Abstract
Purpose: To identify the computed tomography (CT) findings of persistent type II endoleak from the inferior mesenteric artery (IMA) which indicate the need for preoperative IMA embolization.
Materials and Methods: Included were 120 patients (96 males, 49–93 years old, mean: 77.7) who underwent endovascular aortic aneurysm repair (EVAR) between June 2007 and October 2010. The relationship between persistent type II endoleak and CT findings of IMA orifice was examined.
Results: CT showed no type II endoleak from IMA in 106 patients (89%; Group N), and transient type II endoleak from IMA in 10 patients (8.3%; Group T). CT showed persistent type II endoleak from IMA in 4 patients (3.3%; Group P) and three of them underwent reintervention. Univariate Cox-Mantel test analysis indicated that stenosis (p = 0.0003) and thrombus (p = 0.043) in IMA orifice were significant factors for persistent type II endoleak. The ratios of patients with proximal IMA more than 2.5 mm diameter in Groups N, Y, and P were 26/106 (24%), 5/10 (50%) and 4/4 (100%), respectively.
Conclusion: Indicators for embolization of IMA prior to EVAR for the prevention of type II endoleak appear to be: (1) more than 2.5 mm in diameter and (2) no stenosis due to calcification or mural thrombus in IMA orifice.
Keywords: CT, inferior mesenteric artery, type II endoleak, endovascular aneurysm repair
Introduction
Endovascular aortic aneurysm repair (EVAR) is a method for repairing abdominal aortic aneurysms (AAAs) that is increasingly used for patients with suitable anatomy.1,2) EVAR requires continued surveillance because up to 11% of patients need reintervention for major adverse events, in particular endoleak.3) Persistent type II endoleak has been associated with a higher incidence of adverse outcomes, including aneurysmal sac growth, need for reintervention and for conversion to open repair, and rupture.4,5) The most commonly involved branches are the inferior mesenteric artery (IMA) and lumbar arteries (LA), which are usually covered with endografts. Various strategies have been advocated for managing persistent type II endoleak with some investigators recommending preemptive adjunctive procedures such as IMA coil embolization to preclude type II endoleak.6–9)
However, some studies have questioned the effectiveness of preoperative embolization of aortic side branches for the prevention of type II endoleak.9–13) Considering the indications as well as the complications of catheterization of the IMA, computed tomography (CT) findings of the IMA orifice such as stenosis due to mural thrombus or calcification may well be significant. To the best of our knowledge, however, there have been no reports concerning the relationship between persistent type II endoleak from the IMA and CT findings of the IMA orifice. The purpose of the present study was therefore to identify the CT findings of which reveal persistent type II endoleak and constitute indications for preoperative IMA embolization.
Materials and Methods
Of the 173 patients who underwent EVAR using commercially available devices between June 2007 and October 2010, 120 (96 males, 49–93 years old; mean: 77.7) were included in the present study. The reasons for exclusion of the remaining 53 patients were: occlusion of the IMA at its orifice in 36 patients, allergy to contrast media or renal dysfunction in 9 patients, which made evaluation of the IMA with enhanced CT impossible, and the IMA originating from the aorta above the aneurysm in 8 patients.
All clinical data including radiographic data were obtained retrospectively from each of the patient charts.
All CT scans were performed with intravenous contrast material and thin collimation. After an unenhanced scan, a bolus injection of contrast medium (1.5–2.0 ml/s) was administrated after a delay of 20–25 s for preparation. The images were reconstructed from 2 mm-thick slices. Images of the delayed phase were obtained for all patients 3 min after the early phase scan. CT scan with 2-mm slice thickness was used to analyze the anatomical factors of the IMA such as diameter, stenosis, thrombus, and calcification at its orifice. Stenosis of the IMA orifice was defined as 75% stenosis due to mural thrombus and/or calcification seen on the axial and curved multiplaner image of thin-slice CT. Mural thrombus was defined as a dense (more than 5 mm) thrombus around the IMA orifice. Calcification was defined as protrusion of calcified plaque around the IMA orifice. These CT findings for the IMA were determined by two radiological specialists.
All patients included in this study underwent an enhanced CT scan before and one week after the initial EVAR procedure. Subsequently, patients were clinically evaluated with follow-up CT scans 6 and 12 months after EVAR, and at least yearly thereafter. The presence of type II endoleak was determined only from the CT scan for this study.
When the increase in size of the aneurysm sac was 5 mm more than the preoperative maximal sac diameter, selective angiography of the IMA via the superior mesenteric artery and meandering artery or the lumbar arteries was indicated to determine the source of type II endoleak and the indicators of embolization.
