Abstract
Objective: Major side effects after endovenous laser ablation (EVLA) are pain and bruising. The aim of this study was to compare outcome and side effects after EVLA for primary varicose veins with 1470 nm diode laser using bare-tip orradial fiber.
Methods: From October 2007 to December 2010, 385 patients (453 limbs) with primary varicose veins treated with 1470 nm laser were studied. Bare-tip fiber was used in 215 patients (242 limbs) (BF group) and radial fiber (ELVeSTMRadial, Biolitec AG, Germany) was used in 177 patients (211 limbs) (RF group). This study is a retrospective study and radial fiber was started for use from November 2008. Laser energy was administered at 6–12 W of power in the BF group and 10 W of power in the RF group with constant pullback of laser fiber under tumescent local anesthesia. The patients were assessed by clinical examination and venous duplex ultrasonography at 24–48 h, one week, one month, 4 months and one year follow-up postoperatively.
Results: Mean operating time, length of treated vein and linear endovenous laser energy of all cases were 42.6 min, 36.2 cm and 83.4 J/cm, respectively. Major complications such as deep vein thrombosis and skin burns were not noted. Bruising (1.9% vs. 19.4%) and pain (0.9% vs. 7.4%) were significantly lower in the RF group. Cumulative occlusion rates by Kaplan-Meier method were 100% at 32 months in the RF group and 99.5% at 4 years in the BF group.
Conclusion: EVLA using 1470 nm laser with the radial fiber minimized adverse effects compared with bare-tip laser fiber. (*English translation of Jpn J Vasc Surg 2013; 22: 615-621)
Keywords: varicose vein, radial fiber, 1470 nm laser, endovenous laser ablation
Introduction
Endovenous laser ablation (EVLA) and radiofrequency ablation (RFA) accounts for more than 90% of surgical treatments of varicose veins in the USA. In Japan, EVLA for primary varicose veins has rapidly become popular since 2011 when it was covered by the national health insurance. However, after EVLA, pain, in duration, a pulling sensation and bruising frequently occur along the treated vein and the incidence of recanalization of the treated vein was high in the beginning. To reduce these complications and recanalization, lasers with various wavelengths (810, 940, 980, 1064, 1320, 1470, and 2000 nm) have been used for EVLA.1,2) These wavelengths are classified into 1000-nm or longer water-specific laser wavelengths (WSLW), absorbed mainly by water, and hemoglobin-specific laser wavelengths (HSLW), absorbed mainly by hemoglobin.3,4) WSLW lasers were expected to decrease complications after EVLA to be directly absorbed by water contained in the vein wall through the blood in the vein lumen. Goldman, et al.5) initially used an 1320 nm Nd:YAG laser as WSLW for EVLA, and reported reduction of postoperative pain and bruising. However, even if a WSLW laser is used, it may perforate the vein wall and induce bruising and pain because the laser is emitted from the tip when the conventional bare-tip fiber is used.
In this study, we performed EVLA with a 1470-nm laser using a radial fiber, which emits the laser 360° circumferentially from the side of the fiber, and compared the efficacy with that of EVLA using the conventional bare-tip fiber.
Materials and Methods
1. Patients
Between October 2007 and December 2010, 385 patients (453 legs, male:female = 89:296, mean age: 58. 8 years) with primary varicose veins were performed EVLA with a 1470-nm laser in our clinic (Table 1). The preoperative CEAP (clinical class, etiology, anatomy, pathophysiology) classification was C2 in 378 legs, C3 in 12, C4 in 60, C5 in 3, and C6 in 2. The great (GSV), small (SSV), and accessory (ASV) saphenous veins were treated in 399, 56, and 7 legs, respectively, and 68 patients had bilateral varicose veins. The use of a radial fiber has started in November 2008, and only a bare-tip fiber was available earlier than this. Patients were divided into two groups according to type of an optical fiber used: BF group consisted of 215 patients (242 legs) treated using the conventional bare-tip fiber and RF group consisted of 177 patients (211 legs) treated using a radial fiber. Surgery-related factors, postoperative complications, and occlusion of the treated veins and recurrence were retrospectively surveyed. No significant differences were noted in gender, mean age, or CEAP classification between the two groups.
