Abstract
The spur occasionally seen in a left common iliac vein was investigated by anatomical and histological examination of cadavers so the occurrence mechanism could be discussed. Spurs were found in six cases of the 28 cadavers (21.4%) and they were classified into few different kinds of composition of endosporia, tunica media and adventitia. It is considered that there may be different formation mechanisms and stages even in cases of similar anatomical finding. (*English translation of J Jpn Coll Angiol 2013; 53: 43-47)
Keywords: spur, compression, May-Thurner syndrome, left common iliac vein
Introduction
The presence of a septum or process in the left common iliac vein was reported in 1906 by an anatomist at Michigan University, McMurrich.1) He considered these as congenital remnant structures. May and Thurner2) histologically investigated and named them left common iliac vein spurs in 1956. Since they suggested that spurs are acquired through compression by the right common iliac artery and vertebra, and their presence increases the risk for left iliofemoral venous thrombosis, the condition became widely known as May-Thurner syndrome, Cockett’s syndrome, or iliac vein compression syndrome.3) With the recent advances in intravascular ultrasound tests (IVUS) and intravascular treatment (EVT), spurs have again been attracting attention.4–9) Against this background, we investigated the spur-forming mechanism anatomically and histologically.
Subjects and Methods
Twenty-eight cadavers for autopsy practice used in the 2010 Aichi Medical University Autopsy Seminar were used (12 males and 16 females, mean age at death: 82.5 years old).
The pelvic organs were excised, the distributions of the common iliac artery and vein were macroscopically confirmed, and the inferior vena cava and bilateral common iliac veins were excised en bloc. The right margins of the inferior vena cava and right common iliac vein were incised, and the lumen of the left common iliac vein, mainly the region in which the left common iliac vein joined the inferior vena cava, was observed (Fig. 1). When a spur was present, the region was excised in the transverse direction of the left common iliac vein, fixed in formalin, and embedded in paraffin, and thin sections (3 µm) in the transverse-sectional direction were prepared. These were subjected to hematoxylin-eosin (HE), Masson-trichrome, and Elastica-Van-Gieson (EVG) staining and immunostaining with α-smooth muscle-actin antibody (α SMA) for histological observation. Between-group comparison was performed employing the χ2 test, and a p <0.05 was regarded as significant.
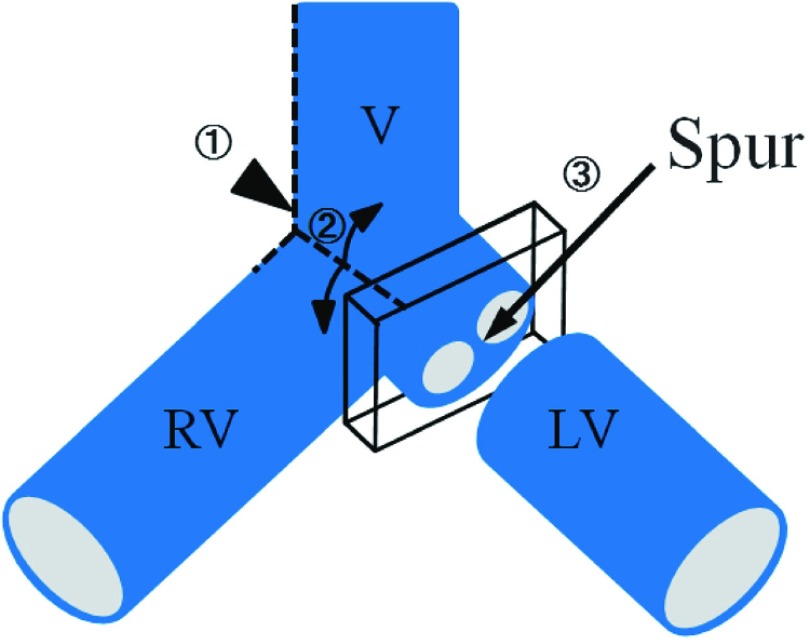
Fig. 1.
