Abstract
Purpose of review
Epigenetic studies are transforming our understanding of a variety of complex pathological conditions including cancer, autoimmune and inflammatory diseases. A selection of the major recent advances in this area will be reviewed focusing on important emerging themes that are relevant to these diseases including inflammatory bowel disease (IBD).
Recent findings
The main current themes that will be addressed are the role of epigenetics in disease pathogenesis, including current understanding of the nature and function of DNA methylation and histone modifications; the expanding research on chromatin readers and their potential as selective therapeutic targets; the connection between epigenetics and metabolic pathways, and new studies on the mechanism of heritability of epigenetic changes. The recent contribution of epigenetic modifications in defining the molecular basis of IBD and how such changes may act as fine-tuners of gene expression in these intestinal disorders are also discussed.
Summary
Published evidence over the last 12–18 months indicates that targeting epigenetic factors can be efficacious in cancer and inflammatory disease. All the indications are that future research will continue to reveal new epigenetic targets and mechanisms that will advance the prospects for selective epigenetic therapy for IBD and other complex diseases.
Keywords: Epigenetics, inflammatory bowel diseases, anti-inflammatory therapy, DNA methylation, histone modifications
Introduction
The established impact of epigenetics on our understanding of cancer [1, 2] and its emerging importance in chronic inflammatory and autoimmune disease pathogenesis [3] [4] suggest that we are at the beginning of a new and exciting era in IBD research. IBD is now recognized as a global disease [5]. The consensus view is that IBD arises due to complex undefined interactions among environmental factors, genetic susceptibility and an abnormal immune response to the gut microbiome that result in a pathological intestinal inflammatory response [6]. The continued increase in widespread occurrence of this heterogeneous group of inflammatory disorders means there is an ever more urgent need for new targets that are translatable to the clinic. Research in cancer [1, 2] indicates that epigenetic mechanisms have also an exceptional potential to classify, diagnose and advance our understanding of IBD [7] [8].
Classically, the term “epigenetics” refers to heritable changes in phenotype that occur independently of changes to the DNA sequence. A broader definition to reflect new advances in epigenetics has been debated but not agreed [9]. Here, I will use the commonly employed definition of epigenetics as indicating events associated with chromatin that regulate a range of DNA-based processes including gene transcription. I will outline current disease-relevant themes in epigenetics, the present status of IBD research with emphasis on recent studies of epigenetics in IBD, and how epigenetics could reveal new insights into IBD pathogenesis through acting as fine tuners of gene expression.
Current themes in role of epigenetics in disease
Basic concepts
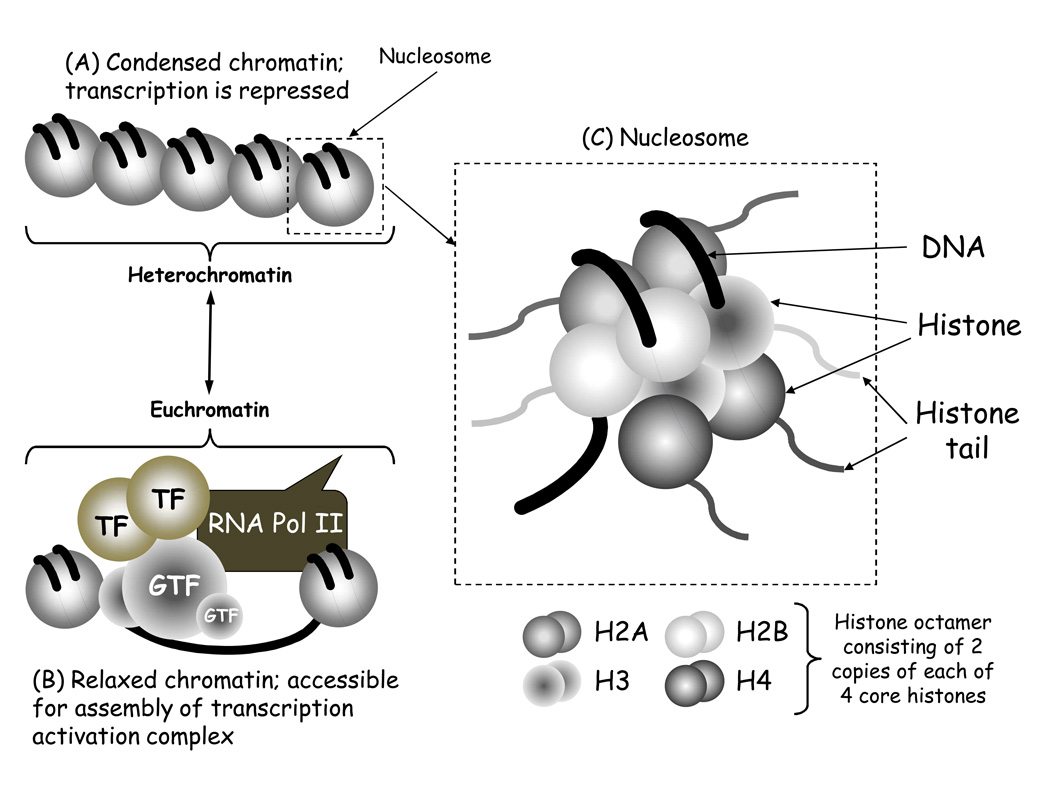
The advances in epigenetics that have occurred over the last two decades have been in large part due to knowledge of the crystal structure of the nucleosome, the basic packaging unit of chromatin [10] (Fig.1). The histone protein core within the nucleosome can be covalently modified in multiple ways to dynamically alter chromatin structure and gene expression in response to environmental cues [11]. Currently four different DNA modifications and 16 classes of histone post- translational modifications have been described and these numbers are likely to increase further [12, 13].
Figure 1. Diagrammatic representation of the structure of chromatin.