The selection of EVLA or other surgical procedures was decided by the patient’s wish with the informed consent. The indication and exclusion criteria of EVLA were set following the ‘Guidelines for endovascular treatment of varicose veins’6) established by the Japanese Society of Phlebology. The indication of EVLA was a primary varicose vein with symptoms or stasis dermatitis. Valvular incompetence in the saphenous vein was diagnosed using duplex scanning. Duplex scanning was performed using XarioTM (Toshiba Medical Systems, Tokyo, Japan) for pre- and postoperative examinations, and iLook25 (SonoSite Japan, Tokyo, Japan) for preoperative marking and intraoperative operation.
2. Laser and optical fibers
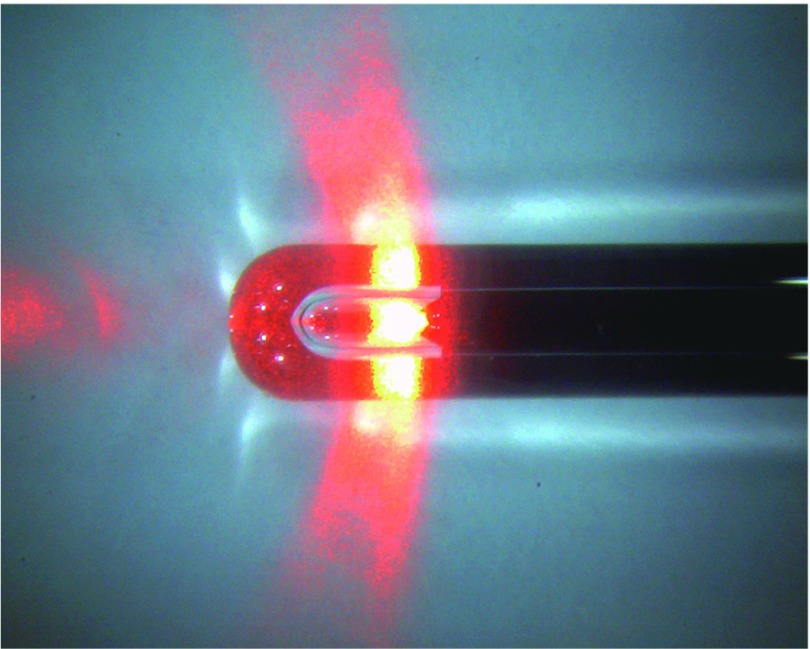
For all cases, the 1470-nm diode laser (ELVeS® PL laser system; Biolitec AG, Jena, Germany, or VenocureTM; Diotech, Busan, Korea) was used. For the optical fiber, a bare-tip fiber with an outer diameter of 600 µm or a radial fiber (ELVeS RadialTM; Biolitec AG, Germany) (Fig. 1) was used. The radial fiber was used in combination with the ELVeS® PL laser system only. It is an optical fiber with an outer diameter of 1.85 mm, and 80% of the laser light is emitted 360° circumferentially and 70° from the fiber axis.7)
Fig. 1.
Radial fiber. A radial fiber (ELVeS RadialTM; Biolitec AG, Germany) emits the laser energy radially around the tip directly into the venous wall.
3. Procedure of EVLA
Procedure of EVLA was as previously reported.8) Briefly, course of the saphenous vein was preoperatively marked by duplexultrasound, and the optical fiber was inserted into the distal side of the saphenous vein via ultrasound-guided percutaneous approachor surgical approach. Tumescent local anesthetic (TLA) solution, which consists of epinephrine-added lidocaine (1% Xylocaine®E) and sodium hydrogen carbonate (7% Meylon®) with saline, was administered perivenously by infiltrating under ultrasound guidance. The optical fiber tip was placed just distal to the orifice of the superficial epigastric vein in the GSV or at a site not contacting the tibial nerve on the distal to the deep vein junction in the SSV. While observing ablation of the vein under ultrasound, the laser energy was delivered by manually pulling the optical fiber so as to adjust the linear endovenous energy density (LEED) to 70–100 J/cm. The laser power using a bare-tip and radial fiber was set at 6–12 and 10 W, respectively. Following EVLA, branch varicosities were resected with the stab avulsion method if necessary. An elastic stocking over an elastic bandage was applied for 24–48 h postoperatively, followed by wearing elastic stocking alone for 3 weeks. Oral analgesics and antibiotics were administered for 3 days.