Illustration of spur excision.  First, the right edge of the right common iliac vein (RV) and the inferior vena cava (V) are incised (dashed line
First, the right edge of the right common iliac vein (RV) and the inferior vena cava (V) are incised (dashed line  ).
).  The ventral wall of the left common iliac vein (LV) are flapped (dashed line
The ventral wall of the left common iliac vein (LV) are flapped (dashed line  ) so as to determine whether there is a spur at the LV.
) so as to determine whether there is a spur at the LV.  When there is spur, the transverse section including spur is removed.
When there is spur, the transverse section including spur is removed.
Results
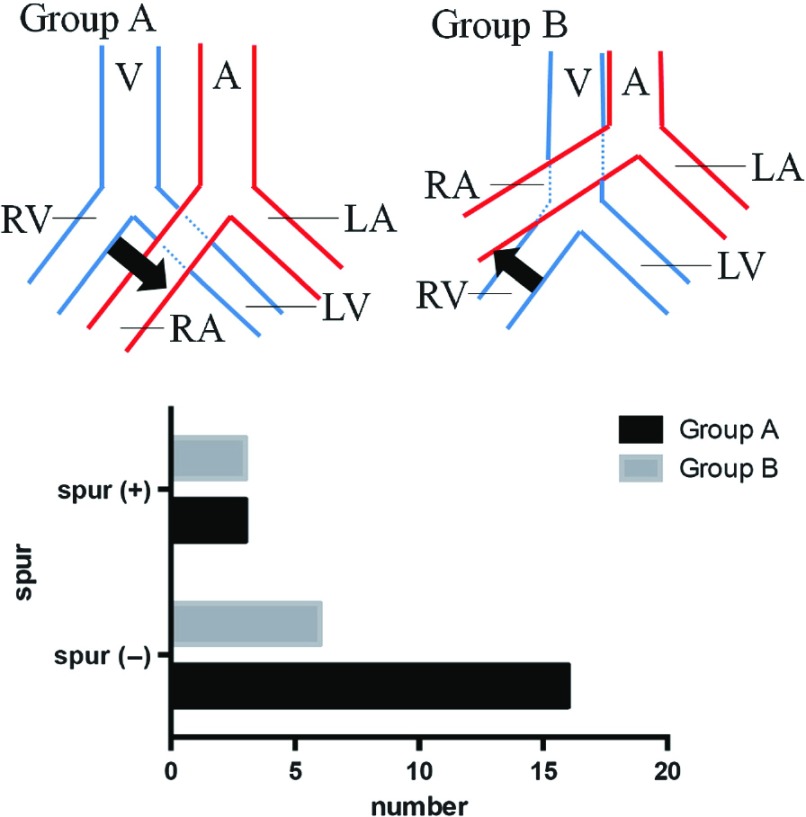
Macroscopically, individual variations were noted in the distributions of common iliac artery and vein. The entirety or a part of the right common iliac artery ran over the left common iliac vein (the aorta bifurcated at a low level, Group A) in 19 (67.9%) of the 28 cadavers, and the right common iliac artery ran over the inferior vena cava (the aorta bifurcated at a high level, Group B) in 9 (32%). Spurs were present in 6 (21.4%) of the 28 cadavers (three males and females). The incidences of spurs were 15.8% and 33.3% in Groups A and B, respectively, but there was no significant difference between these groups (p = 0.351) (Fig. 2).
Fig. 2.
Classification of abdominal aorta by the intersection of the right common iliac artery site. In Group A, all or a part of the right common iliac artery rode across left common iliac vein. In group B, the right common iliac artery overcame the inferior vena cava. A: abdominal aorta; V: inferior vena cava; RA: right common iliac artery; LA: left common iliac artery; RV: right common iliac vein; LV: left common iliac vein.
