Abstract
Objective
The goal of this study was to determine if biomarkers of collagen metabolism in pulmonary arterial hypertension (PAH) identify patients with worse disease and higher risk of death.
Background
The relationship between markers of collagen metabolism, degree of disease, and outcome in PAH is unknown.
Methods
Stable idiopathic, anorexigen-associated and hereditary PAH patients were prospectively enrolled. Collagen biomarkers levels were measured: N-terminal propeptide of type III procollagen (PIIINP), C-terminal telopeptide of collagen type I (CITP), matrix metalloproteinsase 9 (MMP-9) and tissue inhibitor of metalloproteinase 1 (TIMP-1). Patients were divided into mild, moderate, and severe PAH groups. Data was compared between tertiles of each biomarker. Pearson correlation and Spearman rank coefficient analyses were performed. Data on time to death or transplantation was examined by Kaplan-Meier survival curves.
Results
Circulating levels of PIIINP, CITP, MMP9 and TIMP1 were higher in the PAH group (N=68) as compared to age- and gender-matched healthy controls (N=37) (p<0.001 for each). PIIINP levels increased with the severity of disease (p=0.004). PIIINP tertile data indicated that with increasing levels, six-minute walk distance (6MWD) and cardiac index (CI) decreased and WHO FC worsened, and resting heart rate increased. A significant correlation existed between PIIINP with worsening WHO FC (rs=0.319, p=0.008) and a negative correlation with CI and 6MWD (r=-0.304 and -0.361 respectively; p<0.05). PIIINP tertiles showed a trend towards worse outcome in patients with higher tertile (lung transplant or death) (p=0.07, log rank test).
Conclusions
Markers of collagen metabolism were associated with worse disease in PAH patients.
Keywords: vascular remodeling, heart failure, PIIINP, BNP
Introduction
Pulmonary arterial hypertension (PAH) is a terminal disease characterized by pulmonary vascular remodeling resulting in right heart failure and death. Vascular remodeling and fibrosis are among the key pathological features in PAH. One of the main features of vascular remodeling seen in PAH is collagen deposition in the remodeled pulmonary vessels. The best way to quantify collagen deposition in the pulmonary vasculature is by tissues analysis at autopsy or of ex-planted lungs. Antemortem assessment of collagen in the pulmonary vasculature is not possible with current imaging nor is lung biopsy considered safe. Type I and III collagen, the most abundant forms of collagen in the human lungs, provide the architectural support for the alveolar walls, vessels, visceral pleura and the tracheobronchial tree and are primarily synthesized and secreted by lung fibroblasts as procollagen precursor molecules with propeptides at both ends (1). The N-terminal propeptide of type III procollagen (PIIINP) is used as a biological marker of collagen metabolism as it is not completely removed from its procollagen precursor (2). Carboxy-terminal telopeptide of type I collagen (CITP) is a marker of extracellular collagen I degradation. Historically, matrix metalloproteinase 9 (MMP9), a gelatinase that degrades most fibrillar collagen, is considered a marker of extracellular matrix breakdown. However, recent data suggests that MMP-9 may play an important role in the inflammatory response and control of angiogenesis (3-6). Tissue inhibitor of metalloproteinase 1 (TIMP-1) is a ubiquitous inhibitor of all MMPs.
Collagen production and smooth muscle cell proliferation occurs in small pulmonary arteries of patients with severe PAH (7,8). Studies show that the transpulmonary gradient of procollagen III occur in normal subjects undergoing cardiac catheterization suggesting that normal human lungs can actively synthesize collagen (9). It has been established that elevated procollagen III levels in the serum mirror changes in bronchoalveolar lavage of patients with sarcoidosis (10), interstitial pulmonary fibrosis (11), pneumocystis carinii pneumonia (12), acute lung injury (13), and acute respiratory distress syndrome (14,15) indicating that such parenchymal changes are reflected in peripheral blood samples. These studies suggest that ongoing collagen metabolism in the pulmonary vascular can be assessed by measuring circulating levels of collagen metabolites. Accordingly, the objectives of this study were to investigate the relationship between circulating markers of collagen metabolism, degree of disease severity, and outcome in a well characterized PAH cohort.
Methods
Subjects
After obtaining institutional IRB approval and written informed consent, consecutive PAH subjects and age- and gender-matched healthy controls that met inclusion/exclusion criteria were prospectively enrolled in a cross-sectional observational study. PAH subjects were enrolled from the new and established patient population followed at the Pulmonary Hypertension Clinic at Baylor College of Medicine. The diagnosis of PAH was established by the presence of mean pulmonary artery pressure ≥ 25 mm Hg and pulmonary capillary wedge pressure ≤ 15 mm Hg. The mean time between the right heart catheterization and study enrollment was 1.3±1.6 years. Inclusion criteria for PAH patients are were: age of 18 years or older; ability to give written informed consent; stable PAH therapy for 1 month prior to enrollment. Detailed inclusion/exclusion criteria for PAH and controls is outlined in appendix A. PAH patients were followed for up to 61 months (2.8±1.4 years; range 1.2 to 5.1 years) after enrollment, and data on transplant free survival, transplantation, and death was collected. Histologically, the available lungs and heart tissue of 2 PAH patients that underwent transplantation were examined.
Hemoxylin and eosin stain was used to outline the pulmonary vascular remodeling and trichome stain to document collagen staining. To define PAH severity, patients were divided into mild, moderate and severe PAH groups. Mild PAH group was defined by a six-minute walk distance (6MWD) of >440 meters, WHO FC I-II, and RAP ≤10 mmHg. Severe PAH group was defined by a 6MWD of ≤350 meters, WHO FC III-IV, and RAP ≥15 mmHg. Patients in the moderate PAH group fell between the criteria for mild and severe PAH (16).
Biochemical Measurements of Indices of Collagen Metabolism
One time blood was drawn from a peripheral vein for biomarker measurements, transferred immediately into a glass tube, and allowed to clot. Serum was separated from the blood cells by centrifugation at 3000 rpm for 10 minutes; supernatant was collected and immediately stored at -80 °C until simultaneous analysis. Blood was also collected in EDTA collection tubes, centrifuged, and plasma was stored at -20°C for analysis. Enzyme-linked immunosorbent assays (ELISAs) were used to determine serum carboxy-terminal telopeptide of collagen type I levels (CITP EIA). Amino-terminal propeptide of procollagen type III levels were measured using double antibody radioimmunoassays (PIIINP RIA) according to manufacturer's specifications. Serum MMP-9 and TIMP-1 levels were measured using 2-site sandwich ELISAs per manufacturer's protocol. Commercially available immunoassay was used for quantitative brain natriuretic peptide (BNP) determination on an ADVIA Centaur analyzer system according to the manufacturer's recommendation. Detailed methodology for biomarker measurements and ECHO is included in appendix A.
Doppler Echocardiography
All ECHOs were performed by registered diagnostic cardiac sonographers and interpreted by a certified cardiologist. Mean time between ECHO and study enrollment was 2±9 months. A complete ECHO was performed with two-dimensional, spectral Doppler and Doppler tissue imaging (DTI) modes. Tissue Doppler imaging (TDI) of the right ventricular (RV) lateral annulus obtained from the apical four chamber view was used to calculate the peak systolic excursion of the RV annulus (S′) and the calculation of the myocardial performance index (MPI) of the RV as described by Rudski et al (17). Notch ratio was calculated using a PW signal in the RV outflow tract (RVOT) (18). Peak pulmonary artery systolic pressure (PASP) was calculated based of peak tricuspid regurgitation velocity using the formula PASP= 4 (Peak velocity of TR)2 + Estimated Right Atrial Pressure (RAP).
