Abstract
Cannabinoid CB2 receptor is a particularly attractive target for noninvasive imaging of neuroinflammation and monitoring of therapeutic efficacy. Its expression is low to undetectable in healthy brain and induced in resident microglial cells (the macrophage of the brain) after cerebral ischemia, injury, and in neuroinflammatory disease. Additionally, immune cells migrating across the blood–brain barrier typically express CB2 receptors, which adds to the expression pool of this target and provides a reliable indicator of inflammation in the brain. Here, we synthesized a novel conjugable CB2 receptor ligand, mbc94, which has a terminal amino group that allows for facile conjugation to imaging moieties. A near-infrared (NIR) dye labeled mbc94, NIRmbc94, was developed for CB2 targeted imaging. Preliminary evidence, including in vitro fluorescence imaging and a competition study, showed that NIRmbc94 specifically labeled CB2-expressing cells.
Two cannabinoid receptors, CB1 and CB2, have been identified at the molecular level (1, 2). They are G protein-coupled receptors that share an overall 44% amino acid homology and a 68% amino acid homology within their transmembrane domains (1). CB1 receptors are abundantly expressed by neurons, whereas CB2 receptors are abundantly expressed by immune cells (3, 4).
The high level of CB2 receptor expression in immune cells and much lower expression in other cell types, particularly in the CNS, makes this receptor an attractive target for imaging and monitoring of therapy (3, 4) for neurological diseases. Specifically, CB2 receptor expression is high in spleen, tonsils, and thymus and low—or even undetectable—in brain, thyroid, retina, placenta, skeletal muscle, kidney, liver, adrenal gland, heart, prostate, and ovary (4). This expression profile provides great opportunities for imaging with low background. Furthermore, CB2 receptor expression is highly plastic and may be induced under specific disease conditions, for example, in tumor cells (5) and CNS-resident microglia. Accordingly, CB2 receptor has become a predominant target for drug development aimed at treating pain (6, 7), chronic inflammation (8), osteoporosis (9), malignant gliomas (10), tumors of immune origin (11), and immunological disorders (12,13), and thus developing a tool that allows for its precise mapping in tissue is essential.
Together with the characterization of the CB2 receptor, a considerable effort has been made to develop CB2 receptor–ligands. The term cannabinoid was first used to describe terpenophenolic compounds in Cannabis sativa L., among which (−)-trans-Δ9-tetrahydrocanabinol (Δ9-THC) is the main bioactive constituent (14). Many anti-inflammatory effects of Δ9-THC have been described, including inhibition of tumor necrosis factor-α, interleukin-2, nitric oxide, and arachidonic acid production from macrophages and T cells (8, 15).
CB2 receptor ligands can be divided into three main groups: plant-derived, endogenous, and synthetic. The best-known plant-derived cannabinoid is Δ9-THC, but cannabinol and cannabidiol also induce profound biological effects (16). Two endocannabinoids, arachidonoylethanolamide (anandamide) and 2-arachidonoyl glycerol (2-AG), have been identified (Figure 1) (17). Both molecules have greater affinity at CB1 than CB2 receptors (18). 2-AG acts as a full agonist at CB1 and CB2 receptor, and anandamide acts as a partial agonist (showing mixed agonist–antagonist properties) toward these receptors (19). Many synthetic cannabinoid receptor ligands have been developed, including HU-210, CP55940, WIN55212–2, SR141617A, AM630, and SR144528 (Figure 2). HU-210, CP55940, and WIN55212–2 are cannabinoid receptor agonists with no or marginal CB1/CB2 selectivity (18). AM630 and SR144528 are both selective CB2 receptor ligands and behave as inverse agonists rather than “silent” or “neutral” antagonists. The CB2/CB1 affinity ratio is less for AM630 (CB2/CB1 affinity = 165) than for SR144528 (CB2/CB1 affinity > 700) (18). Accordingly, SR144528 has been widely used as a pharmacological tool to determine CB2 receptor-mediated effects (18). However, the use of SR144528 for CB2 receptor-targeted imaging has never been tested directly, since SR144528 is not conjugable. In other words, signaling moieties, such as fluorescent dyes, lanthanide chelates, and nanoparticles, cannot be easily coupled to SR144528. Thus, to further study CB2 receptor and diseases associated with an increase in the expression of this receptor, development of a conjugable SR144528 analogue constitutes an essential step. Here, we synthesized such a conjugable SR144528 analogue, mbc94. To our knowledge, this is the only fully conjugable CB2 receptor ligand in existence. It has a terminal amino group allowing easy conjugation to other molecules, including imaging moieties that can provide opportunities for CB2 receptor-targeted imaging. A near-infrared (NIR) dye, IRDye 800CW NHS ester, was selected to label mbc94 for optical imaging. The resulting imaging agent, NIRmbc94, was used to label CB2-expressing cells. Preliminary fluorescence imaging of live cells and competition study showed that indeed NIRmbc94 specifically labeled CB2-expressing cells.
