Abstract
Cardiovascular complications are the leading causes of morbidity and mortality in individuals with obesity, type 2 diabetes mellitus (T2DM), and insulin resistance. Complications include pathologies specific to large (atherosclerosis, cardiomyopathy) and small (retinopathy, nephropathy, neuropathy) vessels. Common among all of these pathologies is an altered endothelial cell phenotype i.e., endothelial dysfunction. A crucial aspect of endothelial dysfunction is reduced nitric oxide (NO) bioavailability. Hyperglycemia, oxidative stress, activation of the renin-angiotensin system, and increased pro-inflammatory cytokines are systemic disturbances in individuals with obesity, T2DM, and insulin resistance and each of these contribute independently and synergistically to decreasing NO bioavailability. This review will examine the contribution from elevated circulating fatty acids in these subjects that lead to lipotoxicity. Particular focus will be placed on the fatty acid metabolite ceramide.
Keywords: Pathways and mechanisms, Vascular dysfunction, Ceramide, Adiponectin, Nitric oxide, Reactive oxygen species, Insulin resistance, Obesity, Inflammation, Mitochondria, Endothelial dysfunction, Glucotoxicity, Diabetes
1. Introduction
Obesity, type 2 diabetes mellitus (T2DM), and insulin resistance are conditions associated with an altered endothelial cell phenotype i.e., endothelial dysfunction. Eighty percent of patients with these conditions will die from cardiovascular complications. The prevalence of diagnosed and undiagnosed cases of T2DM is estimated to increase to 33% by 2050.[1] Individual and societal costs will increase should this concerning projection be realized, underscoring the need to elucidate effective therapeutic targets and strategies for intervention.
Endothelial dysfunction can be defined as attenuated arterial vasorelaxation in response to pharmacological agonists such as acetylcholine, bradykinin or ATP, or physical stimuli such as increased intraluminal flow. When impaired vasorelaxation to one of these agonists is observed and the integrity of the vascular smooth muscle is verified, a strong case can be made that the endothelium is the source of the defect. Impaired endothelium-dependent vasorelaxation is an early indicator of endothelial dysfunction[2]. A crucial aspect of endothelial dysfunction is reduced nitric oxide (NO) bioavailability. NO bioavailability depends on a delicate balance between factors responsible for the synthesis and degradation of this molecule. In addition to contributing to vasodilation, endothelial cell derived NO has anti-coagulant, anti-inflammatory and anti-proliferative properties [3–5]. Loss of these protective mechanisms contribute importantly to the pathophysiology of micro and macrovascular diseases that characterizes obesity, T2DM, and insulin resistance [2;6]. Much progress has been made in elucidating general mechanisms that precipitate a mismatch between generation and degradation of NO that leads to cardiovascular complications such as hypertension and atherosclerosis that are associated with endothelial dysfunction. Strong evidence exists that hyperglycemia, oxidative stress, activation of the renin angiotensin system, increased pro-inflammatory cytokines, and lipotoxicity contribute independently and synergistically to this process [7;8]. Gaining additional insight into precise mechanisms linking each of these pathways to vascular dysfunction is necessary to design and implement targeted therapeutic strategies with minimal off-target side effects. This report will focus on the contribution from lipotoxicity in general, and recent progress that has been made concerning mechanisms of action whereby the lipid metabolite ceramide might lower NO bioavailability to an extent that precipitates endothelial dysfunction.
2. Lipotoxicity
Impaired tissue homeostasis that is attributable to changes in lipid utilization, or lipid-induced changes in intracellular signaling, have been broadly termed lipotoxicity. Lipotoxicity has the potential to decrease endothelial NO synthase (eNOS) gene expression and eNOS catalytic activity to ultimately result in endothelial cell dysfunction via diverse mechanisms. These include insulin resistance in the vasculature, inflammation, oxidative stress, or the accumulation of lipotoxic metabolites such as ceramide.