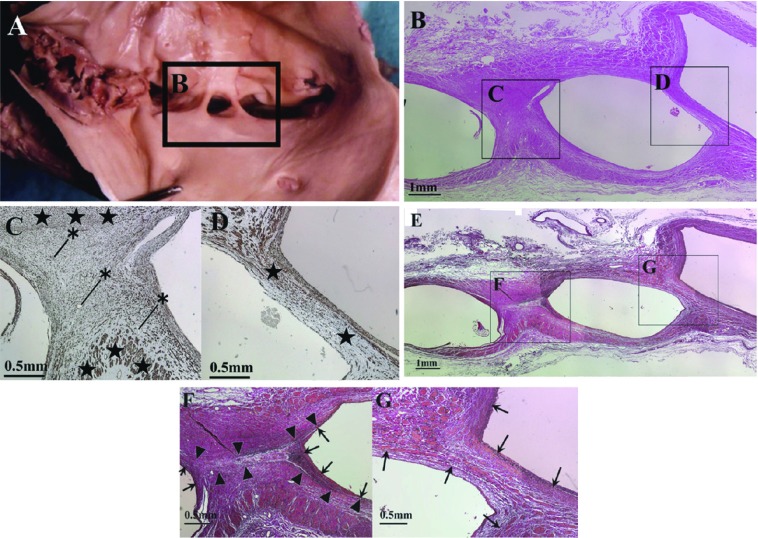
Histologically, αSMA immunostaining-positive structures were noted in all spurs (Fig. 3C and 3D). In addition, the tunica media and internal elastic lamina were present at both ends of spurs in all cases, and these were continuous with the venous wall (Fig. 3F and 3G).
Fig. 3.
A case of macroscopic and histological view of type 2 spur. Panels B–D and panels E–G show different parts of spurs shown in squares in panel (A. B) HE staining. Squares indicate panel C, D respectively. (C) Immunohistochemistry for αSMA. Black stars (⋆) indicate tunica media. Asterisks (*) indicate blood vessels inside the spur. (D) immunohistochemistry for αSMA. No blood vessels were observed in the spur. (E) EVG staining. Squares indicates panel F, G. αSMA-positive cells are identified in each spur. (F) EVG staining. Arrowheads (▲) indicate elastic plate-like structure. Arrows (↑) indicate internal elastic lamina of the spur. Note: there is an area lacking tunica media between the elastic plate-like structure in the spur. (cf. panel C). (G) EVG staining. Arrows (↑) indicate lamina and the inner lining of the vein wall. Note: lamina, the inner lining and tunica media exists in the spur (cf. panel D).
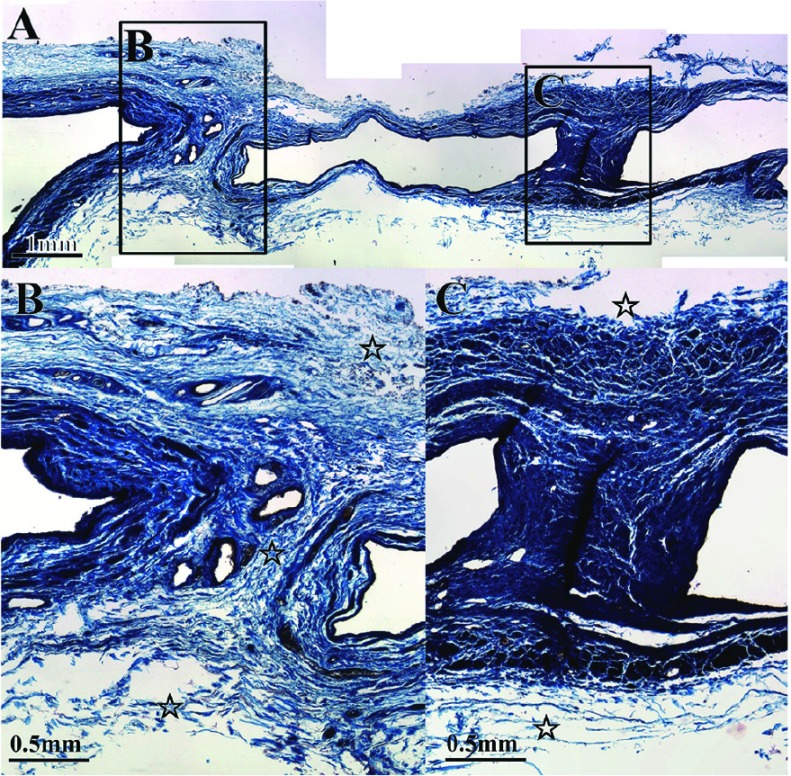
On the other hand, histological variation was noted in the inner structures of spurs: blood vessels were present in some spurs (Fig. 3C) but absent in others (Fig. 3D), and elastic plate-like structures continuous with the inner elastic lamina were present in some spurs (Fig. 3F) but absent in others (Fig. 3G). The tunica media of the opposite venous wall did not continue in spurs with elastic plate-like structures continuous with the inner elastic lamina (Fig. 3C and 3F), but did continue in spurs without an elastic plate-like structure (Fig. 3D and 3G). In spurs in which the tunica media of the venous wall continued, the adventitia also continued in some spurs (Fig. 4B) but not in others (Fig. 4C).