4. Postoperative follow-up
The patients were followed within 48 h, one week, one month, 4 months, and one year after EVLA. Bruising covering 25% or greater of the treated range was defined as “bruising”, and pain requiring treatment with additional analgesics or cooling was defined as “pain”. Duplex scanning was performed within 48 hours, at one and 4 months, and one year after EVLA, and the diameter of the ablated saphenous vein and the occlusion of the treated vein and deep vein thrombosis (DVT) were checked. The average saphenous vein diameter was measured in a standing or sitting position at 5–10 cm distal to the deep vein junction. The treated vein was judged as patent when it had compressibility with the echo probe. When 5 cm or longer patency and presence of blood flow induced by manual limb compression in a standing or sitting position were noted, the condition was judged as recanalization. Endovenous heat-induced thrombus (EHIT) which is extension of thrombus from the saphenous vein into the deep vein, was classified into Class 1 to 4 based on the classification by Kabnic, et al.9)
5. Statistical analysis
The t-test was used for between-group comparison, the χ2 test and Fisher’s exact test were used for comparison of rates between two groups, and p <0.05 was regarded as significant. The cumulative occlusion rate of treated veins was analyzed using the Kaplan-Meier method and log-rank test. Changes in the venous diameter were analyzed using one-way ANOVA and Dunnett’s test.
Results
1. Operative data (Table 2)
The mean operative time in all patients was 42.6 min, the length of the treated vein was 36.2 cm, LEED was 83.4 J/cm, and the volume of TLA solution infiltrated was 345 mL. The most frequent venous access was the percutaneous approach (414 sites, 88%), followed by a surgical approach (53 sites, 11%) and a high ligation approach (5 sites, 1%). Concomitant phlebectomy by stab avulsion method to treat branch varicosities was performed in 409 legs (90%), while sclerotherapy was performed in 12 legs (3%). LEED was significantly lower (81.4 vs. 85.1 J/cm; p = 0.0116) and the volume of TLA was significantly greater (381 vs. 315 mL; p = 0.001) in the RF than in the BF group. No significant differences were noted in the mean operative time, length of treated vein, venous approach, or concurrent procedures between the two groups.
2. Postoperative complications (Table 3)
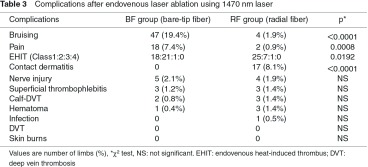
As postoperative complications in all patients, bruising was noted in 51 legs (11.3%), pain in 20 legs (4.4%), EHIT in 73 legs (16.1%), contact dermatitis in 17 (3.8%), nerve injury in nine (2.0%), thrombophlebitis in six (1.3%), peripheral DVT in five (1.1%), hematoma in four (0.9%), and wound infection in one leg (0.2%). No serious complications, such as proximal DVT, pulmonary embolism, and skin burns, were occurred, but peripheral DVT was noted in three and two legs in the RF and BF groups, respectively. All were soleal vein thrombosis and conservatively followed. In cases of EHIT Class 1 and 2, thrombus spontaneously disappeared without any treatments over follow-up. Class 3 was noted in one leg in each group and treated by anticoagulant therapy with heparin and warfarin for 1.5 and 3 months, respectively. The incidences of bruising (1.9% vs. 19.4%; p <0.0001) and pain (0.9% vs. 7.4%; p = 0.0008) were significantly lower in the RF group. The incidence of contact dermatitis associated with postoperative compression therapy was significantly higher in the RF group (8.1% vs. 0%; p <0.0001). The incidences of EHIT were 16.5% and 15.6% in the BF and RF groups, respectively, showing no significant difference, but the incidence of Class 2 was significantly higher in the BF group (p = 0.0192). No significant difference was noted in the other complications between the two groups.