2.1 Insulin resistance in endothelial cells
Insulin receptors exist in endothelial cells, vascular smooth muscle cells, and macrophages. In addition to skeletal muscle, adipose tissue, and liver, impaired insulin-mediated signal transduction in the vasculature may accompany obesity, T2DM, and generalized insulin resistance and might contribute to cardiovascular complications observed in these patients by reducing NO bioavailability.[9] It is generally accepted that a reciprocal relationship exists between insulin resistance in the vasculature and endothelial function,[9] and that this relationship is precipitated by a pathway-selective inhibition of insulin signaling to eNOS via PI3K / Akt whereas MAPK signaling to ET-1 is unaltered or even increased.[10] The resulting endothelial cell dysfunction reduces agonist-induced vasodilation and renders the vascular wall more susceptible to atherosclerosis.[11;12]
Insulin resistance is associated with increased circulating concentrations of fatty acids (FFAs). Studies in cultured cells [13–17], isolated arteries[14;17–19], animal models[14;17;20], and humans[21;22] demonstrate that elevated FFAs lower NO bioavailability. Importantly, when FFAs are experimentally increased in control subjects to levels that circulate in subjects with the metabolic syndrome, endothelium-dependent dysfunction is observed.[23]
Palmitic, oleic, and linoleic acids are the most abundant FFAs in human serum comprising ~70% of the total circulating FFAs.[24] Kim et al. showed that each of these fatty acids impair basal and insulin-stimulated NO production in bovine aortic endothelial cells (BAECs), with palmitic acid exerting the greatest effect [25]. Elevated FFAs can promote de novo synthesis of diacylglycerol which activates certain protein kinase C (PKC) isoforms[7;16]. Tang and Li [16] reported that endothelial cells exposed to 5 or 10 days of palmitate treatment displayed impaired agonist-evoked (i.e., bradykinin, ATP) NO production that was secondary to blunted elevations of intracellular free Ca2+ [Ca2+]i. Co-culture of endothelial cells with PKC inhibitors neutralized the ability of palmitate to lower NO. This finding is consistent with observations that PKC activation selectively inhibits PI3K / Akt signaling in the vasculature [10;26] such that cGMP formation - an estimate of NO bioavailability – is impaired [27]. The PKCβ isoform appears to play an important role in this process.[27–29] To explore mechanisms whereby PKC activation might contribute to vascular insulin resistance, multiple targets in in the insulin signaling pathway were evaluated in endothelial cells [12]. A most interesting finding was that general (phorbol ester) and specific (angiotensin II) PKC activation (particularly the PKCα isoform) phosphorylated a novel site on p85α i.e., Thr-86, which negatively regulates PI3K. As such, phosphorylation of Thr-86 reduced the binding of p85α to IRS1 and decreased insulin and VEGF signaling via PI3K. Mice with germline deletion of insulin receptor substrate-1 (IRS-1) are hypertensive and endothelial specific IRS-1 knockout mice display endothelial dysfunction,[30] demonstrating the importance of intact signaling via this pathway to eNOS. Thus it is reasonable to hypothesize that endothelial dysfunction observed in pathophysiological conditions associated with elevated FFAs might be secondary to PKC mediated phosphorylation of Thr-86 on p85α. Experiments investigating these targets in arteries from experimental models of DIO, T2DM, and insulin resistance are therefore warranted.
Insulin receptor substrate 2 (IRS-2) also plays an important role in transducing IR activation to eNOS [31]. Endothelial cell IRS-2 expression, Akt activation, and p-eNOS were down-regulated and capillary recruitment and insulin delivery were impaired in fat-fed vs. lean mice. These defects were neutralized when fat-fed mice were treated with a prostacyclin analog that increased eNOS expression, and all findings were recapitulated in endothelial specific IRS-2 KO mice. Taken together, these observations suggest a plausible mechanism by which elevated FFAs may impair insulin-mediated signaling to eNOS to an extent that contributes to the pathophysiology of endothelial dysfunction.
Other murine models have been used to demonstrate important functional consequences of disrupted IR-mediated signaling to eNOS. Mice with germ line haploinsufficiency of the IR (i.e., IR+/− mice) display hypertension, mild insulin resistance, reduced basal and insulin-stimulated eNOS phosphorylation in the vasculature, and an age-dependent decrease in arterial vasorelaxation that is associated with an increase in endothelial cell derived NADPH-oxidase-mediated O2•− production[32;33]. Endothelial regeneration in response to wire-induced denudation of the femoral artery was delayed in IR+/− mice, and was rescued by transfusion of angiogenic progenitor cells obtained from insulin-sensitive but not from insulin-resistant animals.[34]. In mice with combined apolipoprotein (apo) E and IR deletion in endothelial cells, atherosclerotic lesion size, endothelium-dependent dysfunction, and VCAM-1 expression were more severe than results obtained from apoE deficient mice with intact insulin receptor signaling [11]. Thus defective IR signaling in the vascular endothelium accelerates the progression of atherosclerotic disease in genetically susceptible animal models. Not all genetic models of IR disruption support the hypothesis that vascular insulin resistance is sufficient to induce arterial dysfunction. For example, we studied IR null mice with transgenic re-expression of the IR in brain, liver, and pancreatic β-cells that was sufficient to preserve glucose homeostasis despite hyperinsulinemia (TTr-IR−/− mice)[35]. No differences in blood pressure, ET-1 mRNA, eNOS mRNA, expression, or phosphorylation were observed between groups. Furthermore, endothelium-dependent vasorelaxation and indices of oxidant stress were similar in vessels from TTr-IR−/− vs. WT mice [14]. Therefore, complex interactions exist between IR haploinsufficiency, loss of IR or IRS isoforms, and the presence or absence of hyperinsulinemia, in the vascular adaptation to genetic disruption of insulin signaling.
2.2 Cross-talk between IRs and insulin-like growth factor 1 receptors (IGF-1Rs)
IGF-1Rs exist in the vasculature and heterodimerize with IRs to form hybrid receptors in insulin resistant conditions associated with obesity and T2DM. Because hybrid receptors bind IGF-1 but not insulin with high affinity, this scenario might contribute to attenuating / preventing insulin-mediated NO production by the endothelium. Arteries from mice with germ line haploinsufficiency of the IGF-1R (IGF-1R+/− mice), or endothelium-specific homozygous or heterozygous deletion of the IGF-1R, display attenuated phenylephrine-induced vasocontraction, increased basal NO production, and greater insulin-mediated NO production.[36] All improvements were neutralized in mice that were heterozygous deficient for the IR and IGF-1R respectively (IR+/− and IGF-1R+/−) [36]. These data provide strong rationale for considering the IGF-1R as a potential therapeutic target for treating vascular diseases associated with insulin-resistance.
