Abstract
We recently developed a protocol for the transcriptome-wide isolation of RNA recognition elements readily applicable to any protein or ribonucleoprotein complex directly contacting RNA (including RNA helicases, polymerases, or nucleases) expressed in cell culture models either naturally or ectopically (Hafner et al., 2010).
Briefly, immunoprecipitation of the RNA-binding protein of interest is followed by isolation of the crosslinked and coimmunoprecipitated RNA. In the course of lysate preparation and immunoprecipitation, the mRNAs are partially degraded using Ribonu-clease T1. The isolated crosslinked RNA fragments are converted into a cDNA library and deep-sequenced using Solexa technology (see Explanatory Chapter: Next Generation Sequencing). By introducing photoreactive nucleosides that generate characteristic sequence changes upon crosslinking (see below), our protocol allows one to separate RNA segments bound by the protein of interest from the background un-crosslinked RNAs.
1. THEORY
Posttranscriptional regulation (PTR) of messenger RNAs (mRNAs) plays important roles in diverse cellular processes (Ambros, 2004; Halbeisen et al., 2008). The fates of mRNAs are determined predominantly by their interactions with RNA-binding proteins (RBPs) and noncoding, guide-RNA-containing ribonucleoprotein complexes (RNPs). Taken together, they form mRNA-containing ribonucleoprotein complexes (mRNPs). The RBPs influence the structure and interactions of the RNAs and play critical roles in their biogenesis, stability, function, transport, and cellular localization (Moore, 2005; Keene, 2007; Glisovic et al., 2008).
Given that hundreds of RBPs and RNPs and their networks remain to be studied and evaluated in a cell-type-dependent manner, the development of powerful tools to determine their binding sites or RNA recognition elements (RREs) is critical to enhance our understanding of PTR. It offers new opportunities for understanding both gene regulation and consequences of genetic variation in transcript regions aside from the open reading frame.
Typically, a combination of genetic, biochemical, and computational approaches has been applied to identify mRNA-RBP or mRNA-RNP interactions. However, each of these methods has limitations. Microarray profiling of mRNA associated with immunopurified RBPs (RIP-ChIP) (Tenenbaum et al., 2000) is limited by incomplete enrichment of bound mRNAs and the difficulty of locating the RRE in the hundreds to thousands of nucleotide (nt) long target mRNA (Gerber et al., 2006; Landthaler et al., 2008).
Some of these problems were addressed by an in vivo UV 254-nm crosslinking and immunoprecipitation (CLIP) protocol (Ule et al., 2003. See also UV crosslinking of interacting RNA and protein in cultured cells) that better defines the interaction site by isolating and sequencing small RNA segments crosslinked to RBPs. However, UV 254-nm crosslinking is not efficient, and the site of crosslinking is not revealed after sequencing of the isolated RNA fragment. To separate crosslinked sites from background noise, additional control crosslinking experiments are needed, including the use of knockout cells of the protein of interest.
To overcome these limitations, we developed a new protocol referred to as PAR-CLIP (Photoactivatable-Ribonucleoside-Enhanced Crosslinking and Immunoprecipitation) (Hafner et al., 2010).
4-Thiouridine (4SU) and 6-thioguanosine (6SG) are readily incorporated into nascent RNAs by simply supplementing the media of cultured cells with the modified nucleoside (Favre et al., 1986; Bezerra and Favre, 1990). At the concentrations used in the presented protocol, neither of the tested photoreactive nucleosides showed any detectable toxic effects based on mRNA profiling or cell count. Irradiation of the cells by UV light of 365 nm leads to crosslinking of photoreactive nucleoside-labeled cellular RNAs to interacting RBPs. Using similar irradiation protocols, 4SU incorporation substantially enhances RNA recovery compared to UV 254-nm crosslinking, 6SG performs in between these two methods.
Most importantly, the sites of crosslinking can be easily identified by mapping characteristic T to C mutations (G to A in the case of 6SG, though less pronounced) in the sequenced cDNA libraries obtained from the recovered RNA initiated by the photocrosslinking itself. We presume that the structural change upon crosslinking of the modified nucleosides to aromatic amino acid side chains directs the incorporation of a noncognate deoxynucleoside during reverse transcription of crosslinked RNAs. The presence of the mutations in sequence reads, together with the observation that multiple positions within a cluster of sequence reads can be altered, facilitates the separation from clusters of unaltered background sequences typically derived from abundant cellular RNAs.
For details on the bioinformatic analyses, please refer to our recent publication (Hafner et al., 2010).
2. EQUIPMENT
| Major equipment | Radioisotope laboratory |
| 365-nm UV-transilluminator | |
| Agarose gel electrophoresis equipment | |
| Protein electrophoresis equipment | |
| Equipment to cast and run 15 × 17 cm × 0.8 mm (or similar) polyacrylamide gels | |
| Balances (e.g., 0.1 mg–64 g and 0.1 g–4.2 kg) | |
| Heating block (90 °C) | |
| CO2 incubator for mammalian cell culture | |
| D-Tube Dialyzer Midi rack (EMD Biosciences, 71511-3) | |
| High-speed floor centrifuge (capable of at least 13 000 × g) | |
| Magnetic rack for 1.5-ml microcentrifuge tubes and 15-ml conical tubes | |
| Multichannel pipettor | |
| pH-meter | |
| Phosphorimager & imaging plates (or developer and X-ray film) | |
| Refrigerated bench top microcentrifuge | |
| Rotating wheel | |
| PCR thermocycler | |
| Thermometer | |
| Thermomixer | |
| UV Stratalinker 2400 equipped with 365-nm light bulbs for crosslinking (Stratagene) | |
| Vortex mixer | |
| Water bath | |
| Water filter; MilliQBiocel water purification system | |
| X-ray exposure cassette | |
| HPLC with a Supelco Discovery C18 (bonded phase silica 5 μM particle, 250 × 4.6 mm) reverse phase column (Bellefonte, PA, USA) |
| Consumables | 1.5-ml polypropylene tubes |
| 1.5-ml siliconized tubes (BIO PLAS Inc., 4165SL) | |
| 15- and 50-ml conical tubes (e.g., Falcon) as well as tubes withstanding high-speed centrifugation (e.g., Sarsted, 13-ml centrifuge tube, 55.518) | |
| 15-cm culture dishes | |
| 10-cm tissue culture dishes | |
| 5-μm Supor membrane syringe filter (Pall Acrodisc) | |
| Cell scraper (Corning) | |
| D-Tube Dialyzer Midi, MWCO 3.5 kDa (EMD Biosciences, 71506-3) | |
| NuPAGE Novex 4–12% BT Midi 1.0 gel (Invitrogen) | |
| pH paper (covering the range between pH 6.5 and 10) | |
| Plastic wrap | |
| Scalpels or razor blades | |
| Strips of 0.2-ml tubes (Thermo Scientific, AB-0264) | |
| Syringes (10 ml) |
3. MATERIALS
| Reagents & Chemicals | Appropriate cell culture medium and selection antibiotics |
| 2-Mercaptoethanol (14.3 M; Sigma, M6250) | |
| Acetonitrile | |
| Agarose, electrophoresis grade (SeaKem LE Agarose, Lonza, 50004) | |
| Agarose, low melting (NuSieve GTG Agarose, Lonza, 50080) | |
| Ammonium persulfate (APS) | |
| Adenosine triphosphate (ATP) | |
| Bacterial Alkaline Phosphatase (Worthington Biochemical, LS006344) | |
| Bromophenol blue | |
| Bovine serum albumin, acetylated (BSA, acetylated; Ambion, AM2614) | |
| Calcium chloride (CaCl2·2H2O) | |
| Calf intestinal alkaline phosphatase (CIP) | |
| Chloroform | |
| Citric acid monohydrate | |
| Complete EDTA-free protease inhibitor cocktail (Roche) | |
| Dimethyl sulfoxide (DMSO) | |
| DNA ladder (25 bp) | |
| dNTPs: dATP, dCTP, dGTP, dTTP (0.1 M each; Fermentas, R0182) | |
| Dithiothreitol (DTT) | |
| Dynabeads Protein G (Invitrogen, 100-03D) | |
| EDTA disodium salt dihydrate (Sigma, E5134) | |
| EGTA (C14H20N2O10Na4; Sigma, E8145-10G) | |
| Ethanol (100%) | |
| Ethidium bromide | |
| Ficoll type 400 | |
| Formamide | |
| [γ-32P]-ATP (10 mCi ml−1, 6000 Ci (222 TBq) mmol−1; Perkin Elmer, NEG002Z500UC) | |
| Glycerol | |
| Glycoblue or glycogen | |
| Hydrochloric acid (HCl) (Fisher Scientific, A144S) | |
| HEPES | |
| Isoamyl alcohol | |
| Isopropyl alcohol | |
| Potassium chloride (KCl) | |
| Potassium hydroxide (KOH) | |
| Magnesium chloride (MgCl2·6H2O) | |
| MOPS SDS running buffer (20×; Invitrogen) | |
| Sodium acetate (NaOAc, Fisher, S210) | |
| Sodium phosphate dibasic (Na2HPO4·7H2O; Sigma, S9390-100G) | |
| Sodium chloride (NaCl) | |
| Sodium fluoride (NaF) | |
| Sodium hydroxide (NaOH) | |
| NP40 substitute (100%; Sigma [74385]) | |
| Phosphate buffered saline (PBS; 10×, commercially available) | |
| Phenol (saturated with 0.1 M citrate buffer, pH 4.3 ± 0.2, Sigma, P4682) | |
| Photoreactive nucleoside (Sigma; 4-thiouridine [T4509]/6-thioguanosine [858412]) | |
| Protein ladder (e.g., Biorad, 161-0374; 10–250 kDa) | |
| Proteinase K (lyophilizate; Roche, 03115801001) | |