3. Postoperative follow-up
The mean duration of postoperative follow-up was 281 days (1–974 days, median: 358 days) in the RF group, and 338 days (1–1457 days, median: 354 days) in the BF group. The treated vein was occluded in all patients in the RF group, but recanalization occurred in one leg (0.4%) 48 days after EVLA in the BF group. The cumulative occlusion rate of treated veins was calculated using the Kaplan-Meier method. The rate was 100% at 2 years and 8 months after EVLA in the RF group and 99.5% 4 years after EVLA in the BF group, showing no significant difference between the two groups. Clinical recurrence was noted in three legs (0.7%) in the BF group: it was due to recanalization of the treated vein in one case, and occurred in a branch of the SFJ (15 months after) and a branch varicosities (2 years after) in one case each. No recurrence was noted in the RF group.
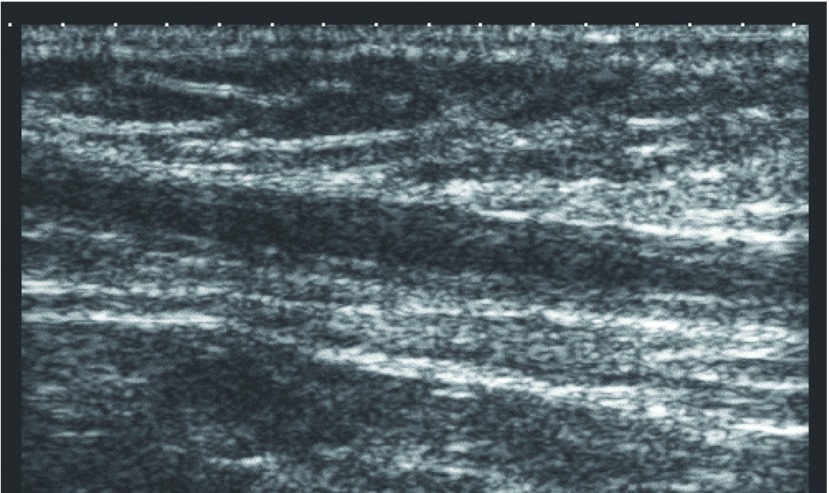
Normally, the treated vein is occluded on duplex scanning 24–48 h after EVLA, the diameter slowly decreases thereafter, and the vein becomes funicular or undetectable on duplex scanning after 4–12 months. Similarly, the venous diameter decreased with time in both groups, and the mean venous diameters 4 months after EVLA were 1.7 and 2.2 mm in the BF and RF groups, respectively (Table 4). However, partial patency of the treated vein without blood flow was frequently noted in the RF group (Fig. 2). This partial patency was a characteristic postoperative ultrasonographic finding of the RF group, but it did not progress to recanalization, and it disappeared as the venous diameter decreased.
Fig. 2.
Typical duplex ultrasound image after endovenous laser ablation using radial fiber. Duplex scanning reveals partial patency of the treated vein which has compressibility without blood flow. Thickening of the vein wall is also seen.
Discussion
Although no studies reported that long-term outcome and occlusion rates of EVLA were different according to laser wavelengths used, in this study, all veins remained occluded except only one leg in the BF group, and the cumulative occlusion rate was 100% at 2 years and 8 months after EVLA in the RF group and 99.5% at 4 years in the BF group, showing that higher occlusion rate than those (98.1% at 7 months after surgery) obtained by EVLA with a 980-nm laser in our clinic.8) High success rate may have been due to the 1470-nm laser specifically absorbed by water, damaging the vein wall more severe, which was demonstrated by the finding that the venous diameter decreased within a short time after EVLA (ca. 2 mm at 4 months).