2.3 Insulin resistance in intact arteries
Pathway-specific resistance to insulin-mediated signal transduction is known to exist in skeletal muscle, adipose tissue, and the liver. Jiang et al. were the first to report selective resistance via the IR-PI3K-Akt pathway in vessels from obese vs. lean rats.[10] This issue has been examined more recently in aortae from lean and fat-fed mice[19;37–39]. Insulin-stimulated Akt phosphorylation was detected in both groups, but was modestly blunted in arteries from obese mice. In contrast, eNOS phosphorylation was completely prevented in vessels from fat-fed animals. Functional repercussions attributable to decreased vascular eNOS phosphorylation and the resulting reduction in NO bioavailability in obese mice included arterial inflammation[19;37;39] and impaired arterial vasorelaxation [38]. We also observed that systemic hypertension, arterial dysfunction, and decreased basal and stimulated eNOS phosphorylation in obese mice occurred in the absence of evidence of deficient signaling via Akt to eNOS in the vasculature. Similar findings were obtained in Akt1 knockout mice. Collectively, these findings indicate that a dissociation exists between insulin mediated signal transduction in the vasculature, and impaired eNOS enzyme function [14;19;37–39].
The apparent dissociation between vascular insulin signaling and impaired eNOS enzyme function in the context of obesity prompted us to explore whether a component of the metabolic environment or altered signaling via another kinase to eNOS might play a role. Because fasting hyperglycemia was mild in fat-fed vs. lean mice, and O2•−, NADPH-oxidase activity, AMPK, and PKA were similar in vessels from both groups, we hypothesized that the 3-fold elevation in FFAs in obese mice might be responsible. [14] We observed that FFAs increased from ~400 uM to ~1200 uM when C57Bl6 mice consumed standard vs. high-fat chow for ~12 weeks.[14] Because palmitic, oleic, and linoleic acids comprise ~70% of the total circulating FFAs,[24] it is reasonable to extrapolate that palmitate concentrations could range from 93 uM – 280 uM after 12 weeks of high-fat feeding. Thus we treated endothelial cells with 500 uM palmitate. Although this level could be deemed elevated, 500 uM lies within the physiological / pathophysiological range [40] and did not lead to cell death or apoptosis in bovine aortic cells treated for 3 h. Compared to vehicle-treated cells, palmitate impaired basal and insulin-stimulated p-eNOS S1177 whereas ERK and Akt phosphorylation was intact. The palmitate-induced reduction of eNOS phosphorylation was sufficient to impair insulin-mediated NO production by BAECs, and endothelium-dependent vasorelaxation but not vascular smooth muscle function of isolated arteries. These in vitro results recapitulated our earlier observations in arteries from obese mice.[14]
3. Palmitate decreases eNOS function in a ceramide-dependent manner in endothelial cells and isolated arteries
When tissues not suited for lipid storage (e.g., skeletal muscle, liver) are exposed to increased circulating levels of FFA, toxic bioactive lipid metabolites can accumulate that might be associated with impaired metabolic homeostasis and increased cardiovascular risk. One such metabolite is the sphingolipid ceramide [41;42]. Obesity and lipid exposure promote sphingolipid accumulation in peripheral tissues of rodents and humans, and recently ceramide was reported to accumulate in arteries from a rat model of uncontrolled type 2 diabetes [43]. Based on our earlier findings in BAECs and isolated arteries that palmitate decreases p-eNOS, NO production, and endothelial but not vascular smooth muscle function, [14] we sought to determine whether ceramide might play a role. A strong rationale exists to hypothesize that ceramide contributes importantly to arterial dysfunction that is secondary to impaired eNOS activity. In several cell types such as adipocytes and C2C12 myotubes, ceramide disrupts signaling kinases that phosphorylate eNOS at positive regulatory sites [44] and potentiates signaling kinases that phosphorylate eNOS at negative regulatory sites [41;45;46]. Short-term incubation with synthetic ceramide impairs endothelium-dependent vasorelaxation [47] and exaggerates vasocontraction of isolated arteries [48] and reduces the bioavailability of NO in human endothelial cells [49]. Based on these data and our own findings in fat-fed mice and endothelial cells treated with palmitate, we tested whether endogenous ceramide accumulation was capable of reducing NO bioavailability in BAECs. Palmitate increased de novo ceramide synthesis, which reduced agonist (e.g., insulin and vascular endothelial cell growth factor)-stimulated eNOS phosphorylation at S1177 and S617, eNOS dimer to monomer formation, eNOS enzyme activity, and NO production [17]. In contrast to findings from other cell types, the changes we observed were not due to impaired upstream signaling to eNOS from Akt, AMPK, or ERK 1/2, or to O2•−-mediated peroxynitrite formation. Importantly, when endogenous ceramide biosynthesis in response to palmitate incubation was inhibited in isolated arteries using pharmacological and genetic approaches, the ability of this FFA to decrease p-eNOS and endothelium-dependent vasorelaxation was prevented.[17] Therefore, ceramide may contribute importantly to palmitate-induced reductions in eNOS enzyme function. We also determined whether the deleterious responses to ceramide observed in endothelial cells and isolated arteries after relatively short term (i.e., 3 h) exposure to palmitate were also present in a clinically relevant rodent model of obesity, T2DM, and insulin resistance.[17] These results are discussed in section 4 (below).