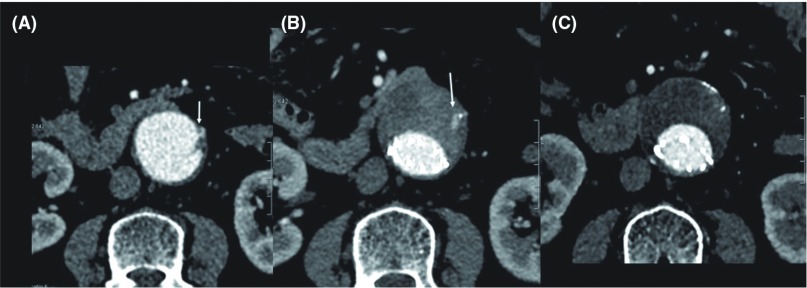
Patients were divided into three follow-up groups based on the CT findings. Group N (106 patients; 89%): no type II endoleak from the IMA; Group T (10 patients; 8.3%): transient type II endoleak from the IMA; Group P (4 patients; 3.3%): persistent type II endoleak from the IMA with or without secondary intervention. When the type II endoleak spontaneously disappeared during the follow-up period, it was defined as transient (Fig. 1).
Fig. 1.
A 74-year-old woman underwent EVAR with Powerlink for treatment of AAA, 46 mm in diameter (A). Stenosis of the orifice of the IMA was caused by mural thrombus and calcification (arrow). Computed tomography (CT) 1 week after EVAR showed type II endoleak from the IMA (B, arrow). However, CT 24 months after EVAR showed spontaneous disappearance of type II endoleak (C). AAA: abdominal aortic aneurysm; IMA: inferior mesenteric artery; EVAR: endovascular aortic aneurysm repair.
The preoperative variables and anatomical factors potentially associated with persistent type II endoleak were assessed using univariate analysis to identify the risk factors for persistent type II endoleak from the IMA.
JMP software (SAS Inc., Cary, North Carolina, USA) was used for all statistical analyses. Univariate comparisons of patient demographic and preoperative risk factors were performed with the Cox-Mantel test.
This study was approved by the institutional review board of the National Cerebral and Cardiovascular Center.
Results
All EVAR procedures were performed successfully with an Excluder (W.L. Gore & Associates, Flagstaff, Arizona, USA) for 55 patients, a Zenith (Cook Medical, Bloomington, Indiana, USA) for 47, and a Powerlink (Endologix, Irvine, California, USA) for 18.
Type II endoleak from the IMA was detected in 14 patients (11.6%) during the follow-up period, but disappeared spontaneously in 10 patients (8.3%) from Group T 2.2–23.9 (mean: 10.6) months after EVAR (Fig. 1). In addition, in 8 of these 10 patients (80%) type II endoleak from the IMA disappeared more than 6 months after EVAR. No significant difference was recognized between three devices in the detection of type II endoleak from IMA (p = 0.47). In addition, no significant difference was recognized in the disappearance of type II endoleak from IMA by the device selection (p = 0.38). Because of the aneurysmal sac enlargement more than 5 mm during the follow up period, transcatheter arterial embolization via the superior mesenteric artery was performed for 3 of the 4 patients of Group P (Fig. 2).
Fig. 2.
A 76-year-old man with AAA, 57 mm in diameter. Preoperative computed tomography (CT) showed the IMA without stenosis or mural thrombus (A, arrow). The size of the aneurysm sac increased from 57 mm (B, 1 week after EVAR) to 65 mm (C, 2 years after EVAR), and selective angiography of the IMA via SMA and meandering artery (D) showed type II endoleak. Coil embolization of the aneurysm sac and IMA was performed (E). AAA: abdominal aortic aneurysm; IMA: inferior mesenteric artery; EVAR: endovascular aortic aneurysm repair; SMA: superior mesenteric artery.
Preoperative anatomical variables related to the IMA are summarized in Table 1. The univariate Cox-Mantel test indicated that the absence of stenosis of the IMA at its orifice was more likely to be a significant factor of persistent type II endoleak (p = 0.0003), and thrombus at the orifice of the IMA was a negative predictor of persistent type II endoleak (p = 0.043).

The diameter of the proximal IMA, near its orifice, tended to be larger in Groups T and P but the differences were not significant (p = 0.096). However, the ratios of patients whose proximal IMA was larger than 2.5 mm in diameter in Groups N, T, and P were 26/106 (24.5%), 5/10 (50%), and 4/4 (100%) respectively (Fig. 3). Moreover, in 8/10 (80%) patients of Group T, CT showed stenosis of the proximal IMA, but no stenosis in any patients of Group P.