Figure 1.
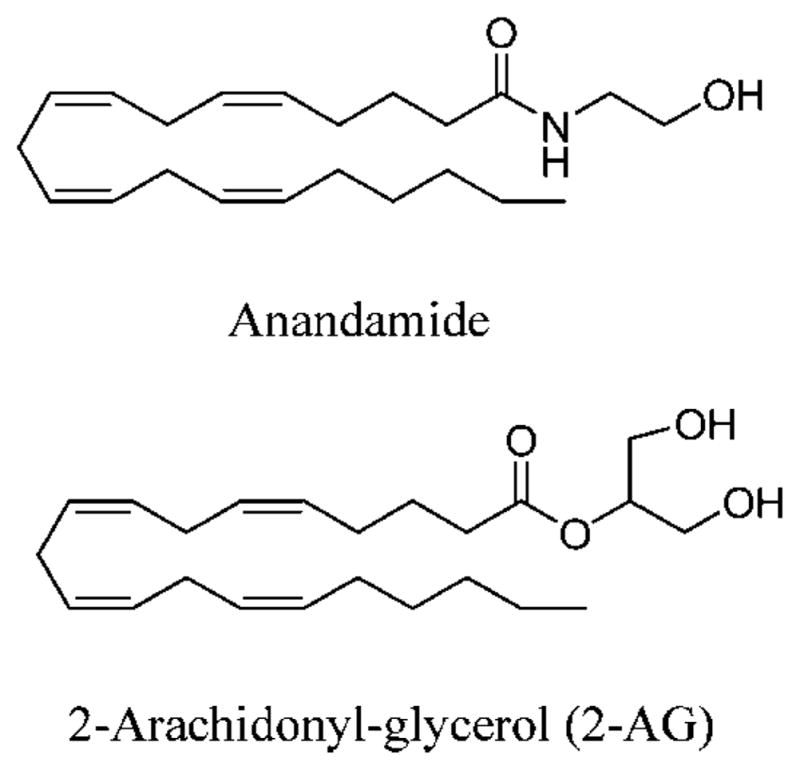
Structures of endogenous CB2 receptor ligands.
Figure 2.
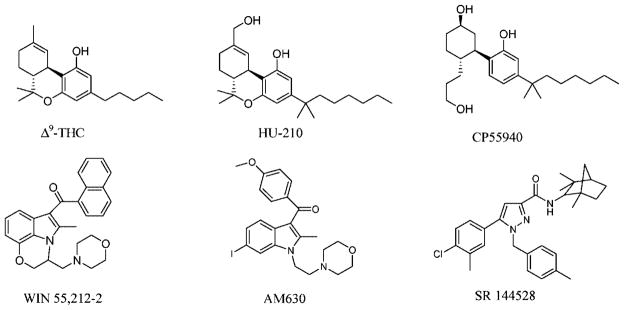
Structures of plant-derived and synthetic CB2 receptor ligands.
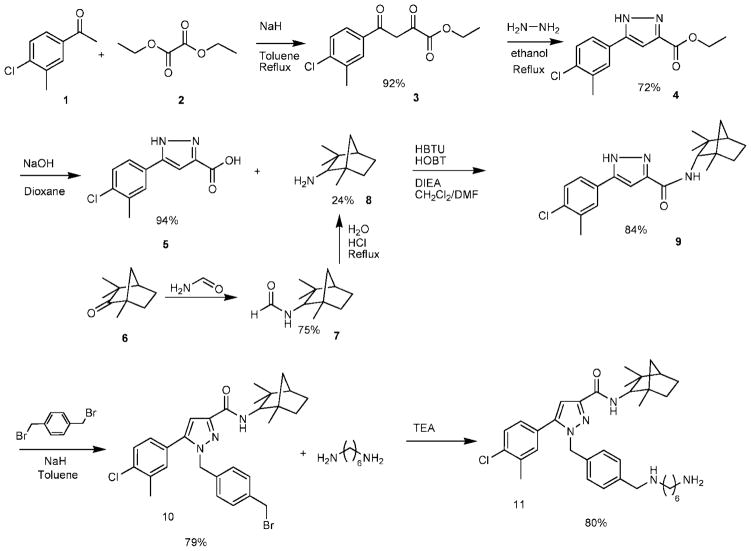
The synthetic pathway of the conjugable SR 144528 analogue, mbc94, is shown in Scheme 1. Compound 3 was prepared from 4′-chloro-3′methylacetophenone and diethyl oxalate as previously described (20). Formation of pyrazoles 4 and 5 was achieved by following literature procedures (21). Fenchylamine 8 was prepared by converting fenchone to a formamide 7, followed by hydrolysis of the amide bond as previously described (22). Compound 9 was prepared using thionyl chloride before, but the yield was relatively low (70%) (21). We synthesized 9 by regular peptide coupling using 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyl uronium hexafluorophosphate (HBTU), and the yield was improved to 84%. The following N-alkylation with α,α-dibromo-p-xylene produced 10, and another N-alkylation with 1,6-hexane diamine yielded the conjugable CB2 receptor ligand, mbc94.