4. Ceramide-induced vascular dysfunction in obese mice is tissue autonomous
In rodent models of lipid oversupply (e.g., fat-feeding, lipid infusion) targeted inhibition of ceramide biosynthesis via pharmacological or genetic approaches attenuates metabolic disturbances [43;50–54] and atherosclerotic lesion formation [55]. Administration of myriocin, an inhibitor of serine palmitoyl transferase, the rate-limiting enzyme responsible for de novo ceramide biosynthesis, to fat-fed streptozotocin-treated rats reduced arterial ceramide content and partially reversed endothelial dysfunction in parallel with amelioration of the metabolic milieu[43]. It is not possible to discern from that study whether improved arterial function resulted from lower vascular ceramide accrual or from less disruption of the amelioration of the metabolic milieu. We used pharmacological and genetic approaches to limit ceramide biosynthesis in fat-fed mice to determine whether our earlier findings from isolated arteries could be recapitulated in vivo. Inhibition of ceramide synthesis with myriocin, or by heterozygous deletion of dihydroceramide desaturase (des1), which catalyzes de novo ceramide synthesis, prevented endothelial dysfunction and systemic hypertension, and preserved eNOS phosphorylation in arteries from fat-fed mice.[17] These studies support the hypothesis that arterial ceramide accumulation precipitates cellular dysfunction in part by impairing NO bioavailability. We will now review molecular mechanisms linking ceramide biosynthesis with endothelial and vascular dysfunction.
5. Evidence linking toll-like receptor 4 (TLR4) signaling and palmitate-mediated endothelial dysfunction
Kim et al. showed in BAECs that palmitate signals via TLR4, a pattern recognition receptor that is essential for initiating inflammatory responses associated with innate immunity.[56;57] When palmitate signals via TLR4, inhibitor of κB-kinase (IKK) is activated, which phosphorylates and degrades IκBα enabling nuclear translocation and expression of NF-κβ, a “master regulator” of inflammation [56]. This increases inflammatory mediators such as IL-6 and ICAM which are associated with decreased endothelial NO production.[13;19] Similar changes in p-Iκ Bα, IL-6, and ICAM were recapitulated in lysates of aorta from fat-fed vs. lean WT but not TLR4−/− mice [19;37]. The Kim group later demonstrated in endothelial cells and confirmed in vascular tissue from obese WT and TLR4−/− mice that the link between palmitate-mediated TLR4 activation and IKK/ p-IκBα/ NF-κβ signaling involves NADPH oxidase-mediated O2•− production.[58] NOX4 is the most abundant isoform of NADPH oxidase in vascular tissue. Bone morphogenic protein 4 (BMP4) induces and activates NADPH oxidase in endothelial cells. Administration of BMP4 to mice induces arterial dysfunction and hypertension in an NADPH-oxidase dependent manner [59]. Maloney et al. showed that endothelial cells treated with palmitate and aortae from fat-fed mice display elevated BMP4 and NOX4 and activated IKK/ p-IκBα/ NF-κβ signaling in a TLR4-dependent manner. NADPH-oxidase-mediated O2•− production can also impair NO bioavailability and vascular function via other mechanisms. For example, if the cellular antioxidant environment is insufficient to scavenge the oxidant load, O2•− may combine with NO to form peroxynitrite leading to disruption of the eNOS dimer, leading to impaired eNOS activity and arterial vasorelaxation. [38;60;61].
Holland et al. provided further insight when they demonstrated that lard oil infusion increased skeletal muscle and liver ceramide synthesis in WT but not TLR4−/− mice, and that palmitate incubation increased ceramide synthesis and transcript levels of enzymes involved in ceramide biosynthesis (sptlc1, sptlc2, des1) in IKK-WT but not IKK-kinase dead cells.[62] To test whether these findings could be translated to the intact animal, mice consumed standard or high-fat chow for 17 weeks. A subgroup of fat-fed mice was then switched to a high-fat diet supplemented with the IKKβ inhibitor sodium salicylate (NS). While body weight was similar between groups, insulin and glucose tolerance were improved, and increases in skeletal muscle and liver ceramide accumulation were prevented by NS treatment. Thus, palmitate signaling via TLR4 has the potential to evoke vascular dysfunction by increasing inflammation via IKK/ p-IκBα/ NF-κβ signaling, NADPH-oxidase-mediated O2•− production which disrupts the balance in the cellular oxidant / antioxidant environment, and ceramide biosynthesis [which might disrupt eNOS enzyme function (see below)]. Although each of these mechanisms can independently impair vascular function, it is highly likely that these pathways synergistically interact. There is some disagreement whether toll like receptors directly bind saturated fatty acids [63], and other studies have suggested that saturated fatty acids might not induce inflammation via TLR mediated signaling at all [64]. FFAs have been shown to amplify the inflammatory responses of monocytes / macrophages to bacterial lipopolysaccharide via a TLR4 - independent mechanism that is dependent upon ceramide generation, which in turn activates PKC and MAPK [57]. In balance, the preponderance of evidence indicates that FFAs (e.g., palmitate) impair endothelial function and increase cardiovascular complications via multiple mechanisms that increase inflammation, oxidant stress, and / or ceramide generation.