| rA, rG, rC, rU, and 4SU (Sigma, T4509) | |
| RNase T1 (Fermentas, EN0541); concentration 1000 U μl−1 | |
| Sodium dodecyl sulfate (SDS; Fisher Scientific, BP166-500) | |
| Snake Venom Phosphodiesterase (Worthington Biochemical, LS003926) | |
| SuperScript III Reverse Transcriptase (Invitrogen, 18080-044); includes 5× First-strand buffer | |
| T4 Polynucleotide Kinase (T4 PNK; NEB, M0201) | |
| T4 RNA Ligase 1 (NEB, M0204L) | |
| T4 RNA Ligase 2, truncated (e.g., NEB, M0242L); or: Rnl2(1-249)K227Q (our plasmid for expression of the his-tagged mutant is available at www.addgene.com, plasmid 14072; however, the purified enzyme will shortly also be available from NEB) | |
| Taq DNA polymerase (5 U μl−1) | |
| Tris-Borate–EDTA buffer solution (TBE) | |
| Acetic acid – triethylamine solution 1:1 (TEAA; Sigma, 09748) | |
| Tetramethylethylenediamine (TEMED) | |
| Tris base (Fisher Scientific, BP152-1) | |
| Tris–HCl (Promega, H5121) | |
| Triton X-100 | |
| TRIzol reagent (Invitrogen, 15596-026) | |
| UreaGel – SequaGel – System, National Diagnostics, EC-833 | |
| Antibody (e.g., for FLAG-tagged RBPs: mouse monoclonal anti-FLAG M2 (Sigma, F1804)) | |
| QIAquick gel purification kit (Qiagen) |
| RNA & DNA oligonucleotides | 3′ adapter (DNA, except for the 5′ riboadenylate (rApp) residue): 5′ rAppTCGTATGCCGTCTTCTGCTTGT |
| 5′ adapter (RNA): 5′ GUUCAGAGUUCUACAGUCCGACGAUC | |
| 3′ PCR primer (DNA): 5′ CAAGCAGAAGACGGCATACGA | |
| 5′ PCR primer (DNA): 5′ AATGATACGGCGACCACCGACAGGTTCAGAGTTCTACAGTCCGA | |
| 19-nt size marker (RNA): 5′ CGUACGCGGGUUUAAACGA | |
| 24-nt size marker (RNA): 5′ CGUACGCGGAAUAGUUUAAACUGU | |
| 33-nt size marker (RNA): 5′CAUCUUGGUCGUACGCGGAAUAGUUUAAACUGU | |
| 35-nt size marker (RNA): 5′CUCAUCUUGGUCGUACGCGGAAUAGUUUAAACUGU | |
| Reference oligoribonucleotides | CGUACGCGGAAUACUUCGA(4SU)U (e.g., from Thermo Scientific) |
| CGUACGCGGAAUACUUCGAUU |
3.1. Solutions & buffers
Step 1 1 M 4-Thiouridine stock solution
| Dissolve 250-mg 4-thiouridine in 960.5-μl DMSO (For a 1 M 6-thioguanosine solution, first dehydrate the powder supplied by Sigma in a vacuum oven at room temperature overnight. Then, dissolve 299.3 mg in 1-ml DMSO) |
4-Thiouridine-containing growth medium
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| 4-thiouridine (in DMSO) | 100 μM | 1 M | 100 μl |
| Cell culture medium | 1 l |
1× PBS
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| PBS | 1× | 10× | 100 ml |
| H2O | 900 ml |
Step 2 NP40 lysis buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| HEPES-KOH, pH 7.5 | 50 mM | 1 M | 50 ml |
| KCl | 150 mM | 1 M | 150 ml |
| EDTA–NaOH, pH 8.0 | 2 mM | 0.5 M | 4 ml |
| NaF | 1 mM | 0.5 M | 2 ml |
| NP40 substitute | 0.5% (v/v) | 100% | 5 ml |
| H2O | 788.5 ml | ||
| DTT (add fresh) | 0.5 mM | 1 M | 0.5 ml |
| Complete EDTA-free protease inhibitor cocktail (add fresh) | 1 tablet/50 ml |
Step 3 Citrate-phosphate buffer, pH 5.0
| Component | Amount |
|---|---|
| Citric acid monohydrate | 4.7 g |
| Na2HPO4·7H2O | 9.2 g |
| H2O to 1 l |
Step 4 IP-wash buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| HEPES-KOH, pH 7.5 | 50 mM | 1 M | 50 ml |
| KCl | 300 mM | 1 M | 300 ml |
| NP40 substitute | 0.05% (v/v) | 100% | 0.5 ml |
| H2O | 649 ml | ||
| DTT (add fresh) | 0.5 mM | 1 M | 0.5 ml |
| Complete EDTA-free protease inhibitor cocktail (add fresh) | 1 tablet/50 ml |
High-salt wash buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| HEPES-KOH, pH 7.5 | 50 mM | 1 M | 50 ml |
| KCl | 500 mM | 1 M | 500 ml |
| NP40 substitute | 0.05% (v/v) | 100% | 0.5 ml |
| H2O | 449 ml | ||
| DTT (add fresh) | 0.5 mM | 1 M | 0.5 ml |
| Complete EDTA-free protease inhibitor cocktail (add fresh) | 1 tablet/50 ml |
10× Dephosphorylation buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Tris–HCl, pH 7.9 | 50 mM | 1 M | 50 ml |
| NaCl | 100 mM | 3 M | 33.3 ml |
| MgCl2·6H2O | 10 mM | 1 M | 10 ml |
| H2O | 906.2 ml | ||
| DTT (add fresh) | 1 mM | 1 M | 0.5 ml |
Step 5 Phosphatase wash buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Tris–HCl, pH 7.5 | 50 mM | 1 M | 50 ml |
| EGTA–NaOH, pH 7.5 | 20 mM | 0.5 M | 40 ml |
| NP40 substitute | 0.5% (v/v) | 100% | 5 ml |
| H2O | 905 ml |
Polynucleotide kinase (PNK) buffer without DTT
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Tris–HCl, pH 7.5 | 50 mM | 1 M | 50 ml |
| NaCl | 50 mM | 3 M | 16.7 ml |
| MgCl2·6H2O | 10 mM | 1 M | 10 ml |
| H2O | 923.3 ml |
PNK buffer with DTT
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Tris–HCl, pH 7.5 | 50 mM | 1 M | 50 ml |
| NaCl | 50 mM | 3 M | 16.7 ml |
| MgCl2·6H2O | 10 mM | 1 M | 10 ml |
| H2O | 918.3 ml | ||
| DTT (add fresh) | 5 mM | 1 M | 5 ml |
1× SDS PAGE loading buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Tris–HCl, pH 6.8 | 50 mM | 1 M | 0.5 ml |
| EDTA–NaOH, pH 8.0 | 2 mM | 0.5 M | 0.04 ml |
| Glycerol | 10% (v/v) | 50% | 2 ml |
| SDS | 2% (v/v) | 20% | 1 ml |
| DTT | 100 mM | 1 M | 1 ml |
| Bromophenol blue | 0.1% (w/v) | 10 mg | |
| H2O to 10 ml |
Step 6 1× MOPS running buffer
| Dilute 1:20 from commercially available 20× buffer (Invitrogen) |
Step 7 Proteinase K storage buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Proteinase K | 20 mg ml−1 | 200 mg | |
| Tris–HCl, pH 8 | 50 mM | 1 M | 0.5 ml |
| CaCl2·2H2O | 30 mM | 1 M | 30 μl |
| Glycerol | 50% | 100% | 5 ml |
| H2O to 10 ml |
2× Proteinase K buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Tris–HCl, pH 7.5 | 100 mM | 1 M | 1 ml |
| EDTA–NaOH, pH 8.0 | 12.5 mM | 0.5 M | 0.25 ml |
| NaCl | 150 mM | 3 M | 0.5 ml |
| SDS | 2% (v/v) | 20% | 1 ml |
| H2O | 7.25 ml |
Acidic Phenol/Chloroform/IAA (25:24:1)
| Combine 25-ml acidic phenol, 24-ml chloroform and 1-ml isoamyl alcohol (overlay with 0.1-M citrate buffer, pH 4.3 ± 0.2 which you can take from the acidic phenol bottle) |
Step 8 50% DMSO
| Mix 1-ml DMSO with 1-ml H2O |
10× RNA ligase buffer without ATP
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Tris–HCl, pH 7.6 | 0.5 M | 1 M | 5 ml |
| MgCl2·6H2O | 0.1 M | 1 M | 1 ml |
| 2-Mercaptoethanol | 0.1 M | 14.3 M | 0.07 ml |
| Acetylated BSA | 1 mg ml−1 | 20 mg ml−1 | 0.5 ml |
| H2O | 3.43 ml |
2× Formamide loading dye
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| EDTA–NaOH, pH 8.0 | 50 mM | 0.5 M | 2 ml |
| Bromophenol blue | 0.05% (w/v) | 5 mg | |
| Formamide | 8 ml |
10× TBE
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Tris base | 445 mM | 53.9 g | |
| Boric acid | 445 mM | 27.5 g | |
| EDTA–NaOH, pH 8.0 | 10 mM | 0.5 M | 20 ml |
| H2O to 1 l |
0.4 M NaCl
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| NaCl | 0.4 M | 3 M | 66.7 ml |
| H2O | 433.3 ml |
Step 9 10× RNA ligase buffer with ATP
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Tris–HCl, pH 7.6 | 0.5 M | 1 M | 5 ml |
| MgCl2·6H2O | 0.1 M | 1 M | 1 ml |
| 2-Mercaptoethanol | 0.1 M | 14.3 M | 0.07 ml |
| Acetylated BSA | 1 mg ml−1 | 20 mg ml−1 | 0.5 ml |
| ATP | 2 mM | 100 mM | 0.2 ml |
| H2O | 3.23 ml |
Step 10 10× dNTP solution
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| dATP | 2 mM | 0.1 M | 0.2 ml |
| dCTP | 2 mM | 0.1 M | 0.2 ml |
| dGTP | 2 mM | 0.1 M | 0.2 ml |
| dTTP | 2 mM | 0.1 M | 0.2 ml |
| H2O | 9.2 ml |
150-mM KOH/20-mM Tris base
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| KOH | 150 mM | 5 M | 30 μl |
| Tris base | 20 mM | 1 M | 20 μl |
| H2O | 950 μl |
150-mM HCl
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| HCl, concentrated | 150 mM | 12.1 M | 12.4 μl |
| H2O | 987.6 μl |
Step 11 10× PCR buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Tris–HCl, pH 8.0 | 100 mM | 1 M | 1 ml |
| KCl | 500 mM | 1 M | 5 ml |
| 2-Mercaptoethanol | 10 mM | 14.3 M | 7 μl |
| Triton X-100 | 1% (v/v) | 100% | 0.1 ml |
| MgCl2·6H2O | 20 mM | 1 M | 2 ml |
| H2O | 1.9 ml |
5× DNA loading dye
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| EDTA–NaOH, pH 8.0 | 50 mM | 0.5 M | 1 ml |
| Bromophenol blue | 0.2% (w/v) | 20 mg | |
| Ficoll type 400 | 20% (w/v) | 2 g | |
| H2O to 10 ml |
Step 12 1 M DTT
| Dissolve 1.54-g DTT in 10-ml water |
3 M NaOAc (pH 5.2)
| Component | Stock | Final concentration | Amount |
|---|---|---|---|
| NaOAc | n/a | n/a | 246.09 g |
| H2O to 1 l |
HPLC buffer A
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Acetonitrile | 3% | 100% | 30 ml |
| TEAA | 0.1 M | 2 M | 50 ml |
| H2O to 1 l |
HPLC buffer B
| Mix 900-ml acetonitrile with 100-ml water |
4. PROTOCOL
4.1. Preparation
4.1.1 Cells
Expand cells in an appropriate growth medium containing selection antibiotics as appropriate to maintain your stable cell line. We usually prepare lysates from 3 to 5 ml of wet cell pellet from crosslinked cells per experiment. This corresponds to 20–50 15-cm cell culture plates (for HEK293). However, if material is limiting, we have performed successful PAR-CLIPs experiments from <0.5 ml of wet cell pellet (200 × 106 HEK293 cells (10 15-cm plates) will yield ~1 ml of wet cell pellet).