EVLA allows application of laser energy into the diseased vein to produce thermal damage of the endothelium and vein wall with subsequent fibrosis. Thermal damage on the tissue occurs through protein coagulation by the photothermal effect of laser. Laser light applied to the tissue is partially reflected on its surface, and the remaining laser light penetrates while being absorbed and scattered.10) The photothermal effect is produced by laser energy absorbed by tissue-specific chromospheres in the tissue. The important chromospheres in the tissue are water and hemoglobin, and the absorption rate markedly varies depending on the wavelength of laser. Laser energy is absorbed by water when the wavelength is above 900 nm, and mainly absorbed by hemoglobin below 1200 nm. Hemoglobin did not absorb laser light at a wavelength 1200 to 1740 nm, and is absorbed only by water.11) On the mechanism of action when the laser light is emitted in a blood vessel, the important chromosphere is blood. Roggan, et al.12) measured the absorbance of diluted blood and water, and observed that their absorption curves were almost the same at a wavelength above 1350 nm. This is reasonable because blood contains both water and hemoglobin. It should be noted that some reports confused hemoglobin and blood and stated that longer wavelengths of laser was absorbed by hemoglobin.13) WSLW laser was usually considered to penetrate blood and induce strong contraction through directly acting on water contained in the vascular wall,5) but this is not the case. HSLW laser does not cause tissue ablation when blood is absent, but WSLW laser causes ablation regardless of the presence or absence of blood. The presence of blood is essential for ablation of veins with an HSLW laser, but a WSLW laser does not necessarily require blood, and it causes strong and reliable ablation when blood is absent.14)
At near-infrared wavelengths, peaks of water absorption are present at about 980, 1470, and 2000 nm. Absorption by water becomes stronger as the wavelength becomes longer. However, the depth of tissue penetration by laser light decreases in inverse proportion to it. Nozoe, et al.15) measured optical properties of 1470-nm and 980-nm lasers with human venous tissue, and observed that the penetration depth of a 1470-nm laser was smaller than that of a 980-nm laser and it induced strong contraction without perforating the vein wall. Water more strongly absorbs 2000-nm than 1470-nm laser light. We previously used a 2000-nm DPSS laser for EVLA,16) but could not obtain the expected clinical result. This may have been due to the fact that an appropriate balance between the absorption by water and the depth of tissue penetration is required for a laser applied in EVLA. A 1470-nm diode laser is compact, which can be simply maintained and causes only mild bruising and pain after EVLA, being superior to conventionally used wavelengths;17,18) it has become widely used in Western countries.
The output power setting of a 1470-nm laser was changed from 6 to 12 W over time in the BF group because the appropriate output power was unclear when the use of a 1470-nm laser was started. A low output power (6 W) was set based on the results of an ex vivo experiment at the beginning, but the retraction speed was low to get an appropriate LEED and ablation took a long time. Thus, the output power was gradually increased while confirming the safety. When the radial fiber was introduced, the output was set at 10 W because the characteristics of a 1470-nm laser had already been clarified. The retraction speed of the optical fiber was adjusted while confirming venous ablation and closure under duplex scan in both groups, from which LEED was calculated. LEED was significantly lower in the RF than in the BF group. The vein may have been closed with a lower energy in the RF group, decreasing LEED.