Statistical Analysis
Baseline characteristics are presented as mean ± standard deviation for continuous variables and as percentages for categorical variables. Comparisons between the control and PAH groups was performed by using Student's t test for most continuous variables except for skewed variables when Wilcoxon Rank sum test was used. Fisher exact test or χ2 test was used for comparing categorical variables. Collagen biomarker levels are presented in box-and-whisker plot where boxes represent the 25th and 75th percentiles, the horizontal line is the median, and bars indicate the lowest 5th and highest 95th percentiles. Clinical parameters and patient baseline characteristics were compared by tertiles of each biomarker and by disease severity using χ2 test for categorical variables and Analysis of Variance (ANOVA) for continuous variables. Each biomarker was also compared by disease etiology using ANOVA.
Further analysis between biomarker levels, and hemodynamic and clinical variables was performed using Pearson correlation coefficient analysis whereas Spearman rank correlation coefficient analysis was used for categorical variables. Survival analysis: Time to death or lung or heart/lung transplantation was estimated using the Kaplan-Meier method with log-rank analysis used to compare differences between biomarker tertiles and between disease severity. All analyses were two sided and significance was judged as p value < 0.05.
Results
Baseline Patient Characteristics
A total of 105 subjects were prospectively enrolled in the study. Of these, 68 were idiopathic PAH, anorexigen associated and hereditary PAH subjects and 37 were healthy controls (Table 1). The mean duration of disease was 2.6 ±2.8 years for PAH subjects. As expected, BNP levels, 6MWD, BDS, WHO FC, and maximum heart rate and minimum oxygen saturation (during the walk test) all showed significant differences between the healthy controls and PAH group (p<0.001). Most notably, circulating levels of PIIINP, CITP, MMP9 and TIMP1 were significantly elevated in the PAH group as compared to controls (p<0.001 for each; Figure 1).
Table 1. Baseline Characteristics of Healthy Controls and PAH Patients.
| Variable | Controls | iPAH, Anx, Hereditary |
P value |
|---|---|---|---|
| N | 37 | 68 | |
| Age (yrs) | 49±14 | 45±15 | 0.175 |
| Gender (F, %) | 89 | 91 | 0.751 |
| Duration of disease (yrs) | - | 2.6±2.8 | |
| Heart rate (beats/min) | 76±10 | 79±16 | 0.278 |
| JVD present (N) | 1 | 17 | <0.001 |
| Edema (N) | 1 | 24 | <0.001 |
| BSA (m2) | 1.83±0.22 | 1.84±0.24 | 0.333 |
| Race (%) | |||
| Caucasian | 23 | 37 | 0.153 |
| Hispanic | 6 | 17 | |
| African American | 2 | 11 | |
| Asian | 4 | 3 | |
| WHO FC (%) | <0.001 | ||
| I-II | 100 | 53 | |
| III-IV | 0 | 47 | |
| Six Minute Walk Test | |||
| Distance (meters) | 469±63 | 414±104 | 0.001 |
| Borg dyspnea score | 0.21±0.31 | 2.59±1.94 | <0.001 |
| Max HR (b/min) | 107±14 | 126±20 | <0.001 |
| Minimum oxygen saturation (%) | 93±5 | 85±9 | <0.001 |
| Laboratory values | |||
| GFR (mL/min/1.73 m2) | 81±19 | 78±19 | 0.407 |
| Platelets (K/μL) | 278±63 | 243±97 | 0.032 |
| BNP (pg/dL) | 20±19 | 130±197 | <0.001 |
| Echocardiogram | |||
| RAP (mm Hg) | 11±5 | ||
| RVSP (mm Hg) | 83±25 | ||
| LVEF (%) | 62±4 | ||
| CI (L/min/m2) | 2.35±0.62 | ||
| Pericardial effusion (N) | 16 | ||
| S′(cm/s)* | 10.68±3.11 | ||
| Notch ratio | 0.95±0.24 | ||
| MPI (Tei index) * | 0.51±0.19 | ||
| PAH Medications: (N) | |||
| PG / ETRA / PDE5-I | 2/14/2010 | ||
| PG & PDE5-I | 2 | ||
| PG & ETRA | 4 | ||
| ETRA & PDE5-I | 17 | ||
| PG & ETRA & PDE5-I | 14 | ||
| Naïve/CCB responder | 2 //1 | ||
| No PAH meds: Study medication | 2 | ||
| Diuretics: (N) | |||
| Aldosterone blocker | 29 | ||
| Other diuretic | 36 | ||
| Other PAH related Meds: (N) | |||
| Anti-coagulation | 31 | ||
| CCB | 15 | ||
| Cardiac glycoside | 31 | ||
| ACE inhibitor/ARBs | 10 | ||
| Beta blocker | 11 |
Data represented as mean±SD for continuous variables and percentages for categorical variables. P values < 0.05 are considered statistically significant. ACE = angiotensin-converting-enzyme; Anx = anorexigen associated PAH; ARB = angiotensin receptor blocker; CCB = calcium channel blocker; CI = cardiac index; CO = cardiac output; ETRA = endothelin receptor antagonist; GFR= glomerular filtration rate; LVEF = left ventricular ejection fraction; ND = not done; NS = not significant; N = number of patients; PG = prostaglandin; PDE5-I = phosphodiesterase-5 inhibitor; RAP = right atrial pressure; RVSP = right ventricular systolic pressure; S′=RV systolic excursion velocity (normal value >11); WHO FC = World Health Organization functional classification. MPI = myocardial performance index (normal value <0.55).
Guidelines for the echocadiographic assessment of the right heart in adults: a report from the American Society of Cardiology (J Am Soc Echocardiogr 2010;23:685-713).
Figure 1. Circulating Collagen Biomarkers in PAH Patients and Healthy Controls.
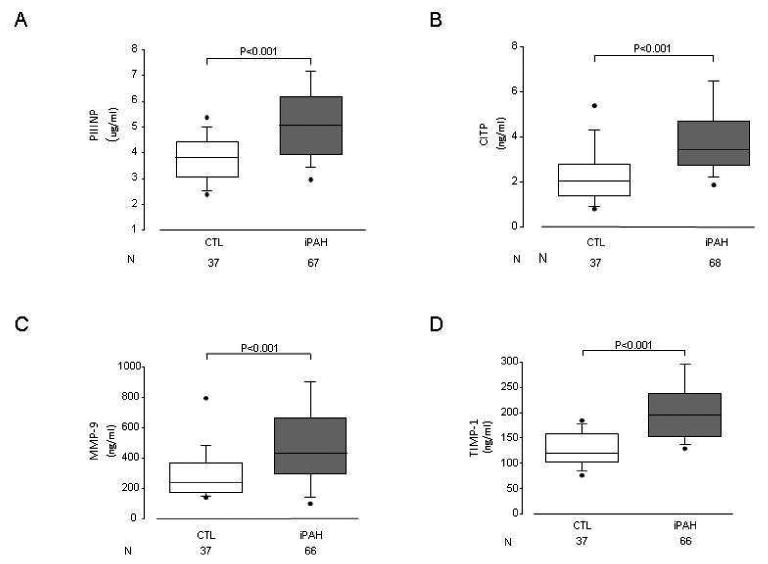
Levels of PIIINP (A), CITP (B), MMP-9 (C), and TIMP-1 (D) were elevated in PAH patients as compared to healthy controls (p <0.001 for all). Data presented as median with 5th and 95th percentiles.