Fig. 4.
Another case of spur. Masson-trichrome staining. Squares indicate panel B, C. (B) Open stars ( ) indicate the outer membrane. The outer membrane (open stars) recognized in the spur. (C) The outer membrane (open stars) did not invade the spur.
) indicate the outer membrane. The outer membrane (open stars) recognized in the spur. (C) The outer membrane (open stars) did not invade the spur.
Discussion
In previous studies on common iliac vein spurs in autopsied patients, spurs near the confluence of the left common iliac vein were observed in 10 of 32 patients (32%) by McMurrich,10) 98 (24%) of 412 by Ehrich,11) 19% of 430 patients by May and Thurner, et al.,12) and 14 (14%) of 100 patients by Negus, et al.13) Vollmar, et al.14) observed spurs after thrombectomy of left iliac vein thrombosis using a vascular endoscope in 50%, and Juhan, et al.15) observed spurs on venography in 62%. Although the frequency of spurs varies among reports, they are not necessarily rare. In Japan, Usui, et al.16) observed hypertrophy and band formation in the tunica media of the iliac vein in 50% of 90 autopsied patients, but, in the right iliac vein, only band formation was noted in one patient.
DiDio17) considered that spurs were formed due to compression of the left common iliac vein anterior to the vertebra by the right common iliac artery from the anterior side and the middle sacral artery from the posterior side. May and Thurner12) stated that spurs are acquired through reactive hyperplasia of the venous wall induced by compression by the right common iliac artery and vertebra because no abnormality was noted in the left iliac vein in 88 fetuses and children.
The incidences of spurs were 15.8% (3/19) in Group A with aortic bifurcation at a low level and 33.3% (3/9) in Group B with aortic bifurcation at a high level. May and Thurner, et al.12) reported that the incidence was high in patients with aortic bifurcation at a high level, but Fretz, et al.18) stated that the incidence was low in these patients. In our study, no significant difference was noted between the two groups, but the incidence was rather higher in those with aortic bifurcation at a low level. The spur location was not completely consistent with the right common iliac artery and left iliac vein crossover site. These findings suggest that there is another cause of spur formation, in addition to compression by the right common iliac artery and vertebra.
The cause of spur formation was compression by organs other than the right common iliac artery and vertebra in several reports. Nagayo, et al.19) assumed that the cause was compression by the pregnant uterus, and Akanuma, et al.20) assumed that the cause was compression of the sigmoid colon by constipation. However, Ferrari21) stated that compression by the pregnant uterus does not cause spur formation because it is temporary and the uterus is soft. As noted in our study, the incidence of spurs in females is not high. Even if the pregnant uterus compresses the vein, it is difficult to explain the presence of a spur in the left common iliac vein. In contrast, spurs were present in the region of the left common iliac artery sandwiched by the sigmoid colon in many cases. Thus, the possibility of the involvement of compression by the artery and pelvic organs in some cases cannot be ruled out.