Grow cells to ~80% confluence. Fourteen hours before crosslinking, add 4-thiouridine (4SU) to a final concentration of 100 μM directly to the cell culture medium. 6-Thioguanosine (6SG, 100 μM) can also be used as the photoactivatable ribonucleoside. Induce expression of protein, if necessary.
4.2. Tip
If you want to add 4SU to 50 15-cm cell culture plates containing 20 ml of growth medium each, place 265 ml of growth medium into a sterile, empty bottle (e.g., an empty media bottle). Add 132.5-μl 1 M 4SU and mix. Additional reagents such as doxycycline (e.g., 1 μg ml−1) to induce protein expression may be added. Dispense 5 ml of the prepared growth medium containing 4SU per 15-cm plate.
4.2.1 Buffers
Buffer recipes and required reagents are listed above. Allow ~1 day for general preparations, including buffer preparation. All pH measurements and adjustments are performed at room temperature. Buffers and all perishable reagents should be refrigerated for storage. We use water purified by a Millipore water purification system.
On the day before you start the PAR-CLIP procedure, fill the required amounts of the individual buffers into 50-ml conical tubes and refrigerate them. The table below gives a rough guide to the required amounts of the individual buffers (but only of those that will be used in quantities above 1 ml on the first 2 days). Add DTT and protease inhibitors on the day of the experiment.
| Buffer | Amount per sample |
|---|---|
| PBS | About 1 l for 20–30 15-cm cell culture plates |
| Citrate–phosphate buffer | 5 ml |
| NP40 lysis buffer | 3 ml per ml cell pellet volume |
| IP wash buffer | 3 ml + 1/10 cell pellet volume |
| High-salt wash buffer | 3 ml |
| Phosphatase wash buffer | 2 ml |
| PNK buffer without DTT | 7 ml |
4.2.2 Antibodies
This protocol was originally developed using anti-FLAG antibodies; use of a different antibody will likely require the optimization of the appropriate IP and wash conditions prior to starting a large-scale experiment. Ensure that the optimal salt concentration for antibody binding is maintained throughout the protocol; washing the immunoprecipitate with high salt may disrupt antibody–antigen interactions.
If in doubt, you can use NP40 lysis buffer instead of the IP and the high-salt wash buffers; however, removal of nonspecifically interacting RNAs might be less efficient.
The table below shows the protocol modifications for an anti-AGO2 antibody (Millipore, 04-642) that we have also used successfully:
| Step | Anti-FLAG antibody | Anti-AGO2 antibody |
|---|---|---|
| Wash after IP | 3× IP wash buffer | 3× NP40 lysis buffer |
| KCl concentration | 300 mM | 150 mM |
| High-salt wash | 3× high-salt wash buffer | 3× NP40 lysis buffer |
| KCl concentration | 500 mM | 150 mM |
Except for these two buffers, all the other washing steps are performed as described.
4.2.3 Radiolabeling
On day 3, you will need 5′-32P-radiolabeled RNA size markers. It is advisable to prepare them before starting the PAR-CLIP experiment. Perform a standard radiolabeling procedure with T4 PNK and [γ-32P]-ATP according to the manufacturer’s guidelines and gel-purify the markers (e.g., phosphorylate 1-μM RNA size marker in a 10-μl reaction volume using 1 μl of [γ-32P]-ATP (see RNA Radiolabeling). Keep radioactive gel pieces from the running front of this gel as markers to implant into gels for alignment of phosphorimager printouts to exposed gels later on.
4.3. Caution
Consult your institute’s Radiation Safety Officer for proper ordering, handling, and disposal of radioactive materials.
4.4. Duration
| Preparation | Expanding cell line(s) | Approximately 2 weeks depending on the desired number of cells |
|---|---|---|
| Antibody testing | Variable | |
| Buffers etc. | 1 day | |
| Radiolabeling of RNA size markers | 1.5 days | |
| Protocol | Total | 7 days |
| Day 1 | 3–4 h | |
| Day 2 | 10–12 h | |
| Day 3 | 5–6 h | |
| Day 4 | 3–4 h | |
| Day 5 | 5–6 h | |
| Day 6 | 6–7 h | |
| Day 7 | 4–5 h |
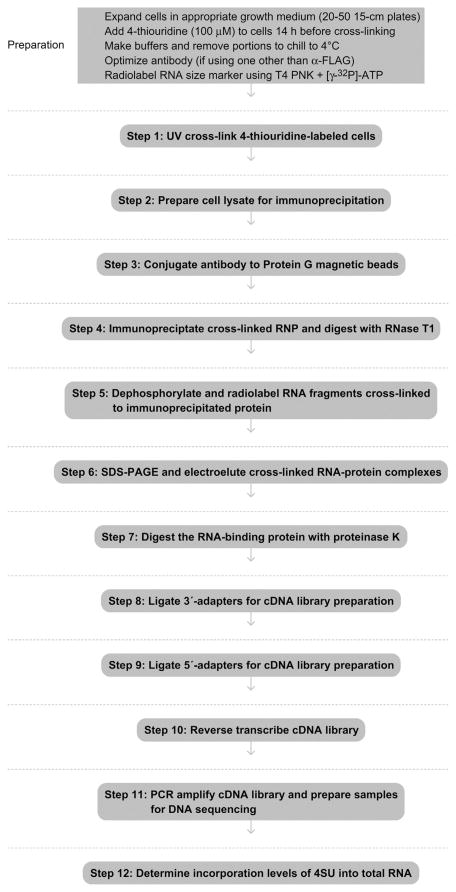
See Figure 8.1 for the flowchart of the complete protocol.
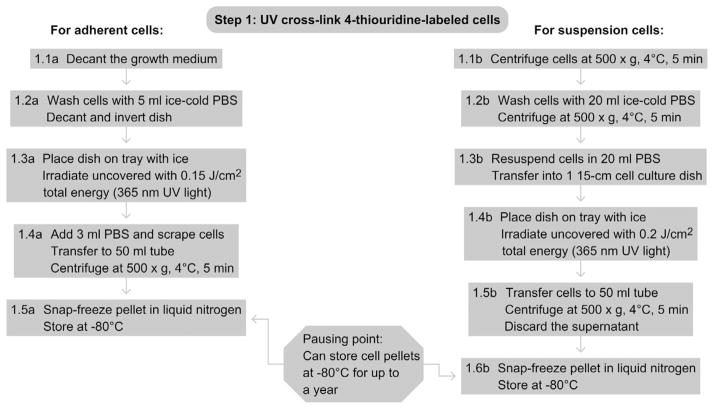
5. STEP 1 UV CROSSLINKING OF 4-THIOURIDINE-LABELED CELLS (DAY 1)
5.1. Overview
In this first step, the RNA-binding protein of interest is cross-linked to its bound mRNAs targets that incorporated the photoactivatable ribonucleoside into nascent transcripts during the labeling step (see also UV crosslinking of interacting RNA and protein in cultured cells). The cells are then collected and the resulting cell pellet will be used as the input for the following PAR-CLIP procedure.
5.2. Duration
About 2–3 h
5.2.1 For adherent cells
-
1.1a
Decant growth medium.
-
1.2a
Wash cells once with 5-ml ice-cold PBS per plate and remove PBS completely by decanting and inverting the cell culture dish.
-
1.3a
Place plates on a tray filled with ice to keep cells cold and irradiate uncovered with 0.15 J cm−2 total energy of 365-nm UV light in a Stratalinker 2400 or similar device.
-
1.4a
Add 3-ml PBS per plate and dislodge cells with a cell scraper. Transfer to prechilled 50-ml centrifugation tubes on ice. After the cells from the last plate have been collected, centrifuge at 500 × g for 5 min at 4 °C and discard the supernatant. Expect to obtain about 5 ml of wet cell pellet from 50 15-cm plates.
-
1.5a
(Optional: Pause point) If you do not want to proceed directly to the cell lysis, snap-freeze the cell pellet in liquid nitrogen and store at 80 °C. Cell pellets can be stored for at least 12 months.
5.2.2 For cells grown in suspension culture
-
1.1b
Collect cells by centrifugation at 500 × g for 5 min at 4 °C.
-
1.2b
Wash cells by resuspending in 20-ml ice-cold PBS and spin again at 500 × g for 5 min at 4 °C.
-
1.3b
Resuspend cells in 20-ml ice-cold PBS and transfer into one 15-cm cell culture plate.
-
1.4b
Place plate on a tray with ice and irradiate uncovered with 0.2 J cm−2 of 365-nm UV light in a Stratalinker 2400 or similar device.
-
1.5b
Transfer cells into a 50-ml centrifugation tube, collect by centrifugation at 500 × g for 5 min at 4 °C, and discard the supernatant.
-
1.6b
(Optional: Pause point) If you do not want to proceed directly to the cell lysis, snap-freeze the cell pellet in liquid nitrogen and store at −80 °C. Cell pellets can be stored for at least 12 months.
5.3. Tip
Keep cell suspensions on ice until centrifugation.
See Fig. 8.2 for the flowchart of Step 1.
Figure 8.2.
Flowchart of Step 1.
6. STEP 2 PREPARATION OF CELL LYSATE FOR IMMUNOPRECIPITATION (DAY 2)
6.1. Overview
The cell pellet obtained on day 1 will be lysed (see also Lysis of mammalian and Sf9 cells) in preparation for immunoprecipitation. Partial RNase T1 digestion of mRNAs facilitates the recovery of cross-linked mRNPs.