When laser is applied to the skin surface, a bare-tip fiber prepared simply by cutting the tip of an optical fiber is used. However, when a bare-tip fiber is used for EVLA, laser light applied in parallel to the vein wall is mostly absorbed by blood, and forms a carbon layer at the fiber tip.19) This carbonized blood absorbs laser energy, and the fiber tip heated to a high temperature directly touches the vein wall, resulting in venous ablation, or boiled blood bubbles damage the endothelium and obstruct the vein.20,21) In this case, there is no major difference in the effect between WSLW and HSLW lasers, and bruising and pain occur due to venous perforation and heterogeneous ablation. Actually, the incidences of bruising and pain were similar to those after treatment with a 980-nm laser in the BF group.8) Fibers with a tip covered with gold or with a rounded shape have been developed, but the clinical outcomes were not markedly improved.22)
The incidences of postoperative bruising and pain were significantly lower in the RF than in the BF group. In addition, the occlusion rate was 100% in the RF group, although LEED was significantly lower. The radial fiber emits laser light circumferentially from the side through a prism contained in the plastic cap attached to the optic fiber tip. The fluence, which is the irradiation energy per unit area, is reduced to 1/5–1/9 of that of the bare-tip fiber because the laser is emitted to a wide area from the side of the fiber, which is less likely to cause perforation and applies the laser vertically to the vein wall, excluding the influence of blood.7) Since the fluence of radial fibers is very low, EVLA without the use of TLA was attempted when it was initially developed. However, when LEED was set at a low level (mean: 22.7 J/cm) to perform laser ablation without TLA, recanalization frequently occurred.3) Thus, EVLA with the radial fiber is now performed under TLA anesthesia. Doganci, et al.23) performed a prospective randomized controlled study on EVLA with 980-nm (bare-tip fiber) and 1470-nm (radial fiber) lasers, and observed that the incidences of postoperative pain and bruising were significantly lower and the Venous Clinical Severity Score (VCSS) at one month after EVLA was significantly improved in the group treated with the 1470-nm laser.
The disadvantages of radial fibers are a large diameter and difficulty in fiber retraction during ablation. The outer diameter is 1.85 mm, which is about three times greater than that of bare-tip fibers, requiring a larger (5 Fr) introducer sheath. Moreover, the vein may not be completely occluded, remaining patent immediately after ablation because of large diameter of the fiber. A partially patent lumen was frequently noted on follow-up duplex scanning, though blood flow was absent. This was also due to the retention of the structure of the vein wall not destroyed by uniform laser irradiation of the radial fiber, and the partial patency disappeared as the venous diameter decreased and recanalization did not occur. Another disadvantage was sticking between the fiber and vein wall during ablation caused by the direct irradiation of the laser in the vein wall resulting in strong contraction of the vein wall. Careful handling of the fiber is needed as the sticking because strong retraction of the fiber may cause falling out of the lesion at once. Contact dermatitis frequently developed in the RF group. The range of varicectomy was extended in the latter period in which the radial fiber was mainly used.
Thus, the dose of TLA anesthesia was increased, and postoperative compression was intensified to prevent bleeding, which may have increased the incidence of contact dermatitis.
Conclusion
The incidence of recanalization after EVLA with a WSWL 1470-nm laser was lower than that after EVLA with a 980-nm laser, and the incidences of bruising and pain were significantly reduced by the use of the radial fiber, which emits the laser light from the side and directly applies laser energy to the vein wall, compared with those after EVLA using the conventional bare-tip fiber, facilitating low-invasive treatment.
Disclosure Statement
Masayuki Hirokawa serves as a consultant to Integral Corporation.
Footnotes
This article is English translation of Jpn J Vasc Surg 2013; 22: 615-621.
References