Biomarker levels According to Disease Severity
We explored the possibility that, since increasing disease severity is associated with progressive vascular remodeling, circulating levels of collagen deposition markers would be elevated in patients with worse disease. Consistent with this hypothesis, PIIINP levels increased with the severity of disease; they were lowest in patients with mild PAH and highest in patients with severe PAH (p=0.002; Figure 2A). Similarly, a trend towards higher CITP levels was also noted with increasing disease severity (p=0.07; Figure 2B) though this was not evident for MMP9 and TIMP-1 data (Figure 2C and 2D). As one would expect for a disease, which often terminates in heart failure, BNP levels followed a similar pattern, increasing with worsening disease (p=0.002). Just as with the BNP, RAP and RVSP increased with disease severity (p<0.01), and a trend towards lower S′ was noted with worsening disease (p=0.053). In addition, and consistent with prior reports, pericardial effusions occurred more frequently in PAH patients designated as severe disease (p=0.021). In our cohort, PAH patients in the severe group were less likely to be on anti-coagulants (p<0.001) and on CCBs (p=0.087). There were no other appreciable differences in the medication profiles of patients based on disease severity. Although the age was not different between the groups, patients in the severe group had shorter disease duration than in the mild or moderate groups (p=0.001).
Figure 2. Collagen Biomarker Levels in Patients with Mild, Moderate, and Severe PAH.
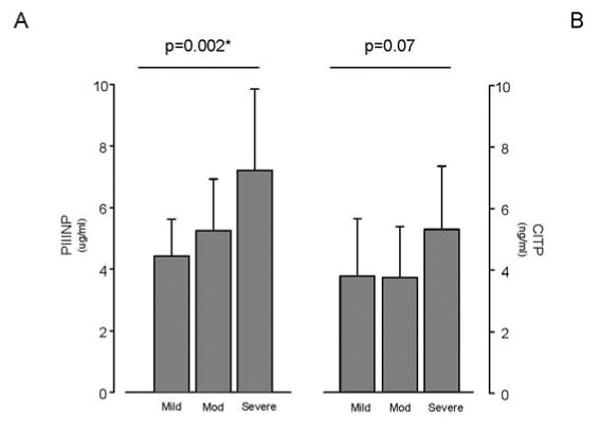
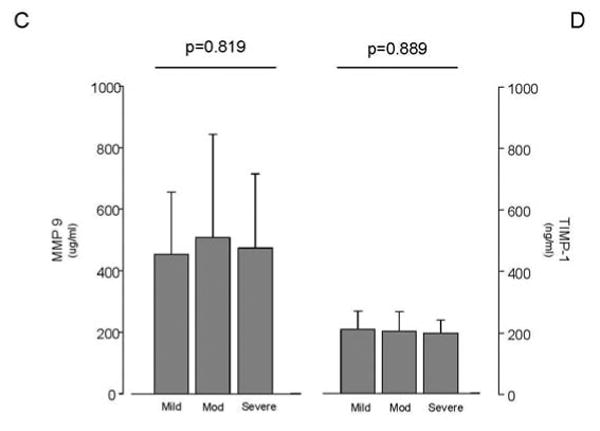
Patients with severe PAH had significantly elevated levels of PIIINP (p=0.002). Mild PAH was defined by a six-minute walk distance of ≥440 m, WHO FC I-II, and RAP ≤10 mmHg. Patients with moderate PAH were between the criteria for severe and mild PAH. Severe PAH was defined by a six-minute walk distance of ≤350 m, WHO FC III-IV, and RAP ≥15 mmHg.
Disease Severity and Biomarker Tertiles
Complimentary to the above findings, higher tertiles of serum PIIINP levels were accompanied by worsening 6MWD (p<0.001), elevated right atrial pressure (p=0.013), and worsening WHO functional class (p=0.024) (Table 2). Contrary to what one would expect, the patients in higher PIIINP tertiles were younger than those in lower tertiles (p<0.001). Similar, though less significant, results were obtained by dividing data into tertiles based on other biomarkers. For example, resting heart rate was significantly higher in the higher tertile groups of PIIINP, CITP and TIMP-1 (p<0.05 for each). The higher CITP tertile group had worse Borg dyspnea score (p=0.040). Also, trends towards higher occurrence rate of pericardial effusion (p=0.058) and worse WHO FC (0.087) were seen with higher CITP tertiles. Similar to PIIINP tertiles, maximum heart rate from the walk test was increased in the higher tertiles of MMP-9 (p=0.016).
Table 2. Healthy Control and PAH Patient data divided across PIIINP tertiles.
| Clinical Parameter | PIIINP Tertile | |||
|---|---|---|---|---|
| I | II | III | P value | |
| N | 28 | 30 | 29 | |
| Age (years) | 57±11 | 39±11 | 45±17 | <0.001 |
| Gender: (F, %) | 96 | 86 | 86 | 0.337 |
| Duration of disease (yrs) | 2.8± 2.4 | 3.4±2.9 | 2.1±1.8 | 0.425 |
| Heart rate (b/min) | 71±9 | 80±15 | 81±15 | 0.016 |
| JVD present (%) | 11 | 27 | 34 | 0.103 |
| Edema (%) | 32 | 30 | 38 | 0.801 |
| BSA (m2) | 1.76±0.28 | 1.87±0.26 | 1.82±0.18 | 0.361 |
| Race (Caucasian %) | 64 | 63 | 45 | 0.337 |
| WHO FC (%) | 0.024 | |||
| I-II | 61 | 63 | 31 | |
| III-IV | 39 | 37 | 69 | |
| Six Minute Walk Test | ||||
| Distance (meters) | 423 ± 84 | 465 ± 90 | 351 ± 106 | <0.001 |
| Borg dyspnea score | 2.35 ± 1.36 | 3.00 ± 2.18 | 2.73 ± 2.00 | 0.488 |
| Maximum Heart Rate (b/min) | 118 ± 20 | 133 ± 21 | 124 ± 18 | 0.040 |
| BNP (pg/dL) | 974± 193 | 138 ± 245 | 184 ± 183 | 0.345 |
| Echocardiogram | ||||
| RAP (mm Hg) | 7 ± 4 | 10 ± 5 | 11 ± 4 | 0.013 |
| RVSP (mm Hg) | 77 ± 26 | 82 ± 24 | 84 ± 25 | 0.597 |
| CI (L/min/m2) | 2.68 ± 0.64 | 2.52 ± 0.91 | 2.31 ± 0.71 | 0.310 |
| Pericardial effusion (%) | 18 | 10 | 31 | 0.121 |
| S′(cm/s) | 10.7 ± 1.9 | 11.0 ± 3.7 | 9.6 ± 2.7 | 0.303 |
| MPI (Tei index) | 0.50 ± 0.13 | 0.52 ± 0.25 | 0.55 ± 0.16 | 0.736 |
| Notch ratio (normal value <0.5) | 0.98 ± 0.17 | 0.88 ± 0.24 | 0.94 ± 0.24 | 0.556 |
Data represented as mean±SD. P-values < 0.05 considered statistically significant.