The classical model of chromatin structure comprises nucleosomes linked together by DNA (A). When the nucleosomes are packed tightly together chromatin is condensed and in this form transcription is repressed, and chromatin is referred to as heterochromatin. When the chromatin is modified and remodeled the nucleosomes are further apart, and in this form chromatin is relaxed making the DNA accessible to the transcription machinery (B). This allows the assembly of an active transcription complex that includes multiple general transcription factors (GTF) and inducible transcription factors (TF) as well as RNA polymerase II (RNA pol II). This form of chromatin is referred to as euchromatin. (C) Each nucleosome contains an octamer of histone proteins made up of two of each type of core histone: Histone 2A (H2A), H2B, H3 and H4. Each of these histones has an amino terminal tail that extends beyond the nucleosome structure and can be modified by chromatin enzymes.
Histone modifications
The multiple modifications of residues within histone tail and core domains are performed by a large number of chromatin modifying enzymes [11] [14]. Those that add chemical groups are called chromatin writers and include histone acetylases, lysine and arginine methyltransferases among many others. The enzymes that catalyze removal of these groups and include histone deacetylases and demethylases are called chromatin erasers. In addition, ATP-dependent chromatin remodeling complexes catalyze the relocation of nucleosomes to promote access of the transcription machinery to DNA. The most studied modifications to date are acetylation and methylation, but there is increasing interest in ubiquitination, phosphorylation and SUMOylation [2]. The function of the majority of the more recently identified modifications remains unknown [13]. The available data indicate that the location (histone tail or core domain) and type of histone modification determine the biological outcome [15] [16]. Importantly, histone modifications also engage in cross talk [16, 17] and in combination provide a platform for the recruitment of chromatin factors and other proteins to modulate transcription in a context specific manner.
DNA methylation
DNA methylation comprising the covalent addition of methyl groups to cytosine residues of CpG dinucleotides is the best-characterized epigenetic hallmark of cancer and several other pathologies [1, 18]. The changes in DNA methylation that are characteristic of chronic inflammatory and autoimmune diseases have only recently begun to be identified [4]. Addition of this modification is catalyzed by DNA methyltransferases (DNMTs) that are recruited to specific loci by a combination of histone modifications, chromatin remodeling enzymes and non-coding RNAs [18]. A new and exciting development has been the identification of demethylating enzymes such as ten-eleven translocation (TET) methylcytosine dioxygenases, activation induced cytidine deaminase (AID) and thymine DNA glycosylase (TDG) [12]. Three new DNA modifications, of unknown function, resulting from consecutive oxidation reactions by the TET proteins have recently been described.
New functions of DNA methylation
A great deal remains poorly understood about the function and timing of DNA methylation. Yet, the loss of global and gene specific DNA methylation; promoter hypermethylation and dysregulated expression of DNMTs in a variety of human cancers is well established [2] [18]. However, whether this modification precedes or follows gene expression to further stabilize the repressed state is unclear. The location of DNA methylation in gene bodies, non-CpG island promoters and enhancers suggests that it may have a variety of functions. In fact, DNA hypermethylation of gene bodies is positively correlated with gene expression [18] and DNA hypermethylation of exons is reported to have a role in regulating splicing [19].