Separately from these compression theories, McMurrich10) and Ehrich, et al.11) stated that spurs are residual structures formed in the process of the confluence of the posterior cardinal vein, which is the main path of return from the caudal region of the fetal body, accompanying the left common iliac vein formation process. Pinsolle, et al.22) reported that 121 abnormal structures including spurs were present in the common iliac vein and inferior vena cava in 88 of 130 cadavers, and 115 of these were present in the left common iliac vein and could be morphologically classified into five types (central spurs, adhesions, bridges, valves and bands). In many reports, the different origin of the left common iliac vein from that of the right common iliac vein is considered to be a cause of the high incidence of abnormal structures in the left common iliac vein, but the cause was discussed from histological viewpoints in only a few reports. Ferrari21) assumed that spurs were congenitally formed because not only remnant collagen and elastic fibers but also smooth muscle cells were observed in spurs. In contrast, May and Thurner2) considered that spurs are not remnants of past thrombi but are pure scar tissue acquired through compression by the right common iliac artery and vertebra because spurs did not contain elastic fiber and hemosiderin, capillary blood vessel-rich collagen tissue was more or less contained, lymphocyte infiltration was observed, and elastic fibers of the tunica media were distributed along the venous wall and localized on the spur surface, not entering spurs. Ehrich, et al.11), Negus, et al.,13) and DiDio17) support this theory.
As described in Results, there was histological variation in the spur structure. In particular, the presence or absence of elastic plate-like structures in spurs may be a finding that should be paid attention. It is difficult to explain the type in which the venous wall tunica media or adventitia continues in spurs (Figs. 3D, 3G and 4B) by the compression theory, and these may be more likely to be congenital structures. In the type in which the inner region of spurs is separated from the venous wall by an elastic plate-like structure (Fig. 3F), the tunica media of the bilateral venous walls may have made contact by an external force, such as compression, and structures filling the space may have been formed. Therefore, it was concluded that there are several spur-forming mechanisms depending on the type.
Negus13) considered four spur-forming mechanisms: (1) congenital, (2) organization of mural thrombi, (3) capture and organization of thrombi formed in the crural veins in the region compressed by the artery, and (4) fibrosis directly caused by compression by the artery. The spurs shown in Figs. 3G and 4B may be congenital, but organization of mural thrombi may also be possible as a formation mechanism of the spur shown in Fig. 3F. Regardless of the spur-forming mechanism being congenital or acquired, clinically, the presence of a spur is likely to cause stagnation of venous blood in the left common iliac vein, and it cannot be ruled out that this condition further promotes capturing thrombi formed in the crural veins in the spur region and leads to iliac vein thrombosis. Further investigation is necessary, particularly for spurs considered to have been acquired.
Conclusion
Macroscopic anatomically, spurs were more frequently noted in cases with aorta bifurcation at a high level, but the difference was not significant. It was considered that spur formation involves not only compression by the right common iliac artery and vertebra but also other factors. In addition, morphological differences were noted histologically in spurs macroscopically appearing to have the same structure, such as differences in the boundary between the venous wall tunica media and spur and invasion of the adventitia into spurs. These findings suggest the presence of several types of spur formed through different mechanisms, and there may be congenital and acquired formation mechanisms.
Acknowledgement
We are grateful to Ms. Seiko Adachi, medical technologist at the Department of Pathology, Aichi Medical University Hospital, for the preparation of pathological samples. We also thank the Aichi Medical University Alumni Association for support over a long period (Grant number: AMU-AARF-2011003).
Disclosure Statement
For the present study, Mitsuoka H received assistance from Aichi Medical University Alumni Association (grant number: AMU-AARF-2011003). The other co-authors have no conflict of interest.
Footnotes
This article is English translation of J Jpn Coll Angiol 2013; 53: 43-47.