PIIINP (ng/ml) tertiles defined by the following values: I (0-4.06), II (4.07-5.65), and III (>5.65). MPI = myocardial performance index (normal value <0.55); NS = Not significant; S′=RV systolic excursion velocity (normal value >11);
Correlations with Collagen Biomarkers
We explored relationships between the collagen biomarkers and the hemodynamic and clinical indicators of PAH (Figure 3). Of note, a weak but significant correlation existed between PIIINP with worsening WHO FC (rs = 0.320, p<0.01), while a weak correlation was seen between CITP and Borg dyspnea score (r = -0.264; p=0.031). In addition, a negative correlation between PIIINP, and cardiac index and six-minute walk distance (r = -0.304 and -0.362 respectively; p<0.05) was noted. Moreover, PIIINP negatively correlated with age (r = -0.394; p<0.01), and duration of disease (r = -0.263; p=0.031), further reinforcing the results of the severity and tertile analyses. This data suggests that PIIINP could be linked to more aggressive and rapid disease progression, and severe disease. BNP was shown to have a weak, positive correlation to PIIINP (rs = 0.243, p=0.055).
Figure 3. Elevated PIIINP Levels Correlated with Clinical Parameters.
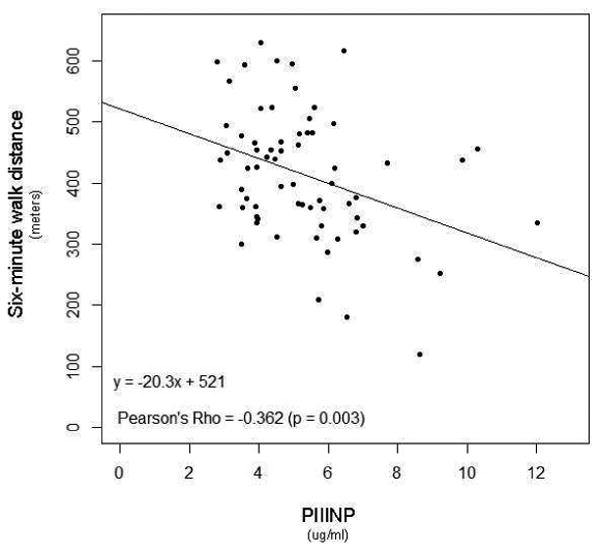
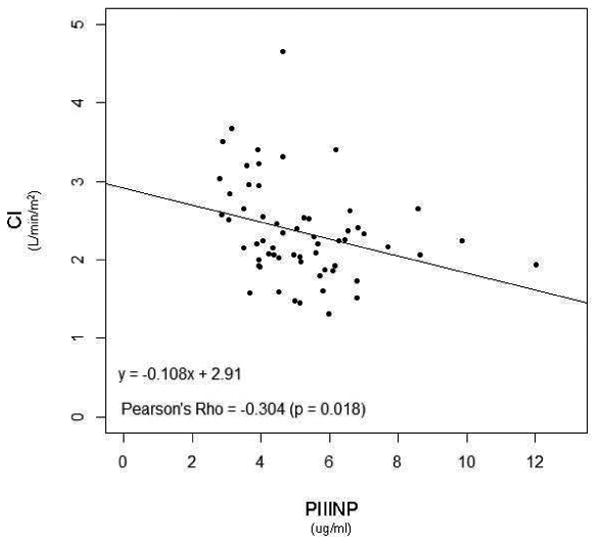
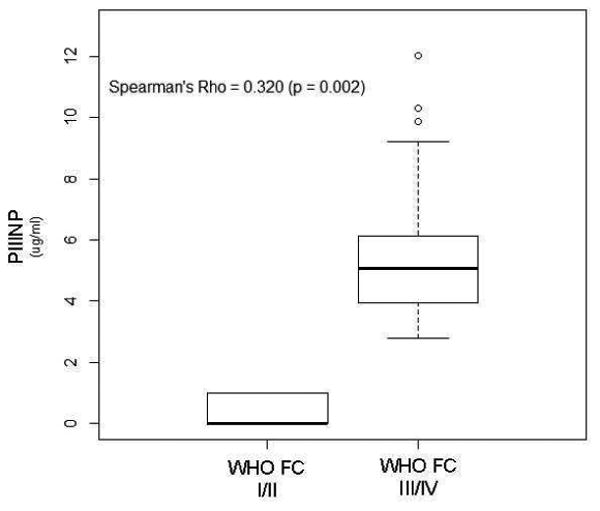
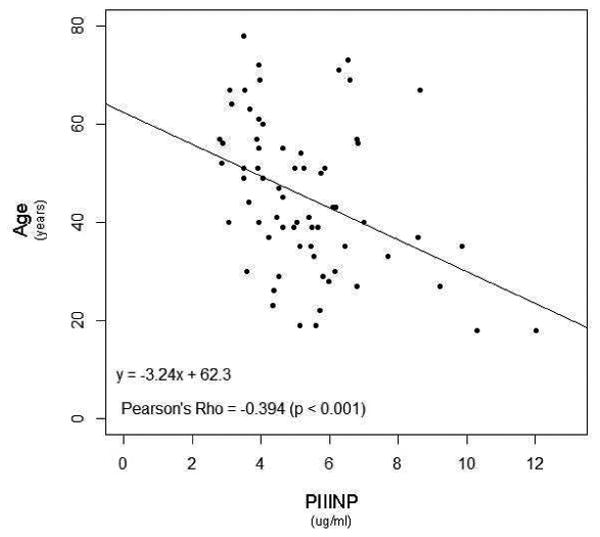
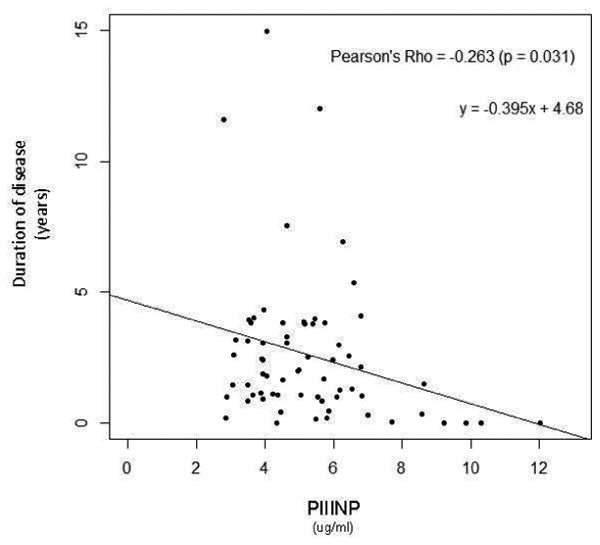
P-values <0.05 considered statistically significant.
Time to death or transplantation
A total of 6 deaths occurred and 7 patients underwent transplantations (5 double lung and 2 heart-double lung transplantations) over the 61-month period of investigation, representing 19% of the sample population. Review of histology slides from transplant recipients showed characteristic lesions of PAH and increased collagen deposition in both heart (in heart-lung transplant recipient) and lungs, as shown in new Figure 4. PIIINP tertiles showed a trend towards worse outcome in patients with higher tertile group (p=0.07, log-rank test, Figure 5) suggesting that increasing levels may be a marker of impending need for lung transplantation or for death. However, unlike those for PIIINP, Kaplan-Meier survival curves for CITP, MMP-9 and TIMP-1 tertiles were not significantly different between tertiles (Figure 5). As expected, the patient group with severe disease had worse outcomes (i.e., lung transplant or death) than the less severe groups (p=0.0009, log rank test).