Histone modifications: Causal, circumstantial and consequential?
Many published studies indicate that modifications of histone proteins correlate with changes in gene transcription but whether these have causal effects, occur incidentally or as a consequence of changes in phenotype is unclear [15] [16] [20]. A major reason for this is the difficulty in distinguishing unambiguously between these possibilities experimentally. Given the diversity and complexity of the epigenome and the environmental factors that can influence it, it is likely that all three scenarios could occur at any time in a particular cellular context. The evidence to date supports the notion that some histone modifications, e.g., H4K16ac are regulators of chromatin structure [21] and gene expression [22]. The rapidly increasing number and possible combinations of modifications that could be functional means the final verdict on causality will require considerable further research.
Histone modifications, like DNA methylation, are associated with functionally distinct regions of the genome [23]. For example, H3K4me1, H3K4me2, H3K4me3, H3K27ac, and H3K9ac have been shown to mark the active enhancer regions of genes in a cell type specific manner [24]. Whether they simply reflect enhancer states or determine them is unknown. Interestingly, levels of H3K4me1 have been used to map gain and loss of enhancer loci in colon cancer and have been linked with a colon cancer specific transcriptome that promotes carcinogenesis [25].
Chromatin readers
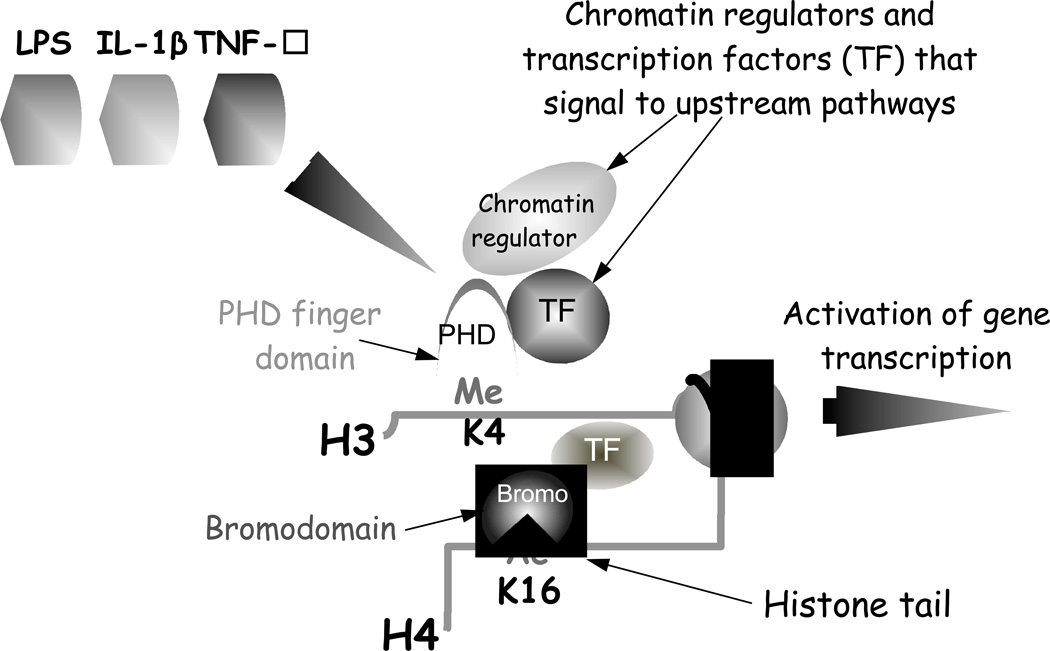
Chromatin readers encompass a variety of chromatin and transcription regulators that contain specialized domains which allow them to recognize specific histone modifications (Fig.2) [14]. These dock at specific sites and provide a platform for the recruitment of other proteins in response to upstream signaling pathways [16]. Residues within the binding pocket of these domains recognize specific modifications and neighboring modified residues in histones can also influence binding specificity. The “readers” of acetylated lysine residues contain evolutionarily conserved binding regions called bromodomains [26]. Some of the major methyl lysine readers include plant homoedomain fingers (PHD) domains, chromo domains, Tudor domains and malignant brain tumor (MBT) domains among others [27] [14]. Importantly, some chromatin readers, e.g., BPTF are “multivalent” [28]. The latter have more than one reading domain and recognize combinations of modified residues to recruit the proteins required for a specific transcriptional outcome. Mutations of these reader domains, e.g. the ING family PHD finger domain which recognizes H3K4me3, have been reported in melanoma and breast cancer [2]. The function and nature of these protein-protein interactions and their potential as therapeutic targets has been demonstrated recently and are areas of intensive research activity [29].