Scheme 1.
Synthesis of mbc94
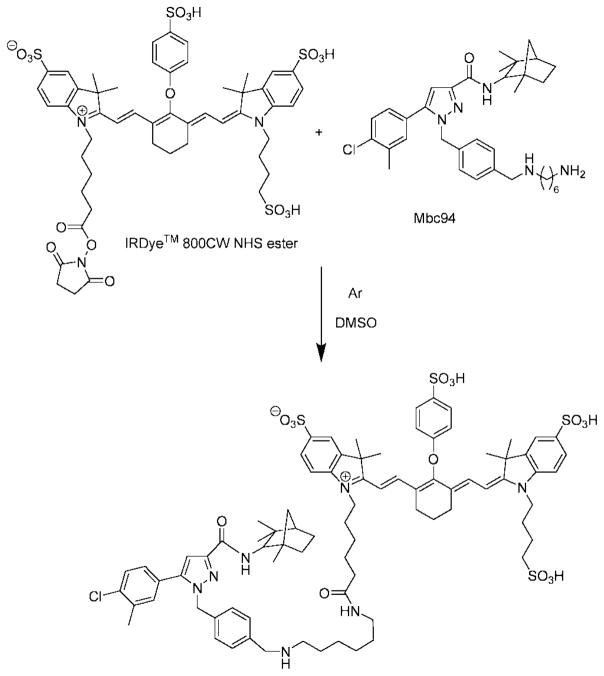
The use of NIR (650–900 nm) light has gained increasingly wide acceptance in molecular imaging during recent years (23) in part because tissues are relatively transparent in this region of the electromagnetic spectrum, with hemoglobin, water, and lipids exhibiting low absorption coefficients (24). Therefore, we labeled mbc94 with a NIR dye, IRDye 800CW NHS ester, for NIR optical imaging. The reaction was monitored by analytical HPLC at 780 nm and the product, IRDye 800CW-mbc94 (NIRmbc94), was purified using a semipreparative HPLC and characterized by NMR and mass spectrometry. The absorption and emission spectra were then taken (Figure 3). NIRmbc94 has maximum absorption at 779 nm and emission at 797 nm (water), allowing deep tissue emission for enhanced in vivo imaging.
Figure 3.
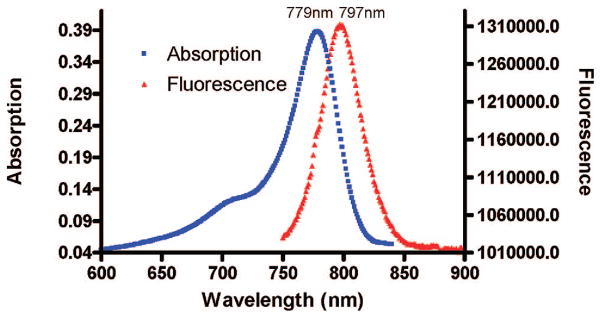
NIRmbc94 absorption and fluorescence in water.