Figure 4. Representative Histology of Pulmonary Artery (PA) and Right Ventricle (RV) Tissue.
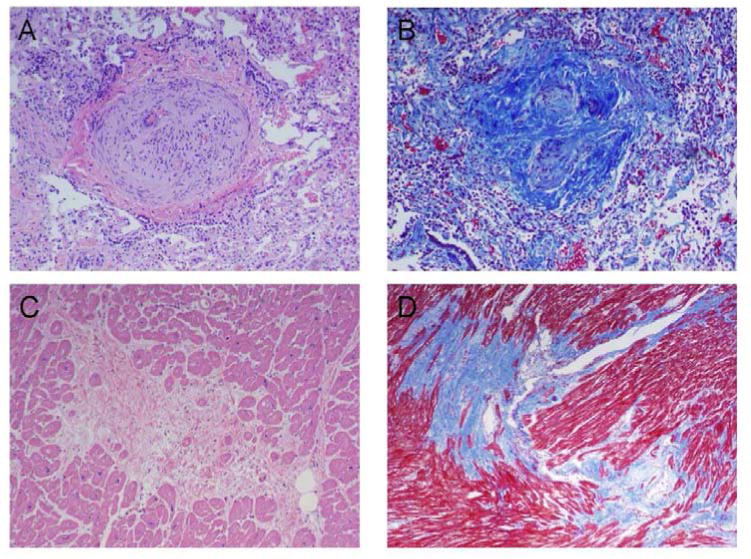
(A) Hematoxylin and eosin stain of PA tissue showing intimal and medial proliferation. (B) Trichrome stain of PA tissue showing dense collagen staining, indicating collagen deposition in the vessel walls. (C) Hematoxylin and eosin stain of RV tissue. (D) Trichrome stain of RV tissue showing dense collagen staining.
Figure 5. Kaplan Meier Survival curves for PIIINP, CITP, MMP9 and TIMP1 tertiles.
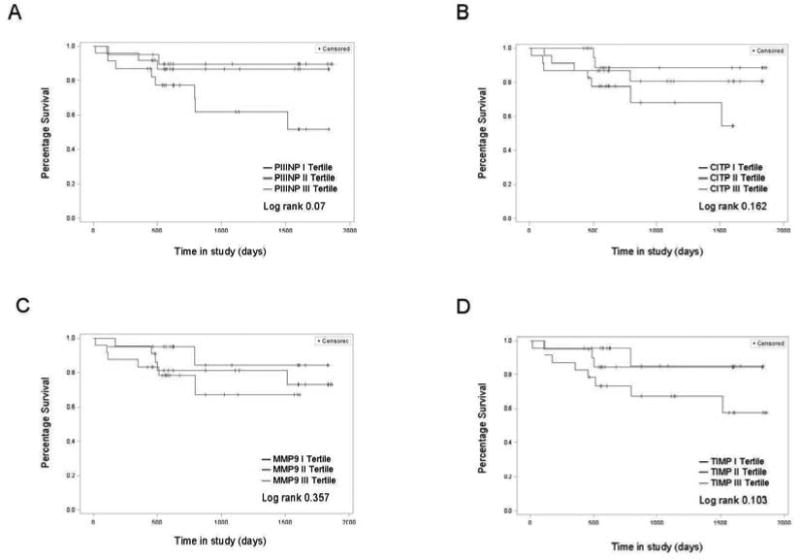
Trend towards higher death or transplantation (lung or heart/lung) with increasing PIIINP levels was noted. Log rank p value = 0.07.
Discussion
We report our novel findings that circulating levels of PIIINP, CITP, MMP-9 and TIMP were elevated in PAH patients as compared to age- and gender-matched healthy controls. In a small number of connective tissue disease associated (N=9) and congenital heart disease associated PAH patients (N=11), levels of PIIINP, CITP, MMP-9 and TIMP-1 were high and not significantly different from the iPAH, hereditary and anorexigen associated PAH group [data not shown]. These results suggest that the elevated levels were markers of disease state rather than markers of the etiology of PAH. In addition, our data suggests that PIIINP levels may be a good indicator of disease severity. Furthermore, circulating markers of new collagen formation, type 1 collagen degradation, elastase (MMP9) activity, and inhibition of matrix metalloproteinase by a ubiquitous inhibitor of MMP's (TIMP1) may be indicative of active vascular remodeling and reflect clinically relevant, peripherally measurable markers of disease and outcome in PAH.
Pulmonary arterial hypertension (PAH) causes significant morbidity and mortality which is commonly due to progressive right heart failure and death (19-21). Our results showed that PIIINP levels are elevated in PAH subjects compared to healthy controls; and these levels correlated with markers of disease severity such as worsening WHO FC, cardiac index and six-minute walk distance. It was interesting that, although PIIINP is shown to increase with age (22) elsewhere, PIIINP levels in our study were negatively correlated with age and duration of disease. We hypothesize that this is likely due to a role in increased collagen turnover due to more aggressive vascular remodeling in patients with more severe disease. This suggestion is supported by the result that PIIINP was higher in patients with worsening disease, and by the fact that PIIINP correlated positively with markers of worsening disease. These findings agree with those from a cohort of systemic hypertensive patients with LVH showing elevated levels of PIIINP and TIMP1 studied by Agrinier el al (23) and presence of excess serum PIIINP and TIMP1 levels were suggested as an indicator of cardiac fibrosis. In addition, Diez et al has explored the role of collagen metabolism in hypertensive subjects with heart failure, specifically the role of MMPs and TIMP-1 (24). True to Diez's findings, we observed increased levels of both MMP 9 and TIMP 1 in our PAH patients, suggesting that their vasculature are undergoing active and possibly excessive remodeling.
Our patients were normotensive and had higher pulmonary artery pressures, reflective of vascular remodeling in the pulmonary arteries rather than the systemic vascular bed. A significant proportion of PAH patients were exposed to MR antagonists, which has been reported to influence collagen biomarkers in heart failure patients (25,26). Consistent with that our results also showed elevated PIIINP levels in patients that were not on a MR blocker (0.028). Furthermore, we are investigating the effects of MR blocker, spironolactone, on circulating collagen biomarkers in PAH patients in an ongoing randomized clinical trial (clinicaltrials.gov; NCT01468571). It is possible the treatment of PAH patients with spironolactone may result in changes in collagen biomarker levels and this study will provide further insight.
As the samples were drawn from peripheral blood, the source of collagen could be the right ventricle, pulmonary vasculature, or the left ventricle. However, based on the ECHO performed as a part of the standard care for each patient, there was no evidence of hypertensive left ventricle hypertrophy or left ventricle systolic or diastolic dysfunction. Collagen metabolites have been shown to be elevated in other conditions associated with RV remodeling such as congenital heart disease, RV pressure overload induced by PA banding and also in animal models of RV hypertrophy (27-30). Therefore, the degree of adverse RV remodeling in more advanced PAH cases could explain increased circulating collagen metabolites. While the source of collagen remains unclear, the disease target and pathology suggest that it likely originated from the RV and/or the pulmonary vascular bed. These claims, however, require further investigation that is beyond the scope of this study.