6.2. Duration
2 h
-
2.1
Thaw the cross-linked cell pellet on ice. Prepare the magnetic beads (see Step 3) while the pellet thaws. Resuspend the cell pellet in 3 cell pellet volumes of NP40 lysis buffer and incubate on ice for 10 min.
-
2.2
Clear the cell lysate by centrifugation at 13 000 × g for 15 min at 4 °C.
-
2.3
Clear the lysate further by filtering it through a 5-μm membrane syringe filter. Attach the syringe filter to a 20-ml syringe, remove the plunger, and transfer the supernatant into the syringe. Be careful to hold the syringe above the 50-ml conical tube since the lysate will start to drip through the filter by gravity at first. Insert the plunger and gently apply pressure until all of the lysate is filtered. Depending on the initial viscosity of the lysate, it might be necessary to exchange a clogged filter for a fresh one.
-
2.4
Add RNase T1 to a final concentration of 1 U μl−1 and incubate in a water bath for 15 min at 22 °C. Invert to mix from time to time. Cool reaction for 5 min on ice before proceeding.
-
2.5
Remove a 10-μl aliquot for immunoblotting as a control for the protein levels used as input and freeze at −20 °C.
6.3. Tip
Take the cell pellet out of the −80 °C freezer and put it on ice first thing in the morning since the thawing process takes a long time.
6.4. Tip
The temperature and duration of the incubation with RNase T1 are both critical for obtaining a controlled partial digestion.
6.5. Tip
Designate a set of micropipettors for working with RNases to avoid contamination at later RNA isolation and cDNA library preparation steps.
See Fig. 8.3 for the flowchart of Step 2.
Figure 8.3.
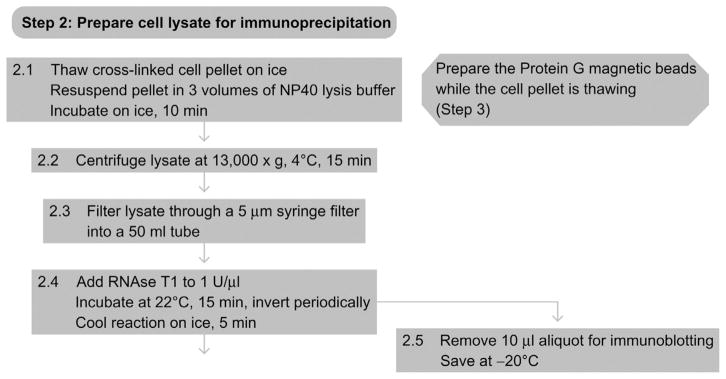
Flowchart of Step 2.
7. STEP 3 PREPARATION OF THE MAGNETIC BEADS (DAY 2)
7.1. Overview
The antibody is conjugated to protein G magnetic beads to be used in the subsequent immunoprecipitation. Protein G is the optimal Ig-binding protein for anti-FLAG antibodies based on species and isotype. The choice of protein A versus protein G should be considered depending on the antibody used.
7.2. Duration
1.5 h
-
3.1
Transfer 10 μl of Protein G magnetic particles per ml cell lysate (typically ~100–150 μl of beads) to a 1.5-ml microtube. Put the magnetic rack on ice. Wash the beads twice with 1 ml of citrate–phosphate buffer.
-
3.2
Resuspend the beads in twice the volume of citrate–phosphate buffer relative to the original volume of bead suspension (i.e., 200–300 μl).
-
3.3
Add antibody to a final concentration of 0.25 mg ml−1 and incubate on a rotating wheel for 40 min at room temperature.
-
3.4
Collect the beads and wash twice in 1 ml of citrate–phosphate buffer to remove unbound antibody.
-
3.5
Resuspend beads in twice the volume of citrate–phosphate buffer relative to the original volume of bead suspension.
7.3. Tip
This step is performed while the cell pellet is thawing.
7.4. Tip
Be careful to not let the magnetic beads dry out.
See Fig. 8.4 for the flowchart of Step 3.
Figure 8.4.
Flowchart of Step 3.
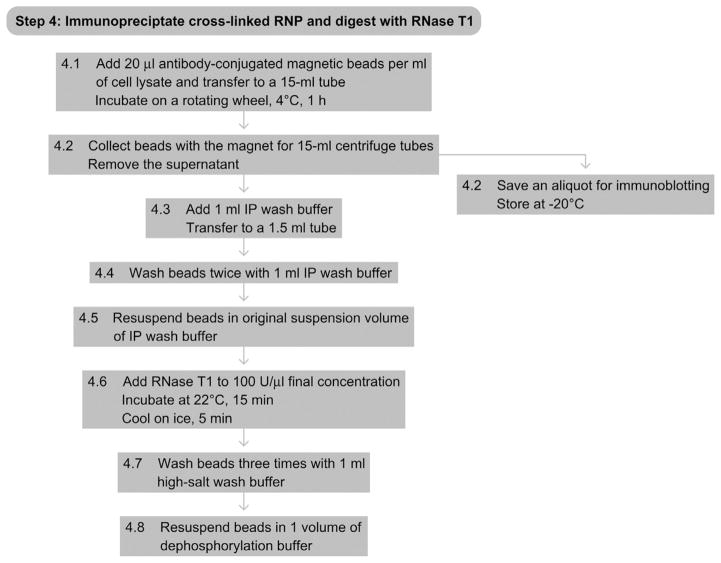
8. STEP 4 IMMUNOPRECIPITATION AND SECOND RNase T1 Treatment (Day 2)
8.1. Overview
The mRNA-RBP complex of choice is isolated from the lysate by immunoprecipitation. A second RNase T1 digestion ensures that only the RNA segment that was bound, crosslinked, and protected by the RBP is recovered and sequenced. This enables the precise definition of the binding sites.
8.2. Duration
2 h
-
4.1
Add 20 μl of freshly prepared antibody-conjugated magnetic beads per ml of the partially RNase T1-treated cell lysate from Step 2 and incubate in a 15-ml centrifuge tube on a rotating wheel for 1 h at 4 °C.
-
4.2
Collect magnetic beads on a magnetic particle collector for 15-ml centrifuge tubes (Invitrogen) and remove the supernatant. Save an aliquot for immunoblotting.
-
4.3
Add 1 ml of IP wash buffer and transfer to 1.5-ml polypropylene tubes.
-
4.4
Wash beads 2 times in 1 ml of IP wash buffer.
-
4.5
Resuspend beads in the original bead volume of IP wash buffer.
-
4.6
Add RNase T1 (Fermentas, 10 000 U μl−1) to a final concentration of 100 U μl−1 and incubate the bead suspension in a 22 °C water bath for 15 min. Cool on ice for 5 min.
-
4.7
Wash beads 3 times with 1-ml high-salt wash buffer.
-
4.8
Resuspend beads in 1 volume of dephosphorylation buffer.
8.3. Tip
The RNase T1 incubation temperature and time are both crucial to avoid over-digestion of RNA. Overdigestion could result in RNA segments that are too short to be mapped uniquely to transcript or genomic sequences.
See Fig. 8.5 for the flowchart of Step 4.
Figure 8.5.
Flowchart of Step 4.
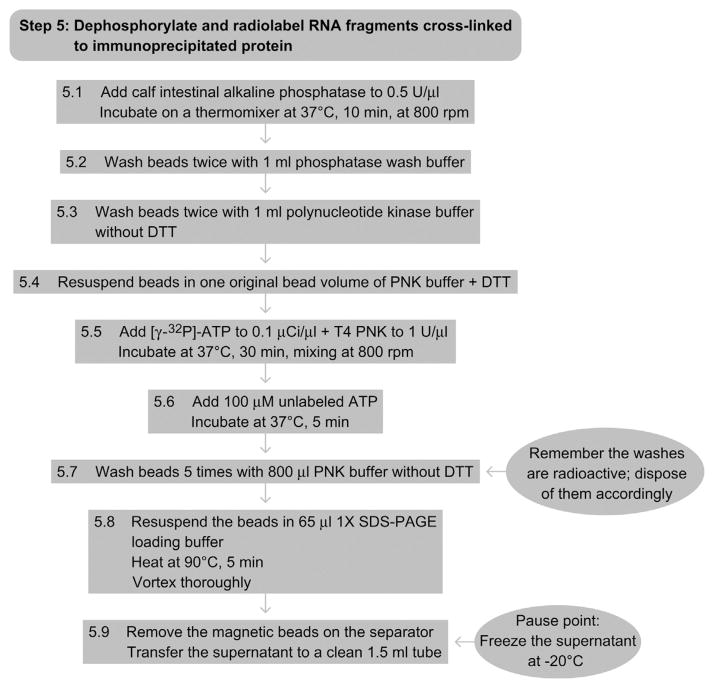
9. STEP 5 DEPHOSPHORYLATION AND RADIOLABELING RNA SEGMENTS CROSSLINKED TO IMMUNOPRECIPITATED PROTEINS (DAY 2)
9.1. Overview
The RNAs crosslinked by the RBP of interest are radiolabeled using T4 PNK and [γ-32P]-ATP in order to visualize them by autoradiography after fractionation by SDS-PAGE (see also RNA Radiolabeling).
9.2. Duration
2 h
-
5.1
Add calf intestinal alkaline phosphatase (CIP from NEB) to a final concentration of 0.5 U μl−1, and incubate the suspension for 10 min at 37°C and mixing at 800 rpm.
-
5.2
Wash beads twice with 1 ml of phosphatase wash buffer.
-
5.3
Wash beads twice with polynucleotide kinase (PNK) buffer without DTT.
-
5.4
Resuspend beads in one original bead volume of PNK buffer containing DTT.
-
5.5
Add [γ-32P]-ATP to a final concentration of 0.1 μCi μl−1 and T4 PNK (NEB) to 1 U μl−1. Incubate the suspension for 30 min at 37 °C and 800 rpm, mixing manually every 5–10 min.
-
5.6
Add 100-μM nonradioactive ATP and incubate for another 5 min at 37 °C. This ensures that all RNAs are fully 5′phosphorylated, which is required for the ligation of the 5′ adapter (Step 9).
-
5.7
Wash the magnetic beads 5 times with 800 μl of PNK buffer without DTT; dispose of the radioactive buffer according to local guidelines.
-
5.8
Resuspend the beads in 65 μl of 1 × SDS-PAGE loading buffer, incubate for 5 min in a heat block at 90 °C to denature, and release the immunoprecipitated RBP with the cross-linked, radiolabeled RNAs from the beads. Vortex.