Figure 2. Chromatin reader domains bind to specific histone modifications to regulate gene transcription.
Chromatin readers contain specialized domains that bind and recognize specific histone modifications. Bromodomains ‘read” acetyl groups (Ac) and PHD finger domains read methyl groups (Me) of modified lysine residues in histone tails (e.g. H4K16 and H3K4 respectively). These chromatin reader proteins dock at histone modified sites and provide platforms for the recruitment of other chromatin regulators or transcription proteins that signal to upstream signaling pathways. IBD-relevant pro-inflammatory stimuli: LPS, IL-1β, TNF-α are shown as examples of pathways that activate gene transcription.
Metabolism
A relatively new avenue of research that is potentially relevant to IBD is investigating the link between the epigenome, metabolic pathways and disease [30]. Cellular metabolites such as acetyl CoA, nictoinamide adenine dinucleotide (NAD) and S adenosyl methionine serve as cofactors for a number of chromatin modifying enzymes. Exciting new studies indicate that metabolites can influence the level of specific histone modifications and exert precise changes in phenotype [31] [32]. These and other studies suggest that metabolic pathways can regulate pathogenic gene expression through their ability to influence the epigenome.
Heritability of epigenetic changes and disease
A fundamental tenet of epigenetic changes is that they are heritable, i.e. they should be stable to DNA replication and be able to rapidly reestablish gene expression patterns in the next generation [33, 34]. The mechanism of heritability is poorly understood but recent compelling data indicate that specific H3K4 and the H3K27 trimethylases can remain associated with newly replicated DNA during cell division, thus retaining the appropriate chromatin state and level of gene expression in the next generation [35].
During development, epigenetic changes are usually erased and reset during gametogenesis. However, if there is perturbation of the epigenome in response to environmental factors, altered epigenetic changes can persist and be passed on to the offspring via the gametes [34]. This is termed multigenerational epigenetic inheritance and has major implications for disease. New data suggest that the HRDE1 (heritable RNAi defective) gene, trimethylated H3K9 associated with gene silencing and H3K9 methyltransferases are involved [36] [37]. The precise mechanism remains to be established. Although a rare event, multigenerational epigenetic inheritance may explain the heritability of diseases such as colon cancer as well as sporadic and familial cases of IBD.
Stochastic noise (random molecular interactions that result in distinct phenotypes) has been recognized as a cause of dysregulated epigenetic changes that can result in disease [38]. For example, hypervariable regions of DNA methylation have been identified in tumors and these may contribute to tumor heterogeneity [39]. These tissue-specific regions exhibit marked stochastic variation across a population and correspond to the same key regions that are reprogrammed during normal cell differentiation or cell fate transitions in development. It will be important to determine if such regions have a role in the etiology of other complex heterogeneous diseases including IBD.
Inhibitors
The expanding families of chromatin regulators have provided a wealth of potential therapeutic targets for new anti-cancer and anti-inflammatory drugs [29]. Many of the inhibitors that have been described to date have targeted chromatin writers and erasers. An example of the latter, the HDAC inhibitors, are proving to be effective in some cancers and to have efficacy in a variety of inflammatory disease models. However, their non-selectivity and consequent side effects are still limiting factors in their broader clinical use. Nevertheless, there remains a great deal of interest in designing small molecule inhibitors of these chromatin enzymes with improved selectivity. An example of this is the first inhibitor of an individual methyltransferase: DOT1-L has been recently reported to be efficacious in mixed lineage leukemia fusions [40]. A number of other effective small molecular inhibitors of individual human methyltransferases and lysine demethylases have been developed [29]. These include new inhibitors with anti-inflammatory activity such as the inhibitor to the H3K27me3 demethylase JMJD3 that ameliorates LPS-induced cytokine production in human macrophages [41]. Inhibition of chromatin reader domains is showing great promise as a selective pharmacological strategy. For example the bromodomains of the BET family of proteins have shown excellent efficacy in several cancers and can ameliorate systemic inflammation [42] [43].