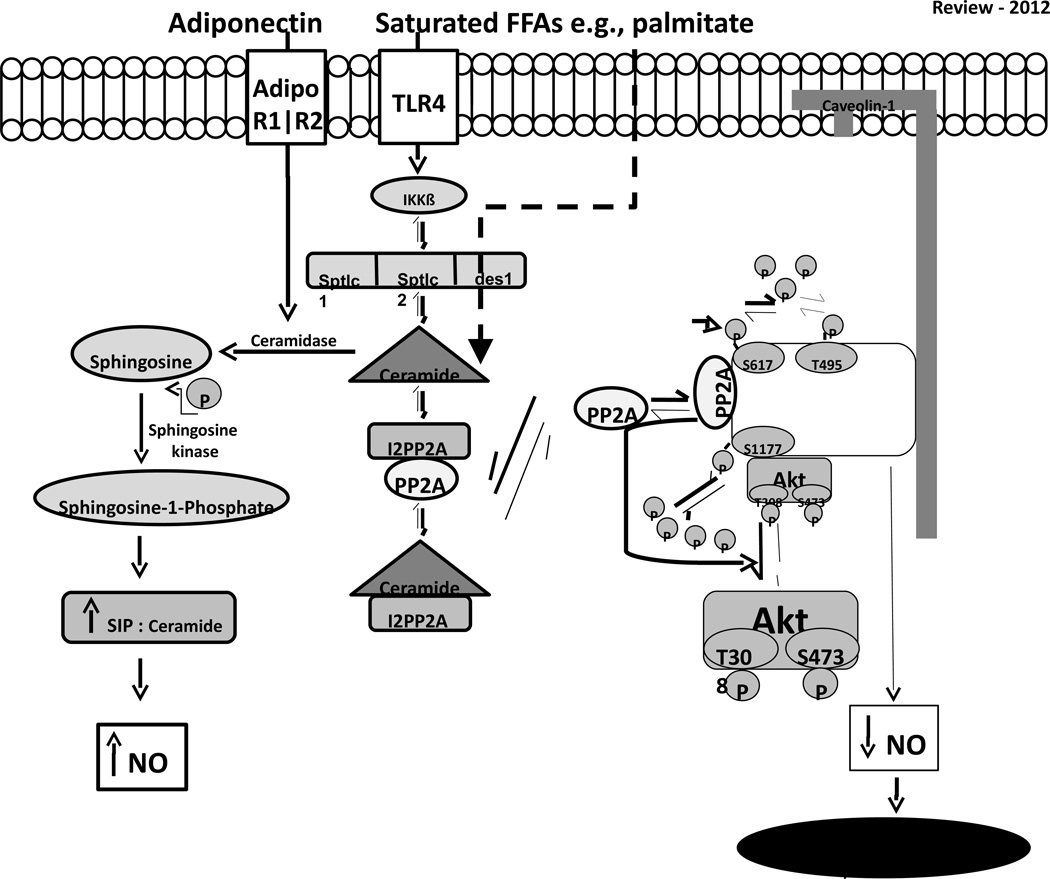
6. Ceramide promotes PP2A association with eNOS
After observing that palmitate and fat-feeding increase endogenous ceramide biosynthesis in endothelial cells and arteries, we used BAECs to elucidate the molecular mechanisms that might be responsible for ceramide-mediated impairment of eNOS phosphorylation, NO production, and arterial vasorelaxation. Palmitate-induced ceramide accumulation induced co-localization of the protein phosphatase 2A (PP2A) with eNOS, prevented eNOS from associating with Akt and Hsp90, decreased the phosphorylation of the pool of Akt that associates directly with eNOS, and impaired full eNOS phosphorylation (Fig). Congruent with these findings in endothelial cells, the association between PP2A and eNOS was increased in arteries from fat-fed mice in a ceramide-dependent manner. Ceramide might initiate PP2A association with eNOS by disrupting the interaction between inhibitor 2 of PP2A (I2PP2A) and PP2A. Rationale for this was provided by results from Mukhopadhyay et al.[65] These authors observed that I2PP2A (but not I1PP2A) is a major ceramide-binding protein in A549 human lung cancer cells. When ceramide binds to I2PP2A, the inhibition of I2PP2A on PP2A is relieved. In BAECs we observed that palmitate-induced ceramide accumulation decreases the association between I2PP2A and PP2A, and translocates PP2A from the cytosol to the membrane where it associates with eNOS. The presence of PP2A in the Akt/Hsp90/eNOS complex impairs Akt phosphorylation (likely by direct dephosphorylation) and decreases eNOS phosphorylation by a similar mechanism, or indirectly as a consequence of reduced Akt activation.[17] While these findings provide initial support for this potential mechanism of action in the context of our experimental conditions, results from ongoing studies using different approaches are required. Collectively, these results contribute to accumulating evidence that ceramide plays a significant role in the pathogenesis of vascular dysfunction, and extend important observations by Wu et al. [44] regarding how ceramide might modulate endothelial cell function.
Figure.