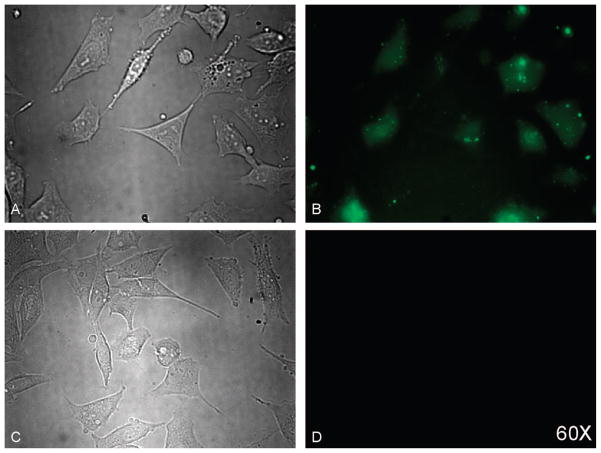
To test whether NIRmbc94 reliably binds to the CB2 receptor, we already had in place a system wherein the receptor was both absent and present. Specifically, the highly malignant mouse astrocytoma cell line, DBT, lacks the targeted receptor (wild-type), and we generated a clone that stably expresses our target, 2D4DBT. First, we demonstrated that NIRmbc94 gave a significant signal (S/N = 1.6), whereas the “free” dye (IRDye 800CW acid) does not produce significant fluorescence, as illustrated by microscopy in the NIR (thus indicating a lack of nonspecific binding due to the dye: Figure 4). Next, as a preliminary indicator of specific targeting of our receptor, both wild-type cells and 2D4 clones were incubated 5 μM concentration of NIRmbc94. Figure 5B,E shows that the fluorescence signal is relatively low in wild-type DBT cells compared to the clone (A,D).
Figure 4.
Fluorescence imaging of DBT cells incubated with NIRmbc94 or free NIR dye: (A) phase contrast microscopy of cells dosed with NIRmbc94; (B) fluorescence imaging of cells dosed with 5 μM NIRmbc94; (C) phase contrast microscopy of cells dosed with free NIR dye; (D) fluorescence imaging of cells dosed with 5 μM free NIR dye (control).
Figure 5.
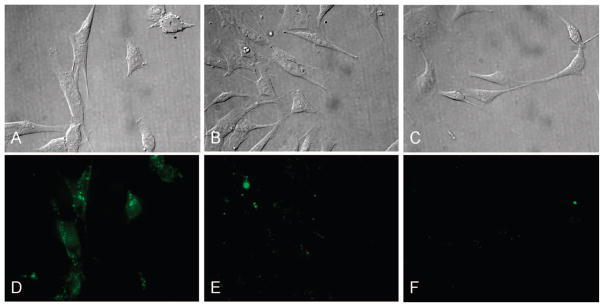
Fluorescence imaging of 2D4 (CB2 expressing) and wild-type (WT, non-CB2 expressing) DBT cells: (A) phase contrast microscopy of 2D4 DBT cells dosed with 5 μM NIRmbc94; (B) phase contrast microscopy of WT DBT cells dosed with 5 μM NIRmbc94; (C) phase contrast microscopy of 2D4 DBT cells dosed with 5 μM NIRmbc94 and 100 nM SR144528; (D) fluorescence imaging of 2D4 DBT cells dosed with 5 μM NIRmbc94; (E) fluorescence imaging of WT DBT cells dosed with 5 μM NIRmbc94; (F) fluorescence imaging of 2D4 DBT cells dosed with 5 μM NIRmbc94 and 100 nM SR144528.
A competition study gave further evidence for specific binding of NIRmbc94 to CB2 receptors. Specifically, in a preliminary competitive binding experiment, the fluorescence signal was significantly reduced when 100 nM SR144528 was added to compete with 5 μM NIRmbc94 at the receptor site. The lack of fluorescence is due to the higher-affinity unlabeled ligand, SR144528 (25), occupying the receptor site and thus inhibiting binding by NIRmbc94. This preliminary pharmacological characterization is typical, and the data indicate that we have indeed labeled our target of interest. More detailed pharmacological and biological characterization, including binding affinity (Kd) and reliable measurement of receptor expression (Bmax) are in order and forthcoming.
In conclusion, we developed a conjugable CB2 receptor ligand, mbc94, which has a terminal amino group, making it universally conjugable. An NIR dye-labeled mbc94, NIRmbc94, specifically labeled CB2-expressing DBT cells, whereas the same cells incubated with same concentration of free NIR dye did not show any significant signal. In addition, the reduced fluorescence signal was observed from non-CB2 expressing wildtype DBT cells incubated with NIRmbc94 compared to CB2-expressing DBT cells incubated with the same concentration of NIRmbc94. Finally, the specific binding of NIRmbc94 to CB2 receptors was confirmed by in vitro competition study. A preliminary competition study in which cells were coincubated with NIRmbc94 and SR144528 showed signal reduction compared to cells incubated with NIRmbc94 only. Overall, mbc94 constitutes a promising conjugable CB2 receptor ligand. NIRmbc94 specifically binds to CB2 receptors and can potentially be used to image CB2-expressing cells in vivo, including immune and cancer cells.
