimer's brains. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, Ye WG, Dannals RF, Ravert HT, Nandi A, Rahmim A, Ming JE, Grachev I, Roy C, Cascella N. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [C-11]OMAR. Neuroimage. 2010;52:1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno F, Brown AK, Zoghbi SS, Krushinski JH, Chernet E, Tauscher J, Schaus JM, Phebus LA, Chesterfield AK, Felder CC, Gladding RL, Hong J, Halldin C, Pike VW, Innis RB. The PET radioligand [11C]MePPEP binds reversibly and with high specific signal to cannabinoid CB1 receptors in nonhuman primate brain. Neuropsychopharmacology. 2008;33:259–269. doi: 10.1038/sj.npp.1301402. [DOI] [PubMed] [Google Scholar]