References
- McMurrich JP. The valves of the iliac vein. Br Med J 1906; 2: 1699-700 [Google Scholar]
- May R, Thurner J. Ein Gefaesssporn in der Vena iliaca communis sinistra als Ursache der ueberwiegend linksseitigen Beckenvenenthrombosen. Z Kreisl–Forsch 1956; 45: 912-22 [PubMed] [Google Scholar]
- Cockett FB, Thomas ML. The iliac compression syndrome. Br J Surg 1965; 52: 816-21 [DOI] [PubMed] [Google Scholar]
- Forauer AR, Gemmete JJ, Dasika NL, et al. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol 2002; 13: 523-7 [DOI] [PubMed] [Google Scholar]
- Mickley V, Schwagierek R, Rilinger N, et al. Left iliac venous thrombosis caused by venous spur: treatment with thrombectomy and stent implantation. J Vasc Surg 1998; 28: 492-7 [DOI] [PubMed] [Google Scholar]
- Patel NH, Stookey KR, Ketcham DB, et al. Endovascular management of acute extensive iliofemoral deep venous thrombosis caused by May-Thurner syndrome. J Vasc Interv Radiol 2000; 11: 1297-302 [DOI] [PubMed] [Google Scholar]
- O’Sullivan GJ, Semba CP, Bittner CA, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol 2000; 11: 823-36 [DOI] [PubMed] [Google Scholar]
- Hurst DR, Forauer AR, Bloom JR, et al. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg 2001; 34: 106-13 [DOI] [PubMed] [Google Scholar]
- Raju S, Neglén P. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg 2009; 50: 360-8 [DOI] [PubMed] [Google Scholar]
- McMurrich JP. The occurence of congenital adhesions in the common iliac veins and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci 1908; 135: 342-6 [Google Scholar]
- Ehrich WE, Krumbhaar EB. A frequent obstructive anomaly of the mouth of the left common iliac vein. Am Heart J 1943; 26: 737-50 [Google Scholar]
- May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957; 8: 419-27 [DOI] [PubMed] [Google Scholar]
- Negus D, Fletcher EW, Cockett FB, et al. Compression and band formation at the mouth of the left common iliac vein. Br J Surg 1968; 55: 369-74 [DOI] [PubMed] [Google Scholar]
- Vollmar JF, Hutschenreiter S. Vascular endoscopy for venous thrombectomy. In: Moore WS Alin SS eds. Endovascular Surgery. Philadelphia: WB Saunders, 1989: 65-73 [Google Scholar]
- Juhan CM, Alimi YS, Barthelemy PJ, et al. Late results of iliofemoral venous thrombectomy. J Vasc Surg 1997; 25: 417-22 [DOI] [PubMed] [Google Scholar]
- Usui N, Muraguchi K, Yamamoto H, et al. Ilium and femoral vein thrombosis. Surgery 1978; 40: 983 (in Japanese) [Google Scholar]
- DiDio LJA. Normal and pathological structures on the inner surface of the left common iliac vein. Arq Cir Clin Experim 1949; 12: 507-616 [Google Scholar]
- Fretz V, Binkert CA. Compression of the inferior vena cava by the right iliac artery: a rare variant of May-Thurner syndrome. Cardiovasc Intervent Radiol 2010; 33: 1060-3 [DOI] [PubMed] [Google Scholar]
- Nagayo M, Nakayama O. Ueber die Stenose bzw. Obliteration der linken V. iliaca an der Einmundungsstelle in die Hohlvene. Dtsch Med Wochenschr 1912; 38: 749-51 [Google Scholar]
- Akanuma J, et al. Stricture of opening in the left common iliac vein in Korean people. Proceedings of the Jpn Soc Pathol 1932; 2: 595-8 (in Japanese) [Google Scholar]
- Ferrari E., Jr Su alcune interessanti malformazioni della vena iliaca commune di sinistra nell`uomo. Briglie congenite tese attraverso illume. Cuore Circol 1940; 24: 455-68 [Google Scholar]
- Pinsolle J, Drouillard J, Grenieret F, et al. Internal arrangement of the union between iliac vein and inferior vena cava. Anatomia Clinica 1982; 4: 295-306 [Google Scholar]