Current status of IBD research including Epigenetics studies
Link between genetics and epigenetics in IBD
IBD can be classified as a multifactorial, complex disease of unknown etiopathogenesis [6, 8]. Genome-wide association studies have identified at least 163 susceptibility loci to date, however, these only explain a minority of the disease risk [44] [45]. The majority of the IBD-associated SNPs or alleles are located in noncoding regions of the genome suggesting that these may have a role in the regulation of gene expression [46]. Intriguingly, links between the genome and the epigenome are beginning to be identified [46]. In the case of type I diabetes for example, disease-associated SNPs have been shown to affect DNA methylation and gene expression [3] Interestingly, a link between the IBD genome and epigenome has recently been reported. Decreased binding of the miRNAs Let-73 and Let-7f to the IBD susceptibility variant of IL-23R resulted in increased levels of mRNA and protein consistent with dysregulation of IL-23R signaling in IBD [47].
Epigenetics and the environment in IBD
Studies of epigenetics have been instrumental in advancing current knowledge of how the environment regulates phenotype and are therefore likely to be pivotal in mediating affects of the intestinal microbiota and dietary factors on intestinal homeostasis. Fascinating experiments in mice have shown that supplementation of the maternal diet of mice with methyl donors was associated with altered DNA methylation at the loci of select immune response genes in the offspring and an increased susceptibility to the development of DSS-induced colitis [48]. Related to these studies are recent observations that dietary additives in processed foods can modulate the phenotype of intestinal bacteria [49]. Conversely, new evidence also indicates that bacteria can regulate epithelial gene expression and the intestinal immune response through epigenetic mechanisms [48, 50].
The first studies of epigenetic factors in IBD showed differential expression of microRNAs (small non-coding RNAs that act as epigenetic regulators of gene expression) in the colonic mucosa samples of UC compared to the mucosa of control patients [51]. Additional reports including analyses of peripheral blood have identified specific miRNAs that could distinguish subtypes of IBD, raising the intriguing possibility that these could be new biomarkers of disease [52] [53, 54].
More recently, global epigenetic profiling studies of tissue from IBD patients have demonstrated the feasibility of identifying DNA methylome signatures for UC and CD [55] [56, 57]. However, only one of these studies systematically correlated gene expression with changes in DNA methylation [57]. Importantly, the function of DNA methylation and its role in IBD pathogenesis in individual cell types remains to be defined. Very recent data from studies of IBD-associated fibrosis have shown that chromatin modifications are linked with transcriptional activation of type I collagen gene expression in intestinal endothelial-to-mesenchymal transition, suggesting epigenetic changes are involved in regulating fibrotic genes and intestinal fibrogenesis [58].
Epigenetics as fine tuners of IBD
Collectively, the compelling studies that have associated epigenetic changes with UC and CD, intestinal homeostasis, the regulation of microbial pathogen gene expression and the host response to the commensal flora suggest that perturbation of epigenetic factors could be a major contributor to the development of IBD [48] [59] [60]. The epigenome may as a “gatekeeper” for the host in the context of the intestine, fine-tuning responses to environmental cues such as alterations in the microbiome, dietary factors and even genetic variants. IBD-associated SNPs could cause subtle changes in accumulation or deposition of histone modifications that result in the differential expression of alleles of inflammatory and fibrotic genes. In fact, in plants [61, 62] SNPs can affect the accumulation of specific histone modifications (H3K27me3) and encode a variant that antagonizes recruitment of chromatin modifying enzymes. This causes incomplete silencing and directly affects allelic expression at specific loci. This demonstrates how small changes in histone modifications could be caused by SNPs associated with IBD that would fine-tune the transcriptional output.
The number and complexity of epigenetic modifications and the combinations required to provide specific biological outputs suggest that each contributing epigenetic factor is important in fine-tuning of the phenotypic outcome. In IBD, particular combinations of epigenetic changes could result in a pathogenic phenotype through activating genes that promote chronic inflammation or inhibiting anti-inflammatory gene expression.
The miRNAs associated with IBD could also serve also as fine tuners of gene expression. The levels of specific mRNAs have been shown to be tightly controlled by miRNAs, whereby slight alterations to the threshold level mRNA level results in marked changes in protein synthesis [63]. Dysregulation of the intestinal inflammatory response could occur through disruption in the balance between miRNA activity and threshold levels of specific target mRNAs, e.g., genes with important functions in intestinal homeostasis resulting in inappropriate gene expression and disease.