PAH Severity
Consistent with the previously observed association between rapid onset and progression of disease with worse outcome, our data indicated that patients with shorter disease duration had more severe PAH, as established by parameters such as lower walk distance, worse hemodynamic indices (RAP, RVSP, pericardial effusion), worse WHO FC and higher BNP levels (16). These findings indicated that patients were correctly assigned to their respective severity group. There was a trend towards lower S′ with increasing disease severity, suggesting worsening RV systolic function as the disease progresses. Elevated BNP levels are considered a poor prognostic indicator (31), a marker of ventricular overload (32,33), and of disease severity in PAH (34). Interestingly, PIIINP levels were positively correlated with BNP levels suggesting that PIIINP may be eligible for use as a potential indicator of disease severity as well. In addition, there was trend towards higher CITP levels as the disease worsened suggesting that circulating collagen I degradation marker maybe reflective of ongoing vascular remodeling. As only a trend was observed, a larger sample size may be needed to further elucidate this finding.
Biomarker tertile data
Published data suggest that PIIINP increases with age (22). Interestingly, we found that our study patients who had higher PIIINP levels were significantly younger. This counterintuitive phenomenon could be due to the potential link between increased levels of the biomarker and rapid disease progression, which we suggest and support in this paper. Accordingly, we found that higher PIIINP tertiles were indeed associated with worse disease, as indicated by low walk distance, higher RAP, and worse WHO FC. The highest tertile group for PIIINP had higher RAP and this parameter tend to be associated with worse renal function, which may in turn mediate circulating levels of these markers. To investigate this possibility, we evaluated GFR for each biomarker tertile and found no significant difference between GFR values across the tertiles.
Outcome analysis
Kaplan-Meier survival analysis showed a trend towards higher rate of lung transplant or death in patients with increasing PIIINP tertiles, which is entirely congruent with our finding that higher PIIINP levels are associated with disease severity. As expected, there was a significantly higher rate of lung transplant or death in the group with severe PAH. It would be interesting to explore whether it is the procollagens themselves or rather the vascular remodeling in patients with worsening disease leading to increased mortality. Perhaps a longer study with larger number of patients could provide additional data on possible links between collagen biomarkers and outcome in PAH.
Limitations of the study
More advanced forms of PAH may lead to reduction in LV mass as a result of impaired filling. It may be the case that a biological response to decreased filling /stretch may also involve activation of collagen synthesis/turnover. However, there is data linking LV remodeling and LV hypertrophy in left heart failure with increased levels of collagen metabolites in the peripheral circulation (27). In fact, there is literature available which states that decreased LV mass may result in decreased collagen levels in the LV (35). Further studies will be needed to explore this possibility. Additional considerations are listed in Appendix B.
Conclusion
This study indicates that circulating collagen biomarkers are elevated in patients with idiopathic, hereditary, and anorexigen associated PAH. These findings suggest that the state of active vascular remodeling in the pulmonary vascular bed or RV may be represented by circulating collagen biomarkers. We propose that the circulating collagen biomarkers provide clinically relevant, peripherally measurable indices of disease severity and outcome, and that elevated PIIINP levels may prove to be a useful marker for the identification of patients with severe disease and poor outcome. However, as this is one of the first studies to investigate these relationships, further research is necessary.
Acknowledgments
Authors wish to thank Ms Dorellyn Lee for help in sample analysis, Praveen Konasagar for sample collection and Ms Janice Brister for editorial support.
Grants: This work was funded in part by National Heart, Lung, and Blood Institute Grant K23 HL093214 to Z. Safdar
List of Abbreviations
- BDS
Borg dyspnea score
- BNP
brain natriuretic peptide
- BSA
body surface area
- CCB
calcium channel blocker
- CITP
C-terminal telopeptide of collagen type I
- ECHO
echocardiogram
- MMP-9
matrix metalloproteinsase 9
- PIIINP
N-terminal propeptide of type III procollagen
- RVSP
right ventricular systolic pressure
- TIMP-1
tissue inhibitor of metalloproteinase 1
- WHO FC
World Health Organization functional classification
Appendix 1
A. Method
Subjects
PAH subjects and healthy controls were excluded if they were hemodynamically unstable, unable to give informed consent, pregnant or breast-feeding, had systemic hypertension, collagen vascular disease congenital heart disease or PH due to left heart disease, significant renal insufficiency (serum creatinine >2.0 mg per deciliter or required hemodialysis) or significant liver dysfunction (AST or ALT more than three times upper limit of normal). In addition, healthy controls were excluded if they were on mineralocorticoid receptor (MR) antagonist (spironolactone or eplerenone) or ACE inhibitors. PAH and healthy controls were excluded if they had an active infection or acute and chronic inflammatory diseases. All subjects had appropriate clinical and laboratory evaluation to identify inclusion/exclusion criteria and suitability for the study.
Thirty-nine age- and gender-matched healthy controls were enrolled; however, 2 controls were excluded when they were found to have systemic hypertension. Controls were matched to the PAH patients so that the two groups would have similar distributions of possible confounders such as age and gender. This way, comparisons between the groups could be made without having to control for these factors.
Biochemical Measurements of Indices of Collagen Metabolism
Our preliminary studies indicated that as compared healthy controls, PICP levels were not elevated (N=22, p=0.828), whereas PIIINP (N=29, p=0.003) and CITP (N=28, p=0.004) levels were elevated in PAH patients. Therefore, we proceeded to measure and analyze these for the remainder of the study. MMP-9 levels were determined as it has a role in vascular remodeling, inflammatory responses and angiogenesis (7-10). The sensitivity (lower detection limit) of both the CITP and the PIIINP assays were approximately 0.3 ng/mL. Coefficients of variation (CV) derived from duplicate measurements were < 10% for both CITP and PIIINP assays. Serum MMP-9 and TIMP-1 levels were measured using 2-site sandwich ELISAs (R & D Systems, Inc., Minneapolis, MN) per manufacturer's protocol. The MMP-9 assay (DMP900, sensitivity < 0.156 ng/mL) was designed to measure both the Pro - 92kDa and the active 82kDa form of MMP-9 in human serum. The TIMP-1 assay (DTM100, sensitivity was < 0.08 ng/mL) measures all forms of serum TIMP-1. CV from duplicate measurements were < 5% for both MMP-9 and TIMP-1 assays. Commercially available immunoassay was used for quantitative brain natriuretic peptide (BNP) determination on an ADVIA Centaur analyzer system according to the manufacturer's recommendation. Sensitivity of the assay was <2.0 to 5000 pg/ml. Normal BNP value at our laboratory was ≤100 pg/ml.
Doppler Echocardiography
All parameters were measured in triplicates and averaged. The echocardiogram was undertaken with subject at rest, lying in the left lateral decubitus position. Ultrasound data was acquired using a Philipds IE-33 scanner (Philips USA, Bothell, WA USA) equipped with a X3-1 transducer. Images were analyzed off line using Digisonics software Version 3.8.1 (Digisonics, Houston, TX USA). Left ventricular ejection fraction was calculated using the Simpson's method of biplane discs (1). Left ventricular cardiac output and cardiac index were calculated using the pulse wave Doppler signal in the left ventricular outflow tract using the continuity equation (2). IVC size and variation with respiration were used to estimate the RAP as described by Lang et al (1).