Saturated free fatty acids (FFAs) such as palmitate not only represent the substrate for de novo ceramide biosynthesis, but also signal via the toll-like receptor 4 (TLR4) to activate inhibitor of kB-kinase (IKKβ) which stimulates transcript levels of enzymes involved in ceramide biosynthesis e.g., serine palmitoyl transferase long chain base 1 (Sptlc1) and 2 (Sptlc2) and dihydroceramide desaturase (des1). The dashed line indicates that ceramide disrupts the association between inhibitor 2 of protein phosphatase 2A (I2PP2A) and protein phosphatase 2A (PP2A) by binding to I2PP2A. PP2A translocates to the cell membrane and associates with endothelial nitric oxide synthase (eNOS). PP2A association with eNOS decreases the association between eNOS and protein kinase B (Akt) and between eNOS and heat shock protein 90 (Hsp90). PP2A promotes the dephosphorylation of Akt that colocalizes with eNOS and /or decreases eNOS phosphorylation at serine (S) 1177 and S617 directly. This impairs NO bioavailability and leads to vascular dysfunction. Adiponectin might modify the effects of ceramide accumulation by binding to adiponectin (Adipo) R1 and Adipo R2 receptors and increasing ceramidase activity. Ceramidases hydrolyze ceramide to form sphingosine leading to an increase sphingosine-1-phosphate (SIP) to ceramide ratio. Evidence exists that signaling via this pathway increases NO production. Neither this pathway, nor the potential metabolism of ceramide via adiponectin, have been evaluated in vivo in the context of obesity and endothelial dysfunction. P; phosphorylation; T; threonine.
7. Cross-talk between adiponectin and ceramide
In addition to its insulin-sensitizing, antiapoptotic, and anti-inflammatory functions, recent findings from Holland et al. describe a novel mechanism whereby adiponectin regulates sphingolipid metabolism. This adiponectin / sphingolipid link has significant potential to influence arterial function (Fig). Once formed, ceramide can be hydrolyzed by ceramidases to produce sphingosine. Sphingosine kinase can then phosphorylate sphingosine to sphingosine-1-phosphate (SIP). In various cell systems ceramide and sphingosine are described as pro-apoptotic whereas SIP is generally regarded as pro-survival. This led to the hypothesis that the ceramide to SIP ratio might be an important determinant of cell survival.[66] Holland et al. showed that lard-oil infusion and / or fat feeding increases hepatic ceramide content in mice and this response can be: (i) normalized by administering recombinant adiponectin; (ii) prevented in mice that transgenically overexpress adiponectin; and (iii) exaggerated in adiponectin null mice [67]. Adiponectin exerts its effects by binding to two receptors, AdipoR1 and AdipoR2., which belong to the progesterone and adiponectin Q receptor (PAQR) family.[68] Because some PAQR receptors enhance ceramidase activity, Holland et al. tested whether AdipoR1 and AdipoR2 might mediate the ability of adiponectin to lower ceramide. Adiponectin increased ceramidase activity in mouse embryonic fibroblasts (MEFs) with intact adiponectin receptors but failed to do so in MEFs in which both isoforms were deleted. Consistent with lower ceramidase activity in AdipoR1 and AdipoR2 deficient MEFs, there was diminished accumulation of SIP such that the ceramide to SIP ratio was 5-fold greater relative to wild type cells. Disruption of the ceramide to SIP balance in knockout MEFs increased their susceptibility to palmitate-induced cell death, and the deleterious effects of palmitate could be reversed when AdipoR1/2 deficient MEFs were treated with SIP. The ratio of S1P to ceramide might also be relevant to endothelial cells. For example, it has been shown in endothelial cells that SIP signals via the endothelial differentiation gene-1 receptor to the heterotrimeric G protein Gi, leading to activation of Akt and eNOS, which increases NO production.[69] Thus it is possible that the balance of SIP and ceramide may influence endothelial function. While adiponectin delivery to mice with T2DM can improve arterial vasorelaxation by increasing NO bioavailability[70], it remains to be determined whether the mechanism is dependent upon generation of SIP from ceramide in the vasculature. Studies investigating this pathway in physiologically relevant scenarios wherein adiponectin is increased (exercise training) or decreased (T2DM) would also be of great interest.
8. Mitochondria
Mitochondria regulate cell survival and ion homeostasis. Endothelial mitochondria are responsible for generating reactive oxygen species and maintaining the cytosolic Ca2+ concentration. Evidence is accumulating that mitochondrial morphological and functional changes may correlate with vascular endothelial cell dysfunction in the context of diabetes.[71;72] Enhanced mitochondrial fission and / or attenuated fusion leads to mitochondrial fragmentation that may precipitate endothelial dysfunction. Putative mechanisms include increased production of reactive oxygen species and Ca2+ overload, which may impair endothelial function via multiple mechanisms as reviewed above or by reducing endothelial cell survival. The mechanisms that impair mitochondrial dynamics in the context of diabetes are incompletely understood but could be a consequence of lipotoxicity. For example, exposure to palmitate, but not hyperglycemia or hyperinsulinemia, induced mitochondrial fragmentation in differentiated C2C12 muscle cells that was associated with increased reactive oxygen species production.[73] Similar changes were observed in skeletal muscle of obese mice. In vitro studies have demonstrated loss of mitochondrial networks when endothelial cells are cultured under hyperglycemic conditions.[72] Increased mitochondrial fragmentation and expression of fission-1 protein were also observed in endothelial cells isolated from patients with T2DM. Additionally, mitochondrial reactive oxygen species generation was greater, and agonist-stimulated NO production was suppressed in these endothelial cells. These changes were correlated with reduced capacity for flow-mediated dilation in the diabetic group. Although these observations in humans together with in vitro results in glucose-exposed endothelial cells, suggest a role for hyperglycemia in mediating endothelial cell mitochondrial fission, a direct contribution of lipid-mediated mechanisms cannot be ruled out.