-
5.9
Remove the magnetic beads on the separator and transfer the supernatant to a clean 1.5-ml microcentrifuge tube. (Pause point: you can freeze the supernatant and continue with the protocol at another time.)
9.3. Tip
Remove the [γ-32P]-ATP from the freezer and place it at room temperature during the dephosphorylation incubation so that it is thawed by the time you need it.
See Fig. 8.6 for the flowchart of Step 5.
Figure 8.6.
Flowchart of Step 5.
10. STEP 6 SDS-PAGE AND ELECTROELUTION OF CROSS-LINKED RNA-PROTEIN COMPLEXES FROM GEL SLICES (DAYS 2 AND 3)
10.1. Overview
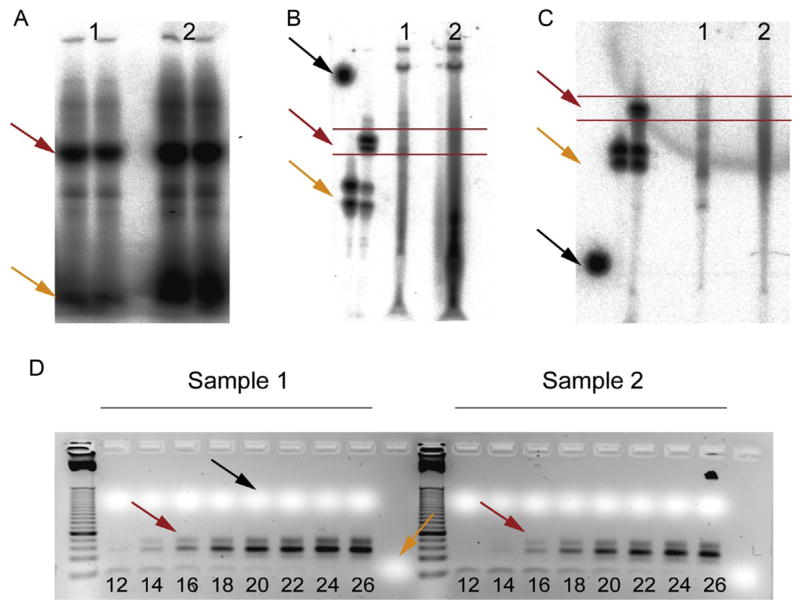
Size fractionation of the radiolabeled and cross-linked RNA protein complexes is achieved by SDS-PAGE (see One-dimensional SDS-Polyacrylamide Gel Electrophoresis (1D SDS-PAGE)). The band corresponding to the expected mass of the protein will be excised and the cross-linked RNA protein complexes electroeluted. This step ensures that only the band corresponding to the correct RBP is isolated and additionally prevents any unbound but labeled RNA from further processing (see Fig. 8.7(a)).
Figure 8.7.
Selected PAR-CLIP experimental steps. (a) SDS-PAGE gel of cross-linked and 5′-radiolabeled RNA-protein complex immunoprecipitates. The red arrow points to the radioactive bands corresponding to the expected size of the RNA-binding protein (FUS, running at 75 kDa), and the yellow to the radioactive running front. (b) 8 M urea poly-acrylamide gel after 3′-adapter ligation. The black arrow indicates one of the inserted little radioactive gel pieces to facilitate alignment of the gel to printout, the red to the 3′-ligated size markers, and the area that was cut from the gel and further processed; the yellow arrows show the unligated 3′-size markers. (c) 8-M urea polyacrylamide gel after 5′-adapter ligation. The black arrow indicates one of the inserted little radioactive gel pieces to facilitate alignment of the gel to printout, the red to the 5′-ligated size markers, and the area which was cut from the gel and further processed; the yellow arrows show the unligated 5′-size markers. (d) Agarose gel after small-scale trial PCR. The black arrow points to the position of migration of the xylene cyanol loading dye, and the yellow to the bromophenol blue loading dye running close to the gel front. Bands of about 75 and 100 bp are detectable, representing insert-less 5′-adapter-3′-adapter PCR side product and expected insert-containing PCR product, respectively. The red arrows indicate the number of cycles chosen for the large-scale experiment. A 25-bp ladder is loaded to the left of each set of experiments; the fourth band from the bottom corresponds to 100 bp. The negative control was performed but is not shown.
10.2. Duration
4.5 h
-
6.1
Load 2 × 30 μl of the supernatant into two adjacent wells on a Novex Bis-Tris 4–12% (Invitrogen) precast SDS-PAGE gel. Leave at least one empty lane between different samples or different proteins of interest. Load a protein ladder on both sides of the gel. Save the remaining 5 μl of the bead eluate for immunoblotting.
-
6.2
Run the gel in 1 × MOPS-SDS running buffer for 45–60 min at 200 V until the loading dye has reached the bottom of the gel.
-
6.3
Disassemble the gel chamber (the buffer will be radioactive!) and carefully dismantle the gel, leaving it mounted on one plate. Cut the protruding bottom of the gel so that the gel will lie flat on the phosphorimager screen.
-
6.4
To facilitate the alignment of the gel to the phosphorimager paper printout later on, place three tiny radioactive gel pieces asymmetrically into three of the four corners of the gel. Radioactive gel pieces could be collected earlier from the bottom of the gel from radiolabeling the size markers (see above).
-
6.5
After placing the gel pieces, wrap the gel in plastic wrap and expose the gel to a phosphorimager screen for 15 min. Visualize it on a phosphorimager. Have a second screen ready and expose it during the scanning process should the first exposure indicate that a longer exposure is necessary.
-
6.6
Print the scanned image file at its original size (100%). Align the transparently wrapped gel on top of the printout guided by the implanted gel pieces for precise positioning. Cut out the bands that correspond to the expected size of the RBP (see Fig. 8.7(a)).
-
6.7
Add 800 μl of water to a D-Tube Dialyzer Midi Tube used for electroelution and let stand at room temperature for 5 min. Remove the water. Take care not to pierce the membrane.
-
6.8
Transfer the excised bands to the dialyzer tube and add 800-μl 1 × MOPS-SDS running buffer.
-
6.9
Place the electroelution rack with the tubes in an agarose gel chamber so that the membrane is exposed to the flow of the current (for details, see manufacturer’s instructions). Add 1 × MOPS-SDS running buffer to the chamber until it covers the tubes.
-
6.10
Electroelute the cross-linked RNA-RBP complex at 100 V for 1.5 h. Reverse the current for 2 min to release any protein attached on the dialysis membrane.
-
6.11
Transfer the solution to two siliconized tubes so that each contains around 350 μl (you will not be able to fully recover the original 800 μl). (Pause point: you can freeze the solution at −20 °C and continue the next day.)
10.3. Tip
To confirm that the correct band was excised from the gel, run a second small-scale SDS-PAGE gel with 1 or 2 μl of the remaining 5 μl of your sample (see above). After transferring the gel to a nitrocellulose membrane, take an autoradiography exposure (exposing for 1–2 h) and then use protein-specific antibodies to perform a standard immunoblot (see Western Blotting using Chemiluminescent Substrates). After overlaying the resulting images, you should be able to establish which band corresponds to your protein of interest and proceed with the protocol.
10.4. Tip
In case you observe more than one band, you can also cut all of them since they should correspond to other co-purifying RNA cross-linked proteins.
10.5. Tip
Make sure that the membrane of the dialyzer tube is aligned correctly to allow flow of current.
10.6. Tip
Use aerosol barrier tips and take general precautions to avoid any RNase contamination since you will be working with RNA from now on until the reverse transcription step on day 6. Clean your pipettors before starting to work with RNA.
10.7. Tip
Use siliconized tubes until you have obtained your cDNA library; at low concentrations, nucleic acids have a tendency to stick to the tube walls.
See Fig. 8.8 for the flowchart of Step 6.
Figure 8.8.
Flowchart of Step 6.
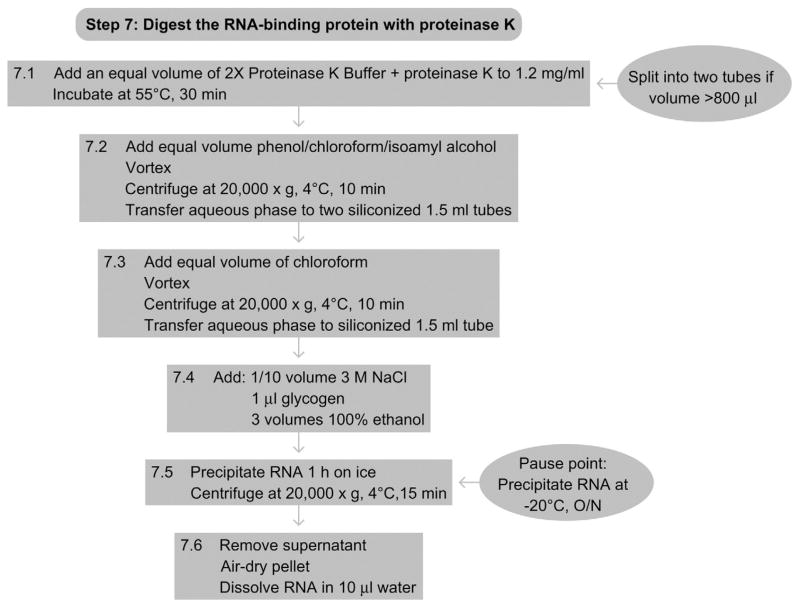
11. STEP 7 PROTEINASE K DIGESTION (DAY 3)
11.1. Overview
In this step, the recovered RBP is proteolyzed and the cross-linked RNA is recovered so that it can serve as the input material for subsequent ligation to adapters and Solexa sequencing.
11.2. Duration
3.5 h
-
7.1
Add 1 volume of 2 × Proteinase K Buffer, followed by the addition of Proteinase K (Roche) to a final concentration of 1.2 mg ml−1. Incubate at 55 °C for 30 min. If the volume per tube exceeds 800 μl at this stage, split the sample into two tubes.
-
7.2
Add 1 volume of acidic phenol/chloroform/isoamyl alcohol, vortex and spin at 20 000 × g for 10 min at 4 °C. Recover the upper aqueous phase without disturbing the interphase and pipette into two siliconized tubes.
-
7.3
Add an equal volume of chloroform, and vortex and spin at 20 000 × g for 10 min at 4 °C. Recover the aqueous phase without disturbing the interphase.
-
7.4
Add 1/10 volume 3 M NaCl, 1 μl of glycogen (10 mg ml−1 stock), and 3 volumes of 100% ethanol.