B. Discussion
We base our hypothesis on the findings from several studies that strongly support a role of extracellular matrix remodeling in the pathophysiology of PAH. For one, an in vitro study by Botney et al showed the presence of procollagen in the media and neointima of small muscular arteries and in plexiform lesions in severe idiopathic PAH subjects (3). More research has been done in which procollagen propeptide was detected in calves and rats exposed to hypoxia (4,5). Another study which implemented a high flow rat model of PAH demonstrated the presence of significantly increased expression of procollagen I and III mRNAs in the lungs of the shunted animals as compared to the control group (6). Moreover, a study in which animals that were exposed to hypoxia after being treated with recombinant adenovirus for the tissue inhibitor of metalloproteinases (TIMP) gene, thereby increasing TIMP expression in the lungs, showed that the animals developed higher pulmonary artery pressures, right ventricular hypertrophy, and an increased degree of muscularization and collagen deposition in their distal pulmonary arteries as compared to control animals (7). A similar finding to these was reported by Bishop et al, who found that collagen turnover rates were increased in the pulmonary arteries of rabbits with chemically induced pulmonary hypertension (8). Furthermore, immunohistochemical studies of fibrotic lungs have confirmed the presence of an increased proportion of type III collagen in early active fibrosis and an overabundance of rigid type I collagen in late fibrosis (9), which is consistent with our findings of increased PIIINP levels. Another investigation, which examined explanted lungs from PAH patients, showed increased TIMP-1 expression and activity in pulmonary arteries and in the smooth muscle cells obtained from those lungs (10). Published research showed that in hypertensive heart failure patients with elevated cardiac filling pressures, an increase in circulating TIMP-1 levels relative to MMP1 levels suggested ongoing collagen synthesis (11). Several studies have shown that cardiac fibrosis histology measures are associated with circulating fibrosis biomarkers (12,13). These and other data established that excessive collagen metabolism due to progressive vascular remodeling plays a distinct role in the pathophysiology of PAH and that collagen content of a tissue may be assessed with the serum analysis of breakdown products of collagen. It is these findings that drive the hypothesis of our investigation.
MMP-9 is present in low quantities in the healthy adult lung, but much more abundant in several lung diseases, including asthma, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease. MMP-9 mediates endothelial cell invasion and its expression by fibrocytes may facilitate angiogenic processes (14). Despite numerous reports of MMP-9 in lung diseases such as asthma and IPF it is unclear whether MMP-9 has a causal role in lung remodeling or it is part of the inflammatory response. The relationship between the collagen biomarkers and/or the individual levels may be useful to determine the effects of vascular remodeling in PAH patients.
As multiple biomarkers were investigated, we conducted Bonferroni adjustment for multiple hypotheses testing on the collagen biomarkers, echocardiogram parameters and walk test separately. The results did not change for biomarkers and walk distance data. However, when we conducted the analysis for echocardiogram parameters, trend towards significance for S′ was not noted.”
Limitations of the study
Higher levels of both PIIINP and CITP have been associated with left heart failure and LV dysfunction. However, in our data PIIINP showed a stronger association with disease severity in PAH. This could be due to the small sample size and to determine the adequacy of sample size, we have performed power calculations using the two-sample T test with unequal variances for comparing PIIINP and CITP between controls and PAHs. If the SD of the control is 1.21 (for CITP) and for the PAH is 2.38 (for CITP), we obtained a power of 80% when the sample size was 34 and 68 respectively. For PIIINP the power was even higher. Therefore, with a sample of 37 for the control, our study is sufficiently powered to detect a difference in both PIIINP and CITP levels between controls and PAH patients.
- 1.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 3.Botney MD, Liptay MJ, Kaiser LR, Cooper JD, Parks WC, Mecham RP. Active collagen synthesis by pulmonary arteries in human primary pulmonary hypertension. Am J Pathol. 1993;143:121–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Crouch EC, Parks WC, Rosenbaum JL, et al. Regulation of collagen production by medial smooth muscle cells in hypoxic pulmonary hypertension. Am Rev Respir Dis. 1989;140:1045–51. doi: 10.1164/ajrccm/140.4.1045. [DOI] [PubMed] [Google Scholar]
- 5.Poiani GJ, Tozzi CA, Yohn SE, et al. Collagen and elastin metabolism in hypertensive pulmonary arteries of rats. Circ Res. 1990;66:968–78. doi: 10.1161/01.res.66.4.968. [DOI] [PubMed] [Google Scholar]
- 6.Wei B, Du J, Li J, Qi J, Tang C. The modulating effect of L-arginine on collagen metabolism of pulmonary artery in pulmonary hypertension induced by a left-to-right shunt. Zhonghua Yi Xue Za Zhi. 2002;82:1273–5. [PubMed] [Google Scholar]
- 7.Vieillard-Baron A, Frisdal E, Eddahibi S, et al. Inhibition of matrix metalloproteinases by lung TIMP-1 gene transfer or doxycycline aggravates pulmonary hypertension in rats. Circ Res. 2000;87:418–25. doi: 10.1161/01.res.87.5.418. [DOI] [PubMed] [Google Scholar]
- 8.Bishop JE, Guerreiro D, Laurent GJ. Changes in the composition and metabolism of arterial collagens during the development of pulmonary hypertension in rabbits. Am Rev Respir Dis. 1990;141:450–5. doi: 10.1164/ajrccm/141.2.450. [DOI] [PubMed] [Google Scholar]
- 9.Low RB, Giancola MS, King TE, Jr, Chapitis J, Vacek P, Davis GS. Serum and bronchoalveolar lavage of N-terminal type III procollagen peptides in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1992;146:701–6. doi: 10.1164/ajrccm/146.3.701. [DOI] [PubMed] [Google Scholar]
- 10.Lepetit H, Eddahibi S, Fadel E, et al. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension. Eur Respir J. 2005;25:834–42. doi: 10.1183/09031936.05.00072504. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez A, Lopez B, Querejeta R, Zubillaga E, Echeverria T, Diez J. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 55:1418–24. doi: 10.1161/HYPERTENSIONAHA.109.149112. [DOI] [PubMed] [Google Scholar]
- 12.Izawa H, Murohara T, Nagata K, et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation. 2005;112:2940–5. doi: 10.1161/CIRCULATIONAHA.105.571653. [DOI] [PubMed] [Google Scholar]
- 13.Bruggink AH, van Oosterhout MF, de Jonge N, et al. Reverse remodeling of the myocardial extracellular matrix after prolonged left ventricular assist device support follows a biphasic pattern. J Heart Lung Transplant. 2006;25:1091–8. doi: 10.1016/j.healun.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Hartlapp I, Abe R, Saeed RW, et al. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. Faseb J. 2001;15:2215–24. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
Footnotes
Disclosures: 1. Zeenat Safdar: United Therapeutics, Gilead Sciences, Acetelion Pharmaceuticals, Bayer Pharmaceuticals
2. Adaani Frost: clinical research: Gilead, Pfizer, Actelioin, United Therapeutics, Lilly, Bayer, Novartis, Intermune, GSK, IKARIA, AIRES; advisory board/consultant: Bayer, Actelion, Gilead, United Therapeutics; steering committee member for study, grant awards or registry: REVEAL(funded by Actelion); ENTELIIGENCE grant award committee (funded by Actelion): endpoint adjudication committee Beraprost study funded by United Therapeutics; steering committee for AMBITION STUDY funded by Gilead, GSK and Lilly; speaker for: Actelion, Gilead, Bayer, Pfizer