Fig. 3.
Prevalence of diameter in inferior mesenteric artery (IMA) orifice. White dots indicate IMA without stenosis, and black dots IMA with stenosis due to thrombus or calcification.
The total number of patients with an IMA more than 2.5 mm diameter and without stenosis was 12 (11.2%) (Fig. 3). The sensitivity and specificity of persistent type II endoleak in patients with an IMA more than 2.5 mm diameter and without stenosis were 4/4 (100%) and 108/116 (93.1%) respectively. In addition, the respective positive and negative predictive values were 4/12 (33%) and 108/108 (100%).
Discussion
The clinical significance of type II endoleak after EVAR has not been established yet and remain controversial.14,15) When compared with type I and III endoleaks, type II is considered to be usually benign because as many as 80% of type II endoleak occurrences are resolved spontaneously within 6 months after EVAR.16,17) However, type II endoleak that persists more than 6 months is less likely to be resolved and is associated with a higher risk of adverse events than is transient type II endoleak.16)
Our data showed spontaneous disappearance of type II endoleak from IMA more than 6 months after EVAR in 8 of 10 patients (80%). This may suggest that type II endoleak from the IMA can be managed conservatively more than 6 months, but three of four patients (75%) with persistent type II endoleak had to undergo secondary intervention. Our data also showed that the natural history of persistent type II endoleak is difficult to define because, even though a majority of such endoleak from the IMA disappeared, if it persists, it often leads to secondary intervention.
Transarterial retrograde embolization as well as translumbar embolization was performed for patients with sac enlargement of more than 5 mm, but the technical success rate was not high (17%–44%) because of the presence of a multitude of collateral networks of aortic side branches.18–20) Identification of patients at increased risk of persistent type II endoleak is still imprecise, and the occurrence of various associated adverse events has prompted some interventionists to come to accept that the best method for handling a type II endoleak is to prevent its development in the first place. Muthu, et al. reported the findings of their study of routine preoperative embolization of the IMA and thrombin injection into the aneurysm sac.21) This technique reduced the occurrence of type II endoleak, but the difference failed to attain statistical significance. Although these preoperative embolization techniques appear attractive, most patients with patent side braches will not develop type II endoleak, so that routine embolization before EVAR exposes many patients to unnecessary procedure-related risks.22)
In this study, only four of 120 patients (3.3%) showed persistent type II endoleak from the IMA. This means that, if all patent IMAs in our study had been subjected to preoperative embolization, 116 (96.7%) embolization procedures would have had to be considered unnecessary. This constitutes a strong argument for selective preoperative endovascular intervention in view of the substantial risk of persistent type II endoleak.23)
To determine the indication of selective intervention of the IMA prior to EVAR, preoperative CT constitutes the most reliable and easily available image source. Univariate analysis of the anatomical variables of preoperative CT revealed that stenosis and thrombus of the IMA orifice is associated with both transient and permanent type II endoleak. Although the proximal diameter of the IMA showed an insignificant p-value, setting the cut-off diameter at 2.5 mm resulted in a sensitivity of 100%, specificity of 93% and negative predictive value of 100%. If embolization had been indicated only for IMA more than 2.5 mm diameter and without stenosis or thrombus, the number of candidate would have been 12 (11.2%), which seems more acceptable than performing preoperative embolization on all patent IMAs.
Small number of cases with persistent type II endoleak from IMA and retrospective design were thought to be the limitation of this study.
In conclusion, the indicators for embolization of the IMA prior to EVAR for the prevention of type II endoleak appear to be: (1) diameter of more than 2.5 mm and (2) no stenosis due to calcification or mural thrombus in the IMA orifice.
Disclosure Statement
Fukuda and the other co-authors have no conflict of interest to declare.