Conclusions
It is clear from the rapid advances made recently in the epigenetics field that future studies in which the epigenome is defined in normal and disease states will be crucial to a full understanding of IBD as well as many other complex human conditions. A great deal of research remains to be done to understand the function and complexity of modifications and chromatin proteins in regulating phenotype in normal and pathophysiological conditions. We are in an exciting phase where advances in the development of inhibitors of chromatin writers, erasers and especially readers provides optimistic prospects for achieving selective and efficacious therapeutic agents that could revolutionize the treatment of many diseases including IBD.
Key points.
Epigenetic changes are central to the pathogenesis of cancer, autoimmune and inflammatory diseases, and early studies suggest this is likely to also apply to IBD.
Epigenetics comprises a plethora of histone modifications, DNA methylation, chromatin enzymes and miRNAs that, in combination, lead to heritable changes in phenotype that do no involve changes to the DNA sequence.
Knowledge of the nature and function of epigenetic modifications and their role in disease pathogenesis is in its infancy but is advancing rapidly.
New insights are emerging into the connection between epigenetics and metabolic pathways, mechanisms of heritability and strategies for selective epigenetic therapeutics with clinical efficacy.
Published evidence reported so far supports the notion that epigenetic factors can act as fine-tuners of gene expression in diseases such as in IBD.
Acknowledgements
SOURCES OF FUNDING
National Institutes of Health, U.S.A [DK50984] and Broad Medical Research Program, U.S.A [IBD-0320R]
The author gratefully acknowledges the funding support of NIH, the Broad Medical Research Program, the Department of Pathobiology and the Digestive Diseases Institute of the Cleveland Clinic.
References
- 1.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11(10):726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Leung A, Schones DE, Natarajan R. Using epigenetic mechanisms to understand the impact of common disease causing alleles. Curr Opin Immunol. 2012;24(5):558–563. doi: 10.1016/j.coi.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballestar E. Epigenetic alterations in autoimmune rheumatic diseases. Nat Rev Rheumatol. 2011;7(5):263–271. doi: 10.1038/nrrheum.2011.16. [DOI] [PubMed] [Google Scholar]
- 5.Molodecky NA, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 6.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 7.Scarpa M, Stylianou E. Epigenetics: Concepts and relevance to IBD pathogenesis. Inflamm Bowel Dis. 2012;18(10):1982–1996. doi: 10.1002/ibd.22934. [DOI] [PubMed] [Google Scholar]
- 8.Jenke AC, Zilbauer M. Epigenetics in inflammatory bowel disease. Curr Opin Gastroenterol. 2012;28(6):577–584. doi: 10.1097/MOG.0b013e328357336b. [DOI] [PubMed] [Google Scholar]
- 9.Berger SL, et al. An operational definition of epigenetics. Genes Dev. 2009;23(7):781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luger K, et al. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 11.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25(23):2436–2452. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan M, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146(6):1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dawson MA, Kouzarides T, Huntly BJ. Targeting epigenetic readers in cancer. N Engl J Med. 2012;367(7):647–657. doi: 10.1056/NEJMra1112635.. An up to the minute topical review that reflects the growing interest in chromatin reader domains and how mutations in these binding regions mediate many of the changes that underlie cancer and other diseases. The review takes you from the basics of epigenetics to the latest on the inhibitors of these domains with therapeutic potential.
- 15.Gardner KE, Allis CD, Strahl BD. Operating on chromatin, a colorful language where context matters. J Mol Biol. 2011;409(1):36–46. doi: 10.1016/j.jmb.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turner BM. The adjustable nucleosome: an epigenetic signaling module. Trends Genet. 2012;28(9):436–444. doi: 10.1016/j.tig.2012.04.003.. A stimulating review discussing the controversies and unanswered questions in the complex area of chromatin modifications and their putative functions.