-
7.5
Precipitate the RNA for 1 h on ice and spin at 20 000 × g for 15 min at 4 °C. (Pause point: precipitate the RNA overnight at 20 °C. See also RNA purification – precipitation methods.)
-
7.6
Remove the supernatant, air-dry the pellets, and dissolve in a total of 10 μl of H2O.
11.3. Tip
Monitor the radioactivity of the supernatant and the pellet to assess the efficiency of the ethanol precipitation.
11.4. Tip
Repeat the phenol/chloroform/IAA extraction until there is no precipitate visible in the interphase (usually, once is sufficient, but two or more times might be needed).
See Fig. 8.9 for the flowchart of Step 7.
Figure 8.9.
Flowchart of Step 7.
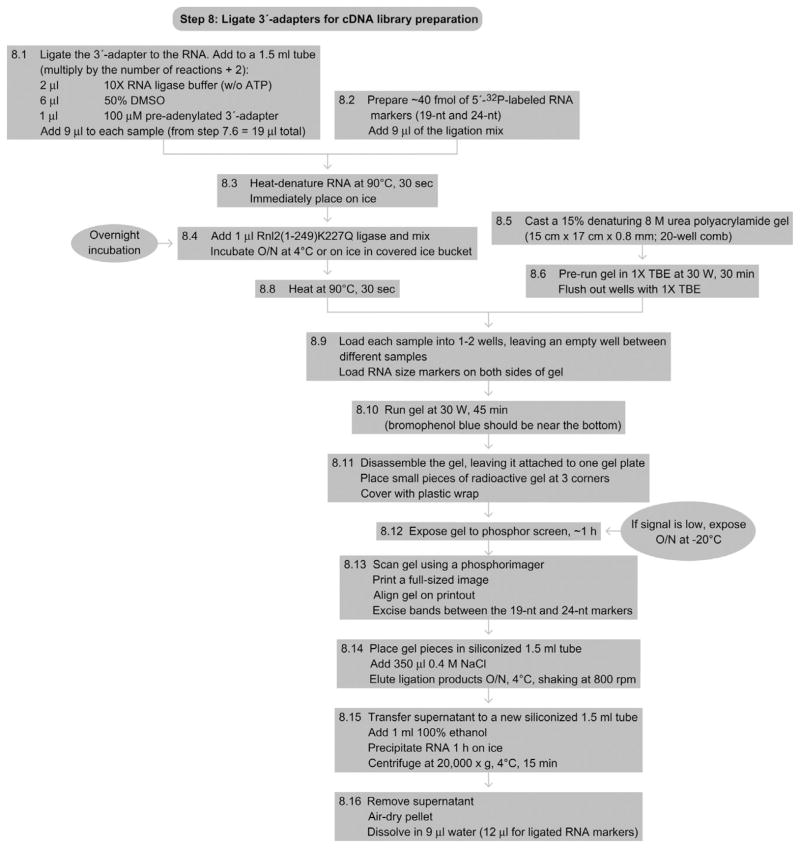
12. STEP 8 3′-ADAPTER LIGATION FOR cDNA Library Preparation (Day 3 overnight, day 4, beginning of day 5)
12.1. Overview
The recovered 5′-32P-phosphorylated RNA is now carried through a standard cDNA library preparation protocol, originally described for the cloning of small regulatory RNA (Hafner et al., 2008). As a first step, a preadenylated 3′-adapter is ligated using T4 Rnl2(1-249)K227Q ligase (see Fig. 8.7(b)).
12.2. Duration
Day 3: 30 min (+ overnight incubation)
Day 4: 4–5 h (highly dependent on required exposure time)
Day 5: 2 h
-
8.1
Prepare the following reaction mixture for ligating the 3′-adapter, multiplying the volumes by the number of ligation reactions plus two (one for the size markers plus another to account for pipetting loss):
2 μl of 10 × RNA ligase buffer (without ATP)
6 μl 50% DMSO
1 μl of 100 μM preadenylated 3′-adapter
Add 9 μl of the reaction mixture to each sample (from Step 7.6, you have 19 μl per tube).
-
8.2
Prepare ~40 fmol of a 1:100 dilution of 5′-32P-labeled RNA size markers (19-nt and 24-nt size marker at equimolar concentrations, see preparation step). This controls for successful ligation and indicates the length of the bands that will be cut out from the gel later on.
-
8.3
Heat-denature the RNA to disrupt secondary structures by incubating at 90 °C for 30 s. Immediately place the tubes on ice for 30 s.
-
8.4
Add 1 μl of Rnl2(1-249)K227Q ligase (1 μg μl−1) to the ligation reactions, mix gently, and incubate overnight on ice in the cold room or in an insulated ice bucket covered with a lid.
-
8.5
The next morning, cast a 15% denaturing 8-M urea polyacrylamide gel (we use the UreaGel system from National Diagnostics. See also RNA purification by preparative polyacrylamide gel electrophoresis) and wait until the polymerization process is complete. Our gels measure 15 × 17 cm × 0.8 mm and contain about 25-ml gel volume with a 20 well comb.
-
8.6
Prerun the gel in 1 × TBE buffer at 30 W for 30 min. After the prerun, flush the wells with 1 × TBE.
-
8.7
Add 20 μl of formamide gel loading solution to the samples to stop the ligation reactions.
-
8.8
Denature the RNA at 90 °C for 30 s.
-
8.9
Load each sample into one well (or two) of the gel. Load the size markers symmetrically on both sides of the gel to allow for approximating the length of the ligated samples between them. Use the center lanes of the gel to guarantee even running of the gel. Make sure to space different samples appropriately, typically at a two-well distance, to avoid cross contamination. Ensure that the overall loading of the gel is asymmetrical.
-
8.10
Run the gel at 30 W for 45 min until the bromophenol blue dye is close to the bottom of the gel.
-
8.11
Dismantle the gel, leaving it attached to one glass plate. To facilitate the alignment of the gel to the phosphorimager paper printout, again implant three tiny radioactive gel pieces asymmetrically at three of the four corners of the gel. Cover the gel with plastic wrap.
-
8.12
Expose the gel to a phosphorimager screen for at least 1 h. If the radioactivity of the recovered RNA is weak, you can expose the gel overnight, placing the exposure cassette in a −20 °C freezer. Allow the cassette to return to room temperature before opening it.
-
8.13
Align the gel on top of a full-scale printout according to the position of the three radioactive gel pieces. Cut out the bands in between the ligated products of the 19-nt and above the 24-nt marker (Note: We do not recommend cutting of RNA that is running below the 19-nt marker line. For our bioinformatic analyses, all sequences shorter than 20 nucleotides are discarded due to the increased probability of mapping to multiple locations and the uncertainty defining its genetic location. Our bioinformatic analysis pipeline discards reads under 20 nucleotides for that reason. In case you would like to cut a larger size range, we also have successfully used two larger-sized markers (33-nt and 35-nt). Cut out the ligated 19- and 24-nt size markers; they will serve once more as a ligation control in the next step (see Figure 8.7(b)).
-
8.14
Place the cut gel pieces in siliconized tubes and add 350-μl 0.4-M NaCl (ensure that the gel pieces are covered by NaCl). Elute the ligation products overnight at 4 °C, shaking at 800 rpm.
-
8.15
Transfer the supernatant into a new siliconized tube and add 1-ml 100% ethanol. Precipitate the RNA for 1 h on ice and spin at 20 000 × g for 15 min at 4 °C.
-
8.16
Remove the supernatant, air-dry the pellets, and dissolve in a total of 9-μl H2O. Dissolve the ligated markers in 12-μl H2O.
12.3. Tip
Keep the supernatant from the ethanol precipitation. In case no pellet should form after the precipitation, you can add 1 μl of glycogen to it and precipitate again. We do not routinely add glycogen at this stage, since the relatively high amount of glycogen might interfere with the subsequent reaction, which is performed in a small volume. The linear acrylamide eluted from the gel is usually a sufficient carrier.
See Fig. 8.10 for the flowchart of Step 8.
Figure 8.10.
Flowchart of Step 8.
13. STEP 9 5′-ADAPTER LIGATION FOR cDNA Library Preparation (Day 5, beginning of day 6)
13.1. Overview
In this step, the 5′-adapter is joined to the 3′-ligated RNA to enable the cDNA synthesis in the next step (see Fig. 8.7(c)).
13.2. Duration
Day 5: about 5 h (highly dependent on required exposure time)
Day 6: 2 h
-
9.1
Prepare the following reaction mixture for the ligation of the 5′-adapter, multiplying the volumes by the number of ligation reactions to be performed plus two (for the positive control plus one extra to account for pipetting loss):
1 μl of 100 μM 5′-adapter
2 μl of 10 × RNA ligase buffer with ATP
-
6 μl 50% DMSO
Combine 9 μl of this mixture with 9 μl of sample.
Remember to also process the ligated markers from the last step. Ligate 9 μl out of the 12 μl and keep 3 μl as an unligated control for the next gel.
-
9.2
Denature the RNA by incubation at 90 °C for 30 s. Immediately place the tube on ice for 30 s.
-
9.3
Add 2 μl of T4 RNA ligase 1 (10 U μl−1), mix gently, and incubate for 1 h at 37 °C.
-
9.4
In the meantime, cast a 12% denaturing 8-M urea polyacrylamide gel and wait until the polymerization process is complete. We again use 0.8-mm spacers and a 20 well comb.
-
9.5
Prerun the gel 1 × TBE buffer at 30 W for 30 min. After the prerun, gently flush the wells with 1 × TBE.
-
9.6
Add 20 μl of formamide gel loading solution to the samples, incubate them at 90 ° C for 30 s, and load the gel. Make sure to space different samples appropriately, typically at a two-well distance, to avoid cross contamination.
Load 50% of the ligated markers on the left side and 50% on the right side. Load the remaining 3-μl unligated marker on one side (see Fig. 8.7(c)).
-
9.7
Run the gel at 30 W for 45 min until the bromophenol blue dye is close to the bottom of the gel. Disassemble and image the gel as described above for the 3′-ligation (start with an exposure roughly twice as long as for the 3′-ligation) and excise the new ligation product (also excise the ligated markers).
-
9.8
Elute the ligation products from the gel slices in 350-μl 0.4-M NaCl. Shake at 800 rpm overnight at 4 °C. Add 1 μl of 100 μM 3′ PCR primer as a carrier to facilitate the recovery of the ligation products.