References
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991; 5: 491-9 [DOI] [PubMed] [Google Scholar]
- Greenhalgh RM, Powell JT. Endovascular repair of abdominal aortic aneurysm. N Engl J Med 2008; 358: 494-501 [DOI] [PubMed] [Google Scholar]
- Conrad MF, Adams AB, Guest JM, et al. Secondary intervention after endovascular abdominal aortic aneurysm repair. Ann Surg 2009; 250: 383-9 [DOI] [PubMed] [Google Scholar]
- van Marrewijk CJ, Fransen G, Laheij RJ, et al. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg 2004; 27: 128-37 [DOI] [PubMed] [Google Scholar]
- Silverberg D, Baril DT, Ellozy SH, et al. An 8-year experience with type II endoleaks: natural history suggests selective intervention is a safe approach. J Vasc Surg 2006; 44: 453-9 [DOI] [PubMed] [Google Scholar]
- Baum RA, Carpenter JP, Stavropoulous SW, et al. Diagnosis and management of type 2 endoleaks after endovascular aneurysm repair. Tech Vasc Interv Radiol 2001; 4: 222-6 [DOI] [PubMed] [Google Scholar]
- Chikazawa G, Yoshitaka H, Hiraoka A, et al. Preoperative coil embolization to aortic branched vessels for prevention of aneurysmal sac enlargement following EVAR: early clinical result. Ann Vasc Dis 2013; 6: 175-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevala T, Biancari F, Manninen H, et al. Inferior mesenteric artery embolization before endovascular repair of an abdominal aortic aneurysm: effect on type II endoleak and aneurysm shrinkage. J Vasc Interv Radiol 2010; 21: 181-5 [DOI] [PubMed] [Google Scholar]
- Nevala T, Biancari F, Manninen H, et al. Type II endoleak after endovascular repair of abdominal aortic aneurysm: effectiveness of embolization. Cardiovasc Intervent Radiol 2010; 33: 278-84 [DOI] [PubMed] [Google Scholar]
- Axelrod DJ, Lookstein RA, Guller J, et al. Inferior mesenteric artery embolization before endovascular aneurysm repair: technique and initial results. J Vasc Interv Radiol 2004; 15: 1263-7 [DOI] [PubMed] [Google Scholar]
- Bonvini R, Alerci M, Antonucci F, et al. Preoperative embolization of collateral side branches: a valid means to reduce type II endoleaks after endovascular AAA repair. J Endovasc Ther 2003; 10: 227-32 [DOI] [PubMed] [Google Scholar]
- Parry DJ, Kessel DO, Robertson I, et al. Type II endoleaks: predictable, preventable, and sometimes treatable. J Vasc Surg 2002; 36: 105-10 [DOI] [PubMed] [Google Scholar]
- Sheehan MK, Hagino RT, Canby E, et al. Type 2 endoleaks after abdominal aortic aneurysm stent grafting with systematic mesenteric and lumbar coil embolization. Ann Vasc Surg 2006; 20: 458-63 [DOI] [PubMed] [Google Scholar]
- Ohki T, Veith FJ, Shaw P, et al. Increasing incidence of midterm and long-term complications after endovascular graft repair of abdominal aortic aneurysms: a note of caution based on a 9-year experience. Ann Surg 2001; 234: 323-34; discussion 334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarins CK, White RA, Hodgson KJ, et al. Endoleak as a predictor of outcome after endovascular aneurysm repair: AneuRx multicenter clinical trial. J Vasc Surg 2000; 32: 90-107 [DOI] [PubMed] [Google Scholar]
- Gelfand DV, White GH, Wilson SE. Clinical significance of type II endoleak after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg 2006; 20: 69-74 [DOI] [PubMed] [Google Scholar]
- Rayt HS, Sandford RM, Salem M, et al. Conservative management of type 2 endoleaks is not associated with increased risk of aneurysm rupture. Eur J Vasc Endovasc Surg 2009; 38: 718-23 [DOI] [PubMed] [Google Scholar]
- Solis MM, Ayerdi J, Babcock GA, et al. Mechanism of failure in the treatment of type II endoleak with percutaneous coil embolization. J Vasc Surg 2002; 36: 485-91 [DOI] [PubMed] [Google Scholar]
- Kasirajan K, Matteson B, Marek JM, et al. Technique and results of transfemoral superselective coil embolization of type II lumbar endoleak. J Vasc Surg 2003; 38: 61-6 [DOI] [PubMed] [Google Scholar]
- Gallagher KA, Ravin RA, Meltzer AJ, et al. Midterm outcomes after treatment of type II endoleaks associated with aneurysm sac expansion. J Endovasc Ther 2012; 19: 182-92 [DOI] [PubMed] [Google Scholar]
- Muthu C, Maani J, Plank LD, et al. Strategies to reduce the rate of type II endoleaks: routine intraoperative embolization of the inferior mesenteric artery and thrombin injection into the aneurysm sac. J Endovasc Ther 2007; 14: 661-8 [DOI] [PubMed] [Google Scholar]
- Rhee SJ, Ohki T, Veith FJ, et al. Current status of management of type II endoleaks after endovascular repair of abdominal aortic aneurysms. Ann Vasc Surg 2003; 17: 335-44 [DOI] [PubMed] [Google Scholar]
- Jones JE, Atkins MD, Brewster DC, et al. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg 2007; 46: 1-8 [DOI] [PubMed] [Google Scholar]