9. Summary
Endothelial dysfunction is a well-established characteristic of insulin resistance and obesity. Multiple synergistic mechanisms impair vascular function in this prevalent condition. Recent findings provide strong evidence that increased circulating lipids may impair vascular function in vivo by disrupting insulin signaling, increasing inflammation, and promoting ceramide accumulation. It is likely that these pathophysiological mechanisms rather than acting independently interact to promote vascular dysfunction. Several emerging mechanisms warrant further study. These include the relative balance between vasculotoxic mechanisms involving ceramide and vasculoprotective mechanisms involving adiponectin in regulating endothelial function; direct examination of the ability of lipids to increase mitochondrial fission or decrease mitochondrial fusion in endothelial cells; and the mechanisms by which loss of mitochondrial networks in the context of lipotoxicity may lead to endothelium-dependent dysfunction.
Acknowledgements
JDS is supported by a National Institutes of Health (NIH) grant 2R15HL091493, American Diabetes Association (ADA) Research Grant 1-12-BS-208, ADA 7-08-RA-164, and the University of Utah College of Health and School of Medicine, EDA is supported by NIH grants R01 DK092065, R01HL108379, U01 HL087947, and is an established investigator of the AHA.
References
- 1.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triggle CR, Ding H. A review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J Am Soc Hypertens. 2010;4:102–115. doi: 10.1016/j.jash.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaul PW. Regulation of endothelial nitric oxide: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 5.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 6.Ding H, Triggle CR. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflugers Arch. 2010;459:977–994. doi: 10.1007/s00424-010-0807-3. [DOI] [PubMed] [Google Scholar]
- 7.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Montagnani M, Koh K, Quon M. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms 9. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 10.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeno Y, Li Q, Park K, Rask-Madsen C, Gao B, Matsumoto M, Liu Y, Wu IH, White MF, Feener EP, King GL. Inhibition of insulin signaling in endothelial cells by protein kinase C-induced phosphorylation of p85 subunit of phosphatidylinositol 3-kinase (PI3K) J Biol Chem. 2012;287:4518–4530. doi: 10.1074/jbc.M111.286591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 14.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mugabo Y, Mukaneza Y, Renier G. Palmitate induces C-reactive protein expression in human aortic endothelial cells. Relevance to fatty acid-induced endothelial dysfunction. Metabolism. 2011;60:640–648. doi: 10.1016/j.metabol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Tang Y, Li G. Chronic exposure to high fatty acids impedes receptor agonist-induced nitric oxide production and increments of cytosolic Ca2+ levels in endothelial cells. J Mol Endocrinol. 2011;47:315–326. doi: 10.1530/JME-11-0082. [DOI] [PubMed] [Google Scholar]
- 17.Zhang QJ, Holland W, Wilson L, Tanner J, Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B, Gale D, Kowalski CA, Deeter N, Nichols A, Deesing M, Arrant C, Ruan T, Boehme C, McCamey DR, Rou J, Ambal K, Narra KK, Summers SA, Abel ED, Symons JD. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61:1848–1859. doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edirisinghe I, McCormick Hallam K, Kappagoda CT. Effect of fatty acids on endothelium-dependent relaxation in the rabbit aorta. Clin Sci. 2006;111:145–151. doi: 10.1042/CS20060001. [DOI] [PubMed] [Google Scholar]
- 19.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 20.Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231–1238. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 24.Knopp RH, Retzlaff B, Walden C, Fish B, Buck B, McCann B. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects. Proc Soc Exp Biol Med. 2000;225:191–199. doi: 10.1046/j.1525-1373.2000.22524.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 26.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 27.Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 28.Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase c in diabetes and insulin resistance. Arterioscler Thromb. 2005;25:487–496. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- 29.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 30.Abe H, Yamada N, Kamata K, Kuwaki T, Shimada M, Osuga J, Shionoiri F, Yahagi N, Kadowaki T, Tamemoto H, Ishibashi S, Yazaki Y, Makuuchi M. Hypertension, hypertriglyceridemia, and impaired endothelium-dependent vascular relaxation in mice lacking insulin receptor substrate-1. J Clin Invest. 1998;101:1784–1788. doi: 10.1172/JCI1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Wheatcroft SB, Shah AM, Li JM, Duncan E, Noronha BT, Crossey PA, Kearney MT. Preserved glucoregulation but attenuation of the vascular actions of insulin in mice heterozygous for knockout of the insulin receptor. Diabetes. 2004;53:2645–2652. doi: 10.2337/diabetes.53.10.2645. [DOI] [PubMed] [Google Scholar]
- 33.Duncan ER, Walker SJ, Ezzat VA, Wheatcroft SB, Li JM, Shah AM, Kearney MT. Accelerated endothelial dysfunction in mild prediabetic insulin resistance: the early role of reactive oxygen species. Am J Physiol Endocrinol Metab. 2007;293:E1311–E1319. doi: 10.1152/ajpendo.00299.2007. [DOI] [PubMed] [Google Scholar]