-
9.9
Pipet the supernatant into a new siliconized tube and add 1-ml 100% ethanol. Precipitate the RNA for 1 h on ice and spin at 20 000 × g at 4°C for 15 min.
-
9.10
Remove the supernatant, air-dry the pellets, and dissolve in 5.6-μl H2O.
13.3. Tip
Make sure that the loading of the gel is asymmetrical.
13.4. Tip
You can recover unligated material by excising the gel region below the ligated 19-nt marker line, since this represents 3′-ligated, 5′-unligated RNA fragments. Freeze these gel pieces. You will have them stored as a backup in case you later wish to perform another 5′-adapter ligation from the RNAs eluted from these gel pieces.
See Fig. 8.11 for the flowchart of Step 9.
Figure 8.11.
Flowchart of Step 9.
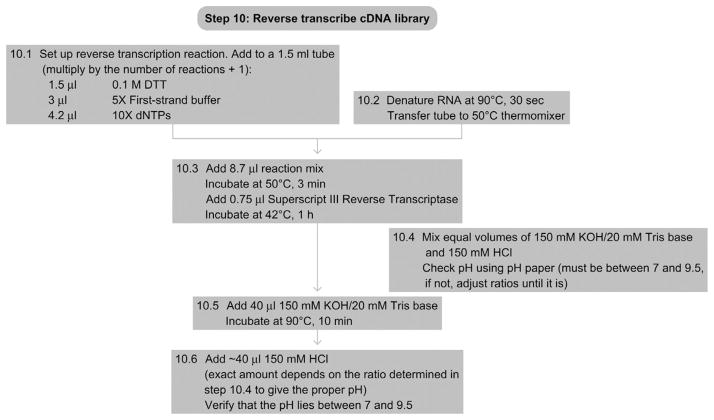
14. STEP 10 cDNA Library Preparation/Reverse Transcription (Day 6)
14.1. Overview
The RNA ligated to both sequencing adapters is reverse-transcribed and will be used for PCR in the subsequent step.
14.2. Duration
1.5 h
-
10.1
Prepare the following reaction mix (multiplied by the number of samples plus one for pipetting loss):
1.5-μl 0.1-M DTT
3-μl 5 × First-strand buffer (Superscript)
4.2-μl 10 × dNTPs
-
10.2
Denature the RNA by incubating the tube at 90 °C for 30 s and transfer the tube to a 50 °C thermomixer.
-
10.3
Add 8.7 μl of the reaction mix to each sample and incubate at 50 °C for 3 min. Add 0.75 μl of Superscript III Reverse Transcriptase and incubate at 42 °C for 1 h.
-
10.4
Prepare 150-mM KOH/20-mM Tris base and 150-mM HCl and use pH paper to verify that a 1:1 mixture results in a pH between 7.0 and 9.5. If not, change the ratios until the pH is within that range.
-
10.5
To hydrolyze the RNA, add 40 μl of 150-mM KOH/20-mM Tris base and incubate at 90 °C for 10 min.
-
10.6
Neutralize the solution by adding 40 μl of 150-mM HCl (the exact volume depends on the ratio determined in Step 10.4) and check the pH of the mixture by spotting 1 μl on pH paper. It should be between 7.0 and 9.5 so that the subsequent PCR is not inhibited. If necessary, readjust the pH by adding more base or acid.
See Fig. 8.12 for the flowchart of Step 10.
Figure 8.12.
Flowchart of Step 10.
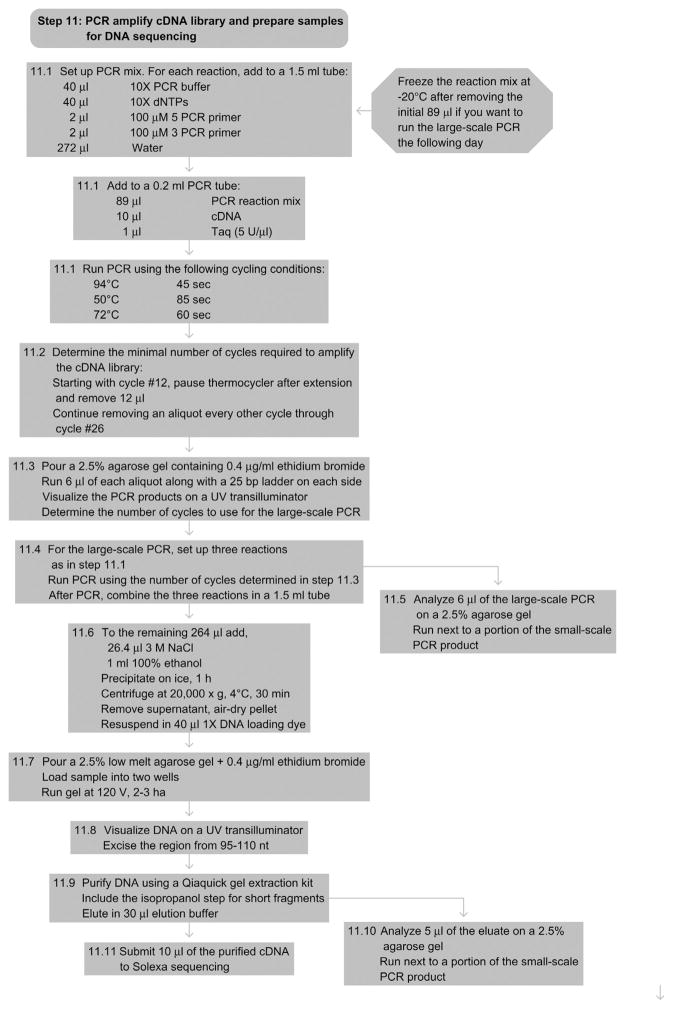
15. STEP 11 PCR AMPLIFICATION OF cDNA Library & Sample Preparation for Sequencing (Day 6)
15.1. Overview
This step concludes the PAR-CLIP protocol. To minimize the distortion of the cDNA library composition by excessive PCR and to recognize possible failure during reverse transcription leading to false positive PCR results, we monitor the accumulation of the PCR product during a pilot PCR. To determine the minimal cycle number, a small-scale trial PCR is performed before the final large-scale PCR. The PCR product is gel fractionated (see Agarose Gel Electrophoresis); the appropriately sized fraction is recovered from the gel and submitted to Solexa sequencing (see Fig. 8.7(d) and Explanatory Chapter: Next Generation Sequencing).
15.2. Duration
8–9 h
-
11.1
Prepare the following mix multiplied by the number of samples (plus one for the negative control):
40-μl 10× PCR buffer
40-μl 10× dNTPs
2 μl of 100-μM 5′ PCR primer
2 μl of 100-μM 3′ PCR primer
-
272-μl H2O
89 μl of the reaction mix will be used in the pilot PCR reaction to determine the minimal cycle number; the remainder will be needed for the large-scale PCR (freeze the reaction mix if you want to run the large-scale PCR on the following day).
-
Add to a 0.2-ml thin-walled PCR tube:
89 μl of the reaction mix
10-μl cDNA
1-μl Taq polymerase (5 U μl−1)
Include a negative control using water instead of cDNA.
-
Use the following cycling conditions:
94°C 45 s
50°C 85 s
72°C 60 s
-
11.2
To determine the necessary number of cycles for amplifying the cDNA library, remove 12-μl aliquots every other cycle starting with cycle number 12 and ending with cycle number 26. To remove aliquots from the PCR tube, temporarily pause the PCR cycler at the end of the 72 °C step. You can use a multichannel pipettor to remove the aliquots.
-
11.3
Analyze 6 μl of each sample on a 2.5% agarose gel containing 0.4 μg ml−1 of ethidium bromide to check for consistency. Load a 25-bp ladder on each side and load all cycles from one sample next to one another in an ascending order.
The PCR products might appear as a double band, with the higher band running at the expected length of about 95–110 nucleotides and a lower band corresponding to the 3′-adapter-to-5′-adapter ligation/template switch products running at about 65 nucleotides. Figure 8.7(d) illustrates a typical small-scale PCR. The red arrows indicate the chosen number of cycles for the large-scale experiment.
Define the minimal cycle number for the cDNA amplification. It should be within the exponential amplification phase of the PCR, about five cycles away from reaching the saturation level of PCR amplification. For a typical PAR-CLIP experiment, the minimal number of cycles is between 16 and 20 (Pause point: you can pause at any time before or after the large-scale PCR).
-
11.4
For the large-scale PCR, set up three 100-μl reactions (as in Step 11.1). Perform the PCR using the determined minimal number of cycles. After the reactions are finished, combine all three PCR reactions. Include a negative control as before.
-
11.5
Analyze 6 μl of the products next to the corresponding products from the pilot PCR on a 2.5% agarose gel containing 0.4 μg ml−1 of ethidium bromide to check for consistency.
-
11.6
To the remaining 264 μl, add 26.4-μl 3 M NaCl and 1-ml 100% ethanol. Precipitate for 1 h on ice and spin at 20 000 × g at 4 °C for 30 min. Remove the supernatant, air-dry the pellet, and dissolve in 40 μl of 1×DNA loading dye (dilute 5× DNA loading dye in 1 × TBE).
-
11.7
Divide the sample into two wells of a 2.5% low melt agarose gel containing 0.4-μg ml−1 ethidium bromide. Run the gel at 120 V for 2–3 h.
Do not overload the gel since that will compromise its ability to separate fragments.
-
11.8
Visualize the DNA on a 365-nm transilluminator and use a clean scalpel to excise the region corresponding to 95–110 nucleotides.
-
11.9
Purify the DNA using the Qiaquick gel extraction kit (Qiagen) according to the manufacturer’s instructions. Include the iso-propranol step as described for short fragments. Elute in 30-μl elution buffer.
-
11.10
Analyze 5 μl of the eluate on a 2.5% agarose containing 0.4 μg ml−1 of ethidium bromide gel to ensure the removal of any unwanted amplified 5′-adapter-3′-adapter PCR products.
-
11.11
Submit 10 μl of the purified cDNA to Solexa sequencing.
15.3. Tip
If you have more than one PAR-CLIP sample, prepare a 96-well plate with 3-μl 5× DNA loading dye in the required number of wells so that you only have to pipet once per cycle using a multichannel pipettor.
15.4. Tip
The main goal of the preparative gel is to reduce noninformative sequence reads of unwanted 5′-adapter-3′-adapter PCR products. Do not overload the gel and run the gel as long as possible to achieve the best separation possible. Check intermittently so that you do not run your samples into the buffer.