- Hirokawa M. Basic of laser. Textbook of Endovenous Laser Ablation. Tokyo: Japan Medical Journal, 2011: 9-21 (in Japanese) [Google Scholar]
- Vuylsteke ME, Mordon SR. Endovenous laser ablation: a review of mechanisms of action. Ann Vasc Surg 2012; 26: 424-33 [DOI] [PubMed] [Google Scholar]
- Almeida J, Mackay E, Javier J, et al. Saphenous laser ablation at 1470 nm targets the vein wall, not blood. Vasc Endovascular Surg 2009; 43: 467-72 [DOI] [PubMed] [Google Scholar]
- Hirokawa M. Mechanisms of action during endovenous laser ablation. JJSLSM 2012; 33: 57-62 (in Japanese) [Google Scholar]
- Goldman MP, Mauricio M, Rao J. Intravascular 1320-nm laser closure of the great saphenous vein: a 6- to 12-month follow-up study. Dermatol Surg 2004; 30: 1380-5 [DOI] [PubMed] [Google Scholar]
- Satokawa H, Sugiyama S, Hirokawa M, et al. Guideline for endovascular treatment of varicose vein of lower extremity. 2009–2010 Subcommittee report. Jpn J Phlebol 2010; 21: 289-309 (in Japanese) [Google Scholar]
- Sroka R, Weick K, Sadeghi-Azandaryani M, et al. Endovenous laser therapy—application studies and latest investigations. J Biophotonics 2010; 3: 269-76 [DOI] [PubMed] [Google Scholar]
- Hirokawa M, Kurihara N. Standard procedure for endovenous laser ablation with 980 nm diode lase. Jpn J Vasc Surg 2012; 21: 583-8 (in Japanese) [Google Scholar]
- Frasier K, Latessa V. Minimally invasive vein therapy and treatment options for endovenous heat-induced thrombus. J Vasc Nurs 2008; 26: 53-7 [DOI] [PubMed] [Google Scholar]
- Awazu K. Chapter II Infrared laser for biomedical engineering. Infrared laser medical engineering. Osaka: Osaka University Press, 2008: 27-51 (in Japanese) [Google Scholar]
- Kuenstner JT, Norris KH. Spectrophotometry of human hemoglobinin the near infrared region from 1000 to 2500 nm. J Near Infrared Spectrosc 1994; 2: 59-65 [Google Scholar]
- Roggan A, Friebel M, Do Rschel K, et al. Optical properties of circulatinghuman blood in the wavelength range 400–2500 nm. J Biomed Opt 1999; 4: 36-46 [DOI] [PubMed] [Google Scholar]
- Sakakibara N, Amano A, Kansaku R, et al. Medical-engineering background of endovenous laser ablation. Jpn J Phlebol 2011; 22: 341-9 (in Japanese) [Google Scholar]
- Vuylsteke M, Van Dorpe J, Roelens J, et al. Endovenous laser treatment: a morphological study in an animal model. Phlebology 2009; 24: 166-75 [DOI] [PubMed] [Google Scholar]
- Nozoe S, Honda N, Ishii K, et al. Comparison of the efficacy and the safety in endovenous laser ablation at wavelengths of 980 nm and 1470 nm laser based on human vein tissue optical properties. JJSLSM 2012; 33: 7-14 (in Japanese) [Google Scholar]
- Hirokawa M, Kondoh K, Miyata T. Two-micron (2000 nm) endovenous laser treatment of the great saphenous vein. Int Angiol 2007; 26: 69 [Google Scholar]
- Pannier F, Rabe E, Maurins U. First results with a new 1470-nm diode laser for endovenous ablation of incompetent saphenous veins. Phlebology 2009; 24: 26-30 [DOI] [PubMed] [Google Scholar]
- Maurins U, Rabe E, Pannier F. Does laser power influence the results of endovenous laser ablation (EVLA) of incompetent saphenous veins with the 1470-nm diode laser? A prospective randomized study comparing 15 and 25 W. Int Angiol 2009; 28: 32-7 [PubMed] [Google Scholar]
- Disselhoff BC, Rem AI, Verdaasdonk RM, et al. Endovenous laser ablation: an experimental study on the mechanism of action. Phlebology 2008; 23: 69-76 [DOI] [PubMed] [Google Scholar]
- Proebstle TM, Sandhofer M, Kargl A, et al. Thermal damage of the inner vein wall during endovenous laser treatment: key role of energy absorption by intravascular blood. Dermatol Surg 2002; 28: 596-600 [DOI] [PubMed] [Google Scholar]
- van der Geld CW, van den Bos RR, van Ruijven PW, et al. The heat-pipe resembling action of boiling bubbles in endovenous laser ablation. Lasers Med Sci 2010; 25: 907-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince EA, Soares GM, Silva M, et al. Impact of laser fiber design on outcome of endovenous ablation of lower-extremity varicose veins: results from a single practice. Cardiovasc Intervent Radiol 2011; 34: 536-41 [DOI] [PubMed] [Google Scholar]
- Doganci S, Demirkilic U. Comparison of 980 nm laser and bare-tipfibre with 1470 nm laser and radial fibre in the treatment of greatsaphenous vein varicosities: a prospective randomised clinical trial. Eur J Vasc Endovasc Surg 2010; 40: 254-9 [DOI] [PubMed] [Google Scholar]