- 34.Kahn MB, Yuldasheva NY, Cubbon RM, Smith J, Rashid ST, Viswambharan H, Imrie H, Abbas A, Rajwani A, Aziz A, Baliga V, Sukumar P, Gage M, Kearney MT, Wheatcroft SB. Insulin resistance impairs circulating angiogenic progenitor cell function and delays endothelial regeneration. Diabetes. 2011;60:1295–1303. doi: 10.2337/db10-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto H, Nakae J, Kitamura T, Park BC, Dragatsis I, Accili D. Transgenic rescue of insulin receptor-deficient mice. J Clin Invest. 2004;114:214–223. doi: 10.1172/JCI21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbas A, Imrie H, Viswambharan H, Sukumar P, Rajwani A, Cubbon RM, Gage M, Smith J, Galloway S, Yuldeshava N, Kahn M, Xuan S, Grant PJ, Channon KM, Beech DJ, Wheatcroft SB, Kearney MT. The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes. 2011;60:2169–2178. doi: 10.2337/db11-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high fat diet fed C57BL/6J mice. Circ Res. 2005;96:1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- 39.Rizzo NO, Maloney E, Pham M, Luttrell I, Wessell H, Tateya S, Daum G, Handa P, Schwartz MW, Kim F. Reduced NO-cGMP Signaling Contributes to Vascular Inflammation and Insulin Resistance Induced by High-Fat Feeding. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.109.199893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watt MJ, Hoy AJ, Muoio DM, Coleman RA. Distinct roles of specific fatty acids in cellular processes: implications for interpreting and reporting experiments. Am J Physiol Endocrinol Metab. 2012;302:E1–E3. doi: 10.1152/ajpendo.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol. 2010;21:128–135. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- 42.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chun L, Junlin Z, Aimin W, Niansheng L, Benmei C, Minxiang L. Inhibition of ceramide synthesis reverses endothelial dysfunction and atherosclerosis in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 2011 doi: 10.1016/j.diabres.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- 45.Chavez JA, Summers SA. Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim Biophys Acta. 2010;1801:252–265. doi: 10.1016/j.bbalip.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol. 2009;587:3911–3920. doi: 10.1113/jphysiol.2009.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang DX, Zou AP, Li PL. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am J Physiol Heart Circ Physiol. 2003;284:H605–H612. doi: 10.1152/ajpheart.00697.2002. [DOI] [PubMed] [Google Scholar]
- 48.Zheng T, Li W, Wang J, Altura BT, Altura BM. Sphingomyelinase and ceramide analogs induce contraction and rises in [Ca(2+)](i) in canine cerebral vascular muscle. Am J Physiol Heart Circ Physiol. 2000;278:H1421–H1428. doi: 10.1152/ajpheart.2000.278.5.H1421. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, Förstermann U. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circ. 2002;106:2250–2256. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- 50.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM, Lopaschuk GD. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole body oxygen consumption. Diabetes. 2010;59:2453–2464. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah C, Yang G, Lee I, Bielawski J, Hannun YA, Samad F. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem. 2008;283:13538–13548. doi: 10.1074/jbc.M709950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bikman BT, Guan Y, Shui G, Siddique MM, Holland WL, Kim JY, Fabrias G, Wenk MR, Summers SA. Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis. J Biol Chem. 2012 doi: 10.1074/jbc.M112.359950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, Kindt EK, Homan R, Karathanasis SK, Rekhter MD. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2004;110:3465–3471. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz EA, Reaven PD. Molecular and signaling mechanisms of atherosclerosis in insulin resistance. Endocrinol Metab Clin North Am. 2006;35:525–49. viii. doi: 10.1016/j.ecl.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz EA, Zhang WY, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol. 2010;30:802–808. doi: 10.1161/ATVBAHA.109.201681. [DOI] [PubMed] [Google Scholar]
- 58.Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, Handa P, Schwartz MW, Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol. 2009;29:1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, Jo H. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation. 2006;113:2818–2825. doi: 10.1161/CIRCULATIONAHA.106.611822. [DOI] [PubMed] [Google Scholar]
- 60.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fonseca FV, Ravi K, Wiseman D, Tummala M, Harmon C, Ryzhov V, Fineman JR, Black SM. Mass spectroscopy and molecular modeling predict endothelial nitric oxide synthase dimer collapse by hydrogen peroxide through zinc tetrathiolate metal-binding site disruption. DNA Cell Biol. 2010;29:149–160. doi: 10.1089/dna.2009.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29:1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 65.Mukhopadhyay A, Saddoughi SA, Song P, Sultan I, Ponnusamy S, Senkal CE, Snook CF, Arnold HK, Sears RC, Hannun YA, Ogretmen B. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J. 2009;23:751–763. doi: 10.1096/fj.08-120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takabe K, Paugh SW, Milstien S, Spiegel S. "Inside-out" signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptormediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holland WL, Scherer PE. PAQRs: a counteracting force to ceramides? Mol Pharmacol. 2009;75:740–743. doi: 10.1124/mol.109.054817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 70.Lee S, Zhang H, Chen J, Dellsperger KC, Hill MA, Zhang C. Adiponectin abates diabetes-induced endothelial dysfunction by suppressing oxidative stress, adhesion molecules, and inflammation in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2012;303:H106–H115. doi: 10.1152/ajpheart.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pangare M, Makino A. Mitochondrial function in vascular endothelial cell in diabetes. J Smooth Muscle Res. 2012;48:1–26. doi: 10.1540/jsmr.48.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32:309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]