15.5. Tip
Bromophenol blue runs roughly at the same position as the samples. Use a DNA gel loading buffer containing xylene cyanol or do not add any dye to it. Run an aliquot containing bromophenol blue next to your samples.
15.6. Tip
Perform a second gel extraction if any 5′-adapter-3′-adapter products are still seen after the first gel extraction.
See Fig. 8.13 for the flowchart of Step 11.
Figure 8.13.
Flowchart of Step 11.
16. STEP 12 DETERMINATION OF INCORPORATION LEVELS OF 4SU INTO TOTAL RNA
16.1. Overview
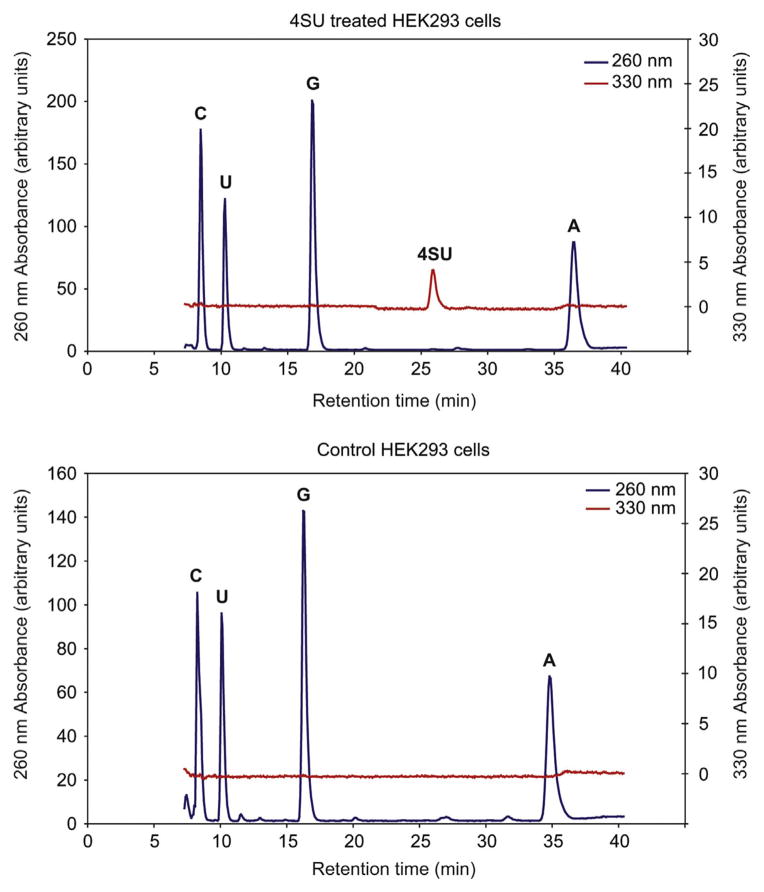
To optimize crosslinking of protein to RNA, it is useful to determine the fraction of substitution of uridine by 4SU. This is especially necessary when changing cell growth conditions or cell type. Total RNA is isolated and enzymatically degraded to monomeric ribonucleosides, which are separated and quantified by HPLC analysis (Andrus and Kuimelis, 2001).
16.2. Duration
Cell labeling and harvesting: 16 h + 20 min
RNA isolation: about 2 h
Dephosphorylation and hydrolysis: 30 min + overnight incubation
Chromatography: as needed
-
12.1
Grow two 10-cm plates of HEK293 cells in regular medium. Add 100-μM 4SU to one plate 16 h prior to harvesting cells.
-
12.2
Decant the growth medium; wash cells once with 1 × PBS.
-
12.3
Add 1 ml of TRIzol reagent directly onto the plate and isolate the total RNA according to the manufacturer’s instructions.
-
12.4
Include 0.1-mM DTT in the isopropanol and ethanol-wash steps as well as in the subsequent reaction to prevent oxidation of the thiocarbonyl group, which yields disulfides or uridine.
-
12.5
Dissolve the RNA pellet in 60-μl H2O containing 1-mM DTT.
-
12.6
Determine the concentration of the obtained RNA. Expect to obtain about 50–100μg total RNA per 10-cm plate.
-
12.7
0.2 OD260 (8.0 μg RNA) of total RNA is digested and dephosphorylated to single nucleosides for HPLC analysis. Set up the following reaction:
Reagent or solution Final concentration Volume RNA 0.2 OD260 x μl 10-mM DTT 0.1 mM 1.3 μl 1 M MgCl2 13.8 mM 1.8 μl 0.5-M Tris–HCl (pH 7.5) 34.6 mM 9.0 μl Bacterial Alkaline Phosphatase 1.6 U x μl Snake Venom Phosphodiesterase 0.2 U x μl H2O to 130 μl -
12.8
Digest for 16 h at 37°C. As an additional control, also digest and analyze synthetic RNAs with and without 4SU.
-
12.9
Add 2.3-μl 100-mM DTT, 4 μl of 3 M NaOAc (pH 5.2), and 100 μl of ice-cold 100% ethanol, incubate on dry ice for 10 min, and centrifuge the sample at 12 500 × g for 5 min at 25 °C.
-
12.10
Collect the supernatant, add 3 μl of 100-mM DTT and 300 μl of ice-cold 100% ethanol, incubate on dry ice for 10 min, centrifuge the sample at 12 500 × g for 5 min at 25 °C, and collect the supernatant.
-
12.11
Evaporate the supernatant in a Speed-vac to complete dryness. If the sample is not completely dried, the retention times during HPLC analysis are affected.
-
12.12
Dissolve the sample in 50 μl of H2O, which is the volume of one HPLC injection.
-
12.13
Separate ribonucleosides on a Supelco Discovery C18 reverse phase column (bonded phase silica 5 μM particles, 250 × 4.6 mm).
-
12.14
Use an isocratic gradient of 0% B for 15 min, 0–10% B for 20 min, and 10–100% B for 30 min with a 5-min 100% B wash between runs (see Fig. 8.14).
-
12.15
Calculate the absorption ratios from the known sequence of the reference oligonucleotides. This is used to estimate the incorporation rate for 4SU (in our experiments, between 1.4 and 2.4% of U is substituted by 4SU).
-
12.16
Confirm U and 4SU retention times by co-injection with standards.
-
12.17Calculate the substitution ratio of 4SU by dividing the area under the curve by the extinction coefficients of rU versus 4SU at 260 nm versus 330 nm.
Nucleoside Extinction coefficient at 260 nm (pH 7.0) Extinction coefficient at 330 nm rA 12 340 0 rC 7020 0 rG 10 240 0 rU 9720 0 4SU 4250 17 000 See Fig. 8.15 for a flowchart of Step 12.
Figure 8.14.
HPLC trace of extracted total RNA to estimate 4SU incorporation into HEK293 cells. Please refer to the main text for a detailed description.
Figure 8.15.
Flowchart of Step 12.
Figure 8.1.
Flowchart of the complete protocol, including preparation.
Footnotes
VIDEO
Please refer to this link (http://www.jove.com/index/Details.stp?ID=2034) for a video illustrating the first day of experiments.
Referenced Protocols in Methods Navigator
Explanatory Chapter: Next Generation Sequencing.
UV crosslinking of interacting RNA and protein in cultured cells.
RNA Radiolabeling.
Lysis of mammalian and Sf9 cells.
One-dimensional SDS-Polyacrylamide Gel Electrophoresis (1D SDS-PAGE).
Western Blotting using Chemiluminescent Substrates.
RNA purification – precipitation methods.
RNA purification by preparative polyacrylamide gel electrophoresis.
Agarose Gel Electrophoresis.
Referenced Literature
- Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Andrus A, Kuimelis RG. Base composition analysis of nucleosides using HPLC. In: Andrus A, Kuimelis RG, editors. Current Protoccols Nucleic Acid Chemistry. Unit 10. New York: Wiley; 2001. p. 16. [DOI] [PubMed] [Google Scholar]
- Bezerra R, Favre A. In vivo incorporation of the intrinsic photolabel 4-thiouridine into Escherichia coli RNAs. Biochemical and Biophysical Research Communications. 1990;166(1):29–37. doi: 10.1016/0006-291x(90)91907-a. [DOI] [PubMed] [Google Scholar]
- Favre A, Moreno G, Blondel MO, Kliber J, Vinzens F, Salet C. 4-Thiouridine photosensitized RNA-protein crosslinking in mammalian cells. Biochemical and Biophysical Research Communications. 1986;141(2):847–854. doi: 10.1016/s0006-291x(86)80250-9. [DOI] [PubMed] [Google Scholar]
- Gerber AP, Luschnig S, Krasnow MA, Brown PO, Herschlag D. Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(12):4487–4492. doi: 10.1073/pnas.0509260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Letters. 2008;582(14):1977–1986. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbeisen RE, Galgano A, Scherrer T, Gerber AP. Post-transcriptional gene regulation: From genome-wide studies to principles. Cellular and Molecular Life Sciences. 2008;65(5):798–813. doi: 10.1007/s00018-007-7447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Landgraf P, Ludwig J, et al. Identification of microRNAs and other small regulatory RNAs using cDNA library sequencing. Methods. 2008;44(1):3–12. doi: 10.1016/j.ymeth.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Landthaler M, Burger L, et al. PAR-CLIP – Transcriptome-wide identification of RNA targets and binding sites of RNA-binding proteins. Cell. 2010;141(1):129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene JD. RNA regulons: Coordination of post-transcriptional events. Nature Reviews Genetics. 2007;8(7):533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- Landthaler M, Gaidatzis D, Rothballer A, et al. Molecular characterization of human Argonaute-containing ribonucleoprotein complexes and their bound target mRNAs. RNA. 2008;14(12):2580–2596. doi: 10.1261/rna.1351608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ. From birth to death: The complex lives of eukaryotic mRNAs. Science. 2005;309(5740):1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- Tenenbaum SA, Carson CC, Lager PJ, Keene JD. Identifying mRNA subsets in messenger ribonucleoprotein complexes by using cDNA arrays. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(26):14085–14090. doi: 10.1073/pnas.97.26.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ule J, Jensen KB, Ruggiu M, Mele A, Ule A, Darnell RB. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302(5648):1212–1215. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
SOURCE REFERENCES
- Hafner M, Landgraf P, Ludwig J, et al. Identification of microRNAs and other small regulatory RNAs using cDNA library sequencing. Methods. 2008;44(1):3–12. doi: 10.1016/j.ymeth.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Landthaler M, Burger L, et al. PAR-CLIP – Transcriptome-wide identification of RNA targets and binding sites of RNA-binding proteins. Cell. 2010;141(1):129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]