- 17.Whitcomb SJ, et al. Histone monoubiquitylation position determines specificity and direction of enzymatic cross-talk with histone methyltransferases Dot1L and PRC2. J Biol Chem. 2012;287(28):23718–23725. doi: 10.1074/jbc.M112.361824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 19.Shukla S, et al. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature. 2011;479(7371):74–79. doi: 10.1038/nature10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henikoff S, Shilatifard A. Histone modification: cause or cog? Trends Genet. 2011;27(10):389–396. doi: 10.1016/j.tig.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Shogren-Knaak M, et al. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311(5762):844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 22.Kapoor-Vazirani P, Kagey JD, Vertino PM. SUV420H2-mediated H4K20 trimethylation enforces RNA polymerase II promoter-proximal pausing by blocking hMOF-dependent H4K16 acetylation. Mol Cell Biol. 2011;31(8):1594–1609. doi: 10.1128/MCB.00524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst J, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473(7345):43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zentner GE, Scacheri PC. The chromatin fingerprint of gene enhancer elements. J Biol Chem. 2012;287(37):30888–30896. doi: 10.1074/jbc.R111.296491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akhtar-Zaidi B, et al. Epigenomic enhancer profiling defines a signature of colon cancer. Science. 2012;336(6082):736–739. doi: 10.1126/science.1217277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippakopoulos P, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149(1):214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez R, Zhou MM. The PHD finger: a versatile epigenome reader. Trends Biochem Sci. 2011;36(7):364–372. doi: 10.1016/j.tibs.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruthenburg AJ, et al. Recognition of a mononucleosomal histone modification pattern by BPTF via multivalent interactions. Cell. 2011;145(5):692–706. doi: 10.1016/j.cell.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arrowsmith CH, et al. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11(5):384–400. doi: 10.1038/nrd3674.. An excellent overview of epigenetic protein families including the individual chromatin enzymes for which small molecule inhibitors have been synthesised. The criteria considered in the design and in improving the efficacy and selectivity of these potential therpeutic molecules are discussed.
- 30.Katada S, Imhof A, Sassone-Corsi P. Connecting threads: epigenetics and metabolism. Cell. 2012;148(1–2):24–28. doi: 10.1016/j.cell.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Shimazu T, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shyh-Chang N, et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339(6116):222–226. doi: 10.1126/science.1226603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moazed D. Mechanisms for the inheritance of chromatin states. Cell. 2011;146(4):510–518. doi: 10.1016/j.cell.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13(3):153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- 35.Petruk S, et al. TrxG and PcG proteins but not methylated histones remain associated with DNA through replication. Cell. 2012;150(5):922–933. doi: 10.1016/j.cell.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckley BA, et al. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature. 2012;489(7416):447–451. doi: 10.1038/nature11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashe A, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150(1):88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pujadas E, Feinberg AP. Regulated noise in the epigenetic landscape of development and disease. Cell. 2012;148(6):1123–1131. doi: 10.1016/j.cell.2012.02.045.. This thought-provoking review explains the concepts of epigenetics and stochastic noise very clearly and addresses how the epigenome may regulate cell plasticity through the modulating the effects of noise in development and disease - concepts of potential importance to IBD pathogenesis.
- 39.Hansen KD, et al. Increased methylation variation in epigenetic domains across cancer types. Nat Genet. 2011;43(8):768–775. doi: 10.1038/ng.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daigle SR, et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011;20(1):53–65. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kruidenier L, et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488(7411):404–408. doi: 10.1038/nature11262.. These authors describe the development of the first anti-inflammatory small molecule catalytic site inhibitor directed at the H3K27 specific demethylases JMJD3 and UTX. They report the synthesis of several inhibitors that decrease LPS induced proinflammatory cytokine production by macrophages and use these to elegantly demonstrate that both JMJD3 and UTX are critical for this process.
- 42.Delmore JE, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zuber J, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–528. doi: 10.1038/nature10334.. An impressive paper that illustrates the the successful targeting of a chromatin reader domain and demonstrates that this is a promising therapeutic strategy. A small molecule inhibitor JQ1, against the bromodomain containing protein BRD4 is identified in an shRNA library screen of chromatin regulators. BRD4 is shown to be critical to disease maintenance and to have anti-leukemic activity in vitro and in vivo.