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fessler JH, Fessler LI. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–62. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- 2.Rojkind Marcos. In: Connective Tissue in Health and Disease. Rojkind Marcos., editor. Taylor & Francis Group; 1990. PD. [Google Scholar]
- 3.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikolova A, Ablasser K, Wyler von Ballmoos MC, et al. Endogenous angiogenesis inhibitors prevent adaptive capillary growth in left ventricular pressure overload hypertrophy. Ann Thorac Surg. 94:1509–17. doi: 10.1016/j.athoracsur.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuenca J, Martin-Sanz P, Alvarez-Barrientos AM, Bosca L, Goren N. Infiltration of inflammatory cells plays an important role in matrix metalloproteinase expression and activation in the heart during sepsis. Am J Pathol. 2006;169:1567–76. doi: 10.2353/ajpath.2006.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stintzing S, Heuschmann P, Barbera L, et al. Overexpression of MMP9 and tissue factor in unstable carotid plaques associated with Chlamydia pneumoniae, inflammation, and apoptosis. Ann Vasc Surg. 2005;19:310–9. doi: 10.1007/s10016-005-0003-7. [DOI] [PubMed] [Google Scholar]
- 7.Botney MD, Liptay MJ, Kaiser LR, Cooper JD, Parks WC, Mecham RP. Active collagen synthesis by pulmonary arteries in human primary pulmonary hypertension. Am J Pathol. 1993;143:121–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Botney MD, Kaiser LR, Cooper JD, et al. Extracellular matrix protein gene expression in atherosclerotic hypertensive pulmonary arteries. Am J Pathol. 1992;140:357–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison NK, Laurent GJ, Evans TW. Transpulmonary gradient of type III procollagen peptides: acute effects of cardio-pulmonary bypass. Intensive Care Med. 1992;18:290–2. doi: 10.1007/BF01706477. [DOI] [PubMed] [Google Scholar]
- 10.Pohl WR, Thompson AB, Kohn H, et al. Serum procollagen III peptide levels in subjects with sarcoidosis. A 5-year follow-up study. Am Rev Respir Dis. 1992;145:412–7. doi: 10.1164/ajrccm/145.2_Pt_1.412. [DOI] [PubMed] [Google Scholar]
- 11.Low RB, Giancola MS, King TE, Jr, Chapitis J, Vacek P, Davis GS. Serum and bronchoalveolar lavage of N-terminal type III procollagen peptides in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1992;146:701–6. doi: 10.1164/ajrccm/146.3.701. [DOI] [PubMed] [Google Scholar]
- 12.Bentsen KD, Nielsen TL, Eaftinck Schattenkerk JK, Jensen BN, Lundgren JD. Serum type III procollagen peptide in patients with Pneumocystis carinii infection. The Copenhagen-Amsterdam PCP-Prednisolone Study Group. Am Rev Respir Dis. 1993;148:1558–62. doi: 10.1164/ajrccm/148.6_Pt_1.1558. [DOI] [PubMed] [Google Scholar]
- 13.Chesnutt AN, Matthay MA, Tibayan FA, Clark JG. Early detection of type III procollagen peptide in acute lung injury. Pathogenetic and prognostic significance. Am J Respir Crit Care Med. 1997;156:840–5. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 14.Entzian P, Huckstadt A, Kreipe H, Barth J. Determination of serum concentrations of type III procollagen peptide in mechanically ventilated patients. Pronounced augmented concentrations in the adult respiratory distress syndrome. Am Rev Respir Dis. 1990;142:1079–82. doi: 10.1164/ajrccm/142.5.1079. [DOI] [PubMed] [Google Scholar]
- 15.Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158:1432–41. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–31. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 17.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 18.Hardziyenka M, Reesink HJ, Bouma BJ, et al. A novel echocardiographic predictor of in-hospital mortality and mid-term haemodynamic improvement after pulmonary endarterectomy for chronic thrombo-embolic pulmonary hypertension. Eur Heart J. 2007;28:842–9. doi: 10.1093/eurheartj/ehl534. [DOI] [PubMed] [Google Scholar]
- 19.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131:1917–28. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 20.Barst RJ, Galie N, Naeije R, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J. 2006;28:1195–203. doi: 10.1183/09031936.06.00044406. [DOI] [PubMed] [Google Scholar]
- 21.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 22.Deng W, Chen QW, Li GQ, et al. Procollagen III N-terminal peptide predicts short-term prognosis and cardiac remodeling in coronary heart disease patients with metabolic syndrome. Am J Med Sci. 341:10–6. doi: 10.1097/MAJ.0b013e3181f080d8. [DOI] [PubMed] [Google Scholar]
- 23.Agrinier N, Thilly N, Boivin JM, Dousset B, Alla F, Zannad F. Prognostic value of serum PIIINP, MMP1 and TIMP1 levels in hypertensive patients: a community-based prospective cohort study. Fundam Clin Pharmacol. 2012 doi: 10.1111/j.1472-8206.2012.01053.x. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez A, Lopez B, Querejeta R, Zubillaga E, Echeverria T, Diez J. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 55:1418–24. doi: 10.1161/HYPERTENSIONAHA.109.149112. [DOI] [PubMed] [Google Scholar]
- 25.Iraqi W, Rossignol P, Angioi M, et al. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation. 2009;119:2471–9. doi: 10.1161/CIRCULATIONAHA.108.809194. [DOI] [PubMed] [Google Scholar]
- 26.Izawa H, Murohara T, Nagata K, et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation. 2005;112:2940–5. doi: 10.1161/CIRCULATIONAHA.105.571653. [DOI] [PubMed] [Google Scholar]
- 27.Bishop JE, Rhodes S, Laurent GJ, Low RB, Stirewalt WS. Increased collagen synthesis and decreased collagen degradation in right ventricular hypertrophy induced by pressure overload. Cardiovasc Res. 1994;28:1581–5. doi: 10.1093/cvr/28.10.1581. [DOI] [PubMed] [Google Scholar]
- 28.Chen CA, Tseng WY, Wang JK, et al. Circulating biomarkers of collagen type I metabolism mark the right ventricular fibrosis and adverse markers of clinical outcome in adults with repaired tetralogy of Fallot. Int J Cardiol. 167:2963–8. doi: 10.1016/j.ijcard.2012.08.059. [DOI] [PubMed] [Google Scholar]
- 29.Turner JE, Oliver MH, Guerreiro D, Laurent GJ. Collagen metabolism during right ventricular hypertrophy following induced lung injury. Am J Physiol. 1986;251:H915–9. doi: 10.1152/ajpheart.1986.251.5.H915. [DOI] [PubMed] [Google Scholar]
- 30.Gardi C, Martorana PA, Calzoni P, et al. Cardiac collagen changes during the development of right ventricular hypertrophy in tight-skin mice with emphysema. Exp Mol Pathol. 1994;60:100–7. doi: 10.1006/exmp.1994.1009. [DOI] [PubMed] [Google Scholar]
- 31.Nagaya N, Nishikimi T, Uematsu M, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–70. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura M, Yasue H, Okumura K, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–9. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- 33.Nagaya N, Nishikimi T, Okano Y, et al. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol. 1998;31:202–8. doi: 10.1016/s0735-1097(97)00452-x. [DOI] [PubMed] [Google Scholar]
- 34.Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol. 2004;43:764–70. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 35.Marchesi C, Essalmani R, Lemarie CA, et al. Inactivation of endothelial proprotein convertase 5/6 decreases collagen deposition in the cardiovascular system: role of fibroblast autophagy. J Mol Med (Berl) 89:1103–11. doi: 10.1007/s00109-011-0776-9. [DOI] [PubMed] [Google Scholar]