Supplementary Material
Scheme 2.
Synthesis of NIRmbc94
Acknowledgments
We thank Eiron Cudaback at the University of Washington for generating and providing CB2-expressing (2D4 cells) and wild-type DBT cells. We also thank Department of Defense (grant # W81XWH-04-1-0432 to D.B.) and NIDA (grant # DA014486 to N.S.) for providing funding for this work.
Footnotes
Supporting Information Available: Details of experimental section. This material is available free of charge via the Internet at http://pubs.acs.org.
LITERATURE CITED
- 1.Munro S, Thomas KL, Abushaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 3.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 4.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Lefur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Ruiz J, Romero J, Velasco G, Tolon RM, Ramos JA, Guzman M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci. 2007;28:39–45. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Malan TP, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol. 2003;3:62–67. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 7.Malan TP, Ibrahim MM, Deng HF, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 8.Iwamura H, Suzuki H, Ueda Y, Kaya T, Inaba T. In vitro and in vivo pharmacological characterization of JTE907, a novel selective ligand for cannabinoid CB2 receptor. J Pharmacol Exp Ther. 2001;296:420–425. [PubMed] [Google Scholar]
- 9.Karsak M, Ofek O, Fogel M, Wright K, Tam J, Gabet Y, Birenboim R, Attar-Namdar M, Muller R, Cohen-Solal M, de Vernejoul M, Shohami E, Mechoulam R, Zimmer A, Bab I. The cannabinoid CB2 receptor: A potential target for the diagnosis and treatment of osteoporosis. J Bone Miner Res. 2004;19:S383–S383. [Google Scholar]
- 10.Sanchez C, de Ceballos ML, del Pulgar TG, Rueda D, Corbacho C, Velasco G, Galve-Roperh I, Huffman JW, Cajal SRY, Guzman M. Inhibition of glioma growth in vivo by selective activation of the CB2 cannabinoid receptor. Cancer Res. 2001;61:5784–5789. [PubMed] [Google Scholar]
- 11.McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu SH, Grant S, Nagarkatti PS, Nagarkatti M. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood. 2002;100:627–634. doi: 10.1182/blood-2002-01-0098. [DOI] [PubMed] [Google Scholar]
- 12.Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pertwee RG. Cannabinoids and multiple sclerosis. Pharmacol Therapeut. 2002;95:165–174. doi: 10.1016/s0163-7258(02)00255-3. [DOI] [PubMed] [Google Scholar]
- 14.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 15.Witting A, Stella N. Cannabinoid signaling in glial cells in health and disease. Curr Neuropharmacol. 2004;2:115–124. [Google Scholar]
- 16.Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: Evidence for new players. AAPS J. 2006;8:E298–E306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Invest Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- 18.Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins, Leukotrienes Essent Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 19.Oka S, Ikeda S, Kishimoto S, Gokoh M, Yanagimoto S, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J Leukocyte Biol. 2004;76:1002–1009. doi: 10.1189/jlb.0404252. [DOI] [PubMed] [Google Scholar]
- 20.Roy AK, Batra S. Facile Baylis-Hillman reaction of substituted 3-isoxazolecarbaldehydes: The impact of a proximal heteroatom within a heterocycle on the acceleration of the reaction. Synthesis-Stuttgart. 2003:2325–2330. [Google Scholar]
- 21.Seltzman HH, Foster MC, Wyrick CD, Burgess JP, Carroll FI. Tritiation of the cannabinoid receptor antagonist SR144528 involving lithium aluminum tritide reduction; assessment of the kinetic isotope effect by H-3-NMR. J Labelled Compds Radiopharm. 2005;48:589–596. [Google Scholar]
- 22.Suchocki JA, May EL, Martin TJ, George C, Martin BR. Synthesis of 2-exo-mecamylamine and 2-endo-mecamylamine analogs - structure-activity-relationships for nicotinic antagonism in the central-nervous-system. J Med Chem. 1991;34:1003–1010. doi: 10.1021/jm00107a019. [DOI] [PubMed] [Google Scholar]
- 23.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 24.Shah K, Weissleder R. Molecular optical imaging: applications leading to the development of present day therapeutics. Am Soc Exp Neuro Therapeutics. 2005;2:215–225. doi: 10.1602/neurorx.2.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere JC, Le Fur G. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Therapeut. 1998;284:644–650. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.