- 44.Jostins L, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brant SR. Promises, delivery, and challenges of inflammatory bowel disease risk gene discovery. Clin Gastroenterol Hepatol. 2013;11(1):22–26. doi: 10.1016/j.cgh.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Hardison RC. Genome-wide epigenetic data facilitate understanding of disease susceptibility association studies. J Biol Chem. 2012;287(37):30932–30940. doi: 10.1074/jbc.R112.352427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zwiers A, et al. Cutting edge: a variant of the IL-23R gene associated with inflammatory bowel disease induces loss of microRNA regulation and enhanced protein production. J Immunol. 2012;188(4):1573–1577. doi: 10.4049/jimmunol.1101494.. A ground-breaking study linking an IBD-associated SNP variant of the IL-23R with increased levels of its mRNA and protein and loss of its binding capacity for specific miRNAs. Hence defects in miRNA activty due to a genetic risk variant contribute to dysregulated IL-23R signaling associated with the chronic inflammatory response in IBD.
- 48.Schaible TD, et al. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum Mol Genet. 2011;20(9):1687–1696. doi: 10.1093/hmg/ddr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nickerson KP, McDonald C. Crohn's Disease-Associated Adherent-Invasive Escherichia coli Adhesion Is Enhanced by Exposure to the Ubiquitous Dietary Polysaccharide Maltodextrin. PLoS One. 2012;7(12):e52132. doi: 10.1371/journal.pone.0052132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin L, Chung WO. Epigenetic regulation of human beta-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol. 2011;4(4):409–419. doi: 10.1038/mi.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu F, et al. Identification of microRNAs associated with ileal and colonic Crohn's disease. Inflamm Bowel Dis. 2010;16(10):1729–1738. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu F, et al. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 2011;17(1):241–250. doi: 10.1002/ibd.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, et al. miR-200b is involved in intestinal fibrosis of Crohn's disease. Int J Mol Med. 2012;29(4):601–606. doi: 10.3892/ijmm.2012.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pekow JR, Kwon JH. MicroRNAs in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(1):187–193. doi: 10.1002/ibd.21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nimmo ER, et al. Genome-wide methylation profiling in Crohn's disease identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. Inflamm Bowel Dis. 2012;18(5):889–899. doi: 10.1002/ibd.21912. [DOI] [PubMed] [Google Scholar]
- 56.Cooke J, et al. Mucosal genome-wide methylation changes in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(11):2128–2137. doi: 10.1002/ibd.22942. [DOI] [PubMed] [Google Scholar]
- 57.Hasler R, et al. A functional methylome map of ulcerative colitis. Genome Res. 2012;22(11):2130–2137. doi: 10.1101/gr.138347.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sadler T, Scarpa M, Rieder F, et al. Cytokine-induced chromatin modifications of the type I collagen alpha 2 gene during intestinal endothelial to mesenchymal transition. Inflamm Bowel Dis. doi: 10.1097/MIB.0b013e318281f37a. in press. This is the first paper to demonstrate the enrichment of specific histone modifications on the cytokine-induced type I collagen alpha 2 gene in primary cells relevant to intestinal fibrosis.
- 59. Olszak T, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–493. doi: 10.1126/science.1219328.. A fascinating paper that presents new evidence for the hygiene hypothesis and the relevance of epigenetic mechanisms to IBD. The results show that early exposure to conventional microbiota leads to protection against the development of mucosal natual killer T cells which is associated with increased expression of the chemokine CXCL16 and increased morbidity in an oxazolone-induced model of colitis. The persistent nature of this effects maybe due to an epigenetic mechanism that involves DNA methylation at specific regions of the CXCL16 gene.
- 60.Takahashi K, et al. Epigenetic control of the host gene by commensal bacteria in large intestinal epithelial cells. J Biol Chem. 2011;286(41):35755–35762. doi: 10.1074/jbc.M111.271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coustham V, et al. Quantitative modulation of polycomb silencing underlies natural variation in vernalization. Science. 2012;337(6094):584–587. doi: 10.1126/science.1221881. [DOI] [PubMed] [Google Scholar]
- 62.Pires ND, Grossniklaus U. How to fine-tune an epigenetic switch. Dev Cell. 2012;23(3):453–454. doi: 10.1016/j.devcel.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 63. Mukherji S, et al. MicroRNAs can generate thresholds in target gene expression. Nat Genet. 2011;43(9):854–859. doi: 10.1038/ng.905.. An intriguing study of miRNA regulation of target mRNA levels employing single cell measurements using quantitative fluorescence microscopy and flow cytometry. The authors show that miRNAs can tightly regulate the levels of mRNA such that a threshold level is maintained below which protein synthesis is markedly repressed. Small changes in the levels of mRNA result in large changes in protein synthesis suggesting that miRNAs can fine-tune gene expression and function as a molecular switch.